Biochar-Derived Electrochemical Sensors: A Green Route for Trace Heavy Metal Detection
Abstract
1. Introduction
2. Biochar: A Green Electrode Material
2.1. Synthesis of BC
2.1.1. Pyrolysis
2.1.2. Hydrothermal Carbonization (HTC)
2.2. Modification of the Synthesized BC
2.2.1. Chemical Activation
2.2.2. Heteroatom Doping
2.2.3. Incorporation of Metal/Metal Oxide Nanoparticles
3. Application of BC for Electrochemical Sensing of Heavy Metal Ions
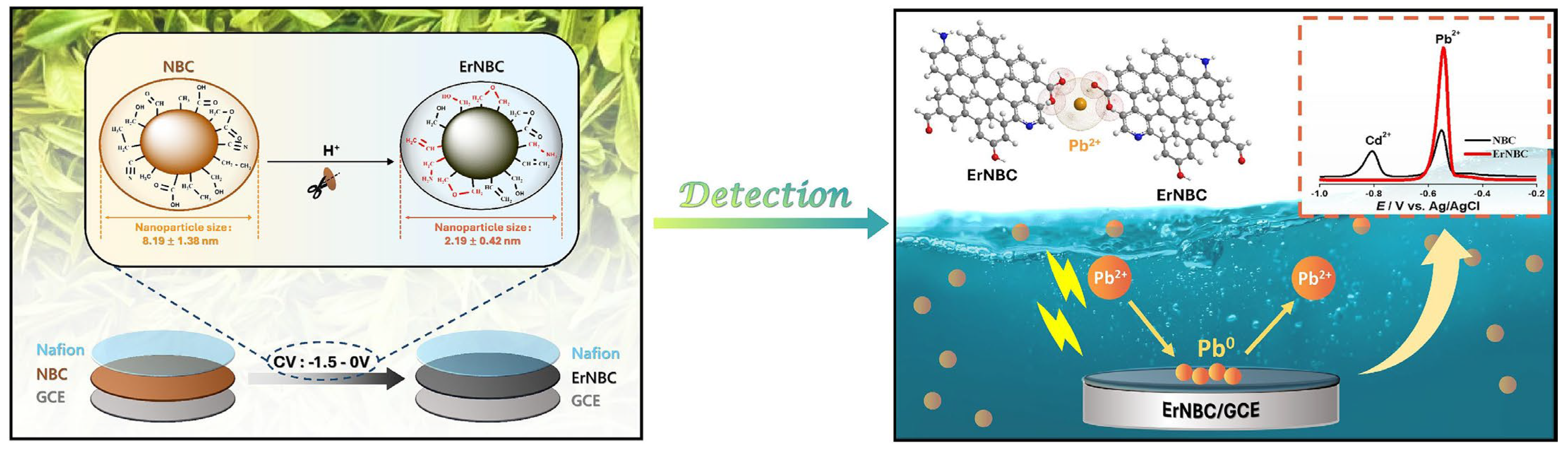
3.1. Lead (Pb2+)
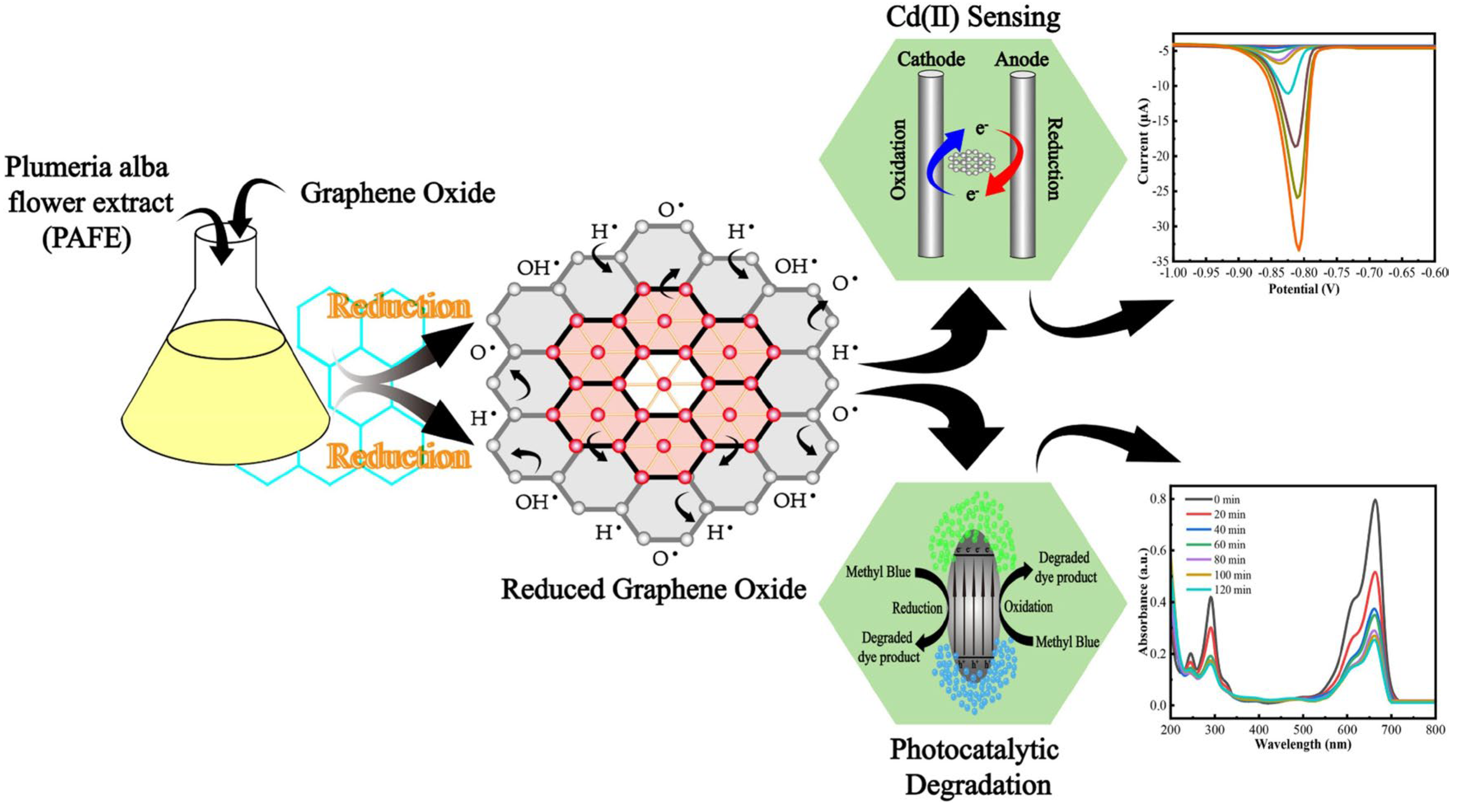
3.2. Cadmium (Cd2+)
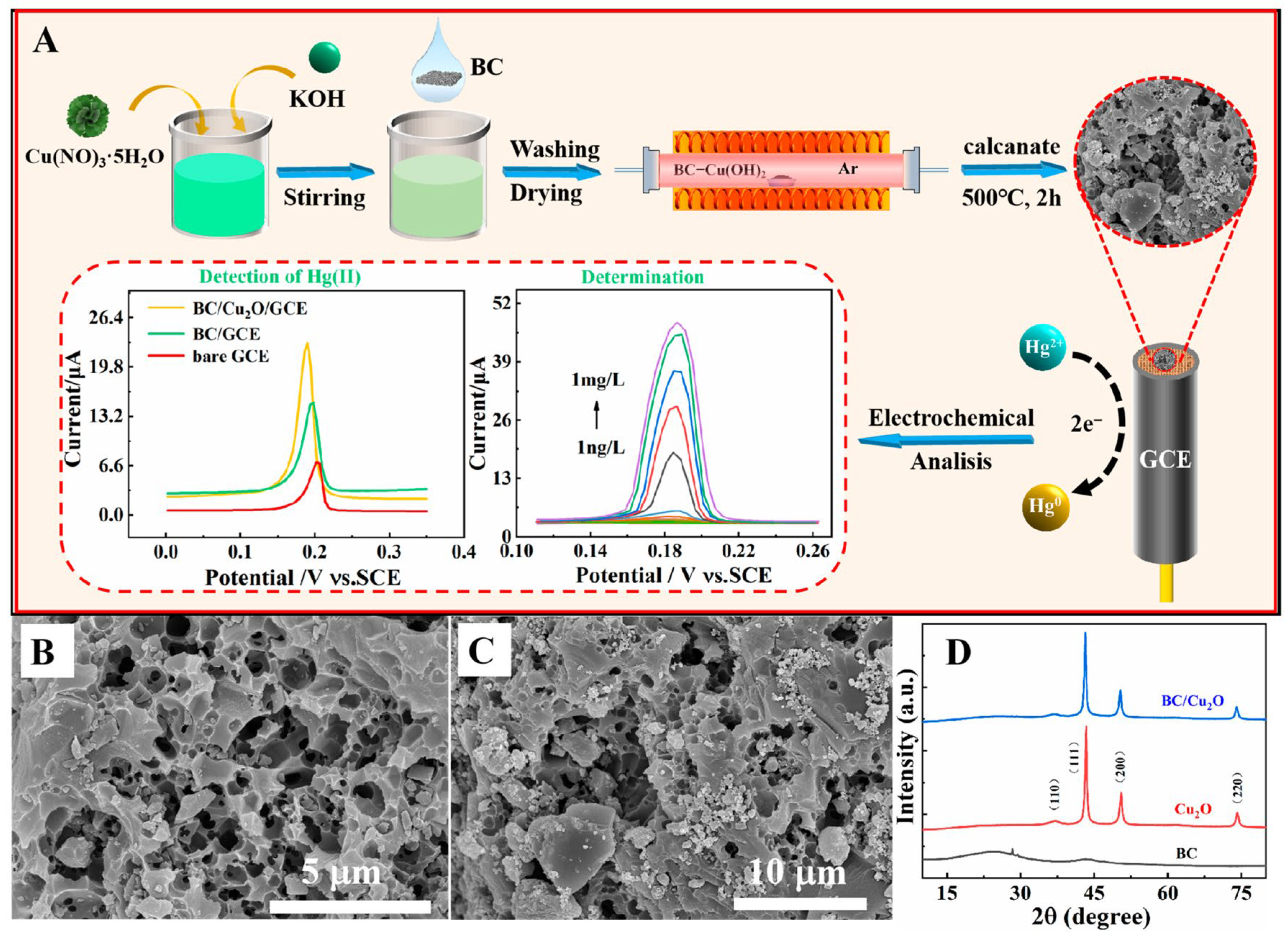
3.3. Mercury (Hg2+)
3.4. Simultaneous Detection of Heavy Metal Ions
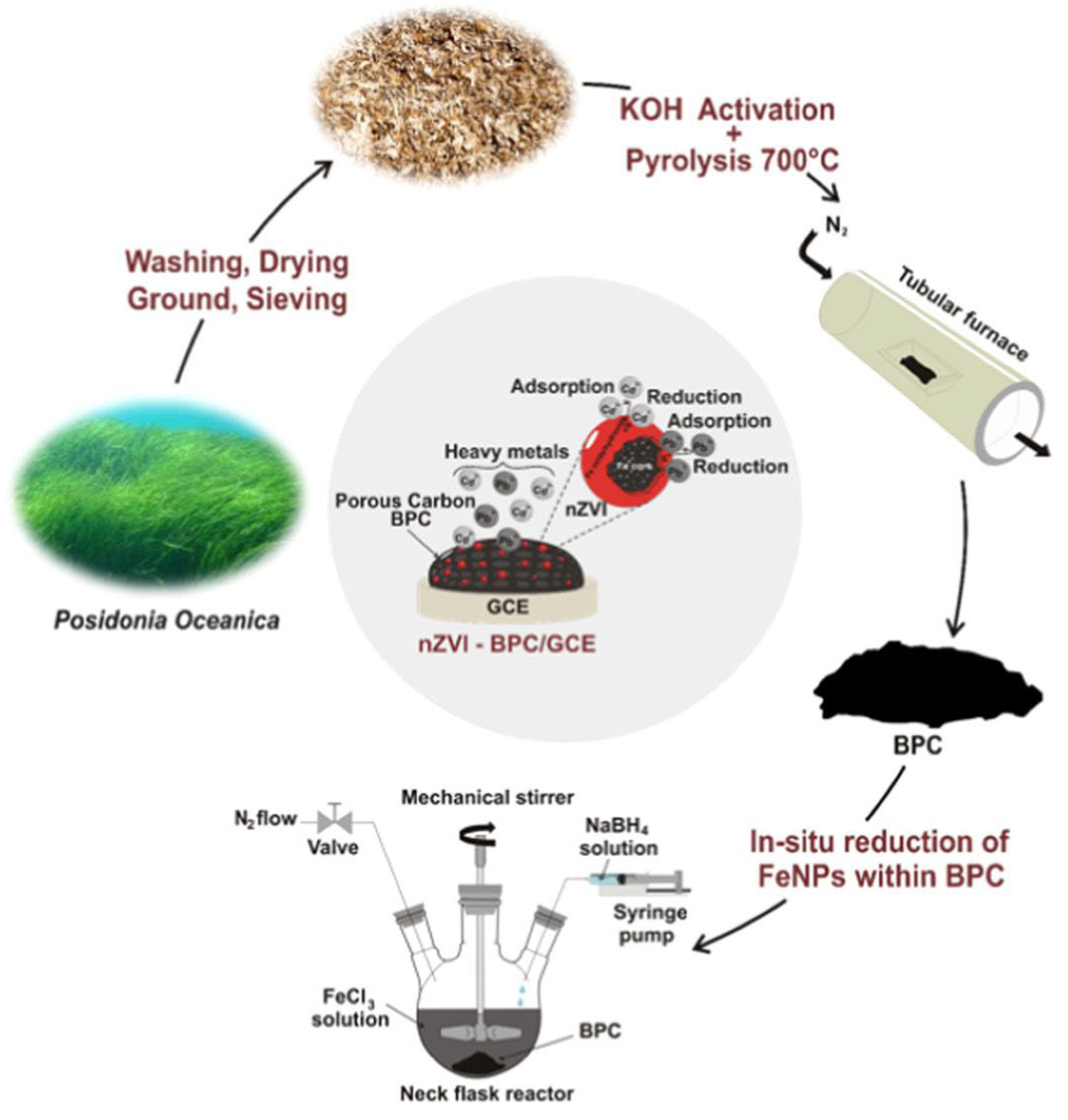
3.4.1. Lead and Cadmium
3.4.2. Lead and Mercury
3.4.3. Cadmium and Mercury
3.4.4. Lead, Cadmium, and Mercury
3.5. Comparative Evaluation of Electrochemical Techniques in Biochar-Based Heavy Metal Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Atomic Absorption Spectroscopy |
| DPAdSV | Adsorptive Stripping Differential Pulse Voltammetry |
| As3+ | Arsenic |
| BC | Biochar |
| B | Boron |
| Cd2+/Cd(II) | Cadmium |
| CPE | Carbon Paste Electrode |
| Cu2O | Copper Oxide |
| CV | Cyclic Voltammetry |
| DPSV | Differential Pulse Stripping Voltammetry |
| DPV | Differential Pulse Voltammetry |
| ErNBC | Electrochemically reshaped nano-BC |
| GCE | Glassy Carbon Electrode |
| GHG | Greenhouse Gas |
| Au | Gold |
| HCl | Hydrochloric Acid |
| HTC | Hydrothermal Carbonization |
| Hap | Hydroxyapatite |
| ICP-MS | Induction-Coupled Plasma-Mass Spectrometry |
| Pb2+/Pb(II) | Lead |
| LCA | Life Cycle Assessment |
| LOD | Limit of Detection |
| Fe3O4 | Magnetic Iron Oxide |
| Hg2+/Hg(II) | Mercury |
| MOF | Metal Organic Framework |
| CeO2 nanoparticles | Nanoceria |
| N/N2 | Nitrogen |
| OPEFB | Oil Palm Empty Fruit Bunches |
| H3PO4 | Phosphoric Acid |
| P | Phosphorus |
| PAFE | Plumeria alba Flower Extract |
| PANI | Polyaniline |
| KOH | Potassium Hydroxide |
| rGO | Reduced Graphene Oxide |
| NaOH | Sodium Hydroxide |
| SWASV | Square Wave Anodic Stripping Voltammetry |
| SWV | Square Wave Voltammetry |
| S | Sulfur |
| H2SO4 | Sulfuric acid |
| TiO2 | Titanium Oxide |
| XRF | X-Ray Fluorescence |
| UiO-66-NH2/BC | Zirconium MOF Incorporated BC |
References
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and Available Heavy Metal Concentrations in Soils of the Thriassio Plain (Greece) and Assessment of Soil Pollution Indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef] [PubMed]
- Gwimbi, P.; Kotelo, T.; Selimo, M.J. Heavy Metal Concentrations in Sediments and Cyprinus Carpio from Maqalika Reservoir–Maseru, Lesotho: An Analysis of Potential Health Risks to Fish Consumers. Toxicol. Rep. 2020, 7, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Dabek-Zlotorzynska, E.; Celo, V.; Ding, L.; Herod, D.; Jeong, C.-H.; Evans, G.; Hilker, N. Characteristics and Sources of PM2.5 and Reactive Gases near Roadways in Two Metropolitan Areas in Canada. Atmos. Environ. 2019, 218, 116980. [Google Scholar] [CrossRef]
- Weber, A.M.; Mawodza, T.; Sarkar, B.; Menon, M. Assessment of Potentially Toxic Trace Element Contamination in Urban Allotment Soils and Their Uptake by Onions: A Preliminary Case Study from Sheffield, England. Ecotoxicol. Environ. Saf. 2019, 170, 156–165. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Anbazhagan, R.; Chinthaginjala, R.; Chitathuru, D.; Ahmad, I.; Kim, T. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef]
- Gonzalez, K.A.; Kazemeini, S.; Weber, D.C.; Cordero, P.A.; Garcia, E.M.; Rusinek, C.A. Electrochemical Sensing of Heavy Metals in Biological Media: A Review. Electroanalysis 2023, 35, e202300098. [Google Scholar] [CrossRef]
- Luo, L. Recent Advances in Functional Nanomaterials for Electrochemical Sensors and Biosensors. Molecules 2023, 28, 6798. [Google Scholar] [CrossRef]
- Kumar, P.; Rajan, R.; Upadhyaya, K.; Behl, G.; Xiang, X.-X.; Huo, P.; Liu, B. Metal Oxide Nanomaterials Based Electrochemical and Optical Biosensors for Biomedical Applications: Recent Advances and Future Prospectives. Environ. Res. 2024, 247, 118002. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, Synthesis and Their Applications in Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Kangmennaa, A.; Forkuo, R.B.; Agorku, E.S. Carbon-Based Electrode Materials for Sensor Application: A Review. Sens. Technol. 2024, 2, 2350174. [Google Scholar] [CrossRef]
- Onfray, C.; Thiam, A. Biomass-Derived Carbon-Based Electrodes for Electrochemical Sensing: A Review. Micromachines 2023, 14, 1688. [Google Scholar] [CrossRef]
- Bhat, V.S.; Supriya, S.; Hegde, G. Review—Biomass Derived Carbon Materials for Electrochemical Sensors. J. Electrochem. Soc. 2020, 167, 037526. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Li, G.; Erk, N. Biomass-Derived Carbon Materials as an Emerging Platform for Advanced Electrochemical Sensors: Recent Advances and Future Perspectives. Ind. Eng. Chem. Res. 2023, 62, 4628–4635. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Biochar-Supported Nanomaterials for Environmental Applications. J. Ind. Eng. Chem. 2019, 78, 21–33. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar-Based Metal Oxide Nanocomposites of Magnesium and Manganese Improved Root Development and Productivity of Safflower (Carthamus tinctorius L.). under Salt Stress. Rhizosphere 2021, 19, 100416. [Google Scholar] [CrossRef]
- Ghaedi, S.; Rajabi, H.; Hadi Mosleh, M.; Sedighi, M. MOF Biochar Composites for Environmental Protection and Pollution Control. Biores. Technol. 2025, 418, 131982. [Google Scholar] [CrossRef]
- Shoudho, K.N.; Khan, T.H.; Ara, U.R.; Khan, M.R.; Shawon, Z.B.Z.; Hoque, M.E. Biochar in Global Carbon Cycle: Towards Sustainable Development Goals. Curr. Res. Green Sustain. Chem. 2024, 8, 100409. [Google Scholar] [CrossRef]
- Samuel Olugbenga, O.; Goodness Adeleye, P.; Blessing Oladipupo, S.; Timothy Adeleye, A.; Igenepo John, K. Biomass-Derived Biochar in Wastewater Treatment- a Circular Economy Approach. Waste Manag. Bull. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, G.; Ran, L.; Liu, M.; Geng, P.; Yi, W. Green Synthesis of Biomass-Derived Porous Carbon for Electrochemical Detection of Heavy Metal Ions: Methods, Properties, and Applications. J. Environ. Chem. Eng. 2024, 12, 113903. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-Derived Biochar: From Production to Application in Removing Heavy Metal-Contaminated Water. Process. Saf. Environ. Prot. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Lichtfouse, E.; Maurer, C.; Liu, H. Life Cycle Assessment of Biochar for Sustainable Agricultural Application: A Review. Sci. Total Environ. 2024, 951, 175448. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Alotaibi, K.D.; EL-Saeid, M.H.; Giesy, J.P. Polycyclic Aromatic Hydrocarbons (PAHs) and Metals in Diverse Biochar Products: Effect of Feedstock Type and Pyrolysis Temperature. Toxics 2023, 11, 96. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, P.; Bojjagani, S.; Srivastava, J.K.; Raj, A. Investigation of the Speciation and Environmental Risk of Heavy Metals in Biochar Produced from Textile Sludge Waste by Pyrolysis at Different Temperatures. Chemosphere 2024, 360, 142454. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of Pyrolysis Temperature on Properties and Environmental Safety of Heavy Metals in Biochars Derived from Municipal Sewage Sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Jiang, Z.; Zou, Y.; Li, Y.; Kong, F.; Yang, D. Environmental Life Cycle Assessment of Supercapacitor Electrode Production Using Algae Derived Biochar Aerogel. Biochar 2021, 3, 701–714. [Google Scholar] [CrossRef]
- Bilge, S.; Karadurmus, L.; Sınağ, A.; Ozkan, S.A. Green Synthesis and Characterization of Carbon-Based Materials for Sensitive Detection of Heavy Metal Ions. TrAC Trends Anal. Chem. 2021, 145, 116473. [Google Scholar] [CrossRef]
- Dhanda, A.; Raj, R.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Graphene and Biochar-Based Cathode Catalysts for Microbial Fuel Cell: Performance Evaluation, Economic Comparison, Environmental and Future Perspectives. Environ. Res. 2023, 231, 116143. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review on the Comparison Between Slow Pyrolysis and Fast Pyrolysis on the Quality of Lignocellulosic and Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and Its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Kamini, G.P.; Tee, K.F.; Gimbun, J.; Chin, S.C. Biochar in Cementitious Material—A Review on Physical, Chemical, Mechanical, and Durability Properties. AIMS Mater. Sci. 2023, 10, 405–425. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Fiorenza, R.; Jlassi, K.; Mekhalif, Z.; Ali, A.M.A.; Chehimi, M.M. The Emerging Role of Biochar in the Carbon Materials Family for Hydrogen Production. Chem. Eng. Res. Des. 2022, 188, 209–228. [Google Scholar] [CrossRef]
- Bughani, A.; Mehtab, S.; Palariya, D.; Maheshwari, J.; Zaidi, M.G.H. Biochar-Derived Nanocomposites for Electrochemical Estimation of Heavy Metals and Organic Pollutants. In Biomass for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2025; pp. 55–80. ISBN 9780443330148. [Google Scholar]
- Arellano, O.; Flores, M.; Guerra, J.; Hidalgo, A.; Rojas, D.; Strubinger, A. Hydrotermal Carbonization (HTC) of Corncob and Characterization of the Obtained Hydrochar. Chem. Eng. Trans. 2016, 50, 235–240. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal Carbonization of Lignocellulosic Biomass for Carbon Rich Material Preparation: A Review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Heikkinen, J.; Keskinen, R.; Soinne, H.; Hyväluoma, J.; Nikama, J.; Wikberg, H.; Källi, A.; Siipola, V.; Melkior, T.; Dupont, C.; et al. Possibilities to Improve Soil Aggregate Stability Using Biochars Derived from Various Biomasses through Slow Pyrolysis, Hydrothermal Carbonization, or Torrefaction. Geoderma 2019, 344, 40–49. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Ahmad, A.; Shekher Giri, B.; Chaturvedi, P. Hydrothermal Liquefaction of Rice Husk and Cow Dung in Mixed-Bed-Rotating Pyrolyzer and Application of Biochar for Dye Removal. Biores. Technol. 2020, 309, 123294. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, S.; Jin, L.; Liu, Y.; Yin, R.; Jiang, Z.; Tao, Y.; Huang, J.; Zhang, Y. Magnetic Porous Biochar with High Specific Surface Area Derived from Microwave-Assisted Hydrothermal and Pyrolysis Treatments of Water Hyacinth for Cr(VI) and Tetracycline Adsorption from Water. Biores. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Wu, L.; Gao, F.; Qi, X.; Zhang, Z. Hydrothermal Synthesis of Magnetic Sludge Biochar for Tetracycline and Ciprofloxacin Adsorptive Removal. Biores. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, Z.; Zhu, S.; Luo, S.; Yan, L.; Dai, Y.; Guo, Y.; Yang, Y. Removal of Cr(VI) from Water by a Biochar-Coupled g-C3N4 Nanosheets Composite and Performance of a Recycled Photocatalyst in Single and Combined Pollution Systems. Appl. Catal. B Environ. 2019, 243, 386–396. [Google Scholar] [CrossRef]
- Rao, L.; Zhu, Y.; Duan, Z.; Xue, T.; Duan, X.; Wen, Y.; Kumar, A.S.; Zhang, W.; Xu, J.; Hojjati-Najafabadi, A. Lotus Seedpods Biochar Decorated Molybdenum Disulfide for Portable, Flexible, Outdoor and Inexpensive Sensing of Hyperin. Chemosphere 2022, 301, 134595. [Google Scholar] [CrossRef]
- Lei, W.; Yang, B.; Sun, Y.; Xiao, L.; Tang, D.; Chen, K.; Sun, J.; Ke, J.; Zhuang, Y. Self-Sacrificial Template Synthesis of Heteroatom Doped Porous Biochar for Enhanced Electrochemical Energy Storage. J. Power Sources 2021, 488, 229455. [Google Scholar] [CrossRef]
- Falco, C.; Perez Caballero, F.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal Carbon from Biomass: Structural Differences between Hydrothermal and Pyrolyzed Carbons via13 C Solid State NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef]
- Mahmood, F.; Ali, M.; Khan, M.; Mbeugang, C.F.M.; Isa, Y.M.; Kozlov, A.; Penzik, M.; Xie, X.; Yang, H.; Zhang, S.; et al. A Review of Biochar Production and Its Employment in Synthesizing Carbon-Based Materials for Supercapacitors. Ind. Crops Prod. 2025, 227, 120830. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Irfan, O.M.; Moustafa, H.M. H3PO4/KOH Activation Agent for High Performance Rice Husk Activated Carbon Electrode in Acidic Media Supercapacitors. Molecules 2022, 28, 296. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, J.; Yılmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-Doped Porous Biochar Derived from Cornstalk for High Performance CO2 Adsorption and Electrochemical Energy Storage. Sep. Purif. Technol. 2022, 299, 121719. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Wang, Z. Electrochemical-Enhanced Fe3O4/Biochar Activates Peroxymonosulfate (E/Nano-Fe3O4/BC/PMS) for Degradation of Oxytetracycline. Chemosphere 2022, 308, 136148. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, H.; Yang, J.; Shi, Z.; Tan, Y.; Jin, J.; Wang, R.; Zhang, S.; Wang, J. Biochar Decorated with Gold Nanoparticles for Electrochemical Sensing Application. Electrochim. Acta 2018, 261, 464–473. [Google Scholar] [CrossRef]
- Ahamed, M.; Singh, S.; Behari, J.R.; Kumar, A.; Siddiqui, M.K.J. Interaction of Lead with Some Essential Trace Metals in the Blood of Anemic Children from Lucknow, India. Clin. Chim. Acta 2007, 377, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Huang, H.; Guan, H.; Tian, Z.-Q.; Chen, M.-M.; Tian, K.-K.; Zhao, F.-J.; Wang, P. Exposure Sources, Intake Pathways and Accumulation of Lead in Human Blood. Soil. Secur. 2024, 15, 100150. [Google Scholar] [CrossRef]
- Ouangpipat, W.; Lelasattarathkul, T.; Dongduen, C.; Liawruangrath, S. Bioaccumulation and Determination of Lead Using Treated-Pennisetum-Modified Carbon Paste Electrode. Talanta 2003, 61, 455–464. [Google Scholar] [CrossRef]
- Mojica, E.-R.E.; Vidal, J.M.; Pelegrina, A.B.; Micor, J.R.L. Voltammetric Determination of Lead (II) Ions at Carbon Paste Electrode Modified with Banana Tissue. J. Appl. Sci. 2007, 7, 1286–1292. [Google Scholar] [CrossRef]
- Agustini, D.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Sensitive Voltammetric Determination of Lead Released from Ceramic Dishes by Using of Bismuth Nanostructures Anchored on Biochar. Talanta 2015, 142, 221–227. [Google Scholar] [CrossRef]
- Tan, D.S.Y.; Impas, M.G.W.; Camacho, D.H.; Palisoc, S.T. Paper-Based Electrode Using Cladophora Cellulosepolyaniline Composite for Electrochemical Quantification of Toxic Lead (II). Cellul. Chem. Technol. 2018, 52, 853–861. [Google Scholar]
- Ajab, H.; Dennis, J.O.; Abdullah, M.A. Synthesis and Characterization of Cellulose and Hydroxyapatite-Carbon Electrode Composite for Trace Plumbum Ions Detection and Its Validation in Blood Serum. Inter. J. Biol. Macromol. 2018, 113, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wan, Z.; Liu, J.; Guliyeva, G. Bayberry-Kernel-Derived Wormlike Micro/Mesoporous Carbon Decorated with Human Blood Vessel-Like Structures and Active Nitrogen Sites as Highly Sensitive Electrochemical Sensors for Efficient Lead-Ion Detection. ACS Omega 2019, 4, 1191–1200. [Google Scholar] [CrossRef]
- Baikeli, Y.; Mamat, X.; Yalikun, N.; Wang, Y.; Qiao, M.; Li, Y.; Hu, G. Differential Pulse Voltammetry Detection of Pb(II) Using Nitrogen-Doped Activated Nanoporous Carbon from Almond Shells. RSC Adv. 2019, 9, 23678–23685. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.K.; Shankar, S.S.; Sajina, N.; Padinjareveetil, A.K.; Pilankatta, R.; Kumar, V.B.S.; Mathew, B.; George, B.; Makvandi, P.; Černík, M.; et al. Fabrication of a Greener TiO2 @Gum Arabic-Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys. ACS Omega 2020, 5, 25390–25399. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Bi, Y.; Ma, C.; Bai, J.; Hu, Z.; Zhou, M. Biomass Derived Worm-like Nitrogen-Doped-Carbon Framework for Trace Determination of Toxic Heavy Metal Lead (II). Anal. Chim. Acta 2020, 1116, 16–26. [Google Scholar] [CrossRef]
- Radotić, K.; Djikanović, D.; Simonović Radosavljević, J.; Jović-Jovičić, N.; Mojović, Z. Comparative Study of Lignocellulosic Biomass and Its Components as Electrode Modifiers for Detection of Lead and Copper Ions. J. Electroanal. Chem. 2020, 862, 114010. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Gevaerd, A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar Obtained from Spent Coffee Grounds: Evaluation of Adsorption Properties and Its Application in a Voltammetric Sensor for Lead (II) Ions. Microchem. J. 2021, 165, 106114. [Google Scholar] [CrossRef]
- Zou, J.; Yu, Q.; Gao, Y.; Chen, S.; Huang, X.; Hu, D.; Liu, S.; Lu, L.-M. Bismuth Nanoclusters/Porous Carbon Composite: A Facile Ratiometric Electrochemical Sensing Platform for Pb2+ Detection with High Sensitivity and Selectivity. ACS Omega 2022, 7, 1132–1138. [Google Scholar] [CrossRef]
- Mamatha, K.M.; Srinivasa Murthy, V.; Ravikumar, C.R.; Murthy, H.C.A.; Kumar, V.G.D.; Kumar, A.N.; Jahagirdar, A.A. Facile Green Synthesis of Molybdenum Oxide Nanoparticles Using Centella Asiatica Plant: Its Photocatalytic and Electrochemical Lead Sensor Applications. Sens. Int. 2022, 3, 100153. [Google Scholar] [CrossRef]
- Su, Z.; Wang, J.; Hu, S.; Cheng, Y.; Yang, Y.; Zhou, S.; Chen, M.; Cao, Q.; Zhang, S.; Yang, L.; et al. In-Situ Reshaping Nano-Biochar on Electrode Surface for Machine Learning Assisted Selective Sensing of Pb2+ in Real Water Samples. Appl. Surf. Sci. 2024, 665, 160294. [Google Scholar] [CrossRef]
- Sharma, R.; Rana, D.S.; Awasthi, A.; Singh, D.; Ibrahim, A.A.; Umar, A.; Baskoutas, S. Nitrogen Functionalized Biomass Derived Mesoporous Carbon Nanomaterials for Electrochemical Detection of Lead (II) Ions. Heliyon 2024, 10, e39090. [Google Scholar] [CrossRef]
- Mampane, L.; Moothi, K.; Ntwampe, O.; Moloto, N.; Ndlovu, G.; Jijana, A.; Tetyana, P.; Mphuthi, N.; Ngqalakwezi, A.; Shumbula, P.; et al. Yerba Mate Tea Mediated Synthesis of Nanoscale Zero Valent Iron Particles and Their Application in Detection of Pb Ions in Water. Sens. Bio-Sens. Res. 2025, 47, 100728. [Google Scholar] [CrossRef]
- Fanfani, A.; Papini, S.; Bortolotti, E.; Vagnoni, G.; Saieva, C.; Bonaccorsi, G.; Caini, S. Cadmium in Biological Samples and Site-Specific Cancer Risk and Mortality: A Systematic Review of Original Articles and Meta-Analyses. Cancer Epidemiol. 2024, 92, 102550. [Google Scholar] [CrossRef] [PubMed]
- Rasin, P.; Ashwathi, A.V.; Basheer, S.M.; Haribabu, J.; Santibanez, J.F.; Garrote, C.A.; Arulraj, A.; Mangalaraja, R.V. Exposure to Cadmium and Its Impacts on Human Health: A Short Review. J. Hazard. Mater. Adv. 2025, 17, 100608. [Google Scholar] [CrossRef]
- Incebay, H.; Aktepe, L.; Leblebici, Z. An Electrochemical Sensor Based on Green Tea Extract for Detection of Cd(II) Ions by Differential Pulse Anodic Stripping Voltammetry. Surf. Interf. 2020, 21, 100726. [Google Scholar] [CrossRef]
- Jangi, S.H.; Khoobi, A. Detection of Cadmium Heavy Metal Ions Using a Nanostructured Green Sensor in Food, Biological and Environmental Samples. Food Chem. 2024, 458, 140307. [Google Scholar] [CrossRef]
- Rani, R.H.; Rahale, C.S.; Prasanthrajan, M.; Girija, S.; Wilson, J.; Sharmila, D.J.; Saranya, N.; Maragatham, S. Eco-Friendly Synthesis of Nanoceria Using Orange Peel Extract for Electrochemical Detection of Selective Cadmium Ions. Microchem. J. 2024, 202, 110794. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, X.; Liao, H.; Gong, S.; Hasan, M.; Zhou, X.; Gunasekaran, S. Bioengineering of Green rGO/Fe3O4 Nanocomposites for Rapid Cadmium Sensing and Dye Decomposition. Ceram. Inter. 2025, 51, 5273–5286. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, H.; Gong, S.; Lan, L.; Hasan, M.; Zhou, X.; Gunasekaran, S. Green Synthesis of Reduced Graphene Oxide Nanosheets: An Efficient Material for Electrochemical Sensor and Photocatalytic Agent. Microchem. J. 2025, 209, 112799. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef]
- Pavithra, K.G.; SundarRajan, P.; Kumar, P.S.; Rangasamy, G. Mercury Sources, Contaminations, Mercury Cycle, Detection and Treatment Techniques: A Review. Chemosphere 2023, 312, 137314. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Y.; Yang, S.; Li, L.; Song, L. Recent Advance of Microbial Mercury Methylation in the Environment. Appl. Microbiol. Biotechnol. 2024, 108, 235. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, J.; Xie, Y.; Peng, G.; Duan, L.; Hu, D.; Chen, S.; Qu, F.; Lu, L. Bifunctional Bismuth Molybdate/Biochar Composite with an Enhanced Photo-Assisted Electrochemical Activity: Memory Effect-Free Hg(II) Detection and Efficient Photocatalytic Reduction of Cr(VI). Chem. Eng. J. 2023, 468, 143849. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Peng, G.; Huang, H.; Wang, L.; Lu, L.; Gao, Y.; Hu, D.; Chen, S. An Electrochemical Sensor Based on a Porous Biochar/Cuprous Oxide (BC/Cu2O) Composite for the Determination of Hg(II). Molecules 2023, 28, 5352. [Google Scholar] [CrossRef] [PubMed]
- Hareesha, N.; Soumya, D.M.; Mounesh; Manjunatha, J.G.; Rohit, R.N.; Manikanta, P.; Varun, D.N.; Ataollahi, N.; Thippeswamy, B.A.; Pramoda, K.; et al. Honeycomb Polypore Biomass-Derived Activated Porous Carbon Nanosheets/Graphite/Nafion Composite: Green and Sensitive Electrocatalyst for Nanomolar Detection of Hg2+ Ions and Water-Splitting Reactions. J. Environ. Chem. Eng. 2024, 12, 113584. [Google Scholar] [CrossRef]
- Zou, J.; Zou, J.; Li, L.; Chen, H.; Liu, S.; Gao, Y.; Huang, X.; Wang, L.; Lu, L. Enhanced Electrocatalytic Activity in MOFs-Derived 3D Hollow NiCo-LDH Nanocages Decorated Porous Biochar for Simultaneously Ultra-Sensitive Electrochemical Sensing of Cu2+ and Hg2+. Talanta 2024, 279, 126624. [Google Scholar] [CrossRef]
- Karazan, Z.M.; Roushani, M.; Hoseini, S.J. Simultaneous Electrochemical Sensing of Heavy Metal Ions (Zn2+, Cd2+, Pb2+, and Hg2+) in Food Samples Using a Covalent Organic Framework/Carbon Black Modified Glassy Carbon Electrode. Food Chem. 2024, 442, 138500. [Google Scholar] [CrossRef]
- Suguihiro, T.M.; De Oliveira, P.R.; De Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. An Electroanalytical Approach for Evaluation of Biochar Adsorption Characteristics and Its Application for Lead and Cadmium Determination. Biores. Technol. 2013, 143, 40–45. [Google Scholar] [CrossRef]
- Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar Prepared from Castor Oil Cake at Different Temperatures: A Voltammetric Study Applied for Pb2+, Cd2+ and Cu2+ Ions Preconcentration. J. Hazard. Mater. 2016, 318, 526–532. [Google Scholar] [CrossRef]
- Zeinu, K.M.; Hou, H.; Liu, B.; Yuan, X.; Huang, L.; Zhu, X.; Hu, J.; Yang, J.; Liang, S.; Wu, X. A Novel Hollow Sphere Bismuth Oxide Doped Mesoporous Carbon Nanocomposite Material Derived from Sustainable Biomass for Picomolar Electrochemical Detection of Lead and Cadmium. J. Mater. Chem. A 2016, 4, 13967–13979. [Google Scholar] [CrossRef]
- Qin, D.; Gao, S.; Wang, L.; Shen, H.; Yalikun, N.; Sukhrobov, P.; Wagberg, T.; Zhao, Y.; Mamat, X.; Hu, G. Three-Dimensional Carbon Nanofiber Derived from Bacterial Cellulose for Use in a Nafion Matrix on a Glassy Carbon Electrode for Simultaneous Voltammetric Determination of Trace Levels of Cd(II) and Pb(II). Microchim. Acta 2017, 184, 2759–2766. [Google Scholar] [CrossRef]
- Dali, M.; Zinoubi, K.; Chrouda, A.; Abderrahmane, S.; Cherrad, S.; Jaffrezic-Renault, N. A Biosensor Based on Fungal Soil Biomass for Electrochemical Detection of Lead (II) and Cadmium (II) by Differential Pulse Anodic Stripping Voltammetry. J. Electroanal. Chem. 2018, 813, 9–19. [Google Scholar] [CrossRef]
- Ajab, H.; Ali Khan, A.A.; Nazir, M.S.; Yaqub, A.; Abdullah, M.A. Cellulose-Hydroxyapatite Carbon Electrode Composite for Trace Plumbum Ions Detection in Aqueous and Palm Oil Mill Effluent: Interference, Optimization and Validation Studies. Environ. Res. 2019, 176, 108563. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, H.; Fang, Y.; Guan, J.; Ma, S.; Pan, Y.; Zhang, M.; Zhu, H.; Liu, X.; Du, M. Detection of Trace Cd2+, Pb2+ and Cu2+ Ions via Porous Activated Carbon Supported Palladium Nanoparticles Modified Electrodes Using SWASV. Mater. Chem. Phys. 2019, 225, 433–442. [Google Scholar] [CrossRef]
- Guan, J.; Fang, Y.; Zhang, T.; Wang, L.; Zhu, H.; Du, M.; Zhang, M. Kelp-Derived Activated Porous Carbon for the Detection of Heavy Metal Ions via Square Wave Anodic Stripping Voltammetry. Electrocatalysis 2020, 11, 59–67. [Google Scholar] [CrossRef]
- Estrada-Aldrete, J.; Hernández-López, J.M.; García-León, A.M.; Peralta-Hernández, J.M.; Cerino-Córdova, F.J. Electroanalytical Determination of Heavy Metals in Aqueous Solutions by Using a Carbon Paste Electrode Modified with Spent Coffee Grounds. J. Electroanal. Chem. 2020, 857, 113663. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, B.; Chen, S.; Wu, L.; Yang, J.; Liang, S.; Xiao, K.; Hu, J.; Hou, H. Ultrasensitive and Simultaneous Electrochemical Determination of Pb2+ and Cd2+ Based on Biomass Derived Lotus Root-Like Hierarchical Porous Carbon/Bismuth Composite. J. Electrochem. Soc. 2020, 167, 087505. [Google Scholar] [CrossRef]
- Djebbi, M.A.; Allagui, L.; El Ayachi, M.S.; Boubakri, S.; Jaffrezic-Renault, N.; Namour, P.; Ben Haj Amara, A. Zero-Valent Iron Nanoparticles Supported on Biomass-Derived Porous Carbon for Simultaneous Detection of Cd2+ and Pb2+. ACS Appl. Nano Mater. 2022, 5, 546–558. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, L.; Guo, H.; Wei, Y.; Feng, H.; Liu, B.; Yu, J.; Wei, Y.; Zhang, X. Controllable Synthesis of Zeolitic Imidazolate Frameworks and the Peanut Shell Carbon Composite for Sensitive and Selective Detection of Pb2+ and Cd2+ Ions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130657. [Google Scholar] [CrossRef]
- Mosquera-Ortega, M.; Figueredo, F.; Fernandez, F.; Arnal, P.; Cortón, E.; Susmel, S. Peanut Shell Biochar for Plastic Electrodes: Green E-Sensors for Sensitive Heavy Metal Detection. Carbon Trends 2025, 20, 100520. [Google Scholar] [CrossRef]
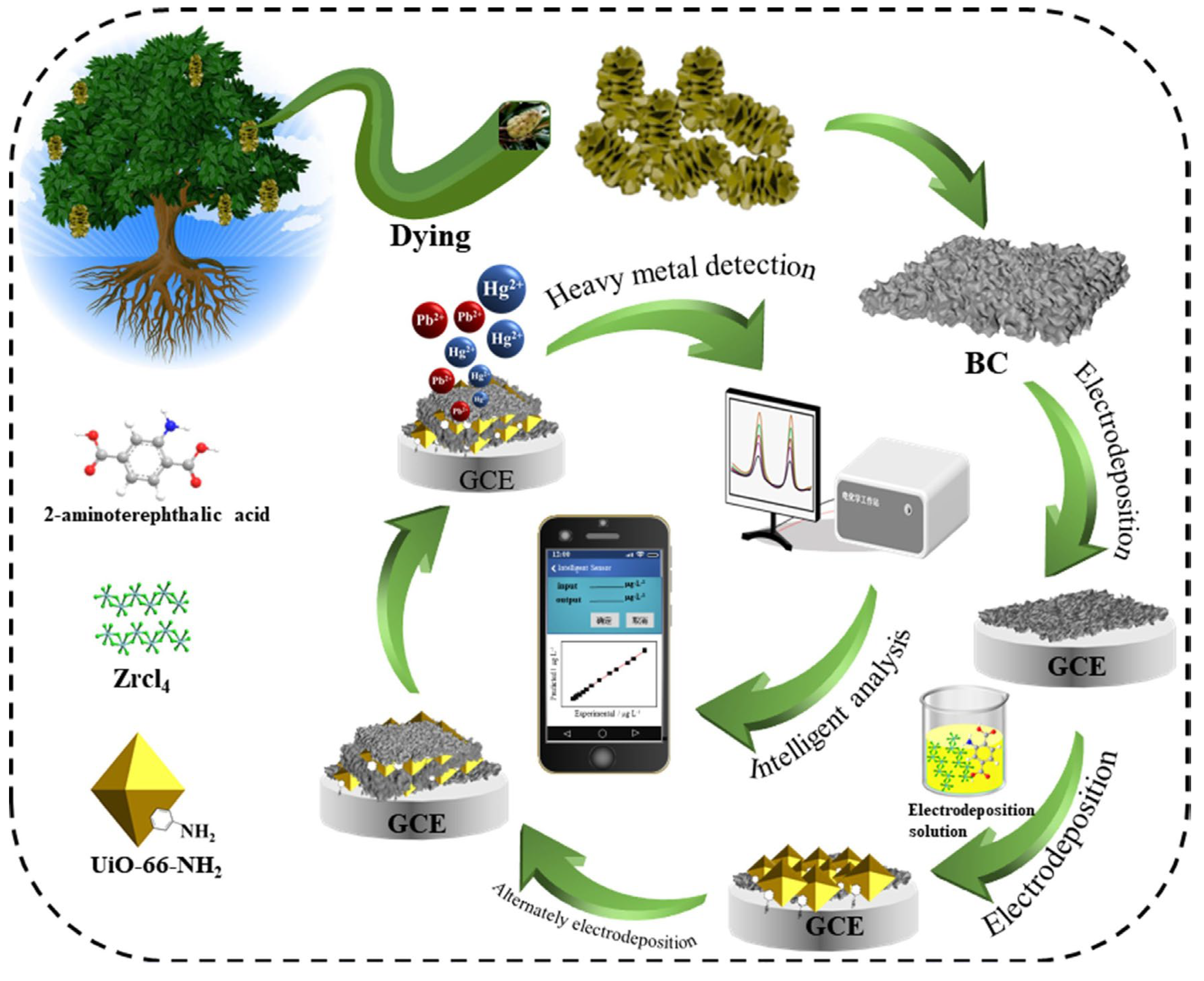
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer Activated Biochar/UiO-66-NH2 Film as Intelligent Sensing Platform for Ultra-Sensitive Electrochemical Detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006. [Google Scholar] [CrossRef]
- Elamin, M.B.; Chrouda, A.; Ali, S.M.A.; Alhaidari, L.M.; Jabli, M.; Alrouqi, R.M.; Renault, N.J. Electrochemical Sensor Based on Gum Arabic Nanoparticles for Rapid and In-situ Detection of Different Heavy Metals in Real Samples. Heliyon 2024, 10, e26364. [Google Scholar] [CrossRef]
- Okpara, E.C.; Fayemi, O.E.; Sherif, E.-S.M.; Ganesh, P.S.; Swamy, B.E.K.; Ebenso, E.E. Electrochemical Evaluation of Cd2+ and Hg2+ Ions in Water Using ZnO/Cu2ONPs/PANI Modified SPCE Electrode. Sens. Bio-Sens. Res. 2022, 35, 100476. [Google Scholar] [CrossRef]
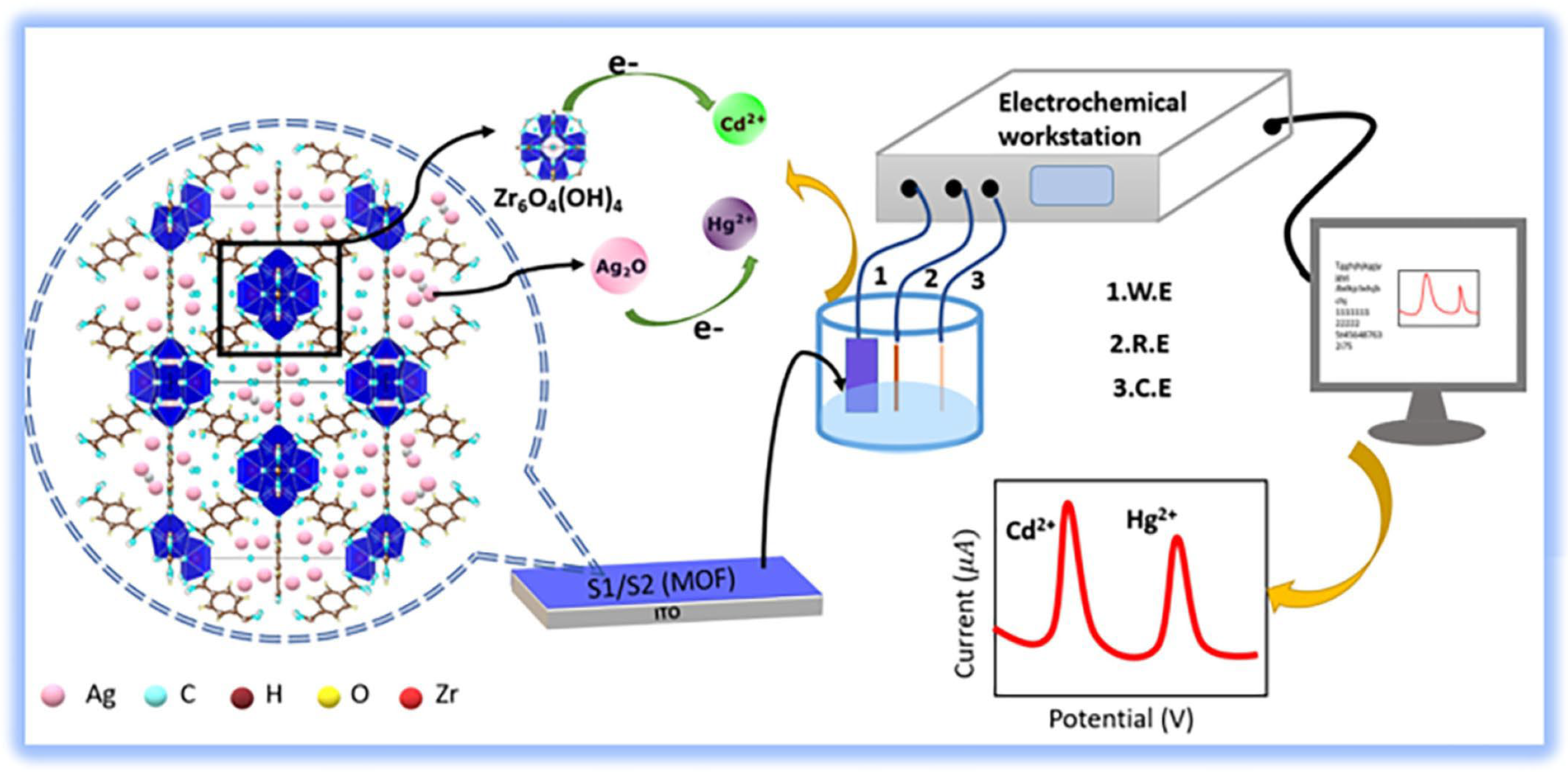
- Doloi, K.; Badhai, N.; Mohanta, D. Nanoscale Ag2O Decorated UiO-66 Metal Organic Framework for Simultaneous Electrochemical Sensing of Heavy Metals, Cd2+ and Hg2+. Mater. Res. Bull. 2024, 170, 112558. [Google Scholar] [CrossRef]
- Madhu, R.; Sankar, K.V.; Chen, S.-M.; Selvan, R.K. Eco-Friendly Synthesis of Activated Carbon from Dead Mango Leaves for the Ultrahigh Sensitive Detection of Toxic Heavy Metal Ions and Energy Storage Applications. RSC Adv. 2014, 4, 1225–1233. [Google Scholar] [CrossRef]
- Djemmoe, L.G.; Njanja, E.; Tchieno, F.M.M.; Ndinteh, D.T.; Ndungu, P.G.; Tonle, I.K. Activated Hordeum vulgare, L. Dust as Carbon Paste Electrode Modifier for the Sensitive Electrochemical Detection of Cd2+, Pb2+ and Hg2+ Ions. Inter. J. Environ. Anal. Chem. 2020, 100, 1429–1445. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabó, T.; Trif, L.; Felhősi, I.; Abbi, K.; Labjar, N.; Harmouche, L.; Shaban, A. Biomass Valorization of Walnut Shell into Biochar as a Resource for Electrochemical Simultaneous Detection of Heavy Metal Ions in Water and Soil Samples: Preparation, Characterization, and Applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- Pu, H.; Ruan, S.; Yin, M.; Sun, Q.; Zhang, Y.; Gao, P.; Liang, X.; Yin, W.; Fa, H. Performance Comparison of Simultaneous Detection Heavy-Metal Ions Based on Carbon Materials Electrochemical Sensor. Microchem. J. 2022, 181, 107711. [Google Scholar] [CrossRef]
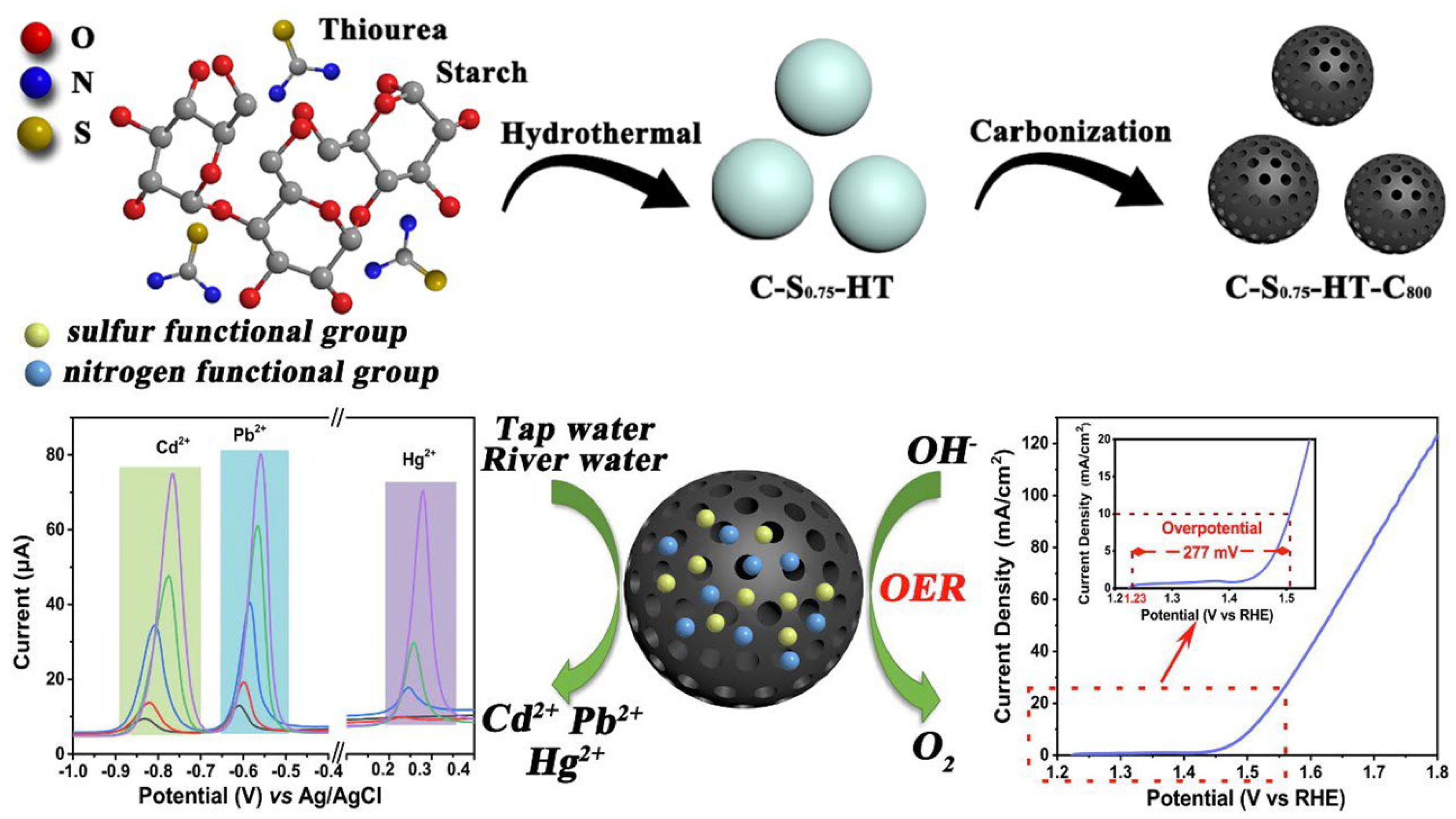
- Li, H.; Li, N.; Zuo, P.; Qu, S.; Qin, F.; Shen, W. Utilization of Nitrogen, Sulfur Co-Doped Porous Carbon Micron Spheres as Bifunctional Electrocatalysts for Electrochemical Detection of Cadmium, Lead and Mercury Ions and Oxygen Evolution Reaction. J. Colloid Interf. Sci. 2023, 640, 391–404. [Google Scholar] [CrossRef]
- Huang, P.; Xiong, Y.; Ge, Y.; Wen, Y.; Zeng, X.; Zhang, J.; Wang, P.; Wang, Z.; Chen, S. Magnetic Fe3O4 Nanoparticles Decorated Phosphorus-Doped Biochar-Attapulgite/Bismuth Film Electrode for Smartphone-Operated Wireless Portable Sensing of Ultra-Trace Multiple Heavy Metal Ions. Microchim. Acta 2023, 190, 94. [Google Scholar] [CrossRef]

| Biomass Feed | Synthesis Method | Technique | E (V) | Linear Range (µM) | LOD (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Pennisetum setosum | Crosslink-xanthate | DPASV | −0.55 a | - | 0.048 | Groundwater, canal water | [59] |
| Banana tissue | Drying | DPASV | −0.5 a | 4.8–96.5 | 0.483 | Water | [60] |
| Castor oil cake | Pyrolysis | DPAdSV | −0.6 a | 0.005–1.0 | 0.001 | Ceramic dishes | [61] |
| Cladophora rupestris | - | DPASV | −0.3 a | 0.96–4.83 | 0.35 | - | [62] |
| Oil palm empty fruit bunches | Pyrolysis | SWASV | −0.5 a | 0.048–0.29 | 0.001 | Blood serum | [63] |
| Bayberry kernel | Pyrolysis | CV | −0.5 | 48.3–3861 | ~0.0002 | - | [64] |
| Almond shells | HTC | DPASV | −0.6 a | 9.65–579.15 | 3.4 | Tap water | [65] |
| Acacia senegal | - | DPV | −0.55 a | 4.826–48.26 | 2.442 | Plastic toys | [66] |
| Benincasa hispida + Holstein Friesian | HTC | DPASV | −0.63 a | 2.4–482.6 | 0.965 | Tap water, lake water | [67] |
| Zea mays L. | - | SWV | −0.5 a | 0.1–5.0 | 0.02 | Tap water | [68] |
| Spent coffee grounds | Pyrolysis | DPAdSV | −0.48 a | 0.128–2.44 | 0.004 | Gunshot residues Hair dye | [69] |
| Litsea cubeba | Pyrolysis | DPASV | −0.2 b | 14.5–4.8 | 0.000005 | Paddy water | [70] |
| Centella Asiatica | Pyrolysis | CV | −0.07 a | - | - | - | [71] |
| Tea residue | Pyrolysis | DPASV | −0.6 a | 0.386–5.791 | 0.002365 | River water, farmland water | [72] |
| Coconut husk | HTC | DPASV | −0.55 a | 0.06–0.14 | 0.022 | Tap water | [73] |
| Yerba-mate tea extracts | HTC | SWV | - | 0.027–0.034 | 0.012 | - | [74] |
| Biomass Feed | Synthesis Method | Technique | E (V) | Linear Range (µM) | LOD (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Green tea extract | Bioextraction | DPASV | −0.75 a | 0.05–6.0 | 0.00101 | River water, drinking water | [77] |
| Sour tea extract | Sol–gel | DPV | −0.9 a | 0.02–20 | 0.00110 | Milk, chocolate | [78] |
| Orange peel | Biological method | SWV | −0.95 a | 10–1000 | 0.00047 | - | [79] |
| Plumeria alba | HTC | DPSV | −0.82 b | 0.00996–149.45 | 8.9 × 10−6 | River water, tap water, rice | [80] |
| Plumeria alba | Freeze drying | DPSV | −0.82 b | 0.0747–9.96 | 4.98 × 10−5 | - | [81] |
| Biomass Feed | Synthesis Method | Technique | E (V) | Linear Range (µM) | LOD (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Litsea cubeba | HTC | DPASV | +0.2 b | 0.000448–4.487 | 0.00015 | Paddy water, apple | [85] |
| Litsea cubeba | HTC | DPASV | +0.2 b | 4.99 × 10−6–4.987 | 1.50 × 10−6 | Lettuce | [86] |
| Honeycomb polypore | HTC | DPV | +0.2 b | 0.05–2 | 0.002309 | Lake water, tap water, sewage water | [87] |
| Magnolia soulangeana leaves | Pyrolysis | DPASV | +0.12 a | 0.0005–3.49 | 0.00015 | Lake water | [88] |
| Biomass Feed | Synthesis Method | Technique | E (V) | Target Ions | Linear Range (µM) | LOD (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Castor oil cake | Ball milling | DPAdSV | −0.6 a −0.8 a | Pb2+ Cd2+ | 0.05–10 0.25–50 | 0.0098 0.069 | Water | [90] |
| Castor oil cake | Pyrolysis | DPAdSV | −0.6 a −0.75 a 0.0 a | Pb2+ Cd2+ Cu2+ | - | - | - | [91] |
| Corn flour | Hydrolysis | SWASV | −0.4 a −0.6 a | Pb2+ Cd2+ | 0.5 × 10−6– 10 × 10−6 10 × 10−6– 100 × 10−6 | 1.72 × 10−6 1.58 × 10−6 | Lake water | [92] |
| Bacterial cellulose | Freeze drying | DPASV | −0.6 a −0.8 a | Pb2+ Cd2+ | 0.0096–0.4825 0.0178–0.8896 | 0.001592 0.003380 | Tap water Waste water | [93] |
| Trichoderma asperellum | Ball milling | DPASV | −0.6 a −0.8 a | Pb2+ Cd2+ | - | 0.01 0.1 | Water | [94] |
| OPEFB | Thermolysis | SWASV | −0.5 a −0.8 a 0.0 a −1.1 a | Pb2+ Cd2+ Cu2+ Zn2+ | 0.0483–0.2413 0.0889–0.4448 0.157–0.787 0.153–0.765 | 0.00053 0.00098 0.00173 0.00168 | Tap water Blood serum Palm oil mill effluent | [95] |
| Pomelo peels | Thermolysis | SWASV | −0.6 b −0.8 b 0.0 b | Pb2+ Cd2+ Cu2+ | 0.025–0.500 0.025–0.500 0.025–0.500 | 0.00919 0.0209 0.01478 | Water | [96] |
| Kelp | Freeze drying | SWASV | −0.6 b −0.8 b | Pb2+ Cd2+ | 0.01–0.5 0.00445–0.4448 | 0.01138 0.0233 | - | [97] |
| Spent coffee grounds | Thermolysis | DPASV | −0.4 a −0.65 a | Pb2+ Cd2+ | 30–189 30–269 | 90 89 | - | [98] |
| Platanus | Microwave-assisted solvothermal pyrolysis | SWASV | −0.55 a −0.8 a | Pb2+ Cd2+ | 0.0024–0.2413 0.0178–0.4448 | 0.000627 0.000712 | Tap water Lake water | [99] |
| Posidonia oceanica | Pyrolysis | SWASV | −0.55 b −0.8 b | Pb2+ Cd2+ | 0.0096–0.2413 0.01–0.45 | 0.001005 0.001713 | Drinking water | [100] |
| Peanut shell | Pyrolysis | DPASV | −0.5 a −0.75 a | Pb2+ Cd2+ | 0.1–8 0.06–9 | 0.008250 0.016 | Domestic sewage | [101] |
| Peanut shell | Pyrolysis | SWASV | −0.55 a −0.7 a | Pb2+ Cd2+ | 0.2413–2.413 0.4448–4.448 | 0.063 0.182 | Seawater | [102] |
| Magnolia grandiflora | Pyrolysis | DPASV | −0.55 b +0.13 b | Pb2+ Hg2+ | 0.000014–4.826 0.000015–4.985 | 0.00000483 0.00000499 | River water Paddy water | [103] |
| Gum arabic | - | DPASV | −0.6 a +0.1 a −0.1 a −1.1 a | Pb2+ Hg2+ Cu2+ Zn2+ | 24.13–1448.55 4.99–997.0 157.4–4723.0 30.58–2293.5 | 20.26 4.48 151.15 29.06 | Farm water | [104] |
| Orange peel Lemon peel | Bioreduction | SWV | −0.9 a +0.05 a | Cd2+ Hg2+ Cd2+ Hg2+ | 2.2–12 2.95–11.8 0.17–1.5 0.12–1.2 | 0.0270 0.0253 0.0096 0.0135 | - | [105] |
| Banana root bulb | Pyrolysis | DPASV | −0.8 a +0.1 a | Cd2+ Hg2+ | 0.01–2 0.06–1.5 | 0.008 0.003 | Lake water | [106] |
| Mangifera indica | Thermolysis | DPV | −0.75 a −0.55 a 0.0 a +0.25 a | Cd2+ Pb2+ Cu2+ Hg2+ | 0.09–4.8 0.09–5.7 0.09–4.8 0.09–0.99 | 24.4 0.0057 23.2 0.0246 | - | [107] |
| Hordeum vulgare L. | Thermolysis | DPASV | −0.5 a −0.75 a +0.1 a | Pb2+ Cd2+ Hg2+ | 0.0002–0.014 0.01–0.085 0.001–0.0115 | 0.000069 0.001820 0.000237 | River water Tap water | [108] |
| Walnut shell | Pyrolysis | SWV | −0.55 b −0.75 b 0.0 b +0.3 b | Pb2+ Cd2+ Cu2+ Hg2+ | 0.001–0.075 0.001–0.075 0.005–0.075 0.005–0.075 | 0.000175 0.000086 0.000246 0.000383 | River water Soil | [109] |
| Oak, pine, and peach wood | Pyrolysis | SWV | −0.5 a −0.78 a 0.22 a | Pb2+ Cd2+ Hg2+ | 0.5–6.0 0.5–6.0 0.5–6.0 | 0.366 0.09 0.489 | Lake water Cucumber | [110] |
| Starch | HTC | SWASV | −0.6 a −0.85 a +0.25 a | Pb2+ Cd2+ Hg2+ | 0.25–2 0.25–2 0.25–2 | 0.00191 0.00138 - | Tap water River water | [111] |
| Cinnamomum camphoras (L.) | HTC | SWASV | −0.55 b −0.75 b +0.2 b | Pb2+ Cd2+ Hg2+ | 0.00001–7 0.0001–5 0.0001–3 | 0.000003 0.000036 0.000011 | Tap water Lake water | [112] |
| Biomass Feed | Target Ions | Linear Range (µM) | LOD (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|
| Pennisetum setosum | Pb2+ | - | 0.048 | Groundwater, canal water | [59] |
| Banana tissue | Pb2+ | 4.8–96.5 | 0.483 | Water | [60] |
| Castor oil cake | Pb2+ | 0.005–1.0 | 0.001 | Ceramic dishes | [61] |
| Cladophora rupestris | Pb2+ | 0.96–4.83 | 0.35 | - | [62] |
| Oil palm empty fruit bunches | Pb2+ | 0.048–0.29 | 0.001 | Blood serum | [63] |
| Bayberry kernel | Pb2+ | 48.3–3861 | ~0.0002 | - | [64] |
| Almond shells | Pb2+ | 9.65–579.15 | 3.4 | Tap water | [65] |
| Acacia senegal | Pb2+ | 4.826–48.26 | 2.442 | Plastic toys | [66] |
| Benincasa hispida + Holstein Friesian | Pb2+ | 2.4–482.6 | 0.965 | Tap water, lake water | [67] |
| Zea mays L. | Pb2+ | 0.1–5.0 | 0.02 | Tap water | [68] |
| Spent coffee grounds | Pb2+ | 0.128–2.44 | 0.004 | Gunshot residues Hair dye | [69] |
| Litsea cubeba | Pb2+ | 14.5–4.8 | 0.000005 | Paddy water | [70] |
| Centella Asiatica | Pb2+ | - | - | - | [71] |
| Tea residue | Pb2+ | 0.386–5.791 | 0.002365 | River water, farmland water | [72] |
| Coconut husk | Pb2+ | 0.06–0.14 | 0.022 | Tap water | [73] |
| Yerba-mate tea extracts | Pb2+ | 0.027–0.034 | 0.012 | - | [74] |
| Green tea extract | Cd2+ | 0.05–6.0 | 0.00101 | River water, drinking water | [77] |
| Sour tea extract | Cd2+ | 0.02–20 | 0.00110 | Milk, chocolate | [78] |
| Orange peel | Cd2+ | 10–1000 | 0.00047 | - | [79] |
| Plumeria alba | Cd2+ | 0.00996–149.45 | 8.9 × 10−6 | River water, tap water, rice | [80] |
| Plumeria alba | Cd2+ | 0.0747–9.96 | 4.98 × 10−5 | - | [81] |
| Litsea cubeba | Hg2+ | 0.000448–4.487 | 0.00015 | Paddy water, apple | [85] |
| Litsea cubeba | Hg2+ | 4.99 × 10−6–4.987 | 1.50 × 10−6 | Lettuce | [86] |
| Honeycomb polypore | Hg2+ | 0.05–2 | 0.002309 | Lake water, tap water, sewage water | [87] |
| Magnolia soulangeana leaves | Hg2+ | 0.0005 to 3.49 | 0.00015 μM | Lake water | [88] |
| Castor oil cake | Pb2+ Cd2+ | 0.05–10 0.25–50 | 0.0098 0.069 | Water | [90] |
| Castor oil cake | Pb2+ Cd2+ Cu2+ | - | - | - | [91] |
| Corn flour | Pb2+ Cd2+ | 0.5 × 10−6- 10 × 10−6 10 × 10−6- 100 × 10−6 | 1.72 × 10−6 1.58 × 10−6 | Lake water | [92] |
| Bacterial cellulose | Pb2+ Cd2+ | 0.0096–0.4825 0.0178–0.8896 | 0.001592 0.003380 | Tap water Waste water | [93] |
| Trichoderma asperellum | Pb2+ Cd2+ | - | 0.01 0.1 | Water | [94] |
| OPEFB | Pb2+ Cd2+ Cu2+ Zn2+ | 0.0483–0.2413 0.0889–0.4448 0.157–0.787 0.153–0.765 | 0.00053 0.00098 0.00173 0.00168 | Tap water Blood serum Palm oil mill effluent | [95] |
| Pomelo peels | Pb2+ Cd2+ Cu2+ | 0.025–0.500 0.025–0.500 0.025–0.500 | 0.00919 0.0209 0.01478 | Water | [96] |
| Kelp | Pb2+ Cd2+ | 0.01–0.5 0.00445–0.4448 | 0.01138 0.0233 | - | [97] |
| Spent coffee grounds | Pb2+ Cd2+ | 30–189 30–269 | 90 89 | - | [98] |
| Platanus | Pb2+ Cd2+ | 0.0024–0.2413 0.0178–0.4448 | 0.000627 0.000712 | Tap water Lake water | [99] |
| Posidoniaoceanica | Pb2+ Cd2+ | 0.0096–0.2413 0.01–0.45 | 0.001005 0.001713 | Drinking water | [100] |
| Peanut shell | Pb2+ Cd2+ | 0.1–8 0.06–9 | 0.008250 0.016 | Domestic sewage | [101] |
| Peanut shell | Pb2+ Cd2+ | 0.2413–2.413 0.4448–4.448 | 0.063 0.182 | Seawater | [102] |
| Magnolia grandiflora | Pb2+ Hg2+ | 0.000014–4.826 0.000015–4.985 | 0.00000483 0.00000499 | River water Paddy water | [103] |
| Gum arabic | Pb2+ Hg2+ Cu2+ Zn2+ | 24.13–1448.55 4.99–997.0 157.4–4723.0 30.58–2293.5 | 20.26 4.48 151.15 29.06 | Farm water | [104] |
| Orange peel Lemon peel | Cd2+ Hg2+ Cd2+ Hg2+ | 2.2–12 2.95–11.8 0.17–1.5 0.12–1.2 | 0.0270 0.0253 0.0096 0.0135 | - | [105] |
| Banana root bulb | Cd2+ Hg2+ | 0.01–2 0.06–1.5 | 0.008 0.003 | Lake water | [106] |
| Mangifera indica | Cd2+ Pb2+ Cu2+ Hg2+ | 0.09–4.8 0.09–5.7 0.09–4.8 0.09–0.99 | 24.4 0.0057 23.2 0.0246 | - | [107] |
| Hordeum vulgare L. | Pb2+ Cd2+ Hg2+ | 0.0002–0.014 0.01–0.085 0.001–0.0115 | 0.000069 0.001820 0.000237 | River water Tap water | [108] |
| Walnut shell | Pb2+ Cd2+ Cu2+ Hg2+ | 0.001–0.075 0.001–0.075 0.005–0.075 0.005–0.075 | 0.000175 0.000086 0.000246 0.000383 | River water Soil | [109] |
| Oak, pine, and peach wood | Pb2+ Cd2+ Hg2+ | 0.5–6.0 0.5–6.0 0.5–6.0 | 0.366 0.09 0.489 | Lake water Cucumber | [110] |
| Starch | Pb2+ Cd2+ Hg2+ | 0.25–2 0.25–2 0.25–2 | 0.00191 0.00138 - | Tap water River water | [111] |
| Cinnamomum camphoras (L.) | Pb2+ Cd2+ Hg2+ | 0.00001–7 0.0001–5 0.0001–3 | 0.000003 0.000036 0.000011 | Tap water Lake water | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikrithika, S.; Kim, Y.-J. Biochar-Derived Electrochemical Sensors: A Green Route for Trace Heavy Metal Detection. Chemosensors 2025, 13, 278. https://doi.org/10.3390/chemosensors13080278
Saikrithika S, Kim Y-J. Biochar-Derived Electrochemical Sensors: A Green Route for Trace Heavy Metal Detection. Chemosensors. 2025; 13(8):278. https://doi.org/10.3390/chemosensors13080278
Chicago/Turabian StyleSaikrithika, Sairaman, and Young-Joon Kim. 2025. "Biochar-Derived Electrochemical Sensors: A Green Route for Trace Heavy Metal Detection" Chemosensors 13, no. 8: 278. https://doi.org/10.3390/chemosensors13080278
APA StyleSaikrithika, S., & Kim, Y.-J. (2025). Biochar-Derived Electrochemical Sensors: A Green Route for Trace Heavy Metal Detection. Chemosensors, 13(8), 278. https://doi.org/10.3390/chemosensors13080278







