Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada
Abstract
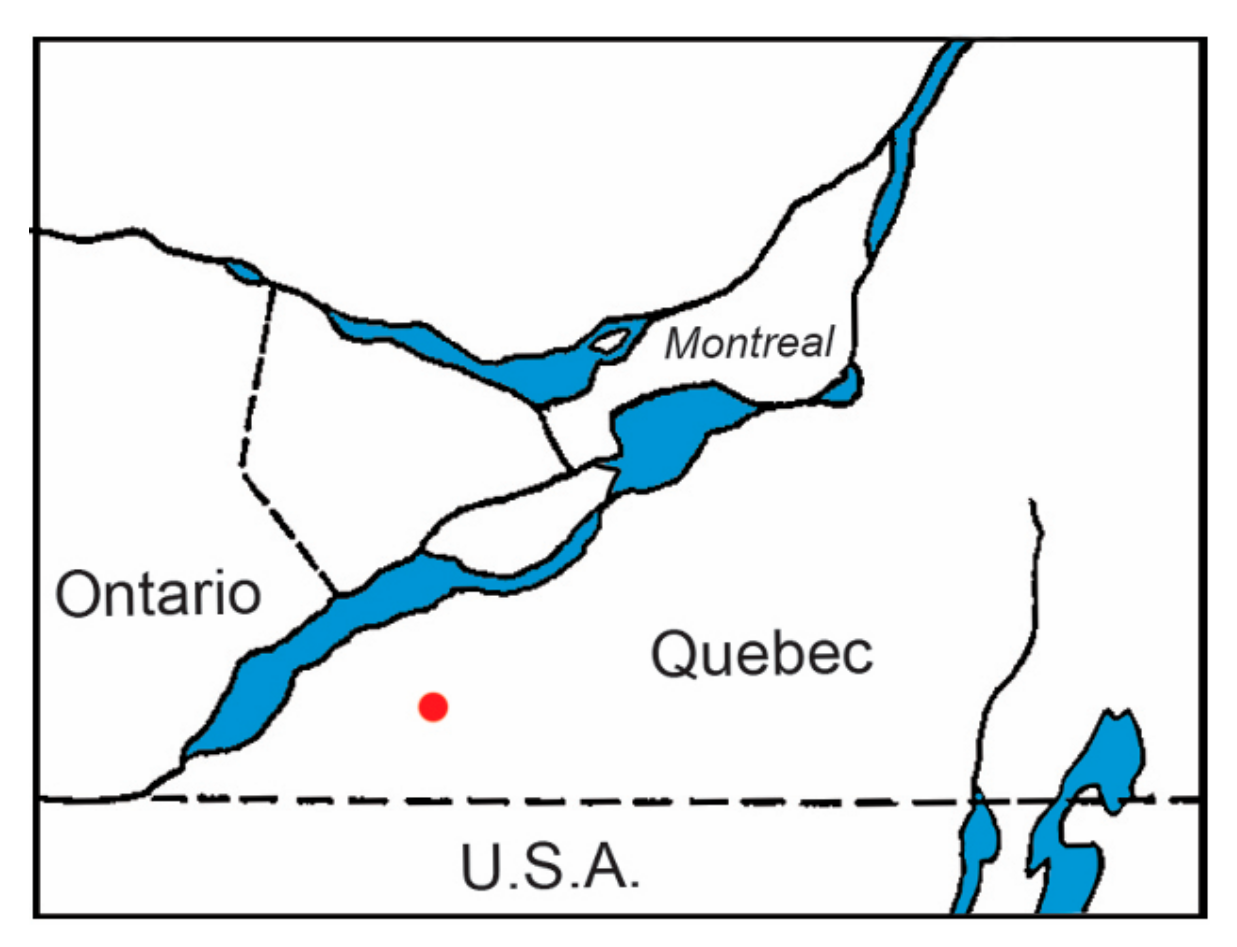
1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. Spirochete Detection
3. Results
3.1. Tick Collection
3.2. Spirochete Detection
4. Discussion
4.1. Nesting Area Marks Established Population of Blacklegged Ticks
4.2. Short-Distance Flights Signify Tick Populations
4.3. Songbirds as Reservoir Hosts
4.4. H. leporispalustris Vector Competency
4.5. Ticks Co-infest Songbirds
4.6. Definition of Lyme Disease Endemic Area
4.7. Clinical Manifestations of Lyme Disease
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicholson, W.A.; Sonenshine, D.E.; Noden, B.H. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press/Elsevier: London, UK, 2019; pp. 603–672. ISBN 978-0-12-814043-7. [Google Scholar]
- Scott, J.D.; Fernando, K.; Banerjee, S.N.; Durden, L.A.; Byrne, S.K.; Banerjee, M.; Mann, R.B.; Morshed, M.G. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol. 2001, 38, 493–500. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of I. scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Durden, L.A.; Jorgensen, D.R.; Mak, S.; Morshed, M.G. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J. Vector Ecol. 2010, 35, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Scott, J.D. Birds Widely Disperse Pathogen-Infected Ticks. In Seabirds and Songbirds: Habitat Preferences, Conservation, Migratory Behavior; Mahala, G., Ed.; Nova Publishers, Inc.: New York, NY, USA, 2015; pp. 1–22. ISBN 978-1-63463-496-0. [Google Scholar]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Bierman, B.C.; Durden, L.A. Far-reaching dispersal of Borrelia burgdorferi sensu lato-infected blacklegged ticks by migratory songbirds in Canada. Healthcare 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef]
- DeLuca, W.V.; Woodworth, B.K.; Rimmer, C.C.; Marra, P.P.; Taylor, P.D.; McFarland, K.P.; Mackenzie, S.A.; Norris, D.R. Transoceanic migration by a 12 g songbird. Biol. Lett. 2015, 11, 20141045. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Kaiser, M.N. Ticks from European-Asiatic birds migrating through Egypt into Africa. Science 1961, 133, 277–278. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. Amblyomma dissimile Koch (Acari: Ixodidae) parasitizes bird captured in Canada. Syst. Appl. Acarol. 2015, 20, 854–860. [Google Scholar]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. First record of Amblyomma rotundatum tick (Acari: Ixodidae) parasitizing a bird collected in Canada. Syst. Appl. Acarol. 2015, 20, 155–161. [Google Scholar]
- Scott, J.D.; Durden, L.A. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int. J. Acarol. 2015, 41, 241–249. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Durden, L.A.; Manord, J.M.; Smith, M.L. First record of Ixodes affinis tick (Acari: Ixodidae) infected with Borrelia burgdorferi sensu lato collected from a migratory songbird in Canada. J. Bacteriol. Parasitol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. Songbird-transported tick Ixodes minor (Ixodida: Ixodidae) discovered in Canada. Can. Entomol. 2015, 147, 46–50. [Google Scholar] [CrossRef]
- Anderson, J.F.; Magnarelli, L.A. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 1984, 57, 627–641. [Google Scholar]
- Anderson, J.F.; Magnarelli, L.A.; Stafford, K.C., III. Bird-feeding ticks transstadially transmit Borrelia burgdorferi that infect Syrian hamsters. J. Wildl. Dis. 1990, 26, 1–10. [Google Scholar] [CrossRef]
- Scott, J.D.; Scott, C.M.; Anderson, J.F. The establishment of a blacklegged tick population by migratory songbirds in Ontario, Canada. J. Veter Sci. Med. 2014, 2, 5. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease—A tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef]
- Baranton, G.; Postic, D.; Saint Girons, I.; Boerlin, P.; Piffaretti, J.-C.; Assous, M.; Grimont, P.A.D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and Group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 1992, 42, 378–383. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Anderson, J.F.; Foley, J.E.; Young, M.R.; Durden, L.A. Lyme disease bacterium, Borrelia burgdorferi sensu lato, detected in multiple tick species at Kenora, Ontario, Canada. J. Bacteriol. Parasitol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Scott, J.D.; Foley, J.E. Detection of Borrelia americana in the avian coastal tick, Ixodes auritulus (Acari: Ixodidae), collected from a bird captured in Canada. Open J. Anim. Sci. 2016, 6, 207–216. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Durden, L.A.; Manord, J.M.; Smith, M.L. Detection of Borrelia genomospecies 2 in Ixodes spinipalpis ticks collected from a rabbit in Canada. J. Parasitol. 2017, 103, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sertour, N.; Cotté, V.; Garnier, M.; Malandrin, L.; Ferguel, E.; Choumet, V. Infection kinetics and tropism of Borrelia burgdorferi sensu lato in mouse after natural (via ticks) or artificial (needle) infection depends on the bacterial strain. Front. Microbiol. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Hynote, E.D.; Mervine, P.C.; Stricker, R.B. Clinical evidence for rapid transmission of Lyme disease following a tickbite. Diagn. Microbiol. Infect. Dis. 2012, 72, 188–192. [Google Scholar] [CrossRef]
- Cook, M.J. Lyme borreliosis: A review of data on transmission time after tick attachment. Int. J. Gen. Med. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef]
- Clifford, C.M.; Anastos, G.; Elbl, A. The larval ixodid ticks of the eastern United States. Misc. Publ. Entomol. Soc. Am. 1961, 2, 213–237. [Google Scholar]
- Keirans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef]
- Durden, L.A.; Keirans, J.E. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. In Monographs: Thomas Say Publications in Entomology; Entomological Society of America: Lanham, MD, USA, 1996; p. 95. ISBN 0-938522-57. [Google Scholar]
- Cooley, R.A. The Genera Boophilus, Rhipicephalus, and Haemaphysalis (Ixodidae) of the New World. In National Institute of Health Bulletin No. 187; U.S. Government: Washington, DC, USA, 1946; p. 54. [Google Scholar]
- Barbour, A.G.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996, 17, 403–409. [Google Scholar] [CrossRef]
- Barbour, A.G.; Bunikis, J.; Travinsky, B.; Hoen, A.G.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef]
- Clark, K.; Hendricks, A.; Burge, D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 2005, 71, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX–Windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tools. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, A.; Kitron, U.; Jones, C.J.; Slajchert, T.L. Influence of season and habitat on Ixodes scapularis infestation on white-footed mice in northeastern Illinois. J. Parasitol. 1994, 80, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Peavey, C.A.; Lane, R.S. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J. Parasitol. 1995, 81, 175–178. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.G.; Ubico, S.R.; Cooksey, L.M. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi). J. Wildl. Dis. 1993, 29, 527–532. [Google Scholar] [CrossRef]
- Rand, P.W.; Lacombe, E.H.; Smith, R.P., Jr.; Rich, S.M.; Kilpatrick, C.A.; Dragoni, C.A.; Caporale, D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in the wild. J. Med. Entomol. 1993, 30, 614–618. [Google Scholar] [CrossRef]
- Anderson, J.F.; Johnson, R.C.; Magnarelli, L.A.; Hyde, F.W. Involvement of birds in the epidemiology of the Lyme disease agent Borrelia burgdorferi. Infect Immun. 1986, 51, 394–396. [Google Scholar] [CrossRef]
- Rand, P.W.; Lacombe, E.H.; Smith, R.P., Jr.; Ficker, J. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. J. Med. Entomol. 1998, 35, 270–276. [Google Scholar] [CrossRef]
- Stafford, K.C., III; Bladen, V.C.; Magnarelli, L.A. Ticks (Acari: Ixodidae) infesting wild birds (Aves) and white-footed mice in Lyme, CT. J. Med. Entomol. 1995, 32, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Spielman, A.; Komar, N.; Matuschka, F.-R. Competence of American Robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.N.; Banerjee, M.; Fernando, K.; Dong, M.Y.; Smith, J.A.; Cook, D. Isolation of Borrelia burgdorferi, the Lyme disease spirochete from rabbit ticks, Haemaphysalis leporispalustris from Alberta. J. Spir. Tick-Borne Dis. 1995, 2, 23–24. [Google Scholar]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Detection and transstadial passage of Babesia species and Borrelia burgdorferi sensu lato in ticks collected from avian and mammalian hosts in Canada. Healthcare 2019, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.N.; Morshed, M.G.; Scott, J.D. Epizootiology of the Lyme Disease Spirochete, Borrelia burgdorferi in Blacklegged Ticks, Ixodes scapularis and Small Mammals at Point Pelee National Park; Point Pelee National Park Library: Leamington, ON, Canada, 2000. [Google Scholar]
- Scott, J.D.; Fernando, K.; Durden, L.A.; Morshed, M.G. Lyme disease spirochete, Borrelia burgdorferi, endemic in epicenter at Turkey Point, Ontario. J. Med. Entomol. 2004, 41, 226–230. [Google Scholar] [CrossRef]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Jorgensen, D.R.; Durden, L.A.; Morshed, M.G. Rapid introduction of Lyme disease spirochete, Borrelia burgdorferi sensu stricto, in Ixodes scapularis (Acari: Ixodidae) established at Turkey Point Provincial Park, Ontario, Canada. J. Vector Ecol. 2008, 33, 64–69. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Banerjee, S.N.; Fernando, K.; Mann, R.; Isaac-Renton, J. First isolation of Lyme disease spirochete, Borrelia burgdorferi from blacklegged tick, Ixodes scapularis, collected at Rondeau Provincial Park, Ontario. Can. Commun. Dis. Rep. 2000, 26, 42–44. [Google Scholar]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Mann, R.B.; Durden, L.A. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J. Med. Entomol. 2003, 40, 91–94. [Google Scholar] [CrossRef]
- Scott, J.D.; Anderson, J.F.; Durden, L.A.; Smith, M.L.; Manord, J.M.; Clark, K.L. Prevalence of the Lyme disease spirochete, Borrelia burgdorferi, in blacklegged ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario. Int. J. Med. Sci. 2016, 13, 316–324. [Google Scholar] [CrossRef]
- Scott, J.D.; Foley, J.E.; Anderson, J.F.; Clark, K.L.; Durden, L.A. Detection of Lyme disease bacterium, Borrelia burgdorferi sensu lato, in blacklegged ticks collected in the Grand River Valley, Ontario, Canada. Int. J. Med. Sci. 2017, 14, 150–158. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, M.; Scott, J.; Lankester, M.; Kubinec, J. Isolation of Borrelia burgdorferi−Thunder Bay District, Ontario. Can. Commun. Dis. Rep. 1996, 22, 138–140. [Google Scholar] [PubMed]
- Banerjee, S.N.; Christensen, C.I.; Scott, J.D. Isolation of Borrelia burgdorferi on mainland Ontario. Can. Commun. Dis. Rep. 1995, 21, 85–86. [Google Scholar] [PubMed]
- Scott, J.D.; Foley, J.E.; Clark, K.L.; Anderson, J.F.; Durden, L.A.; Manord, J.M.; Smith, M.L. Established population of blacklegged ticks with high infection prevalence for the Lyme disease bacterium, Borrelia burgdorferi sensu lato, on Corkscrew Island, Kenora District, Ontario. Int. J. Med. Sci. 2016, 13, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti-Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef]
- Shor, S.; Green, C.; Szantyr, B.; Phillips, S.; Liegner, K.L.; Burrascano, J., Jr.; Bransfield, R.; Maloney, E.L. Chronic Lyme disease: An evidence-based definition by the ILADS Working Group. Antibiotics 2019, 8, 269. [Google Scholar] [CrossRef]
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the mask of average treatment effects in chronic Lyme disease research using Big Data and subgroup analysis. Healthcare 2018, 6, 124. [Google Scholar] [CrossRef]
- Berger, B.W. Dermatologic manifestations of Lyme disease. Rev. Infect. Dis. 1989, 11 (Suppl. 6), S1475–S1481. [Google Scholar] [CrossRef]
- Stonehouse, A.; Studdiford, J.S.; Henry, C.A. An update on the diagnosis and treatment of early Lyme disease: “focusing on the bull’s eye, you may miss the mark”. J. Emerg. Med. 2010, 39, e147–e151. [Google Scholar] [CrossRef]
- Johnson, L.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: A quality of life survey. PeerJ 2014, 2, e322. [Google Scholar] [CrossRef]
- Sapi, E.; MacDonald, A. Biofilms of Borrelia burgdorferi in chronic cutaneous borreliosis. Am. J. Clin. Pathol. 2008, 129, 988–989. [Google Scholar]
- MacDonald, A.B. Concurrent neocortical borreliosis and Alzheimer’s disease: Demonstration of a spirochetal cyst form. Ann. NY Acad. Sci. 1988, 539, 468–470. [Google Scholar] [CrossRef]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamäki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Inflammatory brain changes in Lyme borreliosis: A report on three patients and review of literature. Brain 1996, 119, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Alzheimer’s neuroborreliosis and trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med. Hypotheses 2007, 68, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, J. Alzheimer’s disease—A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 2011, 8, 90. [Google Scholar] [CrossRef]
- Sapi, E.; Kasliwala, R.S.; Ismail, H.; Torres, J.P.; Oldakowski, M.; Markland, S.; Gaur, G.; Melillo, A.; Eisendle, K.; Liegner, K.B.; et al. The long-term persistence of Borrelia burgdorferi antigens and DNA in the tissues of a patient with Lyme disease. Antibiotics 2019, 8, 183. [Google Scholar] [CrossRef]
- Oksi, J.; Mertsola, J.; Reunanen, M.; Marjamäki, M.; Viljanen, M.K. Subacute multiple-site osteomyelitis caused by Borrelia burgdorferi. Clin. Infect. Dis. 1994, 19, 891–896. [Google Scholar] [CrossRef]
- Preac-Mursic, V.; Pfister, H.W.; Spiegel, H.; Burk, R.; Wilske, B.; Reinhardt, S.; Böhmer, R. First isolation of Borrelia burgdorferi from an iris biopsy. J. Clin. Neuroophthalmol. 1993, 13, 155–161. [Google Scholar]
- Häupl, T.; Hahn, G.; Rittig, M.; Krause, A.; Schoerner, C.; Schönherr, U.; Kalden, J.R.; Burmester, G.R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthr. Rheum. 1993, 36, 1621–1626. [Google Scholar] [CrossRef]
- Müller, M.E. Damage of collagen and elastic fibres by Borrelia burgdorferi―Known and new clinical histopathogical aspects. Open Neurol. J. 2012, 6 (Suppl. S1), S179–S186. [Google Scholar]
- Preac-Mursic, V.; Marget, W.; Busch, U.; Pleterski Rigler, D.; Hagl, S. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection 1996, 24, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Jaulhac, B.; Piemont, Y.; Marcellin, L.; Boohs, P.M.; Vautravers, P.; Jesel, M.; Kuntz, J.L.; Monteil, H.; Sibilia, J. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am. J. Med. 1998, 104, 591–594. [Google Scholar] [CrossRef]
- Girschick, H.J.; Huppertz, H.I.; Rüssmann, H.; Krenn, V.; Karch, H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Int. 1996, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monco, J.C.; Villar, F.F.; Alen, J.C.; Benach, J.L. Borrelia burgdorferi in the central nervous system: Experimental and clinical evidence for early invasion. J. Infect. Dis. 1990, 161, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Luft, B.J.; Steinman, C.R.; Neimark, H.C. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 1992, 267, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Livengood, J.A.; Gilmore, R.D., Jr. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Borda, J.T.; Dufour, J.; Kaushal, D.; Ramamoorthy, R.; Lackner, A.A.; Philipp, M.T. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol. 2008, 173, 1415–1427. [Google Scholar] [CrossRef]
- Ramesh, G.; Santana-Gould, L.; Inglis, F.M.; England, J.D.; Philipp, M.T. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J. Neuroinflamm. 2013, 10, 88. [Google Scholar] [CrossRef]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B. Counterpoint: Long-Term antibiotic therapy improves persistent symptoms associated with Lyme disease. Clin. Infect. Dis. 2007, 45, 149–157. [Google Scholar] [CrossRef]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Liegner, K.B.; Duray, P.; Agricola, M.; Rosenkilde, C.; Yannuzzi, L.A.; Ziska, M.; Tilton, R.C.; Hulinska, D.; Hubbard, J.; Fallon, B.A. Lyme disease and the clinical spectrum of antibiotic responsive chronic meningoencephalomyelitides. J. Spir. Tick-Borne Dis. 1997, 4, 61–73. [Google Scholar]
- Middelveen, M.J.; Sapi, E.; Burke, J.; Filush, K.R.; Franco, A.; Fesler, M.C.; Stricker, R.B. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare 2018, 6, 33. [Google Scholar] [CrossRef]
- Miklossy, J.; Kasas, S.; Zurn, A.D.; McCall, S.; Yu, S.; McGeer, P.L. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreiosis. J. Neuroinflamm. 2008, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K.; Schutzer, S.E.; Deng, Z.; Krupp, L.B.; Belman, A.L.; Benach, J.L.; Luft, B.J. Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 1995, 45, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Mikkilä, H.O.; Seppälä, I.J.; Viljanen, M.K.; Peltomaa, M.P.; Karma, A. The expanding clinical spectrum of ocular Lyme borreliosis. Ophthalmology 2000, 107, 581–587. [Google Scholar] [CrossRef]
- Preac-Mursic, V.; Weber, K.; Pfister, H.W.; Wilske, B.; Gross, B.; Baumann, A.; Prokop, J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989, 17, 355–359. [Google Scholar] [CrossRef]
- Battafarano, D.F.; Combs, J.A.; Enzenauer, R.J.; Fitzpatrick, J.E. Chronic septic arthritis caused by Borrelia burgdorferi. Clin. Orthop. Relat. Res. 1993, 297, 238–241. [Google Scholar]
- Chancellor, M.B.; McGinnis, D.E.; Shenot, P.J.; Kiilholma, P.; Hirsch, I.H. Urinary dysfunction in Lyme disease. J. Urol. 1993, 149, 26–30. [Google Scholar] [CrossRef]
- Lawrence, C.; Lipton, R.; Lowy, R.; Coyle, P.K. Seronegative chronic relapsing neuroborreliosis. Eur. Neurol. 1995, 35, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.J.; Stewart, M.; Lennox, V.A.; Fukunaga, M.; Yabuki, M.; Macorison, H.; Kitchener-Smith, J. Culture-positive Lyme borreliosis. Med. J. Aust. 1998, 168, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Nikoskelainen, J.; Hiekkanen, H.; Lauhio, A.; Peltomaa, M.; Pitkäranta, A.; Nyman, D.; Granlund, H.; Carlsson, S.A.; Seppälä, I.; et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: A double-blind, randomized, placebo-controlled, multicenter clinical study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.D.; Kong, L.I.; Miller, F.W. Molecular detection of persistent Borrelia burgdorferi in a man with dermatomyositis. Clin. Exp. Rheumatol. 1992, 10, 387–390. [Google Scholar]
- Stricker, R.B.; Fesler, M.C. Chronic Lyme disease: A working case definition. Am. J. Infect. Dis. 2018, 14, 1–44. [Google Scholar] [CrossRef]
- Davidsson, M. The financial implications of a well-hidden and ignored chronic Lyme disease pandemic. Healthcare 2018, 6, 16. [Google Scholar] [CrossRef]
- Fallon, B.A.; Pavlicova, M.; Coffino, S.W.; Brenner, C. A comparison of Lyme disease serologic test results from four laboratories in patients with persistent symptoms after antibiotic treatment. Clin. Infect. Dis. 2014, 59, 1705–1710. [Google Scholar] [CrossRef]
- Stricker, R.B.; Johnson, L. Lyme disease diagnosis and treatment: Lessions from the AIDS epidemic. Minerva Med. 2010, 101, 419–425. [Google Scholar]
- Schubert, H.D.; Greenebaum, E. Cytologically proven seronegative Lyme choroiditis and vitriitis. Retina 1994, 14, 39–42. [Google Scholar] [CrossRef]
- Schutzer, S.E.; Coyle, P.K.; Belman, A.L.; Golightly, M.G.; Drulle, J. Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet 1990, 335, 312–315. [Google Scholar] [CrossRef]
- Schmidli, J.; Hunziker, T.; Moesli, P.; Schaad, U.B. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J. Infect. Dis. 1988, 158, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Berndtson, K. Review of evidence from immune evasion and persistent infection in Lyme disease. Int. J. Gen. Med. 2013, 6, 291–306. [Google Scholar] [CrossRef]
- Bransfield, R.C. Suicide and Lyme and associated diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C. Aggressiveness, violence, homocidality, homicide, and Lyme disease. Neuropsychiatr. Dis. Treat. 2018, 14, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C.; Cook, M.J.; Bransfield, D.R. Proposed Lyme disease guidelines and psychiatric illnesses. Healthcare 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Quezado, M.M.; Ghatak, N.R.; Duray, P.H. Lyme-associated parkinsonism: A neuropathologic case study and review of the literature. Arch. Pathol. Lab. Med. 2003, 127, 1204–1206. [Google Scholar]
- Bertrand, E.; Szpak, G.M.; Pilkowska, E.; Habib, N.; Lipczyńska-Lojkowska, W.; Rudnicka, A.; Tylewska-Wierzbanowska, S.; Kulczycki, J. Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol. 1999, 37, 43–51. [Google Scholar]
- Fesler, M.C.; Middelveen, M.J.; Burke, J.M.; Stricker, R.B. Erosive vulvovaginitis associated with Borrelia burgdorferi infection. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619842901. [Google Scholar] [CrossRef]
- Lavoie, P.E.; Lattner, B.P.; Duray, P.H.; Barbour, A.G.; Johnson, P.C. Culture positive seronegative transplacental Lyme borreliosis infant mortality. Arthr. Rheum. 1987, 3, S50. [Google Scholar]
- MacDonald, A.B. Gestational Lyme Borreliosis: Implication for the Fetus. In Rheumatic Disease Clinics of North America; Johnson, R.C., Ed.; Saunders: Philadelphia, PA, USA, 1989; Volume 15, pp. 657–677. Available online: http://www.molecularalzheimer.org/files/Gestational_Lyme_Borreliosis_-_Annotated_1989.pdf (accessed on 14 October 2017).
- Trevisan, G.; Stinco, G.; Cinco, M. Neonatal skin lesions due to spirochetal infection: A case of congenital Lyme borreliosis? Int. J. Dermatol. 1997, 36, 677–680. [Google Scholar]
- Horowitz, R.I. Lyme Disease and Pregnancy: Implication of Chronic Infection, PCR Testing, and Prenatal Treatment. In Proceedings of the 16th International Scientific Conference on Lyme Disease & Other Tick-Borne Disorders, Hartford, CT, USA, 7–8 June 2003. [Google Scholar]
- MacDonald, A.B.; Benach, J.L.; Burgdorfer, W. Stillbirth following maternal Lyme disease. NY State J. Med. 1987, 87, 615. [Google Scholar] [PubMed]
- Gardner, T. Lyme Disease. In Infectious Diseases of the Fetus and Newborn Infant, 5th ed.; Remington, J.S., Klein, J.O., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 2001; pp. 519–641, Chapter 11; ISBN 0-7216-7976-5. [Google Scholar]



| Bird Species | No. of Hosts | No. of Ticks | No. of Ticks Pos./No. Ticks Tested | ||||
|---|---|---|---|---|---|---|---|
| Hlp | Isc | I. scapularis Nymphs (%) | |||||
| Larva | Nymphs | Larva | Nymphs | ||||
| American Robin, Turdus migratorius L. | 3 | 14 * | 0/0 | 0/0 | 0/0 | 5/5, 2NT | 5/5 (100) |
| Common Yellowthroat, Geothlypis trichas (L.) | 6 | 19 | 0/0 | 0/1 | 0/0 | 2/6, 12NT | 2/6 (33) |
| Rose-breasted Grosbeak, Pheucticus ludovicianus (L.) | 2 | 3 | 0/0 | 0/0 | 0/0 | 1/1, 2NT | 1/1 (100) |
| Chestnut-sided Warbler, Setophaga pensylvanica (L.) | 1 | 1 | 0/0 | 0/0 | 0/0 | 0/0, 1NT | 0/0 (0) |
| Veery, Catharus fuscescens (Stephens) | 8 | 26 | 0/1 | 0/2 | 1NT | 6/19, 3NT | 6/19 (32) |
| Song Sparrow, Melospiza melodia (Wilson) | 1 | 3 | 0/0 | 0/0 | 0/0 | 0/0, 3NT | 0/0 (0) |
| Gray Catbird, Dumetella carolinensis (L.) | 1 | 1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 (0) |
| Nashville Warbler, Oreothlypsis ruficapilla (Wilson) | 1 | 1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 (0) |
| Total: 8 bird species | 23 | 68 | 0/1 | 0/3 | 1NT | 14/33, 23NT | 14/33 (42) |
| Bird Species | Reference Strain | flaB Gene Sequence |
|---|---|---|
| American Robin * | CN19-71 | MT039724 |
| American Robin | CN19-75 | MT039725 |
| Common Yellowthroat | CN19-76B | MT039726 |
| Rose-breasted Grosbeak | CN19-77 | MT039727 |
| Veery | CN19-109A | MT039728 |
| American Robin † | CN19-112A-1 | MT039729 |
| American Robin † | CN19-112A-2 | MT039730 |
| American Robin † | CN19-112B-1 | MT039731 |
| American Robin † | CN19-112B-2 | MT039732 |
| Veery | CN19-113A | MT039733 |
| Veery § | CN19-117A-2 | MT039735 |
| Veery § | CN19-117B-1 | MT039736 |
| Veery | CN19-118B | MT039737 |
| Common Yellowthroat | CN19-121 | MT093795 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.D.; Pascoe, E.L.; Sajid, M.S.; Foley, J.E. Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada. Healthcare 2020, 8, 59. https://doi.org/10.3390/healthcare8010059
Scott JD, Pascoe EL, Sajid MS, Foley JE. Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada. Healthcare. 2020; 8(1):59. https://doi.org/10.3390/healthcare8010059
Chicago/Turabian StyleScott, John D., Emily L. Pascoe, Muhammad S. Sajid, and Janet E. Foley. 2020. "Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada" Healthcare 8, no. 1: 59. https://doi.org/10.3390/healthcare8010059
APA StyleScott, J. D., Pascoe, E. L., Sajid, M. S., & Foley, J. E. (2020). Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada. Healthcare, 8(1), 59. https://doi.org/10.3390/healthcare8010059





