The Classification of Synthetic- and Petroleum-Based Hydrocarbon Fluids Using Handheld Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydraulic Fluid Samples
2.2. Use of Handheld Raman Spectroscopy
2.3. Pre-Processing and Chemometric Analysis
3. Results and Discussion
3.1. Pre-Processed Raman
3.1.1. Spectra Peak Characteristics
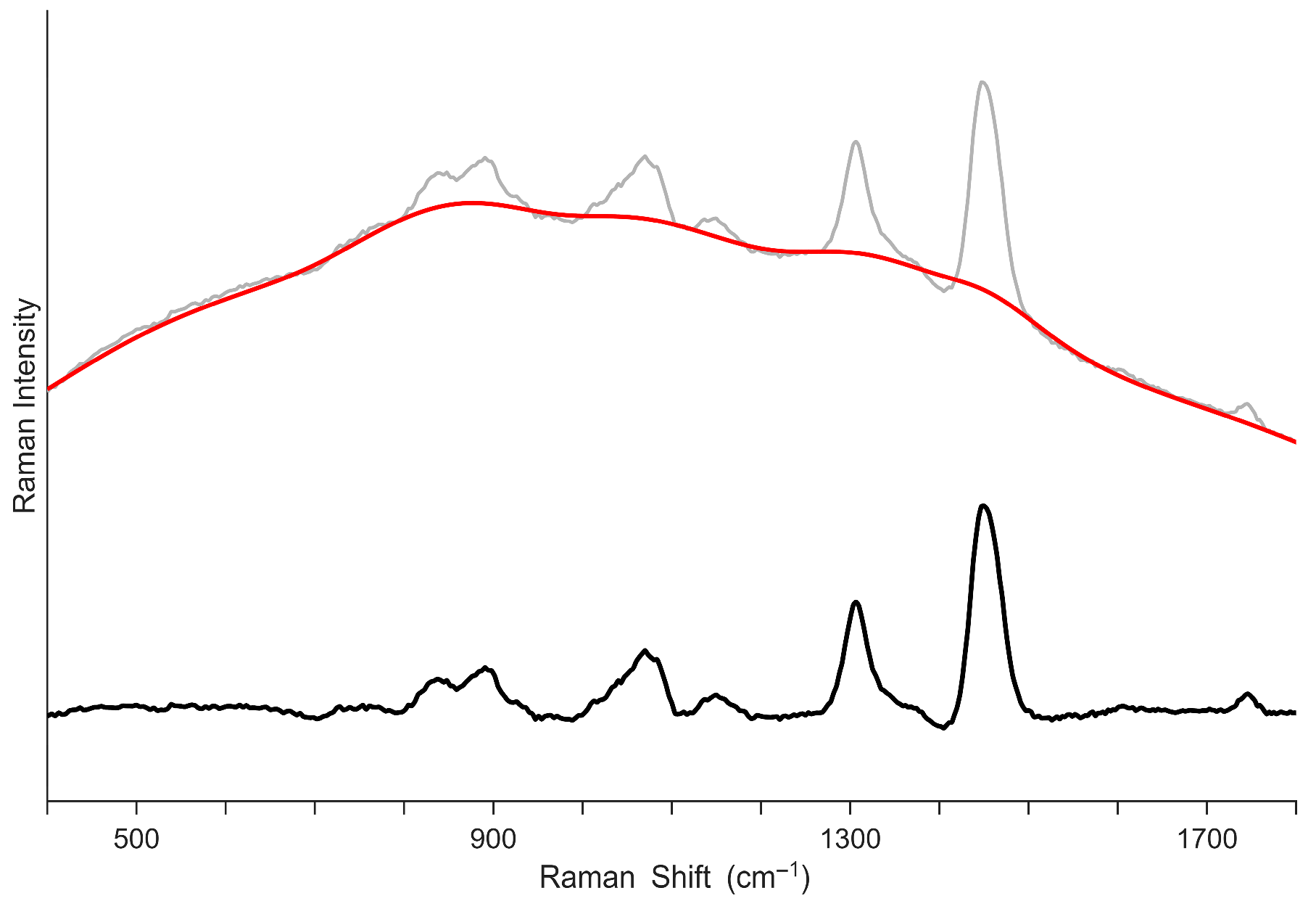
3.1.2. Baseline Intensities
3.1.3. Pre-Processing Approach
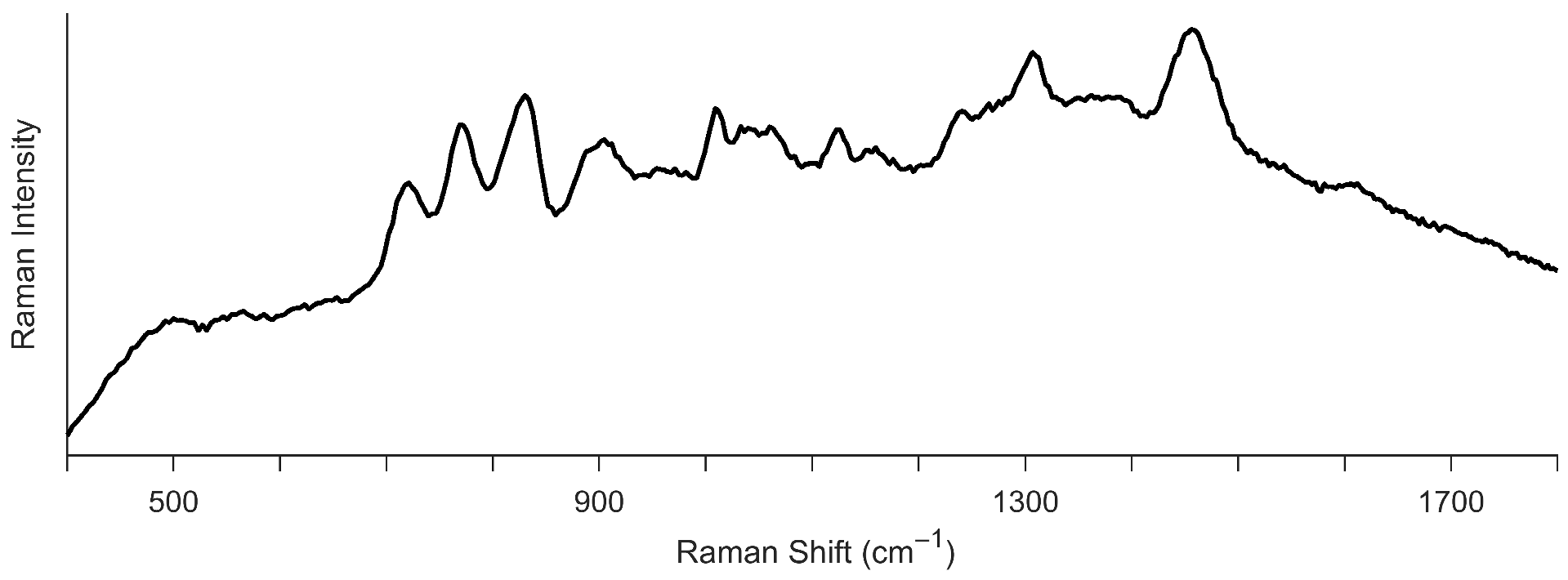
3.1.4. Phosphate Ester-Based Hydraulic Fluid
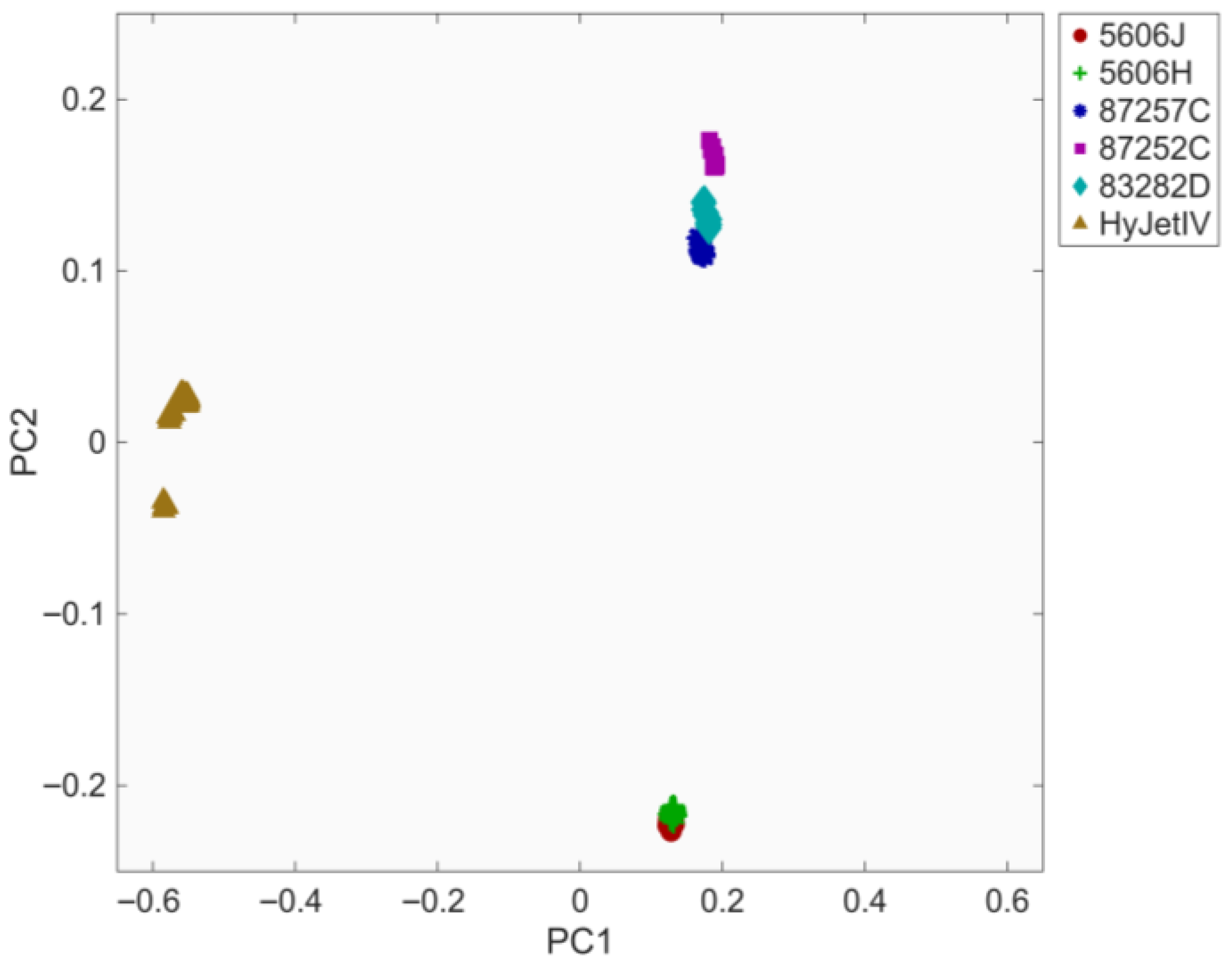
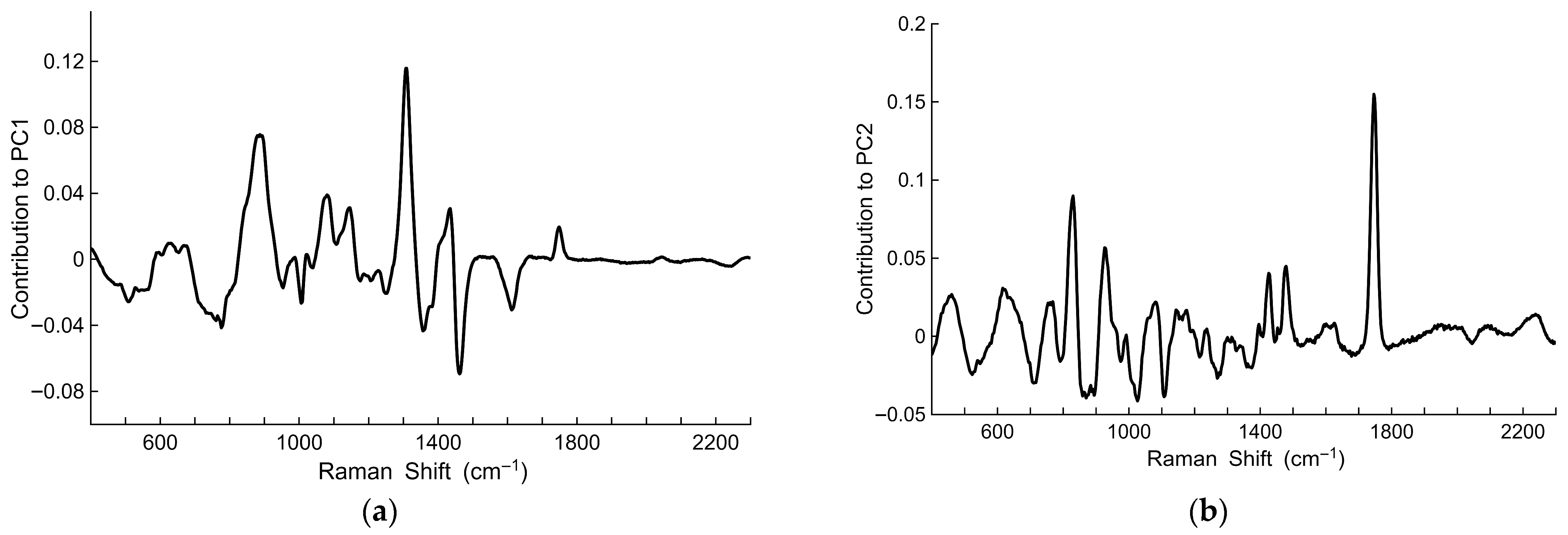
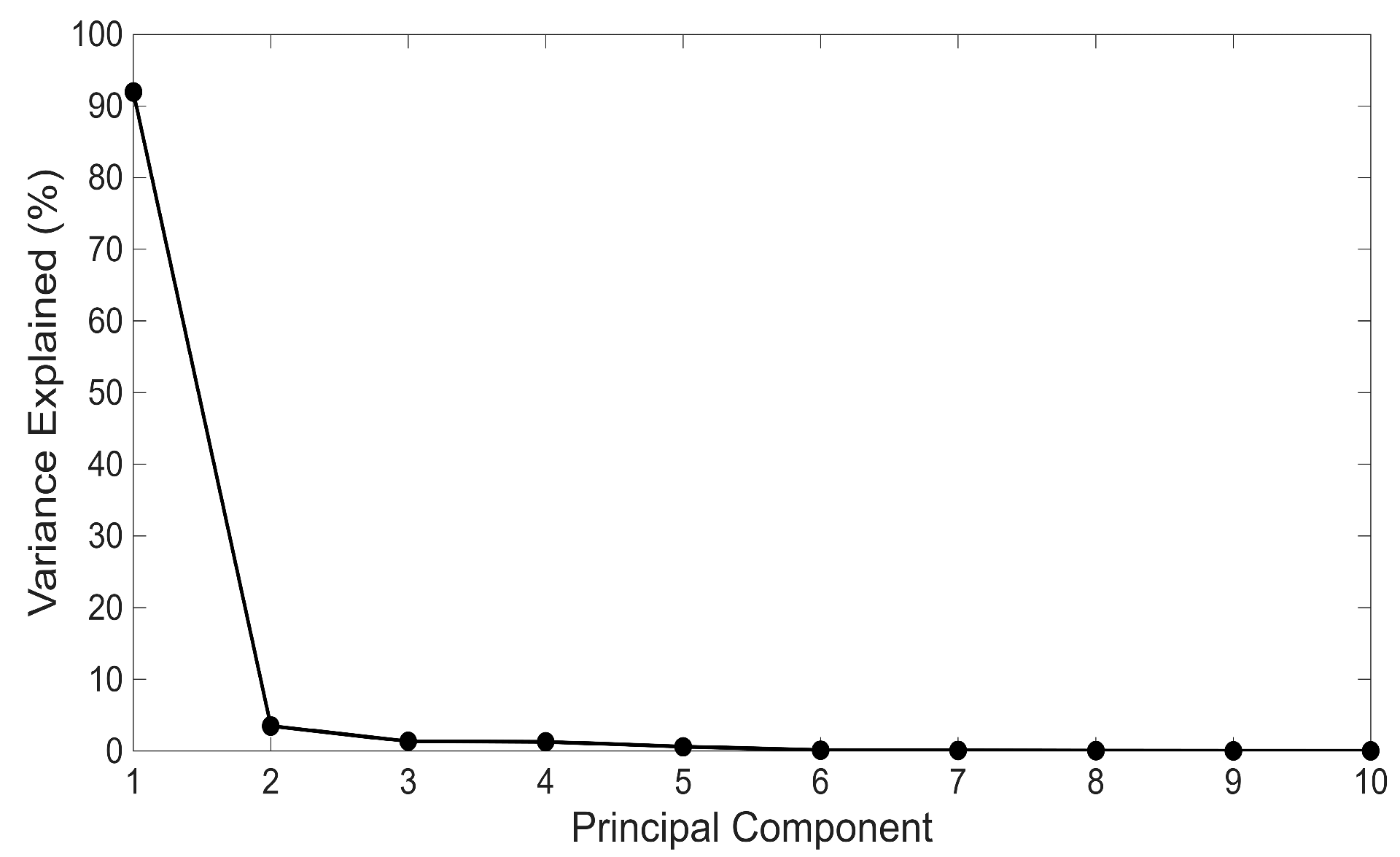
3.2. Principal Component Analysis of Processed Spectra
3.2.1. Principal Component Analysis with a Phosphate Ester-Based Hydraulic Fluid
3.2.2. Principal Component Analysis Without a Phosphate Ester-Based Hydraulic Fluid
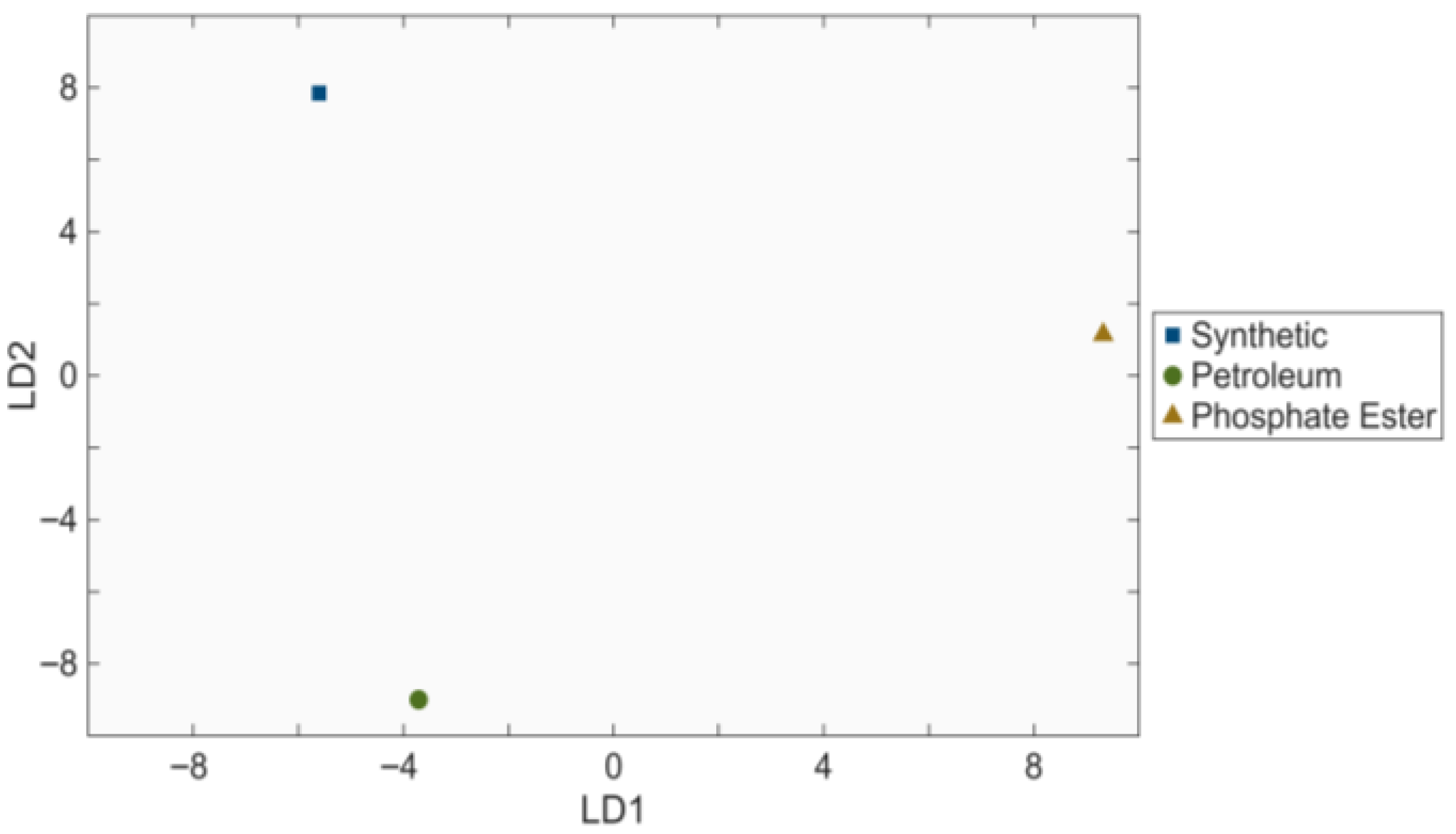
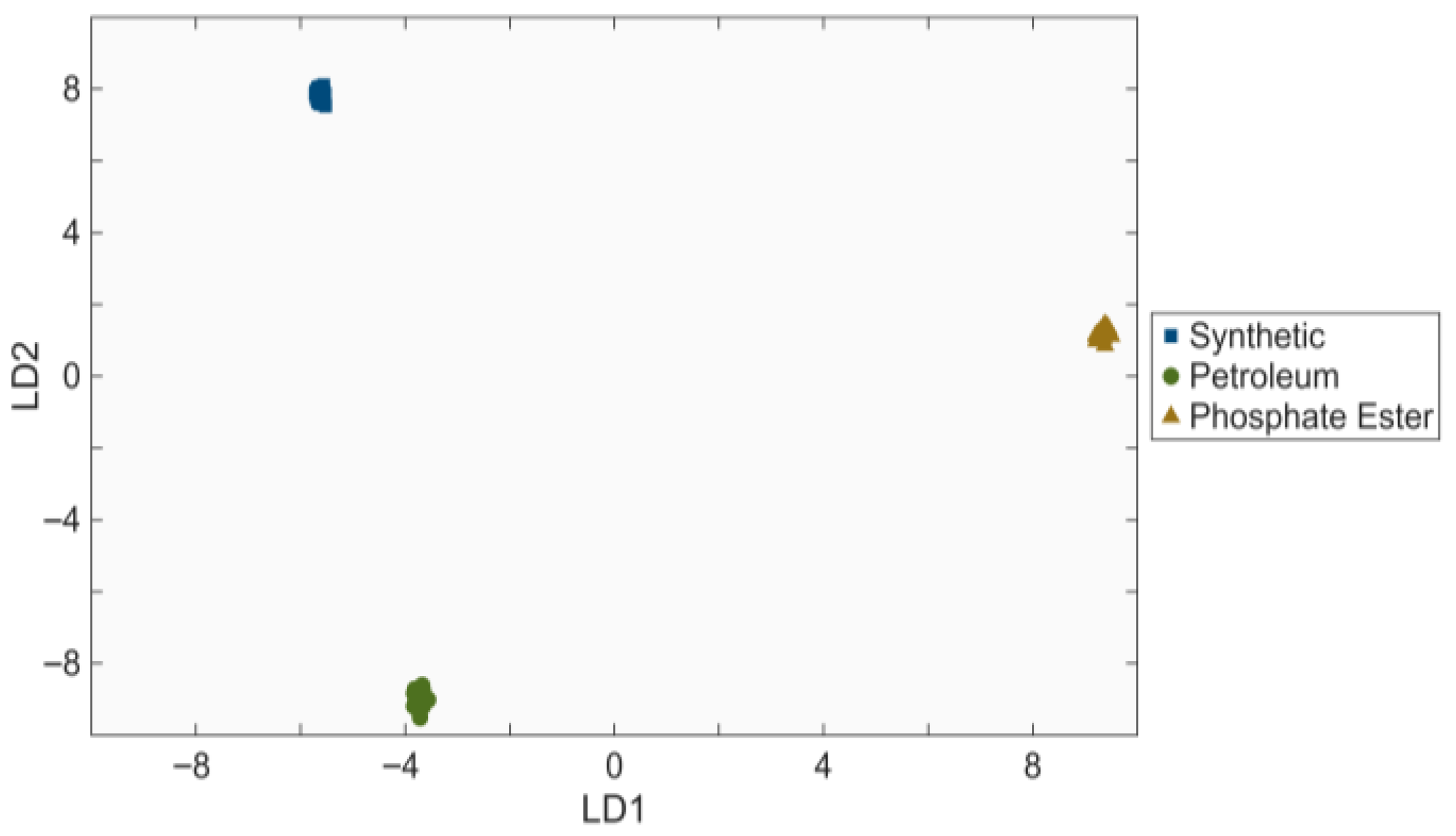
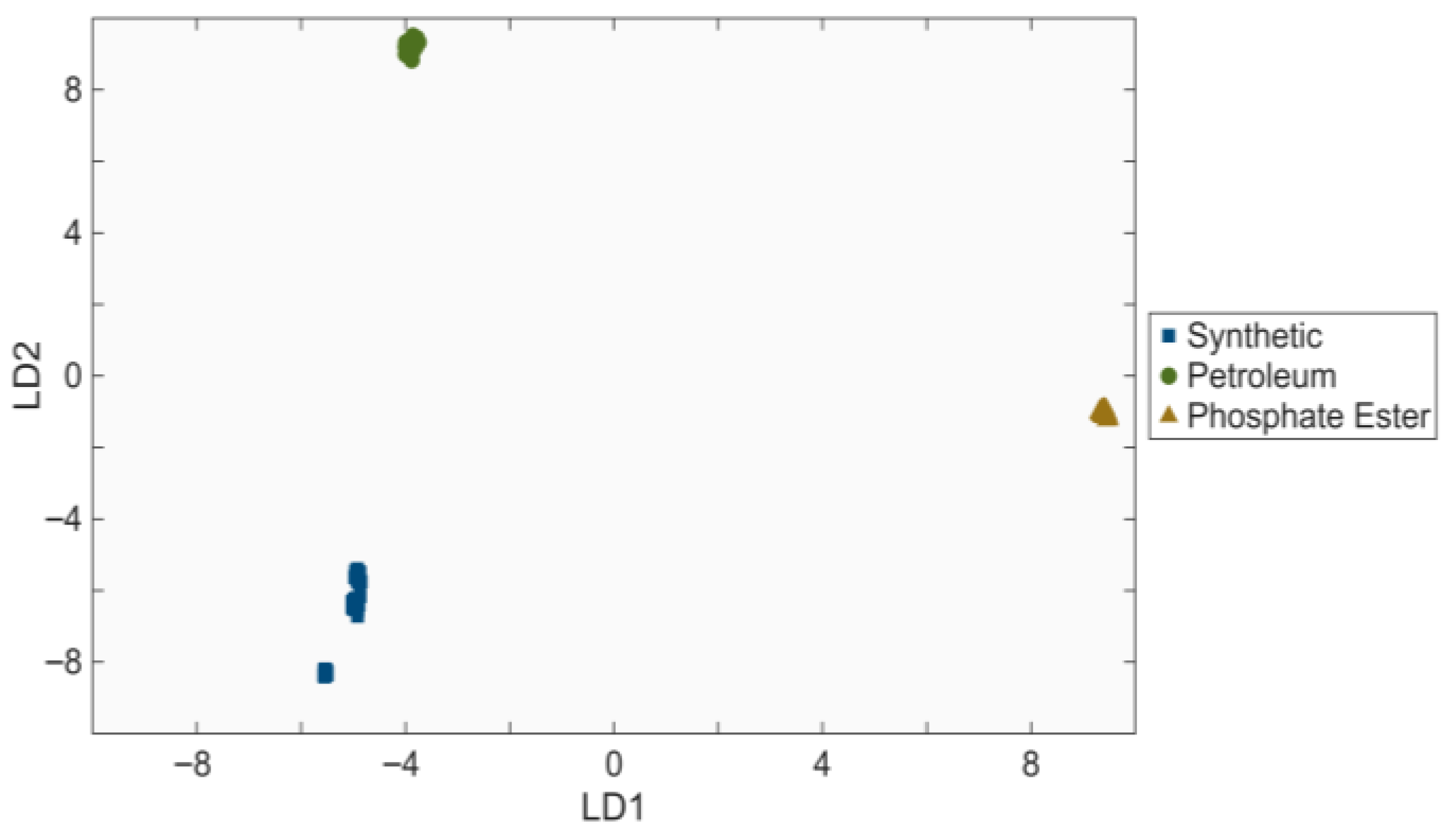
3.3. Linear Discriminant Analysis of Processed Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forsberg, C. What Is the Long-Term Demand for Liquid Hydrocarbon Fuels and Feedstocks? Appl. Energy 2023, 341, 121104. [Google Scholar] [CrossRef]
- Bart, J.; Gucciardi, E.; Cavallaro, S. Bioloubricant Product Groups and Technological Applications. In Biolubricants; Woodhead Publishing: Sawdust, UK, 2013; pp. 565–711. ISBN 978-0-85709-263-2. [Google Scholar]
- Stauffer, E.; Dolan, J.A.; Newman, R. Fire Debris Analysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-12-663971-1. [Google Scholar]
- Picardi, G.; Cattaruzza, F.; Mangione, D.; Manzo, F.; Terracciano, A.; Proposito, A. Rapid Screening of Designer Fuel Frauds by Raman Spectroscopy. Talanta Open 2024, 9, 100333. [Google Scholar] [CrossRef]
- Doble, P.; Sandercock, M.; Du Pasquier, E.; Petocz, P.; Roux, C.; Dawson, M. Classification of Premium and Regular Gasoline by Gas Chromatography/Mass Spectrometry, Principal Component Analysis and Artificial Neural Networks. Forensic Sci. Int. 2003, 132, 26–39. [Google Scholar] [CrossRef]
- Ryan, P.; Denne, D.; Wakefield, C.; Warburton, G.; Hazelby, D. An Investigation of the Reproducibility of Results of an Automatic GC/MS/DS Method for the Detection of Organic Contaminants. Int. J. Mass Spectrom. Ion Phys. 1983, 48, 283–286. [Google Scholar] [CrossRef]
- Nowak, P.; Bis, A.; Rusin, M.; Woźniakiewicz, M. Carbon Footprint of the Analytical Laboratory and the Three-Dimensional Approach to Its Reduction. Green Anal. Chem. 2023, 4, 100051. [Google Scholar] [CrossRef]
- Woodrow, J. The Laboratory Characterization of Arco Jet Fuel Vapor and Liquid; University of Nevada: Reno, NV, USA, 2000. [Google Scholar]
- Mana Kialengila, D.; Wolfs, K.; Bugalama, J.; Van Schepdael, A.; Adams, E. Full Evaporation Headspace Gas Chromatography for Sensitive Determination of High Boiling Point Volatile Organic Compounds in Low Boiling Matrices. J. Chromatogr. A 2013, 1315, 167–175. [Google Scholar] [CrossRef]
- Pires, A.; Han, Y.; Kramlich, J.; Garcia-Perez, M. Chemical Composition and Fuel Properties of Alternative Jet Fuels. BioResources 2018, 13, 2632–2657. [Google Scholar] [CrossRef]
- Fujihara, K.; Fujita, Y.; Yamamoto, T.; Nishimoto, N.; Kimura-Kataoka, K.; Kurata, S.; Takinami, Y.; Yasuda, T.; Takeshita, H. Blood Identification and Discrimination between Human and Nonhuman Blood Using Portable Raman Spectroscopy. Int. J. Leg. Med. 2017, 131, 319–322. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Yu, J.C.-C. Development of Crime Scene Intelligence Using a Hand-Held Raman Spectrometer and Transfer Learning. Anal. Chem. 2021, 93, 8889–8896. [Google Scholar] [CrossRef]
- Hargreaves, M.; Page, K.; Munshi, T.; Tomsett, R.; Lynch, G.; Edwards, H. Analysis of Seized Drugs Using Portable Raman Spectroscopy in an Airport Environment—A Proof of Principle Study. J. Raman Spectrosc. 2008, 39, 873–880. [Google Scholar] [CrossRef]
- Navin, C.; Tondepu, C.; Toth, R.; Lawson, L.; Rodriguez, J. Quantitative Determinations Using Portable Raman Spectroscopy. J. Pharm. Biomed. Anal. 2017, 136, 156–161. [Google Scholar] [CrossRef]
- Wang, J.; Koo, K.; Trau, M. Tetraplex Immunophenotyping of Cell Surface Proteomes via Synthesized Plasmonic Nanotags and Portable Raman Spectroscopy. Anal. Chem. 2022, 94, 14906–14916. [Google Scholar] [CrossRef]
- Daoust, F.; Nguyen, T.; Orsini, P.; Bismuth, J.; de Denus-Baillargeon, M.; Veilleux, I.; Wetter, A.; Mckoy, P.; Dicaire, I.; Massabki, M.; et al. Handheld Macroscopic Raman Spectroscopy Imaging Instrument for Machine-Learning-Based Molecular Tissue Margins Characterization. J. Biomed. Opt. 2021, 26, 022911. [Google Scholar] [CrossRef]
- Lauwers, D.; Hutado, A.; Tanevska, V.; Moens, L.; Bersani, D.; Vandenabeele, P. Characterisation of a Portable Raman Spectrometer for In Situ Analysis of Art Objects. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 294–301. [Google Scholar] [CrossRef]
- Gueli, A.; Galvagno, R.; Incardona, A.; Pappalardo, E.; Politi, G.; Paladini, G.; Stella, G. Correlation of Visible Reflectance Spectrometry and Portable Raman Data for Red Pigment Identification. Heritage 2024, 7, 2161–2175. [Google Scholar] [CrossRef]
- Jehlička, J.; Vítek, P.; Edwards, H.; Heagreaves, M.; Čapoun, T. Application of Portable Raman Instruments for Fast and Non-Destructive Detection of Minerals on Outcrops. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Culka, A.; Jehlička, J.; Opluštil, S. Evaluation of Carbonification of Coals Using a Portable Raman Spectrometer. J. Raman Spectrosc. 2023, 54, 1220–1232. [Google Scholar] [CrossRef]
- Pereira, R.; Skrobot, V.; Castro, E.; Fortes, I.; Pasa, V. Determination of Gasoline Adulteration by Principal Components Analysis-Linear Discriminant Analysis Applied to FTIR Spectra. Energy Fuels 2006, 20, 1097–1102. [Google Scholar] [CrossRef]
- Barbeira, P.; Pereira, R.; Corgozinho, C. Identification of Gasoline Origin by Physical and Chemical Properties and Multivariate Analysis. Energy Fuels 2007, 21, 2212–2215. [Google Scholar] [CrossRef]
- Li, S.; Dai, L. Classification of Gasoline Brand and Origin by Raman Spectroscopy and a Novel R-Weighted LSSVM Algorithm. Fuel 2012, 96, 146–152. [Google Scholar] [CrossRef]
- Yousefinejad, S.; Aalizadeh, L.; Honarasa, F. Application of ATR-FTIR Spectroscopy and Chemometrics for the Discrimination of Furnace Oil, Gas Oil and Mazut Oil. Anal. Methods 2016, 8, 4640–4647. [Google Scholar] [CrossRef]
- Khanmohammadi Khorrami, M.; Sadrara, M.; Mohammadi, M. Quality Classification of Gasoline Samples Based on Their Aliphatic to Aromatic Ratio and Analysis of PONA Content Using Genetic Algorithm Based Multivariate Techniques and ATR-FTIR Spectroscopy. Infrared Phys. Technol. 2022, 126, 104354. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Gao, M.; Zuo, Y. Classification of Lubricating Oil Types Using Mid-Infrared Spectroscopy Combined with Linear Discriminant Analysis–Support Vector Machine Algorithm. Lubricants 2023, 11, 268. [Google Scholar] [CrossRef]
- Biaktluanga, L.; Lalhruaitluanga, J.; Lalramnghaka, J.; Thanga, H. Analysis of Gasoline Quality by ATR-FTIR Spectroscopy with Multivariate Techniques. Results Chem. 2024, 8, 101575. [Google Scholar] [CrossRef]
- Shang, L.; Bao, Y.; Tang, J.; Ma, D.; Fu, J.; Zhao, Y.; Wang, X.; Yin, J. A Novel Polynomial Reconstruction Algorithm-based 1D Convolutional Neural Network Used for Transfer Learning in Raman Spectroscopy Application. J. Raman Spectrosc. 2022, 53, 237–246. [Google Scholar] [CrossRef]
- Zhou, W.; Qian, Z.; Ni, X.; Tang, Y.; Guo, H.; Zhuang, S. Dense Convolutional Neural Network for Identification of Raman Spectra. Sensors 2023, 23, 7433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, X.; Zhang, D.; Lee, H. Machine Learning Empowered Coherent Raman Imaging and Analysis for Biomedical Applications. Comms. Eng. 2025, 4, 8. [Google Scholar] [CrossRef]
- Wang, Z.; Ranasinghe, J.; Wu, W.; Chan, D.; Gomm, A.; Tanzi, R.; Zhang, C.; Zhang, N.; Allen, G.; Huang, S. Machine Learning Interpretation of Optical Spectroscopy Using Peak-Sensitive Logistic Regression. ACS Nano 2025, 19, 15457–15473. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Wang, W.; Zhou, W.; Jin, M.; Vikesland, P. Machine Learning-Assisted Surface-Enhanced Raman Spectroscopy Detection for Environmental Applications: A Review. Environ. Sci. Technol. 2024, 58, 20830–20848. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.; Gupta, D. Deep Machine Learning and Neural Networks: An Overview. IAES Int. J. Artif. Intell. IJ-AI 2017, 6, 66–73. [Google Scholar] [CrossRef]
- Amouzgar, M.; Glass, D.; Baskar, R.; Averbukh, I.; Kimmey, S.; Tsai, A.; Hartmann, F.; Bendall, S. Supervised Dimensionality Reduction for Exploration of Single-Cell Data by HSS-LDA. Patterns 2022, 3, 100536. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, K.; Brusey, J.; Hunt, A.; Gaura, E. Linear Classifier Design under Heteroscedasticity in Linear Discriminant Analysis. Expert Syst. Appl. 2017, 79, 44–52. [Google Scholar] [CrossRef]
- Krzanowski, W.; Jonathan, P.; McCarthy, W.; Thomas, M. Discriminant Analysis with Singular Covariance Matrices: Methods and Applications to Spectroscopic Data. J. R. Stat. 1995, 44, 101. [Google Scholar] [CrossRef]
- Barreñada, L.; Dhiman, P.; Timmerman, D.; Boulesteix, A.; Van Calster, B. Understanding Overfitting in Random Forest for Probability Estimation: A Visualization and Simulation Study. Diagn. Progn. Res. 2024, 8, 14. [Google Scholar] [CrossRef]
- Mirjalili, S.; Powell, P.; Strunk, J.; James, T.; Duarte, A. Evaluation of Classification Approaches for Distinguishing Brain States Predictive of Episodic Memory Performance from Electroencephalography. Neuroimage 2022, 247, 118851. [Google Scholar] [CrossRef]
- Federal Aviation Administration. Jet Fuel Contamination with Diesel Exhaust Fluid (DEF); Federal Aviation Administration: Washington, DC, USA, 2023. [Google Scholar]
- Stamker, D.G.; Tartakovsky, K.; Rabaev, M. Identification and Quantification of Phosphate Ester-Based Hydraulic Fluid in Jet Fuel. SAE Int. J. Fuels Lubr. 2019, 12, 43–50. [Google Scholar] [CrossRef]
- U.S. Air Force. Department of the Air Force (DAF) 23.3 Small Business Innovation Research (SBIR) Direct to Phase II (D2P2) Proposal Submission Instructions Amendment 2. Available online: https://media.defense.gov/2023/Aug/22/2003285647/-1/-1/0/AF_SBIR_233_DP2.PDF (accessed on 17 January 2023).
- Secretary of the Air Force. The Department of the Air Force in 2050; Department of Defense: Arlington, VA, USA, 2024. [Google Scholar]
- MATLAB R2025a, version 25.1.0.2943329; MathWorks: Natick, MA, USA, 2025.
- Eilers, P.; Boelens, H. Baseline Correction with Asymmetric Least Squares Smoothing; Leiden University Medical Centre: Leiden, The Netherlands, 2005. [Google Scholar]
- Kanno, N.; Kato, S.; Ohkuma, M.; Matsui, M.; Iwasaki, W.; Shigeto, S. Machine Learning-Assisted Single-Cell Raman Fingerprinting for In Situ and Nondestructive Classification of Prokaryotes. iScience 2021, 24, 102975. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, X.; Qiu, W.; Bian, J.; Zhang, X.; Chen, Q. Surface-Enhanced Raman Scattering Spectroscopy Reveals the Phonon Softening of Yttrium-Doped Barium Zirconate Thin Films. J. Phys. Chem. C 2022, 126, 10722–10728. [Google Scholar] [CrossRef]
- Wise, B.; Gallagher, N. The Process Chemometrics Approach to Process Monitoring and Fault Detection. J. Process Control 1996, 6, 329–348. [Google Scholar] [CrossRef]
- Kuptsov, A.; Arbuzova, T. A Study of Heavy Oil Fractions by Fourier-Transform near-Infrared Raman Spectroscopy. Pet. Chem. 2011, 51, 203–211. [Google Scholar] [CrossRef]
- Böke, J.; Popp, J.; Krafft, C. Optical Photothermal Infrared Spectroscopy with Simultaneously Acquired Raman Spectroscopy for Two-Dimensional Microplastic Identification. Sci. Rep. 2022, 12, 18785. [Google Scholar] [CrossRef]
- Moosavinejad, S.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of Degradation in Chemical Compounds of Wood in Historical Buildings Using FT-IR and FT-Raman Vibrational Spectroscopy. Maderas Cienc. Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef]
- Gieleciak, R.; Hall, A.; Michaelian, K.; Chen, J. Exploring the Potential of Raman Spectroscopy for Characterizing Olefins in Olefin-Containing Streams. Energy Fuels 2023, 37, 13698–13709. [Google Scholar] [CrossRef]
- Ruxton, G. The Unequal Variance t-Test Is an Underused Alternative to Student’s t-Test and the Mann-Whitney U Test. Behav. Ecol. 2006, 17, 688–690. [Google Scholar] [CrossRef]
- Genkawa, T.; Shinzawa, H.; Kato, H.; Ishikawa, D.; Murayama, K.; Komiyama, M.; Ozaki, Y. Baseline Correction of Diffuse Reflection Near-Infrared Spectra Using Searching Region Standard Normal Variate (SRSNV). Appl. Spectrosc. 2015, 69, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, Q. Denoising Raman Spectra by Wiener Estimation with a Numerical Calibration Dataset. Biomed. Opt. Express 2020, 11, 200. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Vangrieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Dobbin, K.; Simon, R. Optimally Splitting Cases for Training and Testing High Dimensional Classifiers. BMC Med. Genom. 2011, 4, 31. [Google Scholar] [CrossRef]
- Gholamy, A.; Kreinovich, V.; Kosheleva, O. Why 70/30 or 80/20 Relation Between Training and Testing Sets: A Pedagogical Explanation; University of Texas at El Paso: El Paso, TX, USA, 2018. [Google Scholar]
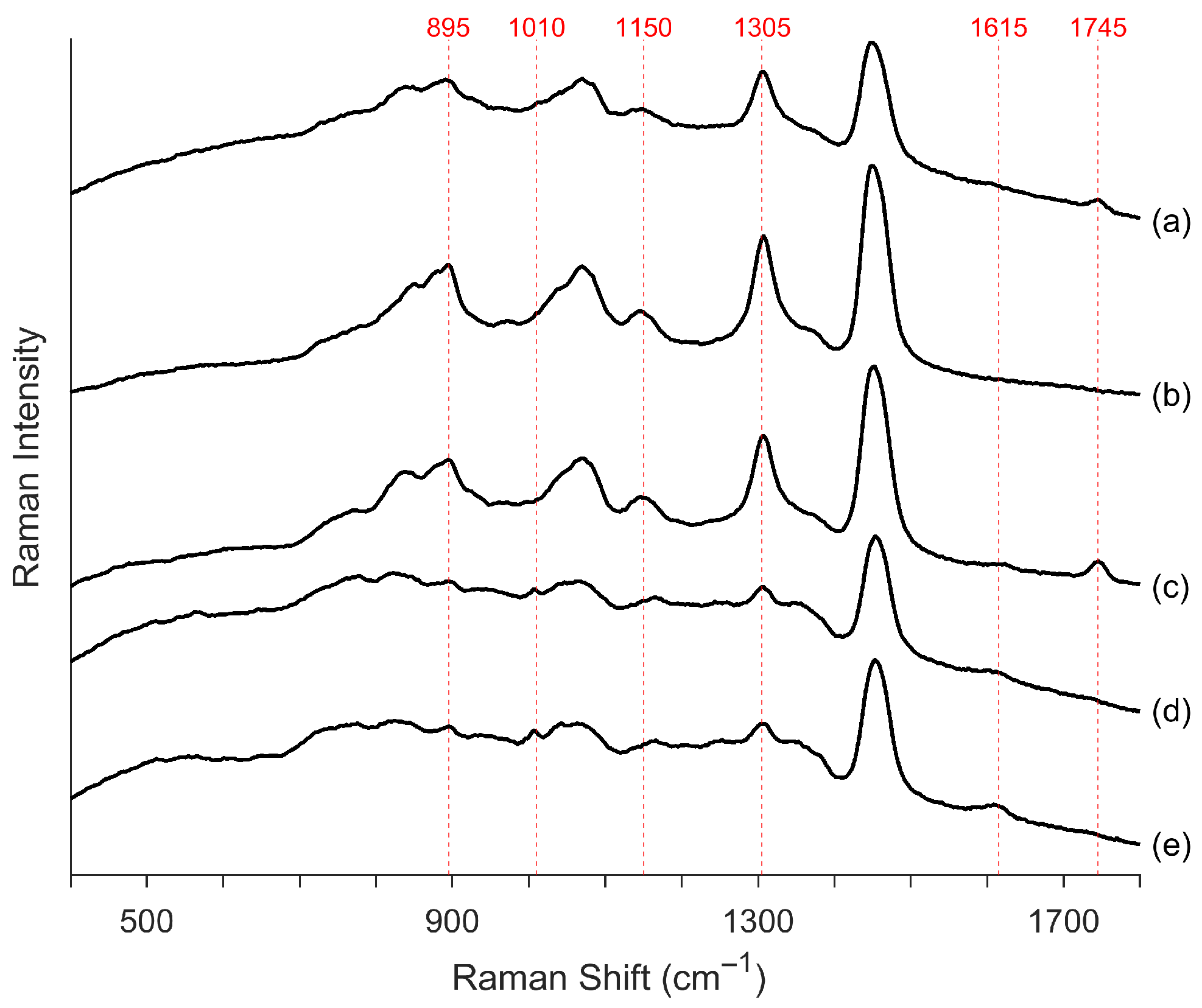


| Spectra Difference | Peak Wavenumber (cm−1) |
|---|---|
| Larger peak in synthetic-based spectra | 895 |
| 1070 | |
| 1150 1 | |
| 1305 | |
| 1455 | |
| 1745 2 | |
| Larger peak in petroleum-based spectra | 1615 3 |
| Sharper minimum in the synthetic-based spectra | 860 |
| Unique peak in petroleum-based spectra | 1010 |
| 1350 |
| Molecular Vibration | Peak Wavenumber (cm−1) |
|---|---|
| Alkane C-C stretching [48,49] | 860 |
| 1070 | |
| In-plane H-C-H scissoring [50] | 895 |
| Monocyclic aromatic H breathing [48] | 1010 |
| Iso-alkane C-C skeletal stretching [48] | 1150 |
| Alkane bending [48] | 1305 |
| 1455 | |
| PAH 1 H [48] | 1350 |
| Alkene C=C stretching [51] | 1615 |
| Carbonyl C=O stretching [49] | 1745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodges, J.E.; Marchand, K.; Monjardez, G.; Yu, J.C.-C. The Classification of Synthetic- and Petroleum-Based Hydrocarbon Fluids Using Handheld Raman Spectroscopy. Chemosensors 2025, 13, 327. https://doi.org/10.3390/chemosensors13090327
Hodges JE, Marchand K, Monjardez G, Yu JC-C. The Classification of Synthetic- and Petroleum-Based Hydrocarbon Fluids Using Handheld Raman Spectroscopy. Chemosensors. 2025; 13(9):327. https://doi.org/10.3390/chemosensors13090327
Chicago/Turabian StyleHodges, Javier E., Kailee Marchand, Geraldine Monjardez, and Jorn Chi-Chung Yu. 2025. "The Classification of Synthetic- and Petroleum-Based Hydrocarbon Fluids Using Handheld Raman Spectroscopy" Chemosensors 13, no. 9: 327. https://doi.org/10.3390/chemosensors13090327
APA StyleHodges, J. E., Marchand, K., Monjardez, G., & Yu, J. C.-C. (2025). The Classification of Synthetic- and Petroleum-Based Hydrocarbon Fluids Using Handheld Raman Spectroscopy. Chemosensors, 13(9), 327. https://doi.org/10.3390/chemosensors13090327







