HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Measurement of FC and CEs
2.3. Statistical Analysis
3. Results
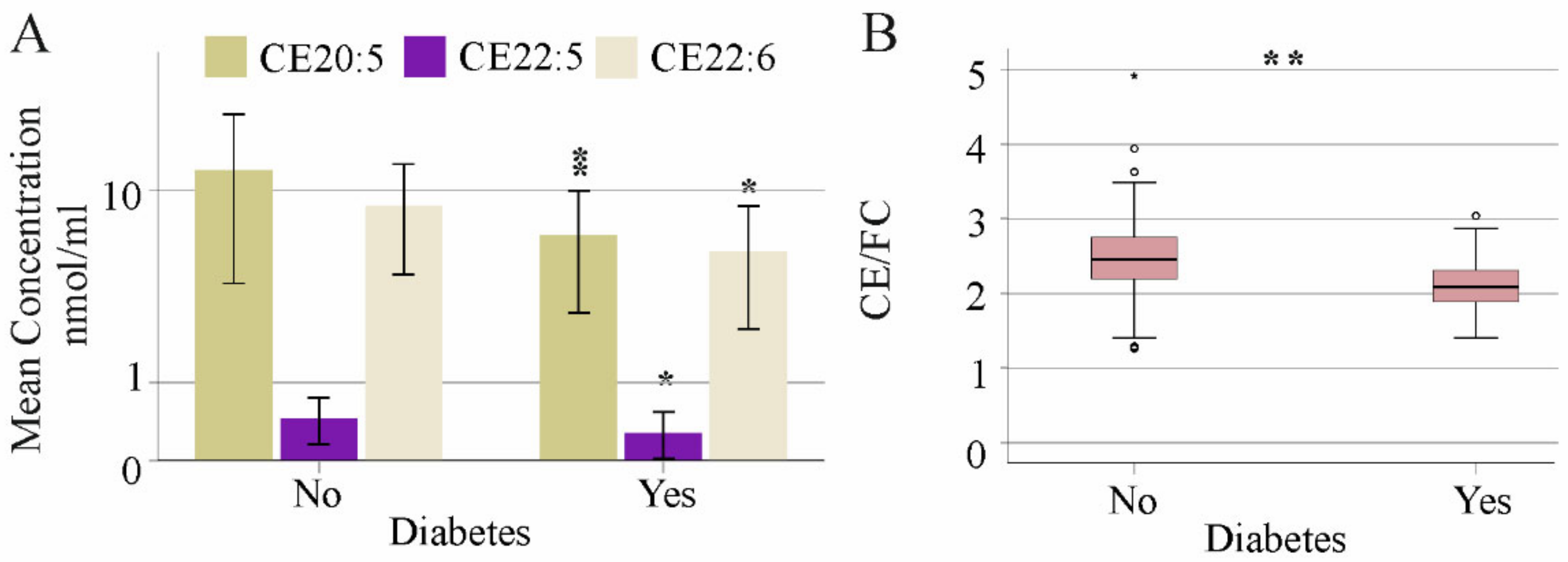
3.1. Association of Age, Gender, Body Mass Index, Liver Steatosis and Diabetes with Serum FC and CE Species
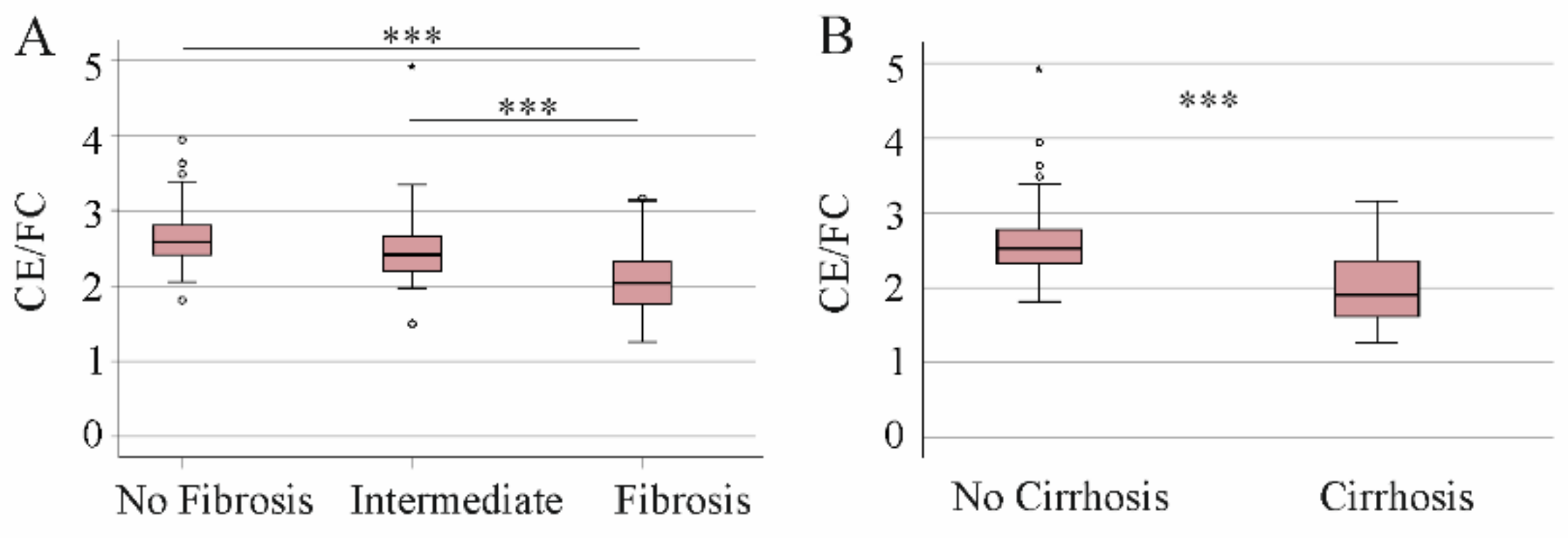
3.2. CE Species Levels and Distribution in Patients with Advanced Liver Disease
3.3. Correlation of CE Species with the MELD Score and Laboratory Measures
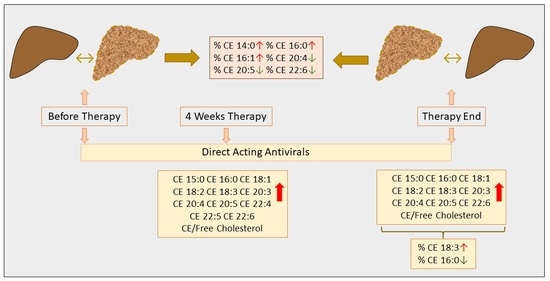
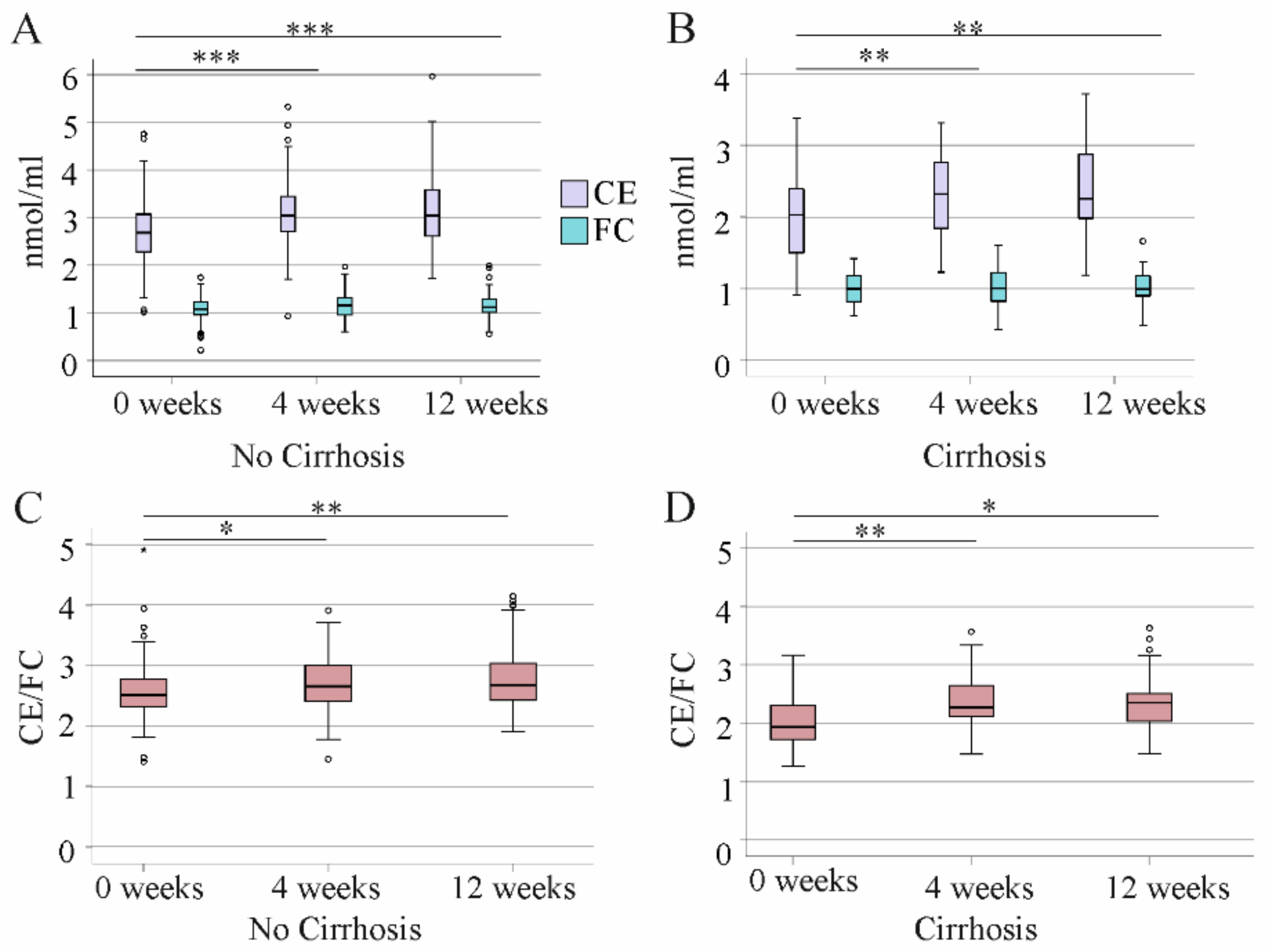
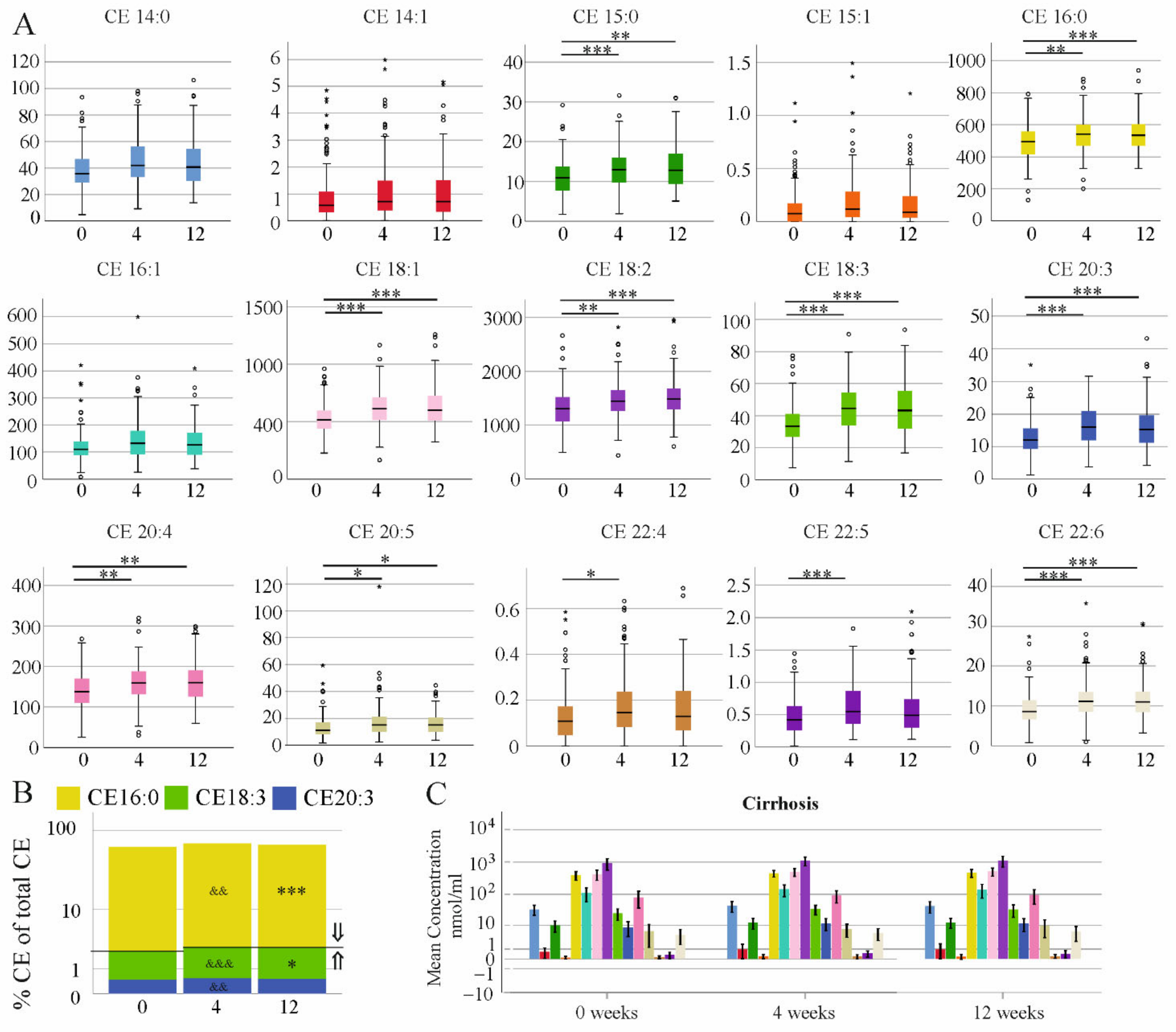
3.4. Effect of DAA Therapy for HCV on Serum CE Species
3.5. Association of CE Species with Non-Invasive Measures of Liver Cirrhosis at Therapy End
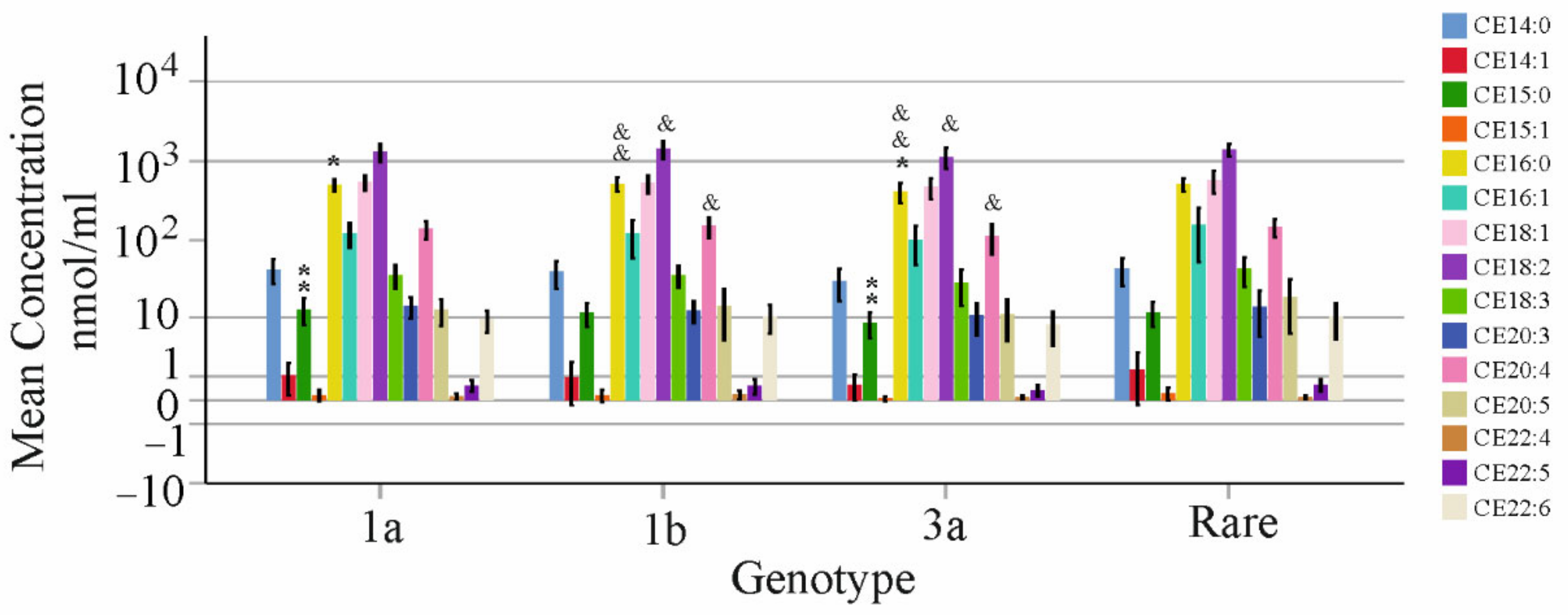
3.6. Association of CE Species with Viral Titer and Genotype
3.7. Correlation of CE Species with HDL and LDL
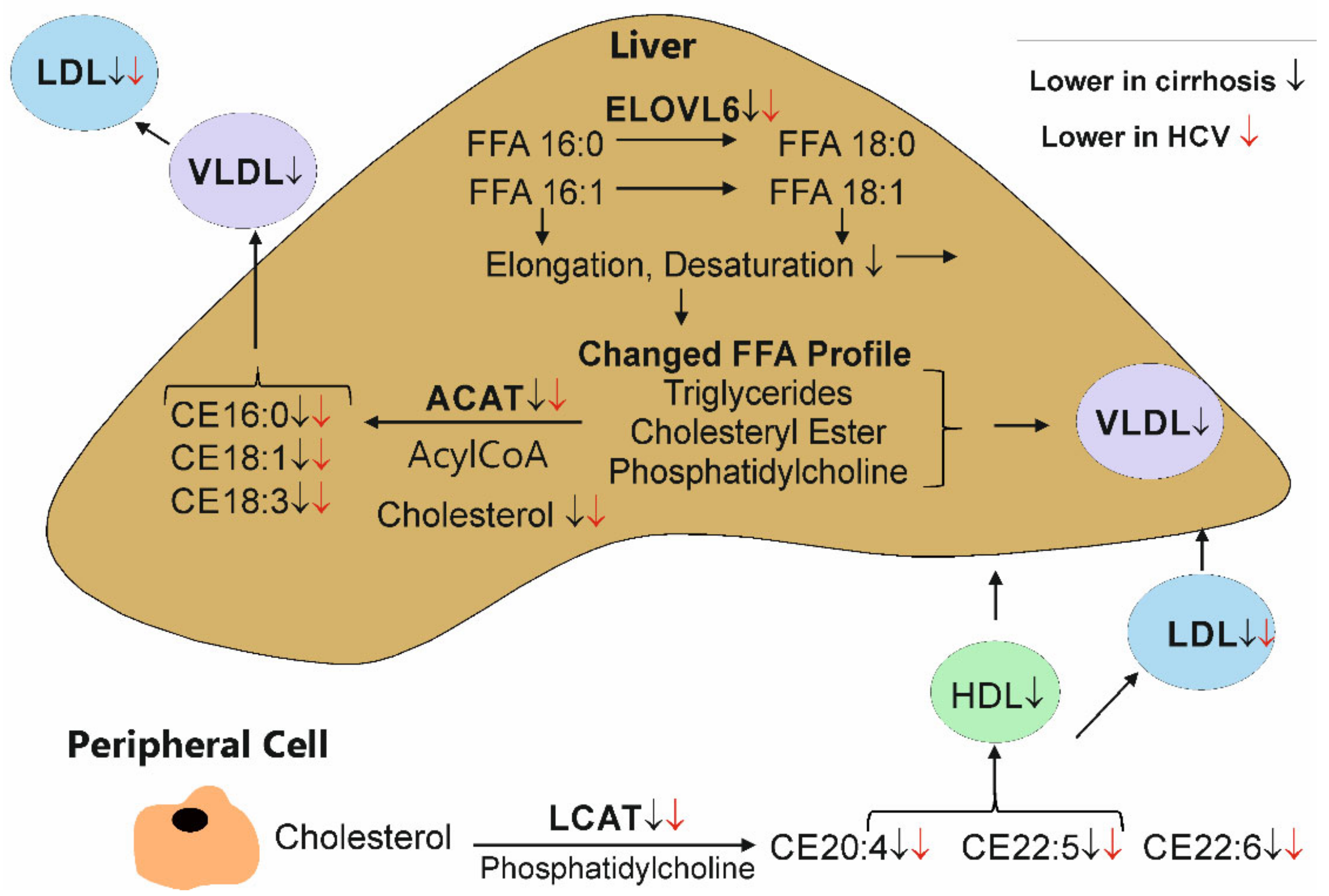
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casiraghi, M.A.; De Paschale, M.; Romano, L.; Biffi, R.; Assi, A.; Binelli, G.; Zanetti, A.R. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Fouda, S.; Sainu, A.; Pappachan, J.M. Metabolic complications of hepatitis C virus infection. World J. Gastroenterol. 2021, 27, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, M.L.; Gunn, N.T. Impact of hepatitis C virus therapy on metabolism and public health. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37 (Suppl. 1), 13–18. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel; EASL Governing Board representative. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.J.; Thurairajah, P.H.; Kumar, R.; Fock, K.M.; Law, N.M.; Chong, S.Y.; Manejero, F.G.; Ang, T.L.; Teo, E.K.; Tan, J. The impact of unrestricted access to direct-acting antiviral among incarcerated hepatitis C virus-infected patients. Clin. Mol. Hepatol. 2021, 27, 474–485. [Google Scholar] [CrossRef]
- Alshuwaykh, O.; Kwo, P.Y. Current and future strategies for the treatment of chronic hepatitis C. Clin. Mol. Hepatol. 2021, 27, 246–256. [Google Scholar] [CrossRef]
- Buechler, C.; Haberl, E.M.; Rein-Fischboeck, L.; Aslanidis, C. Adipokines in Liver Cirrhosis. Int. J. Mol. Sci. 2017, 18, 1392. [Google Scholar] [CrossRef]
- Peschel, G.; Grimm, J.; Gulow, K.; Muller, M.; Buechler, C.; Weigand, K. Chemerin Is a Valuable Biomarker in Patients with HCV Infection and Correlates with Liver Injury. Diagnostics 2020, 10, 974. [Google Scholar] [CrossRef]
- Verna, E.C.; Morelli, G.; Terrault, N.A.; Lok, A.S.; Lim, J.K.; Di Bisceglie, A.M.; Zeuzem, S.; Landis, C.S.; Kwo, P.; Hassan, M.; et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J. Hepatol. 2020, 73, 540–548. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, C.Y.; Su, W.W.; Liu, C.J.; Lo, C.C.; Huang, K.J.; Chen, J.J.; Tseng, K.C.; Chang, C.Y.; Peng, C.Y.; et al. Sofosbuvir/velpatasvir plus ribavirin for Child-Pugh B and Child-Pugh C hepatitis C virus-related cirrhosis. Clin. Mol. Hepatol. 2021, 27, 575–588. [Google Scholar] [CrossRef]
- Sandmann, L.; Schulte, B.; Manns, M.P.; Maasoumy, B. Treatment of Chronic Hepatitis C: Efficacy, Side Effects and Complications. Visc. Med. 2019, 35, 161–170. [Google Scholar] [CrossRef]
- Wiegand, J.; Berg, T. The etiology, diagnosis and prevention of liver cirrhosis: Part 1 of a series on liver cirrhosis. Dtsch. Arztebl. Int. 2013, 110, 85–91. [Google Scholar] [CrossRef]
- Carmona, I.; Cordero, P.; Ampuero, J.; Rojas, A.; Romero-Gomez, M. Role of assessing liver fibrosis in management of chronic hepatitis C virus infection. Clin. Microbiol. Infect. 2016, 22, 839–845. [Google Scholar] [CrossRef]
- Ragazzo, T.G.; Paranagua-Vezozzo, D.; Lima, F.R.; de Campos Mazo, D.F.; Pessoa, M.G.; Oliveira, C.P.; Alves, V.A.F.; Carrilho, F.J. Accuracy of transient elastography-FibroScan(R), acoustic radiation force impulse (ARFI) imaging, the enhanced liver fibrosis (ELF) test, APRI, and the FIB-4 index compared with liver biopsy in patients with chronic hepatitis C. Clinics 2017, 72, 516–525. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Endo, D.; Satoh, K.; Shimada, N.; Hokari, A.; Aizawa, Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J. Gastroenterol. 2017, 23, 2355–2364. [Google Scholar] [CrossRef][Green Version]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef]
- Villani, R.; Di Cosimo, F.; Romano, A.D.; Sangineto, M.; Serviddio, G. Serum lipid profile in HCV patients treated with direct-acting antivirals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 13944. [Google Scholar] [CrossRef]
- Horing, M.; Ejsing, C.S.; Hermansson, M.; Liebisch, G. Quantification of Cholesterol and Cholesteryl Ester by Direct Flow Injection High-Resolution Fourier Transform Mass Spectrometry Utilizing Species-Specific Response Factors. Anal. Chem. 2019, 91, 3459–3466. [Google Scholar] [CrossRef]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef]
- Glomset, J.A. Lecithin: Cholesterol acyltransferase. An exercise in comparative biology. Prog. Biochem. Pharm. 1979, 15, 41–66. [Google Scholar]
- Jonas, A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta 2000, 1529, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Furbee, J.W., Jr.; Francone, O.; Parks, J.S. In vivo contribution of LCAT to apolipoprotein B lipoprotein cholesteryl esters in LDL receptor and apolipoprotein E knockout mice. J. Lipid Res. 2002, 43, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Eriksson, M.; Parini, P. Cholesteryl esters and ACAT. Eur. J. Lipid Sci. Techbnol. 2012, 114, 624–633. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Jiang, X.C.; Belikova, N.A.; Aizezi, B.; Huang, Z.H.; Reardon, C.A. Regulation of plasma cholesterol esterification by sphingomyelin: Effect of physiological variations of plasma sphingomyelin on lecithin-cholesterol acyltransferase activity. Biochim. Biophys. Acta 2012, 1821, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Buhman, K.K.; Accad, M.; Novak, S.; Choi, R.S.; Wong, J.S.; Hamilton, R.L.; Turley, S.; Farese, R.V., Jr. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 2000, 6, 1341–1347. [Google Scholar] [CrossRef]
- Lee, R.G.; Kelley, K.L.; Sawyer, J.K.; Farese, R.V., Jr.; Parks, J.S.; Rudel, L.L. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 2004, 95, 998–1004. [Google Scholar] [CrossRef]
- Erkkila, A.T.; Manninen, S.; Fredrikson, L.; Bhalke, M.; Holopainen, M.; Ruuth, M.; Lankinen, M.; Kakela, R.; Oorni, K.; Schwab, U.S. Lipidomic changes of LDL after consumption of Camelina sativa oil, fatty fish and lean fish in subjects with impaired glucose metabolism-A randomized controlled trial. J. Clin. Lipidol. 2021, 15, 743–751. [Google Scholar] [CrossRef]
- Tahara, D.; Nakanishi, T.; Akazawa, S.; Yamaguchi, Y.; Yamamoto, H.; Akashi, M.; Chikuba, N.; Okuno, S.; Maeda, Y.; Kusumoto, Y.; et al. Lecithin-Cholesterol Acyltransferase and Lipid Transfer Protein Activities in Liver-Disease. Metab-Clin. Exp. 1993, 42, 19–23. [Google Scholar] [CrossRef]
- Read, S.A.; Tay, E.; Shahidi, M.; George, J.; Douglas, M.W. Hepatitis C virus infection mediates cholesteryl ester synthesis to facilitate infectious particle production. J. Gen. Virol. 2014, 95, 1900–1910. [Google Scholar] [CrossRef]
- Lacerda, G.S.; Medeiros, T.; Rosario, N.F.D.; Peralta, R.H.S.; Cabral-Castro, M.J.; Esberard, E.B.C.; Andrade, T.G.; Xavier, A.R.; Silva, A.A. Exploring lipid and apolipoprotein levels in chronic hepatitis C patients according to their response to antiviral treatment. Clin. Biochem. 2018, 60, 17–23. [Google Scholar] [CrossRef]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010, 10, 285–288. [Google Scholar]
- Valkov, I.; Ivanova, R.; Alexiev, A.; Antonov, K.; Mateva, L. Association of Serum Lipids with Hepatic Steatosis, Stage of Liver Fibrosis and Viral Load in Chronic Hepatitis C. J. Clin. Diagn. Res. 2017, 11, OC15–OC20. [Google Scholar] [CrossRef]
- Vere, C.C.; Streba, C.T.; Streba, L.; Rogoveanu, I. Lipid serum profile in patients with viral liver cirrhosis. Med. Princ. Pract. 2012, 21, 566–568. [Google Scholar] [CrossRef]
- Kaiser, T.; Kinny-Koster, B.; Bartels, M.; Berg, T.; Scholz, M.; Engelmann, C.; Seehofer, D.; Becker, S.; Ceglarek, U.; Thiery, J. Cholesterol esterification in plasma as a biomarker for liver function and prediction of mortality. BMC Gastroenterol. 2017, 17, 57. [Google Scholar] [CrossRef]
- Krautbauer, S.; Weiss, T.S.; Wiest, R.; Schacherer, D.; Liebisch, G.; Buechler, C. Diagnostic Value of Systemic Cholesteryl Ester/Free Cholesterol Ratio in Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 3527–3535. [Google Scholar] [CrossRef]
- Tabas, I. Free cholesterol-induced cytotoxicity a possible contributing factor to macrophage foam cell necrosis in advanced atherosclerotic lesions. Trends Cardiovasc. Med. 1997, 7, 256–263. [Google Scholar] [CrossRef]
- Meikle, P.J.; Mundra, P.A.; Wong, G.; Rahman, K.; Huynh, K.; Barlow, C.K.; Duly, A.M.; Haber, P.S.; Whitfield, J.B.; Seth, D. Circulating Lipids Are Associated with Alcoholic Liver Cirrhosis and Represent Potential Biomarkers for Risk Assessment. PLoS ONE 2015, 10, e0130346. [Google Scholar] [CrossRef]
- Chen, W.X.; Li, J.Z. Correlation of serum cholesteryl ester fatty acid composition with susceptibility to atherosclerosis in different species. Chin. Med. J. (Engl.) 1993, 106, 163–166. [Google Scholar]
- Werner, R.M.; Pearson, T.A. LDL-cholesterol: A risk factor for coronary artery disease--from epidemiology to clinical trials. Can. J. Cardiol. 1998, 14 (Suppl. B), 3B–10B. [Google Scholar]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Peschel, G.; Grimm, J.; Buechler, C.; Gunckel, M.; Pollinger, K.; Aschenbrenner, E.; Kammerer, S.; Jung, E.M.; Haimerl, M.; Werner, J.; et al. Liver stiffness assessed by shear-wave elastography declines in parallel with immunoregulatory proteins in patients with chronic HCV infection during DAA therapy. Clin. Hemorheol. Microcirc. 2021, 79, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Hui, V.W.; Au, C.L.; Lam, A.S.M.; Yip, T.C.; Tse, Y.K.; Lai, J.C.; Chan, H.L.; Wong, V.W.; Wong, G.L. Drug-drug interactions between direct-acting antivirals and co-medications: A territory-wide cohort study. Hepatol. Int. 2022, 16, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Kuo, F.Y.; Chen, C.H.; Hu, T.H.; Lu, S.N.; Wang, J.H.; Hung, C.H. Ultrasound is highly specific in diagnosing compensated cirrhosis in chronic hepatitis C patients in real world clinical practice. Medicine 2019, 98, e16270. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef]
- Horing, M.; Peschel, G.; Grimm, J.; Krautbauer, S.; Muller, M.; Weigand, K.; Liebisch, G.; Buechler, C. Serum Ceramide Species Are Associated with Liver Cirrhosis and Viral Genotype in Patients with Hepatitis C Infection. Int. J. Mol. Sci. 2022, 23, 9806. [Google Scholar] [CrossRef]
- Andrade, V.G.; Yamashiro, F.D.S.; Oliveira, C.V.; Kurozawa, L.L.; Moreira, A.; Silva, G.F. Increase of Lipids during Hcv Treatment: Virus Action or Medication? Arq. De Gastroenterol. 2018, 55, 184–187. [Google Scholar] [CrossRef]
- Doyle, M.A.; Galanakis, C.; Mulvihill, E.; Crawley, A.; Cooper, C.L. Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters. Cells 2019, 8, 252. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Taura, N.; Motoyoshi, Y.; Akahoshi, H.; Nakamura, J.; Takahashi, Y.; Honda, T.; Yajima, H.; et al. Changes in serum LDL, PCSK9 and microRNA-122 in patients with chronic HCV infection receiving Daclatasvir/Asunaprevir. Biomed. Rep. 2019, 10, 156–164. [Google Scholar] [CrossRef]
- Inoue, T.; Goto, T.; Iio, E.; Matsunami, K.; Fujiwara, K.; Shinkai, N.; Matsuura, K.; Matsui, T.; Nojiri, S.; Tanaka, Y. Changes in serum lipid profiles caused by three regimens of interferon-free direct-acting antivirals for patients infected with hepatitis C virus. Hepatol. Res. 2018, 48, E203–E212. [Google Scholar] [CrossRef]
- Jones, D.P.; Sosa, F.R.; Shartsis, J.; Shah, P.T.; Skromak, E.; Beher, W.T. Serum cholesterol esterifying and cholesteryl ester hydrolyzing activities in liver diseases: Relationships to cholesterol, bilirubin, and bile salt concentrations. J. Clin. Investig. 1971, 50, 259–265. [Google Scholar] [CrossRef][Green Version]
- Mazzaro, C.; Quartuccio, L.; Adinolfi, L.E.; Roccatello, D.; Pozzato, G.; Nevola, R.; Tonizzo, M.; Gitto, S.; Andreone, P.; Gattei, V. A Review on Extrahepatic Manifestations of Chronic Hepatitis C Virus Infection and the Impact of Direct-Acting Antiviral Therapy. Viruses 2021, 13, 2249. [Google Scholar] [CrossRef]
- Sheridan, D.A.; Shawa, I.T.; Thomas, E.L.; Felmlee, D.J.; Bridge, S.H.; Neely, D.; Cobbold, J.F.; Holmes, E.; Bassendine, M.F.; Taylor-Robinson, S.D. Infection with the hepatitis C virus causes viral genotype-specific differences in cholesterol metabolism and hepatic steatosis. Sci. Rep. 2022, 12, 5562. [Google Scholar] [CrossRef]
- Van der Velde, A.E. Reverse cholesterol transport: From classical view to new insights. World J. Gastroenterol. 2010, 16, 5908–5915. [Google Scholar] [CrossRef]
- Cansancao, K.; Silva Monteiro, L.; Carvalho Leite, N.; Davalos, A.; Tavares do Carmo, M.D.G.; Arantes Ferreira Peres, W. Advanced Liver Fibrosis Is Independently Associated with Palmitic Acid and Insulin Levels in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 1586. [Google Scholar] [CrossRef]
- Arranz, M.I.; Lasuncion, M.A.; Perales, J.; Herrera, E.; Lorenzo, I.; Carcamo, C.; Concostrina, L.; Villar, J.; Gasalla, R. Fatty acid composition of lipoprotein lipids in hepatobiliary diseases. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 701–709. [Google Scholar] [CrossRef][Green Version]
- Biagi, P.; Hrelia, S.; Stefanini, G.; Zunarelli, P.; Bordoni, A. Delta-6-desaturase activity of human liver microsomes from patients with different types of liver injury. Prostaglandins Leukot. Essent. Fat. Acids 1990, 39, 39–42. [Google Scholar] [CrossRef]
- Kawakami, M.; Koda, M.; Murawaki, Y. The prevalence of atherosclerosis in cirrhotic patients: Assessment of two aspects of atherosis and sclerosis. Hepatol. Res. 2007, 37, 152–157. [Google Scholar] [CrossRef]
- Boursier, J.; Anty, R.; Carette, C.; Cariou, B.; Castera, L.; Caussy, C.; Fontaine, H.; Garioud, A.; Gourdy, P.; Guerci, B.; et al. Management of diabetes mellitus in patients with cirrhosis: An overview and joint statement. Diabetes Metab. 2021, 47, 101272. [Google Scholar] [CrossRef]
- Van Woudenbergh, G.J.; Kuijsten, A.; Van der Kallen, C.J.; Van Greevenbroek, M.M.; Stehouwer, C.D.; Blaak, E.E.; Feskens, E.J. Comparison of fatty acid proportions in serum cholesteryl esters among people with different glucose tolerance status: The CoDAM study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 133–140. [Google Scholar] [CrossRef]
- Johnston, L.W.; Harris, S.B.; Retnakaran, R.; Zinman, B.; Giacca, A.; Liu, Z.; Bazinet, R.P.; Hanley, A.J. Longitudinal Associations of Phospholipid and Cholesteryl Ester Fatty Acids With Disorders Underlying Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Noordam, R.; Kooijman, S.; Jansen, S.W.M.; Akintola, A.A.; Slagboom, P.E.; Pijl, H.; Rensen, P.C.N.; Biermasz, N.R.; van Heemst, D. Familial longevity is characterized by high circadian rhythmicity of serum cholesterol in healthy elderly individuals. Aging Cell 2017, 16, 237–243. [Google Scholar] [CrossRef] [PubMed]



| CE | MELD | INR | Albumin | ALT | AST | Creatinine | Bilirubin | CRP | Leukocytes |
|---|---|---|---|---|---|---|---|---|---|
| 14:0 | −0.082 | −0.066 | −0.113 | −0.037 | 0.024 | 0.040 | 0.008 | −0.061 | −0.115 |
| 14:1 | −0.080 | −0.076 | −0.134 | −0.013 | 0.022 | 0.021 | −0.058 | 0.030 | −0.014 |
| 15:0 | −0.108 | −0.114 | −0.008 | −0.124 | −0.110 | −0.030 | 0.034 | 0.024 | −0.180 |
| 15:1 | −0.093 | −0.048 | −0.197 | −0.166 | −0.134 | −0.006 | 0.089 | 0.068 | −0.114 |
| 16:0 | −0.212 | −0.254 | 0.048 | −0.095 | −0.087 | 0.116 | 0.083 | 0.076 | −0.079 |
| 16:1 | −0.123 | −0.088 | −0.099 | −0.070 | 0.056 | −0.042 | 0.003 | 0.166 | 0.088 |
| 18:1 | −0.198 | −0.220 | 0.011 | −0.159 | −0.119 | 0.036 | 0.054 | 0.087 | 0.101 |
| 18:2 | −0.231 | −0.282 * | 0.149 | −0.121 | −0.172 | 0.027 | 0.105 | 0.105 | −0.176 |
| 18:3 | −0.243 | −0.243 | −0.117 | −0.193 | −0.200 | 0.098 | −0.026 | 0.148 | −0.021 |
| 20:3 | −0.121 | −0.154 | −0.051 | −0.006 | −0.006 | 0.031 | 0.017 | −0.012 | 0.025 |
| 20:4 | 0.176 | −0.255 | −0.015 | −0.102 | −0.164 | 0.117 | 0.106 | 0.175 | −0.049 |
| 20:5 | −0.222 | −0.214 | −0.063 | −0.146 | −0.186 | 0.073 | 0.071 | 0.261 | 0.008 |
| 22:4 | −0.061 | −0.108 | −0.021 | −0.071 | −0.144 | 0.108 | 0.161 | 0.063 | −0.147 |
| 22:5 | 0.025 | 0.022 | −0.198 | 0.019 | −0.026 | 0.004 | 0.161 | 0.026 | 0.010 |
| 22:6 | −0.120 | −0.149 | 0.054 | −0.004 | −0.029 | 0.062 | 0.116 | 0.200 | −0.133 |
| CE | MELD | INR | Albumin | ALT | AST | Creatinine | Bilirubin | CRP | Leukocytes |
|---|---|---|---|---|---|---|---|---|---|
| 14:0 | −0.225 | −0.332 | 0.276 | 0.100 | 0.005 | −0.347 | −0.128 | −0.023 | 0.221 |
| 14:1 | −0.270 | −0.350 | 0.209 | 0.179 | 0.011 | −0.201 | −0.112 | 0.041 | 0.425 |
| 15:0 | −0.261 | −0.362 | 0.377 | 0.176 | −0.044 | −0.182 | −0.125 | 0.146 | 0.349 |
| 15:1 | −0.175 | −0.231 | 0.075 | 0.199 | 0.067 | −0.161 | −0.015 | 0.042 | 0.319 |
| 16:0 | −0.494 | −0.530 | 0.639 ** | 0.010 | −0.268 | −0.313 | 0.424 | −0.130 | 0.389 |
| 16:1 | −0.172 | −0.235 | 0.048 | −0.011 | 0.066 | −0.065 | 0.017 | 0.095 | 0.483 |
| 18:1 | −0.484 | −0.524 | 0.510 | 0.084 | −0.139 | −0.235 | −0.377 | −0.158 | 0.400 |
| 18:2 | −0.593 * | −0.573 * | 0.650 ** | 0.105 | −0.252 | −0.315 | −0.578 * | −0.189 | 0.419 |
| 18:3 | 0.434 | −0.507 | 0.337 | 0.108 | −0.046 | −0.223 | −0.251 | −0.117 | 0.468 |
| 20:3 | −0.616 ** | −0.604 * | 0.482 | 0.253 | −0.030 | −0.237 | −0.476 | −0.073 | 0.521 |
| 20:4 | −0.739 *** | −0.733 *** | 0.744 *** | 0.130 | −0.226 | −0.258 | −0.610 ** | −0.162 | 0.524 |
| 20:5 | −0.468 | 0.519 | 0.519 | 0.031 | −0.271 | −0.262 | −0.375 | 0.029 | 0.372 |
| 22:4 | −0.310 | −0.289 | 0.237 | 0.061 | 0.098 | −0.058 | −0.132 | 0.060 | 0.391 |
| 22:5 | −0.327 | −0.331 | 0.388 | 0.102 | −0.043 | −0.218 | −0.123 | 0.085 | 0.312 |
| 22:6 | −0.702 *** | −0.708 *** | 0.693 ** | 0.197 | −0.150 | −0.270 | −0.574 ** | −0.118 | 0.472 |
| No Fibrosis | Fibrosis | ||||||
|---|---|---|---|---|---|---|---|
| CE nmol/mL | Median | Minimum | Maximum | Median | Minimum | Maximum | p-Value |
| 16:0 | 538.29 | 349.67 | 873.71 | 398.63 | 280.04 | 697.76 | ** |
| 18:1 | 591.08 | 381.86 | 1258.74 | 469.63 | 272.86 | 760.45 | *** |
| 18:2 | 1496.90 | 845.63 | 2926.86 | 856.50 | 312.47 | 1911.60 | *** |
| 18:3 | 45.55 | 16.66 | 93.63 | 28.69 | 11.51 | 49.09 | *** |
| 20:3 | 15.27 | 4.24 | 43.19 | 8.65 | 2.64 | 15.20 | *** |
| 20:4 | 159.33 | 77.53 | 296.05 | 77.58 | 27.60 | 180.98 | *** |
| 20:5 | 14.57 | 3.65 | 44.58 | 6.20 | 1.41 | 39.61 | ** |
| 22:6 | 10.96 | 3.77 | 30.68 | 4.59 | 0.92 | 18.33 | *** |
| No Cirrhosis | Cirrhosis | ||||||
|---|---|---|---|---|---|---|---|
| CE nmol/mL | Median | Minimum | Maximum | Median | Minimum | Maximum | p-Value |
| 16:0 | 536.44 | 328.26 | 939.53 | 427.61 | 280.04 | 697.76 | * |
| 18:1 | 600.85 | 323.54 | 1258.74 | 494.44 | 272.86 | 750.15 | ** |
| 18:2 | 1480.27 | 603.00 | 2955.23 | 1045.26 | 312.47 | 1911.60 | *** |
| 18:3 | 44.10 | 16.66 | 93.63 | 30.26 | 11.51 | 69.24 | ** |
| 20:3 | 15.15 | 4.24 | 43.19 | 10.32 | 2.64 | 21.97 | ** |
| 20:4 | 159.91 | 60.10 | 298.83 | 95.27 | 27.60 | 180.98 | *** |
| 20:5 | 14.99 | 3.65 | 44.58 | 7.26 | 1.41 | 19.71 | *** |
| 22:6 | 11.03 | 3.69 | 30.68 | 5.18 | 0.92 | 13.16 | *** |
| Before Therapy | Therapy End | |||||||
|---|---|---|---|---|---|---|---|---|
| No Cirrhosis | Cirrhosis | No Cirrhosis | Cirrhosis | |||||
| CE | LDL | HDL | LDL | HDL | LDL | HDL | LDL | HDL |
| 14:0 | 0.514 *** | 0.149 | 0.439 | 0.554 | 0.559 *** | 0.157 | 0.440 | 0.651 ** |
| 14:1 | 0.449 *** | 0.013 | 0.393 | 0.336 | 0.306 *** | 0.075 | 0.429 | 0.501 |
| 15:0 | 0.523 *** | 0.263 | 0.642 ** | 0.626 ** | 0.537 *** | 0.247 | 0.544 | 0.539 |
| 15:1 | 0.408 *** | 0.234 | 0.310 | 0.345 | 0.360 ** | 0.213 | 0.427 | 0.389 |
| 16:0 | 0.752 *** | 0.246 | 0.862 *** | 0.554 | 0.878 ** | 0.119 | 0.799 *** | 0.414 |
| 16:1 | 0.464 *** | 0.157 | 0.415 | 0.205 | 0.447 *** | 0.193 | 0.438 | 0.539 |
| 18:1 | 0.585 *** | 0.184 | 0.821 *** | 0.510 | 0.707 *** | 0.169 | 0.773 *** | 0.433 |
| 18:2 | 0.745 *** | 0.348 ** | 0.884 *** | 0.563 * | 0.791 *** | 0.229 | 0.838 *** | 0.38 |
| 18:3 | 0.597 *** | 0.220 | 0.684 ** | 0.328 | 0.562 *** | 0.149 | 0.639 ** | 0.465 |
| 20:3 | 0.593 *** | 0.047 | 0.723 *** | 0.485 | 0.679 *** | 0.038 | 0.727 *** | 0.483 |
| 20:4 | 0.616 *** | 0.208 | 0.820 *** | 0.506 | 0.576 *** | −0.016 | 0.701 ** | 0.401 |
| 20:5 | 0.498 *** | 0.250 | 0.684 ** | 0.266 | 0.388 *** | 0.160 | 0.589 * | 0.244 |
| 22:4 | 0.244 | 0.390 *** | 0.415 | 0.574 * | 0.292 ** | −0.043 | 0.290 | 0.086 |
| 22:5 | 0.306 ** | 0.159 | 0.237 | 0.631 ** | 0.288 ** | 0.141 | 0.248 | 0.553 * |
| 22:6 | 0.543 *** | 0.147 | 0.825 *** | 0.464 | 0.473 *** | 0.088 | 0.712 *** | 0.247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigand, K.; Peschel, G.; Grimm, J.; Müller, M.; Höring, M.; Krautbauer, S.; Liebisch, G.; Buechler, C. HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV. Biomedicines 2022, 10, 3152. https://doi.org/10.3390/biomedicines10123152
Weigand K, Peschel G, Grimm J, Müller M, Höring M, Krautbauer S, Liebisch G, Buechler C. HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV. Biomedicines. 2022; 10(12):3152. https://doi.org/10.3390/biomedicines10123152
Chicago/Turabian StyleWeigand, Kilian, Georg Peschel, Jonathan Grimm, Martina Müller, Marcus Höring, Sabrina Krautbauer, Gerhard Liebisch, and Christa Buechler. 2022. "HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV" Biomedicines 10, no. 12: 3152. https://doi.org/10.3390/biomedicines10123152
APA StyleWeigand, K., Peschel, G., Grimm, J., Müller, M., Höring, M., Krautbauer, S., Liebisch, G., & Buechler, C. (2022). HCV Infection and Liver Cirrhosis Are Associated with a Less-Favorable Serum Cholesteryl Ester Profile Which Improves through the Successful Treatment of HCV. Biomedicines, 10(12), 3152. https://doi.org/10.3390/biomedicines10123152