High Variation in Resource Allocation Strategies among 11 Indian Wheat (Triticum aestivum) Cultivars Growing in High Ozone Environment
Abstract
1. Introduction
2. Materials and Methods
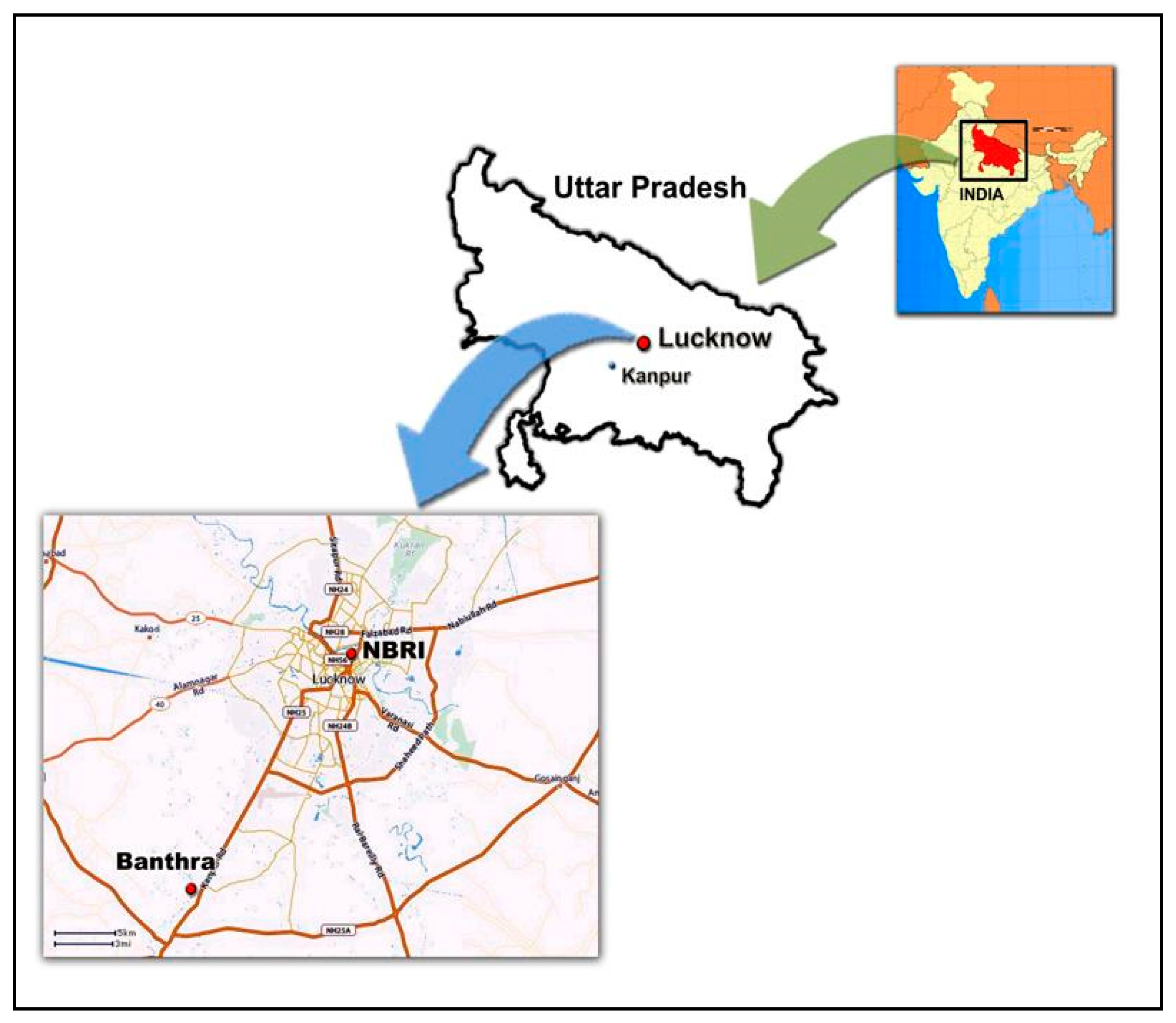
2.1. Experimental Sites and Plant Material
2.2. Experimental Design and EDU Application
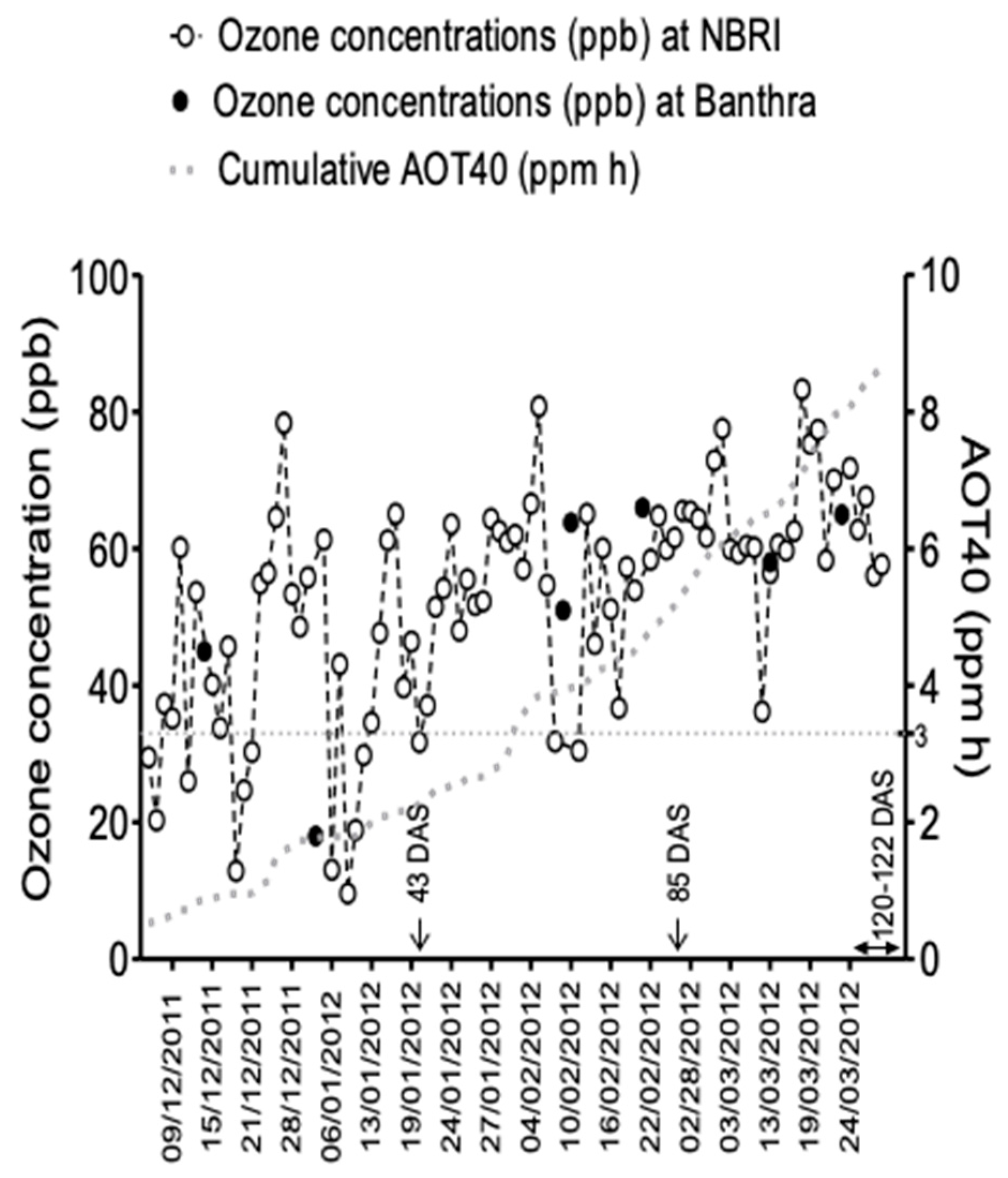
2.3. Ozone Monitoring
2.4. Biomass and Yield Attributes
2.5. Physiological and Biochemical Measurements
2.6. Statistical Analyses
3. Results
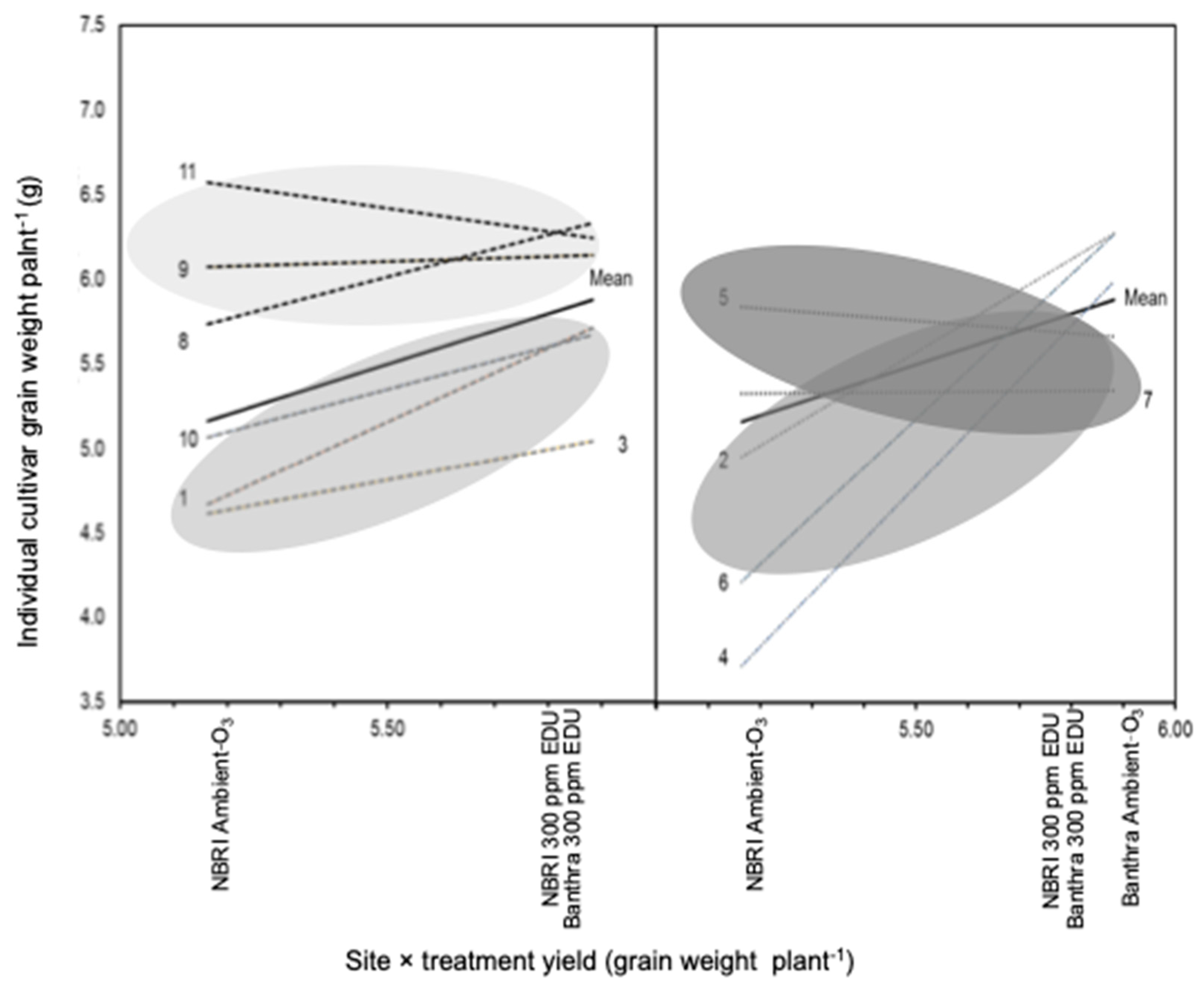
3.1. Yield and Biomass in Response to EDU Treatment
3.2. Gas Exchange and Pigments
3.3. Biochemical Measurements
3.4. Correlations of Measured Parameters and Grain Yield
4. Discussion
4.1. Biomass, Allocation Strategies and Grain Yield
4.2. EDU as a Tool to Reveal Ozone Impact
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agathokleous, E.; Saitanis, C.J.; Koike, T. Tropospheric O3, the nightmare of wild plants: A review study. J. Agric. Meteorol. 2015, 71, 142–152. [Google Scholar] [CrossRef]
- Agathokleous, E. Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. Ecotoxicol. Environ. Saf. 2017, 142, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Emberson, L.D.; Büker, P.; Ashmore, M.R.; Mills, G.; Jackson, L.S.; Agrawal, M.; Atikuzzaman, M.D.; Cinderby, S.; Engardt, M.; Jamir, C.; et al. A comparison of North American and Asian exposure–response data for ozone effects on crop yields. Atmos. Environ. 2009, 43, 1945–1953. [Google Scholar] [CrossRef]
- Tai, A.P.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817. [Google Scholar] [CrossRef]
- Fuhrer, J. Ozone risk for crops and pastures in present and future climates. Naturwissenschaften 2009, 96, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.P.K.; Val Martin, M. Impacts of ozone air pollution and temperature extremes on crop yields: Spatial variability, adaptation and implications for future food security. Atmos. Environ. 2017, 169, 11–21. [Google Scholar] [CrossRef]
- Avnery, S.; Mauzerall, D.L.; Fiore, A.M. Increasing global agricultural production by reducing ozone damages via methane emission controls and ozone-resistant cultivar selection. Glob. Chang. Biol. 2013, 19, 1285–1299. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, E.; Wang, X.; Jiang, L.; Liu, X. Ground-level O3 pollution and its impacts on food crops in China: A review. Environ. Pollut. 2015, 199, 42–48. [Google Scholar] [CrossRef]
- Tiwari, S.; Rai, R.; Agrawal, M. Annual and seasonal variations in tropospheric ozone concentrations around Varanasi. Int. J. Remote Sens. 2008, 29, 4499–4514. [Google Scholar] [CrossRef]
- Tiwari, S. Ethylenediurea as a potential tool in evaluating ozone phytotoxicity: A review study on physiological, biochemical and morphological responses of plants. Environ. Sci. Pollut. Res. 2017, 24, 14019–14039. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M. The global burden of disease from air pollution. In Proceedings of the 2016 AAAS Annual Meeting, Washington, DC, USA, 11–15 February 2016. [Google Scholar]
- Oksanen, E.; Pandey, V.; Pandey, A.K.; Keski-Saari, S.; Kontunen-Soppela, S.; Sharma, C. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013, 177, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Effect of elevated ozone and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: Growth, gas-exchange, antioxidant status, grain yield and quality. Ecotoxicol. Environ. Saf. 2018, 158, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Agrawal, M. Effect of Ozone on Physiological and Biochemical Processes of Plants. In Tropospheric Ozone and its Impacts on Crop Plants; Springer: Cham, Switzerland, 2018; pp. 65–113. [Google Scholar]
- United Nations Population Division (2010) World Population Prospects, the 2010 Revision. Available online: http://esa.un.org/unpd/wpp/ (accessed on 20 April 2018).
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic damage under two scenarios of O3 pollution. Atmos. Environ. 2011, 45, 2297–2309. [Google Scholar] [CrossRef]
- Castagna, A.; Ranieri, A. Detoxification and repair process of ozone injury: From O3 uptake to gene expression adjustment. Environ. Pollut. 2009, 157, 1461–1469. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Booker, F.L.; Burkey, K.O. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzõrjova, I.; Brosché, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.S.; Lindgren, O.; Salojärvi, J.; Loog, M. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef]
- Black, V.J.; Black, C.R.; Roberts, J.A.; Stewart, C.A. Tansley Review No. 115 Impact of ozone on the reproductive development of plants. New Phytol. 2000, 147, 421–447. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos. Environ. 2009, 43, 1510–1519. [Google Scholar] [CrossRef]
- Tomer, R.; Bhatia, A.; Kumar, V.; Kumar, A.; Singh, R.; Singh, B.; Singh, S.D. Impact of elevated ozone on growth, yield and nutritional quality of two wheat species in Northern India. Aerosol Air Qual. Res. 2015, 15, 329–340. [Google Scholar] [CrossRef]
- Ghude, S.D.; Jena, C.; Chate, D.M.; Beig, G.; Pfister, G.G.; Kumar, R.; Ramanathan, V. Reductions in India’s crop yield due to ozone. Geophys. Res. Lett. 2014, 41, 5685–5691. [Google Scholar] [CrossRef]
- Mills, G.; Buse, A.; Gimeno, B.; Bermejo, V.; Holland, M.; Emberson, L.; Pleijel, H. A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos. Environ. 2007, 41, 2630–2643. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, S.B.; Agrawal, M. Differential protection of ethylenediurea (EDU) against ambient ozone for five cultivars of tropical wheat. Environ. Pollut. 2009, 157, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.; Agrawal, S.B.; Agrawal, M. Use of Ethylenediurea (EDU) in identifying indicator cultivars of Indian clover against ambient ozone. Ecotoxicol. Environ. Saf. 2018, 147, 1046–1055. [Google Scholar] [CrossRef]
- Carnahan, J.E.; Jenner, E.L.; Wat, E.K.W. Prevention of ozone injury to plants by a new protectant chemical. Phytopathology 1978, 68, 1229. [Google Scholar] [CrossRef]
- Paoletti, E.; Contran, N.; Manning, W.J.; Ferrara, A.M. Use of the antiozonant ethylenediurea (EDU) in Italy: Verification of the effects of ambient ozone on crop plants and trees and investigation of EDU’s mode of action. Environ. Pollut. 2009, 157, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Paoletti, E.; Sandermann Jr, H.; Ernst, D. Ethylenediurea (EDU): A research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ. Pollut. 2011, 159, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M.; Haque, Z.; Ali, B.; Mathew, B.; Yu, P.; Hochholdinger, F.; de Abreu Neto, J.B.; McGillen, M.R.; Ensikat, H.J.; Manning, W.J.; et al. Ethylenediurea (EDU) mitigates the negative effects of ozone in rice: Insights into its mode of action. Plant Cell Environ. 2018, 41, 2882–2898. [Google Scholar] [CrossRef]
- Pandey, A.K.; Majumder, B.; Keski-Saari, S.; Kontunen-Soppela, S.; Mishra, A.; Sahu, N.; Pandey, V.; Oksanen, E. Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: Results from ethylenediurea studies. Sci. Total Environ. 2015, 532, 230–238. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, S.; Szantoi, Z.; Chen, S.; Wang, X. Protection of plants from ambient ozone by applications of ethylenediurea (EDU): A meta-analytic review. Environ. Pollut. 2010, 158, 3236–3242. [Google Scholar] [CrossRef]
- Paoletti, E.; Castagna, A.; Ederli, L.; Pasqualini, S.; Ranieri, A.; Manning, W.J. Gene expression in snapbeans exposed to ozone and protected by ethylenediurea. Environ. Pollut. 2014, 193, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.A.A.M.; Van Zantvoort, E.D.G. Mapping of exceedances of ozone critical levels for crops and forest trees in the Netherlands: Preliminary results. Environ. Pollut. 1997, 96, 89–98. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Picchi, V.; Iriti, M.; Quaroni, S.; Saracchi, M.; Viola, P.; Faoro, F. Climate variations and phenological stages modulate ozone damages in field-grown wheat. A three-year study with eight modern cultivars in Po Valley (Northern Italy). Agric. Ecosyst. Environ. 2010, 135, 310–317. [Google Scholar] [CrossRef]
- Pandey, A.K.; Majumder, B.; Keski-Saari, S.; Kontunen-Soppela, S.; Pandey, V.; Oksanen, E. Differences in responses of two mustard cultivars to ethylenediurea (EDU) at high ambient ozone concentrations in India. Agric. Ecosyst. Environ. 2014, 196, 158–166. [Google Scholar] [CrossRef]
- Brosché, M.; Merilo, E.B.E.; Mayer, F.; Pechter, P.; Puzõrjova, I.; Brader, G.; Kangasjärvi, J.; Kollist, H. Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant Cell Environ. 2010, 33, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Dizengremel, P.; Le Thiec, D.; Bagard, M.; Jolivet, Y. Ozone risk assessment for plants: Central role of metabolism-dependent changes in reducing power. Environ. Pollut. 2008, 156, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Felzer, B.S.; Cronin, T.; Reilly, J.M.; Melillo, J.M.; Wang, X. Impacts of ozone on trees and crops. C. R. Geosci. 2007, 339, 784–798. [Google Scholar] [CrossRef]
- Singh, A.A.; Fatima, A.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environ. Monit. Assess. 2018, 190, 190. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sharma, M.; Majumder, B.; Maurya, V.K.; Lohani, M.; Deeba, F.; Pandey, V. Impact of Ethylene diurea (EDU) on growth, yield and proteome of two winter wheat varieties under high ambient ozone phytotoxicity. Chemosphere 2017, 196, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.; Keski-Saari, S.; Keinänen, M.; Cohen, D.; Ningre, N.; Kontunen-Soppela, S.; Baldet, P.; Gibon, Y.; Dizengremel, P.; Vaultier, M.N.; et al. Ozone affects ascorbate and glutathione biosynthesis as well as amino acid contents in three Euramerican poplar genotypes. Tree Physiol. 2014, 34, 253–266. [Google Scholar] [CrossRef]
- Pleijel, H.; Uddling, J. Yield vs. Quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2012, 18, 596–605. [Google Scholar] [CrossRef]
- Mishra, A.K.; Rai, R.; Agrawal, S.B. Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J. Biochem. Biophys. 2013, 50, 139–149. [Google Scholar]
- Saitanis, C.J.; Bari, S.M.; Burkey, K.O.; Stamatelopoulos, D.; Agathokleous, E. Screening of Bangladeshi winter wheat (Triticum aestivum L.) cultivars for sensitivity to ozone. Environ. Sci. Pollut. Res. 2014, 21, 13560–13571. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Q.; Yao, F.; Chen, Z.; Feng, Z.; Manning, W.J. Assessing the impact of ambient ozone on growth and yield of a rice (Oryza sativa L.) and a wheat (Triticum aestivum L.) cultivar grown in the Yangtze Delta, China, using three rates of application of ethylenediurea (EDU). Environ. Pollut. 2007, 148, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Calatayud, V.; Jiang, L.; Manning, W.J.; Hayes, F.; Tian, Y.; Feng, Z. Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ. Pollut. 2015, 205, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Flagler, R.B.; Frenkel, M.A. Assessing plant response to ambient ozone: Growth of ozone-sensitive loblolly pine seedlings treated with ethylenediurea or sodium erythorbate. Environ. Pollut. 2003, 126, 73–81. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, S.B. Use of ethylene diurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivars of Indian wheat (Triticum aestivum L.). Environ. Monit. Assess. 2009, 159, 125. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M.; Lubna, F.A.; Holtkamp, F.; Manning, W.J.; Kraska, T.; Frei, M. Diagnosing ozone stress and differential tolerance in rice (Oryza sativa L.) with ethylenediurea (EDU). Environ. Pollut. 2017, 230, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.A.; Bell, J.N.B.; Marshall, F.M. Effects of air filtration on Egyptian clover (Trifolium alexandrinum L. cv. Messkawy) grown in open-top chambers in a rural site in Egypt. Res. J. Biol. Sci. 2007, 2, 395–402. [Google Scholar]
- Oksanen, E.; Rousi, M. Differences of Betula origins in ozone sensitivity based on open-field experiment over two growing seasons. Can. J. For. Res. 2001, 31, 804–811. [Google Scholar] [CrossRef]
- Akhtar, N.; Yamaguchi, M.; Inada, H.; Hoshino, D.; Kondo, T.; Fukami, M.; Funada, R.; Izuta, T. Effects of ozone on growth, yield and leaf gas exchange rates of four Bangladeshi cultivars of rice (Oryza sativa L.). Environ. Pollut. 2010, 158, 2970–2976. [Google Scholar] [CrossRef]
- Meier, S.; Tzfadia, O.; Vallabhaneni, R.; Gehring, C.; Wurtzel, E.T. A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst. Biol. 2011, 5, 77. [Google Scholar] [CrossRef]
- Ranieri, A.; Soldatini, G. Detoxificant systems in bean plants grown in polluted air: Effects of the antioxidant EDU. Mediterr. Agric. 1995, 125, 375–386. [Google Scholar]
- Agathokleous, E.; Mouzaki-Paxinou, A.-C.; Saitanis, C.J.; Paoletti, E.; Manning, W.J. The first toxicological study of the antiozonant and research tool ethylene diurea (EDU) using a Lemna minor L. bioassay: Hints to its mode of action. Environ. Pollut. 2016, 213, 996–1006. [Google Scholar] [CrossRef] [PubMed]

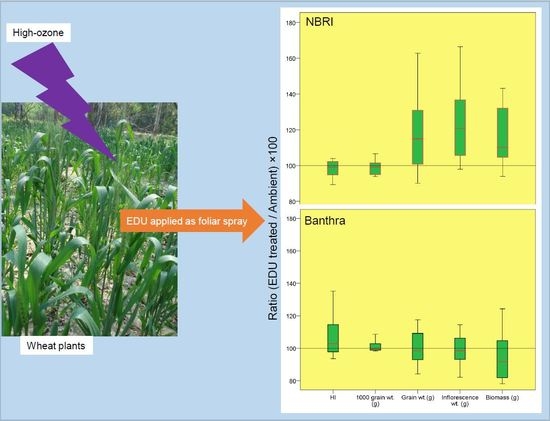
| NBRI | Banthra | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vegetative | Flowering | Vegetative | Flowering | |||||||||||||||||||||
| Cultivar | Treatment (EDU) | Cultivar × treatment (EDU) | Cultivar | Treatment (EDU) | Cultivar × treatment (EDU) | Cultivar | Treatment (EDU) | Cultivar × treatment (EDU) | Cultivar | Treatment (EDU) | Cultivar × treatment (EDU) | |||||||||||||
| SOD | 23.36 | ** | 175.10 | ** | 25.63 | ** | 1.86 | 0.03 | 1.81 | ** | 48.16 | ** | 956.17 | ** | 28.25 | ** | 45.78 | ** | 0.04 | 35.04 | ** | |||
| CAT | 27.26 | ** | 46.27 | ** | 25.94 | ** | 17.56 | ** | 24.40 | ** | 14.79 | ** | 67.83 | ** | 0.34 | 17.77 | ** | 169.41 | ** | 307.79 | ** | 108.07 | ** | |
| GSH | 4.71 | ** | 1.82 | 6.67 | ** | 14.86 | ** | 16.82 | ** | 19.63 | ** | 10.78 | ** | 699.47 | ** | 12.57 | ** | 11.36 | ** | 15.86 | ** | 18.38 | ** | |
| GSSG | 10.80 | ** | 25.17 | ** | 19.12 | ** | 7.62 | ** | 76.36 | ** | 14.48 | ** | 26.24 | ** | 82.41 | ** | 7.02 | ** | 36.15 | ** | 24.56 | ** | 21.41 | ** |
| MDA | 12.97 | ** | 3.56 | 8.45 | ** | 46.62 | ** | 12.06 | ** | 14.02 | ** | 17.69 | ** | 238.44 | ** | 26.32 | ** | 18.05 | ** | 82.85 | ** | 5.71 | ** | |
| T Chl | 41.92 | ** | 58.73 | ** | 26.84 | ** | 10.60 | ** | 8.53 | ** | 15.20 | ** | 1614.38 | ** | 2919.22 | ** | 2101.01 | ** | 327.17 | ** | 3314.84 | ** | 128.99 | ** |
| Caro | 25.94 | ** | 27.69 | ** | 15.56 | ** | 24.52 | ** | 2.47 | 12.26 | ** | 1549.28 | ** | 1622.39 | ** | 2027.76 | ** | 514.43 | ** | 1993.56 | ** | 158.96 | ** | |
| A | 2.08 | * | 1.15 | 4.63 | ** | 17.43 | ** | 18.21 | ** | 23.80 | ** | 11.19 | ** | 33.61 | ** | 11.16 | ** | 25.52 | ** | 2.38 | 9.98 | ** | ||
| gs | 3.22 | ** | 0.37 | 1.65 | 13.89 | ** | 3.03 | 11.31 | 6.33 | ** | 31.85 | ** | 5.93 | ** | 39.34 | ** | 7.13 | ** | 3.30 | ** | ||||
| FvFm | 1.79 | 0.08 | 1.09 | 2.97 | ** | 2.98 | 1.69 | 0.65 | 4.10 | * | 1.51 | 0.96 | 0.23 | 0.93 | ||||||||||
| Harvest parameters | Harvest parameters | |||||||||||||||||||||||
| HI | 16.04 | ** | 6.03 | ** | 2.74 | ** | HI | 67.73 | ** | 22.33 | ** | 15.31 | ** | |||||||||||
| 1000_grain wt | 401.47 | ** | 55.75 | ** | 51.52 | ** | 1000_grain wt | 331.58 | ** | 62.61 | ** | 31.57 | ** | |||||||||||
| Inflorescence wt | 12.58 | ** | 96.61 | ** | 6.80 | ** | Inflorescence wt | 12.56 | ** | 0.66 | 3.44 | ** | ||||||||||||
| Grain_wt | 10.01 | ** | 34.61 | ** | 3.93 | ** | Grain_wt | 10.78 | ** | 0.00 | 3.39 | ** | ||||||||||||
| Biomass | 14.65 | ** | 81.30 | ** | 8.24 | ** | Biomass | 12.95 | ** | 20.95 | ** | 12.12 | ** | |||||||||||
| Parameters | Cultivars 8,9,11 (Well-adapted) | Cultivars 1,3,10 (Poorly Adapted) | Cultivars 5,7 (Low-yield Condition) | Cultivars 2,4,6 (High-yield Condition) | All Cultivars |
|---|---|---|---|---|---|
| Vegetative | |||||
| SOD | −0.151 | 0.011 | 0.166 | −0.614 ** | −0.313 * |
| CAT | 0.005 | 0.294 | 0.244 | 0.156 | 0.311 * |
| GSH | 0.125 | 0.060 | −0.240 | −0.149 | 0.033 |
| GSSG | 0.277 | −0.542 ** | 0.192 | 0.490 ** | 0.325 * |
| MDA | −0.246 | 0.163 | 0.087 | −0.007 | −0.024 |
| Chl | −0.263 | 0.230 | −0.472 * | −0.079 | −0.087 |
| Car | −0.320 | 0.294 | −0.401 | −0.221 | −0.12 |
| A | −0.285 | 0.441 ** | −0.204 | 0.132 | 0.144 |
| gs | −0.281 | 0.344 * | −0.218 | 0.245 | 0.26 |
| Fv/Fm | 0.049 | −0.055 | 0.116 | 0.062 | 0.06 |
| Flowering | |||||
| SOD | −0.033 | 0.155 | −0.364 | 0.590 ** | 0.137 |
| CAT | 0.222 | −0.472 ** | −0.408 * | −0.629 ** | −0.373 * |
| GSH | 0.266 | 0.067 | 0.236 | −0.203 | 0.191 |
| GSSG | −0.132 | −0.379 * | 0.401 | −0.543 ** | −0.322 * |
| MDA | 0.010 | −0.618 ** | −0.222 | −0.178 | −0.251 |
| Chl | 0.202 | −0.010 | 0.132 | 0.384 * | 0.236 |
| Car | −0.117 | 0.116 | 0.053 | 0.410 * | 0.198 |
| A | 0.383 * | 0.147 | −0.078 | −0.533 ** | −0.141 |
| gs | 0.189 | −0.093 | −0.140 | −0.653 ** | −0.257 |
| FvFm | 0.284 | 0.012 | −0.125 | −0.098 | 0.107 |
| Final harvest | |||||
| HI | 0.209 | 0.064 | 0.327 | 0.659 ** | 0.426 ** |
| 1000_grain wt | −0.082 | −0.425 ** | −0.097 | 0.243 | −0.246 |
| Inflorescence wt | 0.614 ** | 0.599 ** | −0.033 | 0.695 ** | 0.737 ** |
| Biomass | 0.472 ** | 0.618 ** | 0.138 | 0.694 ** | 0.776 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.K.; Majumder, B.; Keski-Saari, S.; Kontunen-Soppela, S.; Pandey, V.; Oksanen, E. High Variation in Resource Allocation Strategies among 11 Indian Wheat (Triticum aestivum) Cultivars Growing in High Ozone Environment. Climate 2019, 7, 23. https://doi.org/10.3390/cli7020023
Pandey AK, Majumder B, Keski-Saari S, Kontunen-Soppela S, Pandey V, Oksanen E. High Variation in Resource Allocation Strategies among 11 Indian Wheat (Triticum aestivum) Cultivars Growing in High Ozone Environment. Climate. 2019; 7(2):23. https://doi.org/10.3390/cli7020023
Chicago/Turabian StylePandey, Ashutosh K., Baisakhi Majumder, Sarita Keski-Saari, Sari Kontunen-Soppela, Vivek Pandey, and Elina Oksanen. 2019. "High Variation in Resource Allocation Strategies among 11 Indian Wheat (Triticum aestivum) Cultivars Growing in High Ozone Environment" Climate 7, no. 2: 23. https://doi.org/10.3390/cli7020023
APA StylePandey, A. K., Majumder, B., Keski-Saari, S., Kontunen-Soppela, S., Pandey, V., & Oksanen, E. (2019). High Variation in Resource Allocation Strategies among 11 Indian Wheat (Triticum aestivum) Cultivars Growing in High Ozone Environment. Climate, 7(2), 23. https://doi.org/10.3390/cli7020023