Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses
Abstract
1. Introduction
2. General Information on the Species
3. Phytochemical Characteristics
4. Importance of A. absinthium in the History of Medicine
5. Application in Traditional Medicine
6. Position in Modern Allopathy and Homeopathy
7. Biological Activities Confirmed by Scientific Research
7.1. Long-Known Possible Applications Confirmed by Modern Scientific Research
7.1.1. Effect of Stimulating Digestion
7.1.2. Anthelmintic Effect
7.2. New Possible Applications Substantiated by Scientific Research
7.2.1. Antiprotozoal Effect
7.2.2. Antimicrobial and Antifungal Activities
7.2.3. Anti-Ulcer Effect
7.2.4. Hepatoprotective Effect
7.2.5. Anti-Inflammatory Effect
7.2.6. Immunomodulatory Effect
7.2.7. Cytotoxic Effect
7.2.8. Analgesic Effect
7.2.9. Neuroprotective Effect
7.2.10. Antidepressant Effect
7.2.11. Procognitive Activity
7.2.12. Neurotrophic Action
7.2.13. Cell Membrane Stabilizing Effect
7.2.14. Antioxidant Effect
7.3. Importance in Veterinary Pharmacology
8. Applications in Cosmetology
9. Applications in the Food Industry
10. Safety of Use
11. Biotechnological Research
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahamad, J.; Mir, S.R.; Amin, S.A. Pharmacognostic review on Artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Akram, A.; Iftikhar, A.; Ashfaq, D.; Yasmeen, F.; Mazhar, R.; Imran, M.; Iqbal, M.A. Status review on the pharmacological implications of Artemisia absinthium: A critically endangered plant. Asian Pac. J. Trop. Dis. 2017, 7, 185–192. [Google Scholar] [CrossRef]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 12 March 2020).
- Hassler, M.; Roskov, Y.; Ower, G.; Orrell, T.; Nicolson, D.; Bailly, N.; Kirk, P.M.; Bourgoin, T.; DeWalt, R.E.; Decock, W.; et al. World Plants: Synonymic Checklists of the Vascular Plants of the World (Version November 2018). In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist. 2019. Available online: www.catalogueoflife.org/annual-checklist/2019 (accessed on 1 January 2020).
- Wikispecies, Free Species Directory. Available online: https://species.wikimedia.org/wiki/Main_Page (accessed on 24 March 2020).
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: Berlin, Germany, 2007. [Google Scholar]
- Amidon, C.; Barnett, R.; Cathers, J.; Chambers, B.; Hamilton, L.; Kellett, A.; Kennel, E.; Montowski, J.; Thomas, M.A.; Watson, B. Artemisia—An Essential Guide from the Herb Society of America; Caroline, A., Thomas, M., Kennel, E., Eds.; The Herb Society of America: Kirtland, OH, USA, 2014. [Google Scholar]
- Missouri Botanical Garden. Tropicos.org. Available online: http://www.tropicos.org/ (accessed on 5 January 2020).
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed.; Medpharm: Marburg, Germany, 2004. [Google Scholar]
- Van Wyk, B.E.; Wink, M. Medicinal Plants of the World; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- European Medicines Agency European Union. Herbal Monograph on Artemisia absinthium L. Herba. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-Artemisia-absinthium-l-herba-revision-1_en.pdf (accessed on 4 April 2020).
- Prezes Urzędu Rejestracji Produktów Leczniczych Wyrobów Medycznych i Produktów Biobójczych. In Farmakopea Polska XI. Tom II.; Polskie Towarzystwo Farmaceutyczne: Warsaw, Poland, 2017.
- Hayat, M.Q.; Ashraf, M.; Ajab Khan, M.; Yasmin, G.; Shaheen, N.; Jabeen, S. Diversity of foliar trichomes and their systematic implications in the genus Artemisia (Asteraceae). Int. J. Agric. Biol. 2009, 11, 542–546. [Google Scholar]
- GBIF.org. GBIF Home Page. 2020. Available online: https://www.gbif.org (accessed on 12 March 2020).
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M. Potential of wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. Pharma Innov. J. 2017, 6, 176–187. [Google Scholar]
- Geszprych, A. Diversity of wormwood (Artemisia absinthium L.) growing wild in Mazury area in respect of the content and composition of the essential oil. Adv. Agric. Sci. Probl. Issues 2007, 517, 317–324. [Google Scholar]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J.M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161. [Google Scholar] [CrossRef]
- García-Rodríguez, J.J.; Andrés, M.F.; Ibañez-Escribano, A.; Julio, L.F.; Burillo, J.; Bolás-Fernández, F.; González-Coloma, A. Selective nematocidal effects of essential oils from two cultivated Artemisia absinthium populations. Zeitschrift für Naturforschung C 2015, 70, 275–280. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Äorević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Artemisia absinthium Linn. and Artemisia asiatica Nakai: A Review. J. Pharm. Res. 2010, 3, 325–328. [Google Scholar]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crop. Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Hadi, A.; Hossein, N.; Shirin, P.; Najmeh, N.; Abolfazl, M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 237–244. [Google Scholar]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; de las Heras, L.; del Prado, G.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. Inst. Oswaldo Cruz. 2015, 110, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 6. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia, L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka, India. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy. ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products; Thieme: New York, NY, USA, 2003. [Google Scholar]
- European Food Safety Authority. Outcome of the consultation with Member States and EFSA on the basic substance application for Artemisia absinthiumforuse in plant protection asfungicide in wheat and asnematicide and insecticide in vegetables. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/sp.efsa.2014.EN-665 (accessed on 19 August 2020).
- Word, K. Homoditerpene peroxideds from Artemisia absinthium. Phytochemistry 1992, 10, 340–342. [Google Scholar]
- Safayhi, H.; Sabieraj, J.; Sailer, E.; Ammon, H. Chamazulene: An antioxidant-type inhibitor of leukotriene B4 formation. Planta Med. 1994, 60, 410–413. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuñiga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind. Crop. Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Ihsan-ul-haq. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crop. Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Amat, N.; Upur, H.; Blažeković, B. In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol. 2010, 131, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.; Omer, T.N.; Omer, B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease—A controlled clinical trial. Phytomedicine 2010, 17, 305–309. [Google Scholar] [CrossRef]
- Hatziieremia, S.; Gray, A.I.; Ferro, V.A.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFκB signalling pathways in monocytes/macrophages. Br. J. Pharm. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Javed, A.; Kamran, J.N.; Mohammed, A.; Showkat, R.M. New glycoside esters from the aerial parts of Artemisia absinthium Linn. Nat. Prod. J. 2013, 3, 260–267. [Google Scholar]
- Zeng, K.W.; Liao, L.X.; Song, X.M.; Lv, H.N.; Song, F.J.; Yu, Q.; Dong, X.; Jiang, Y.; Tu, P.F. Caruifolin D from Artemisia absinthium L. inhibits neuroinflammation via reactive oxygen species-dependent c-jun N-terminal kinase and protein kinase c/NF-κB signaling pathways. Eur. J. Pharmacol. 2015, 767, 82–93. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, H.; Oh, W.K.; Yu, K.A.; Choe, Y.K.; Ahn, J.S.; Kim, D.S.; Kim, S.H.; Dinarello, C.A.; Kim, K.; et al. Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor κB. Ann. N. Y. Acad. Sci. 2004, 1030, 555–568. [Google Scholar] [CrossRef]
- Danilets, M.G.; Bel’skiĭ, I.P.; Gur’ev, A.M.; Belousov, M.V.; Bel’skaia, N.V.; Trofimova, E.S.; Uchasova, E.G.; Alhmedzhanov, R.R.; Ligacheva, A.A.; Iusbov, M.S.; et al. Effect of plant polysaccharides on TH1-dependent immune response: Screening investigation. Eksp. Klin. Farmakol. 2010, 73, 19–22. [Google Scholar]
- Hegi, G. Illustrierte Flora von Mittel-Europa; Band VI/2.; J. F. Lehmanns Verlag: Munich, Germany, 1908. [Google Scholar]
- Unterkircher, F. Der Wiener Dioskoriodes: Codex Medicus Graecus 1 der Ősterreichischen Nationalbibliothek Teil 1; Mazal, O., Ed.; Akademische Druck u. Verlagsanstalt: Graz, Austria, 1998. [Google Scholar]
- Caius, P.S.K. Pliniusza Starszego Historyi Naturalnej Ksiąg XXXVII; Księgarnia i drukarnia J. Łukaszewicza: Poznan, Poland, 1845. [Google Scholar]
- Stoll, U. Das Lorscher Arzneibuch (Codex Bambergis Medicinalis 1); Franz Steiner Verlag: Stuttgart, Germany, 1992. [Google Scholar]
- Pahlow, M. Das Grosse Buch der Heilpflanzen; Gräfe und Unzer Verlag: Augsburg, Germany, 2001. [Google Scholar]
- Rätsch, C. Enzyklopädie der Psychoaktiven Pflanzen, 11 Auflage; AT Verlag: Aarau, Switzerland, 2013. [Google Scholar]
- Dümmler, E. Poetae Latini medii aevi 2: Poetae Latini aevi Carolini (II); Weidmann: Berlin, Germany, 1884. [Google Scholar]
- Müller, I. Die Pflanzlichen Heilmittel bei Hildegard von Bingen/Heilwissen aus der Klostermedizin; Herder: Munich, Germany, 2008. [Google Scholar]
- Lonitzer, A.; Uffenbach, P. Kräuter-Buch und Künstliche Conterfeyungen der Bäumen, Stauden, Hecken, Kräuter; Verlag Bartholomae: Ulm, Germany, 1703. [Google Scholar]
- Fuchs, L. Das Kräuterbuch von 1543; Taschen Verlag: Cologne, Germany, 2001. [Google Scholar]
- Falimirz, S. O Ziołach i o Moczy Ich; Florian Ungler: Krakow, Poland, 1534. [Google Scholar]
- Urzędow, M. Herbarz Polski to jest o Przyrodzeniu Ziół i Drzew Rozmaitych; Januszowski, Jan (1550–1613); Druk: Krakow, Poland, 1595. [Google Scholar]
- Joannicy, G.; Makowski, T.; Skalski, B.; Syreński, S. Zielnik Herbarzem z Ięzyka Łacinskiego Zowią; Drukarnia Bazylego Skalskiego: Krakow, Poland, 1613. [Google Scholar]
- Tabernaemontanus, J.T. Neu Vollkommen Kräuterbuch/Mit Schönen und Künstlichen Figuren/Aller Gewächs der Bäumen / Stauden und Kräutern/so in Denen Teutschen und Welschen Landen / Auch in Hispanien/Ost-und West-Indien/Oder in der Neuen Welt. Gedruckt zu Basel/in Verl; Johann Ludwig König and Johann Brandmyller: Basel, Switzerland, 1687. [Google Scholar]
- Zedler, J.H. Grosses Vollständiges Universal-Lexicon Aller Wissenschafften und Künste; Band 1.; Hrsg. v. Johann Heinrich Zedler: Halle/Leipzig, Germany, 1732. [Google Scholar]
- Roth, L.; Daunderer, M.; Kornmann, K. Giftpflanzen, Pflanzengifte; Nikol: Hamburg, Germany, 2012. [Google Scholar]
- Monographie BGA/BfArM Kommission, E. Bundesanzeiger 1984, Heftnummer, ATC-Code: A09A. Available online: https://buecher.heilpflanzen-welt.de/BGA-Kommission-E-Monographien/ (accessed on 19 August 2020).
- Bäumler, S. Heilpflanzen Praxis Heute; Urban & Fischer: Munich, Germany, 2007. [Google Scholar]
- Piątkowska, I. Lecznictwo ludowe w okolicach Sieradza. Wisła 1894, 8, 138. [Google Scholar]
- Jastrzębski, J. Tradycje Lecznictwa Ziołowego Zwierząt/Historia Leków Naturalnych. Vol. II, Natura i Kultura—Współzależności w Dziejach Lekoznawstwa; Kuźnicka, B., Ed.; Polska Akademia Nauk: Warszawa, Poland, 1989; pp. 168–171. [Google Scholar]
- Parekh, H.S.; Liu, G.; Wei, M.Q. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol. Cancer 2009, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prezes Urzędu Rejestracji Produktów Leczniczych Wyrobów Medycznych i Produktów Biobójczych. In Farmakopea Polska XI. Tom III.; Polskie Towarzystwo Farmaceutyczne: Warsaw, Poland, 2017.
- Medizinprodukte, B. für A. In German Commission E Monograph; Blaumenthal, M.T., Hall, R., Rister, B., Eds.; American Botanical Council: Austin, TX, USA, 1984. [Google Scholar]
- Medizinprodukte, B. für A. In German Commission D monograph; Blaumenthal, M.T., Hall, R., Rister, B., Eds.; American Botanical Council: Austin, TX, USA, 1994. [Google Scholar]
- German Pharmacopoeia; German Federal Institute for Drugs and Medical Devices: Bonn, Germany, 1872.
- Council of Europe. European Pharmacopoeia 10. Available online: https://www.edqm.eu/sites/default/files/pheur_latin_index_10.0.pdf (accessed on 23 April 2020).
- Agence Nationale de Sécurité du Médicament et des Produits de Santé Absinthium for Homoeopathic Preparations. Available online: https://www.ansm.sante.fr/var/ansm_site/storage/original/application/27e6ac1e270d6995df0756d4fa0dc4ab.pdf (accessed on 17 April 2020).
- Lockie, A. Encyclopedia of Homeopathy; DK Publishing: New York, NY, USA, 2006; ISBN 9780756618711. [Google Scholar]
- McMullen, M.K.; Whitehouse, J.M.; Whitton, P.A.; Towell, A. Bitter tastants alter gastric-phase postprandial haemodynamics. J. Ethnopharmacol. 2014, 154, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Adesogan, A.T.; Kim, J.H.; Ko, Y.D. Influence of replacing rice straw with wormwood (Artemisia montana) silage on feed intake, digestibility and ruminal fermentation characteristics of sheep. Anim. Feed Sci. Technol. 2006, 128, 1–13. [Google Scholar] [CrossRef]
- Kim, S.C.; Adesogan, A.T.; Shin, J.H. Effects of dietary addition of wormwood (Artemisia montana Pampan) silage on growth performance, carcass characteristics, and muscle fatty acid profiles of beef cattle. Anim. Feed Sci. Technol. 2012, 177, 15–22. [Google Scholar] [CrossRef]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef]
- Caner, A.; Döşkaya, M.; Deǧirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp. Parasitol. 2008, 119, 173–179. [Google Scholar] [CrossRef]
- Urban, J.; Kokoska, L.; Langrova, I.; Matejkova, J. In vitro anthelmintic effects of medicinal plants used in Czech Republic. Pharm. Biol. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Singh, O.P.; Tiwari, S.K.; Ojha, D. Pilyriasis versicolor vis-a-vis sidhma and its ayurvedic management. Sadvitra Ayurveda 1994, 46, 920. [Google Scholar]
- Zafar, M.M.; Hamdard, M.E.; Hameed, A. Screening of Artemisia absinthium for antimalarial effects on Plasmodium berghei in mice: A preliminary report. J. Ethnopharmacol. 1990, 30, 223–226. [Google Scholar]
- Ramazani, A.; Sardari, S.; Zakeri, S.; Vaziri, B. In vitro antiplasmodial and phytochemical study of five Artemisia species from Iran and in vivo activity of two species. Parasitol. Res. 2010, 107, 593–599. [Google Scholar] [CrossRef]
- Tahir, M.; Siddiqui, M.M.H.; Khan, A.B. Effect of Afsanteen (Artemisia absinthium Linn.) in acute intestinal amoebiasis. Hamdard Med. 1997, 40, 24–27. [Google Scholar]
- Valdés, A.F.C.; Martínez, J.M.; Lizama, R.S.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L. and Artemisia absinthium L. Mem. Inst. Oswaldo Cruz. 2008, 103, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodivers. 2011, 8, 614–623. [Google Scholar] [CrossRef]
- Mendiola, J.; Bosa, M.; Perez, N.; Hernandez, H.; Torre, D. Extracts of Artemisia abrotanum and Artemisia absinthium inhibit growth of Naegleria fowleri in vitro. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 78–79. [Google Scholar] [CrossRef]
- Hernández, H.; Mendiola, J.; Torres, D.; Garrido, N.; Pérez, N. Effect of aqueous extracts of Artemisia on the in vitro culture of Plasmodium falciparum. Fitoterapia 1990, 41, 540–541. [Google Scholar]
- Moslemi, H.R.; Hoseinzadeh, H.; Badouei, M.A.; Kafshdouzan, K.; Fard, R.M.N. Antimicrobial activity of Artemisia absinthium against surgical wounds infected by Staphylococcus aureus in a rat model. Indian J. Microbiol. 2012, 52, 601–604. [Google Scholar] [CrossRef]
- Habibipour, R.; Rajabi, M. Antibacterial effects of Arctium lappa and Artemisia absinthium extracts in laboratory conditions. J. Herbmed Pharm. 2015, 4, 133–137. [Google Scholar]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia Spicig. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [CrossRef]
- Shafi, N.; Khan, G.A.; Ghauri, E.G. Antiulcer effect of Artemisia absinthium L. in rats. Pak. J. Sci. Ind. Res. 2004, 47, 130–134. [Google Scholar]
- Gilani, A.U.H.; Janbaz, K.H. Preventive and curative effects of Artemisia absinthium on acetaminophen and CCl4-induced hepatotoxicity. Gen. Pharmacol. 1995, 26, 309–315. [Google Scholar] [CrossRef]
- Mohammadian, A.; Moradkhani, S.; Ataei, S.; Shayesteh, T.H.; Sedaghat, M.; Kheiripour, N.; Ranjbar, A. Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J. Herbmed Pharm. 2016, 5, 29–32. [Google Scholar]
- Ahmad, F.; Khan, R.; Rasheed, S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J. Islam. Acad. Sci. 1992, 5, 111–114. [Google Scholar]
- Nalbantsoy, A.; Erel, Ş.B.; Köksal, Ç.; Göçmen, B.; Yildiz, M.Z.; Karabay Yavaşoĝlu, N.Ü. Viper venom induced inflammation with Montivipera xanthina (Gray, 1849) and the anti-snake venom activities of Artemisia absinthium L. in rat. Toxicon 2013, 65, 34–40. [Google Scholar] [CrossRef]
- Shahnazi, M.; Azadmehr, A.; Hajiaghaee, R.; Mosalla, S.; Latifi, R. Effects of Artemisia absinthium L. extracts on the maturation and function of dendritic cells. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e20163. [Google Scholar] [CrossRef]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium: A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharmacol. 2010, 129, 403–409. [Google Scholar] [CrossRef]
- Sansar, W.; Gamrani, H. The pharmacological effect of Artemisia absinthium extract in protecting adult rats against lead neurotoxicity. J. Neurol. Sci. 2013, 333, e598. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Ansaroudi, F.; Nabavi, S.F.; Nabavi, S.M. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr. J. Biotechnol. 2009, 8, 7170–7175. [Google Scholar] [CrossRef]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef]
- Li, Y.; Ohizumi, Y. Search for constituents with neurotrophic factor-potentiating activity from the medicinal plants of Paraguay and Thailand. J. Pharm. Soc. Jpn. 2004, 124, 417–424. [Google Scholar] [CrossRef]
- De Freitas, M.V.; de Cássia, M.; Netto, R.; da Costa Huss, J.C.; de Souza, T.M.T.; Costa, J.O.; Firmino, C.B.; Penha-Silva, N. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol. Vitr. 2008, 22, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L.) extracts. J. Sci. Food Agric. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm. Biol. 2011, 49, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Burke, J. Management of Barber Pole Worm in Sheep and Goats in the Southern U.S. Available online: https://attra.ncat.org/downloads/goat_barber_pole.pdf (accessed on 7 April 2020).
- Squires, J.M.; Ferreira, J.F.S.; Lindsay, D.S.; Zajac, A.M. Effects of artemisinin and Artemisia extracts on Haemonchus contortus in gerbils (Meriones unguiculatus). Vet. Parasitol. 2011, 175, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef]
- Hallal, N.; Kharoubi, O.; Benyettou, I.; Tair, K.; Ozaslan, M.; Aoues, A.E.K. In vivo amelioration of oxidative stress by Artemisia absinthium L. administration on mercuric chloride toxicity in brain regions. J. Biol. Sci. 2016, 16, 167–177. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.H.; Ko, Y.D. Influence of dietary addition of dried wormwood (Artemisia sp.) on the performance and carcass characteristics of Hanwoo steers and the nutrient digestibility of sheep. Asian Australas. J. Anim. Sci. 2002, 15, 549–554. [Google Scholar] [CrossRef]
- Cosmetics—CosIng. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 4 April 2020).
- Padosch, S.A.; Lachenmeier, D.W.; Kröner, L.U. Absinthism: A fictitious 19th century syndrome with present impact. Subst. Abus. Treat. Prev. Policy 2006, 1, 14. [Google Scholar] [CrossRef][Green Version]
- European Commission Health and Consumer Protection Directorate-General. Opinion of the Scientific Committee on Food on Thujone. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_flavourings-out162.pdf (accessed on 4 April 2020).
- Panesar, P.S.; Kumar, N.; Marwaha, S.S.; Joshi, V.K. Review paper vermouth production technology—An overview. Nat. Prod. Radiance 2009, 8, 334–344. [Google Scholar]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Höld, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. α-Thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Pollens—Weeds and Garden Plants. Available online: https://www.fda.gov/media/81288/download (accessed on 4 April 2020).
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kour, B.; Kour, G.; Kaul, S.; Dhar, M.K. In vitro mass multiplication and assessment of genetic stability of in vitro raised Artemisia absinthium L. plants using ISSR and SSAP molecular markers. Adv. Bot. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
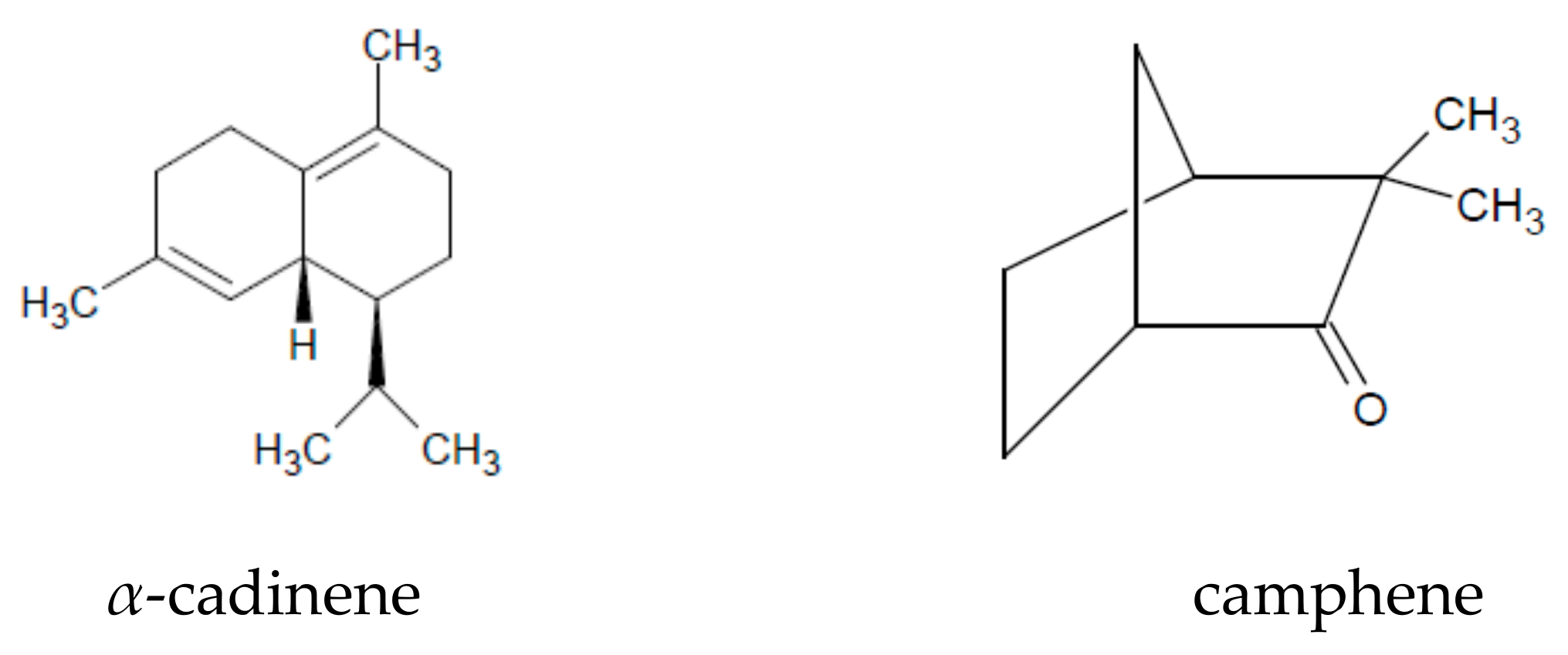
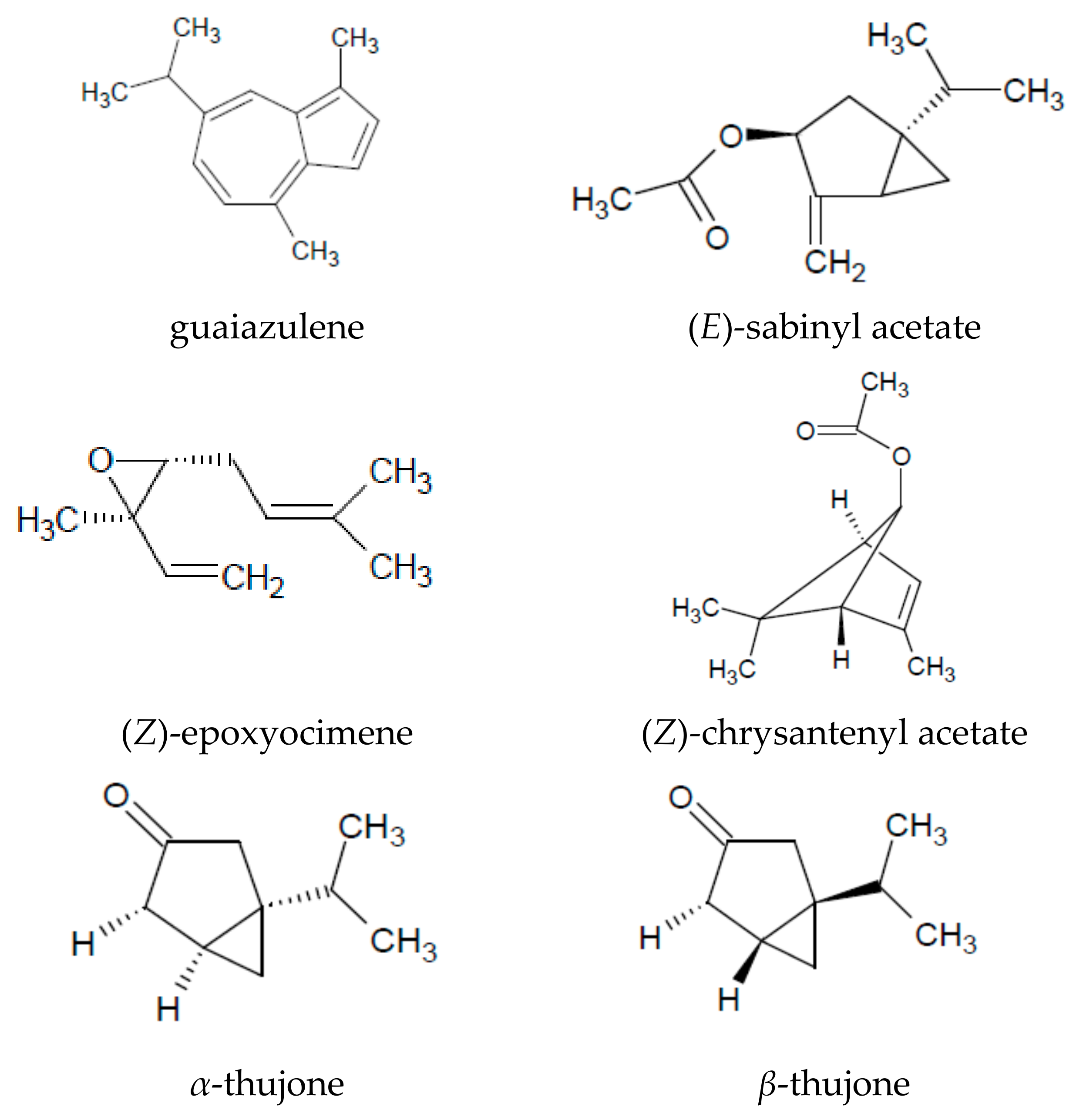
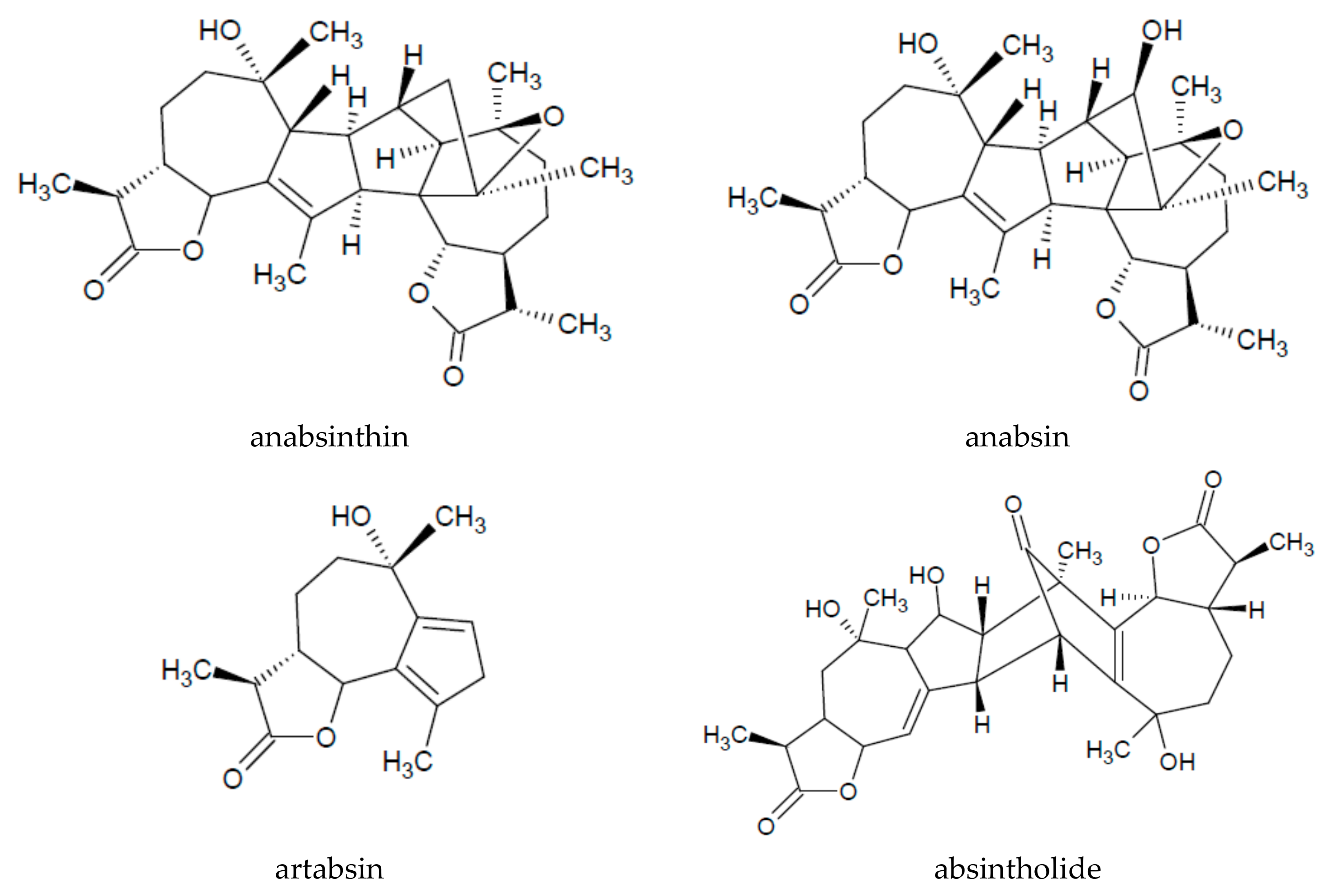
| Chemical Groups/Compounds | References |
|---|---|
| Monoterpenoids | |
| (E)-6,7-epoxyocimene, (Z)-6,7-epoxyocimene, (Z)-carveol, carvacrol, geranyl pentanoate, geranial, p-menth-3-en-9-ol, neryl acetate | [18] |
| (E)-epoxyocimene | [19] |
| (Z)-epoxyocimene | [16,19] |
| 1,8-cineole | [1,16,17,18,19,20,21,22,23,24,25] |
| geranyl 2-methylbutanoate, neryl 2-methylpropanoate, linalyl 3-methylbutanoate, geranyl 3-methylbutanoate, bornyl 3-methylbutanoate, linalyl butanoate, (Z)-β-epoxyocimene, fenchone, (E)-sabinene hydrate, isobornyl acetate, isobornyl propanoate, pulegone, α-fenchene | [23] |
| neryl 2-methylbutanoate | [21,23] |
| 2-β-pinene, lyratyl acetate | [25] |
| linalyl 3-methylbutanoate | [18,23] |
| neryl 3-methylbutanoate | [21,23] |
| terpinene-4-ol, (E)-sabinene hydrate, (E)-sabinol | [22,23] |
| thujyl alcohol | [1,21] |
| allo-ocimene | [18,20] |
| Artemisia ketone, 3-methylbutanoate, (E)-thujone, phellandrene, isothujyl acetate, pinene, (E)-verbenol, (Z)-thujone | [21] |
| borneol, (Z)-nerolidol, (Z)-verbenol, (E)-β-ocimene, (Z)-sabinene hydrate, α-terpinyl acetate, p-cymen-8-ol, terpinolene, α-terpinene | [22] |
| chrysanthenol | [19,24] |
| (Z)-chrysanthenol | [15,26] |
| (Z)-expoxyocimene | [15,21,24,26] |
| phellandrene epoxide, thujol | [18,24] |
| eugenol | [18,22,23,27] |
| geraniol | [1,18,22,27] |
| iso-3-thujanol | [27] |
| geranyl isovalerate, lavandulyl acetate, allo-ocimene, β-linalool | [20] |
| camphene | [1,19,22,23] |
| camphor | [16,22,23,24,26,28] |
| carvone | [21,22] |
| lavandulol | [20,22,23,24] |
| limonene | [22,23,24,25] |
| linalool | [18,19,21,22,23,24,26] |
| myrcene | [16,21] |
| neral, geranyl acetate, neryl acetate, (Z)-β-ocimene | [18,22] |
| nerol | [16,18,21,22,27] |
| bornyl acetate | [15,22,23,24] |
| chrysanthenyl acetate | [15,16,21,24,26,27] |
| (Z)-chrysanthenyl acetate | [16,18] |
| linalyl acetate | [16,22,23,24] |
| sabinyl acetate | [15,18,20,22,24] |
| (E)-sabinyl acetate | [16,21,23,28] |
| thujyl acetate | [1] |
| p-cymene | [1,16,18,22,23] |
| linalyl propionate | [23,24] |
| sabinene | [18,20,21,22,23,24] |
| thymol | [24] |
| santolinatriene | [23,25] |
| (Z)-linalooloxide | [22,23,24] |
| (E)-linalool oxide | [22,24] |
| epoxyocymene | [21,24] |
| tricyclene | [29] |
| α-phellandrene | [16,18,20,23] |
| α-pinene | [1,16,18,19,22,23,24,25] |
| α-terpineol | [1,18,20,24,25] |
| α-thujene | [18,22,23] |
| α-thujone | [1,15,16,18,23,24,30] |
| β-phellandrene | [1,18,22] |
| β-myrcene | [18,19,20,23,28] |
| β-pinene | [16,21,22,23,28] |
| β-thujone | [1,15,18,20,23,24,27,28,30,31] |
| γ-terpinene | [18,20,22,23] |
| Sesquiterpenoids | |
| (E)-nerolidol, ar-curcumene, diepi-α-cedrene, bisabolol oxide, α-copaene, β-gurjunene | [18] |
| (E,E)-farnesyl acetate, (E,E)-farnesal, (Z,E)-α-farnesene, (E,E)-farnesyl 3-methylbutanoate, 7-α-silphiperfol-5-ene, allo-aromadendrene, bicyclogermacrene, (Z)-α-bisabolene, cyperene, epi-β-santalene, hexahydrofarnesyl acetone, petasitene, pethybrene, presilphiperfol-7-ene, (E)-nerolidyl propanoate, silfinen-1-en, silphiperfol-6-ene, humulene oxide II, α-cedrene, α-gurjunene, α-isocomene, α-santalene, α-(E)-bergamotene, β-bisabolene, β-eudesmol, β-isocomene, β-santalene, γ-humulene | [23] |
| elemol, guaiazulene, cadinene, α-himachalene | [1] |
| germacrene D | [16,19,22,26] |
| caryophyllene | [1,24] |
| curcumene | [21] |
| nerolidol, (E)-β-farnezene | [25] |
| spathulenol | [18,27] |
| bisabololoxide B | [27] |
| caryophyllene oxide | [1,21,22,23,24,25,27] |
| (E)-caryophyllene | [26] |
| α-bisabolene, α-calacorene, γ-curcumene, γ-muurolene | [22] |
| α-bisabolol | [18,22,23,27] |
| α-humulene | [18,22,23] |
| α-copaen | [22,23] |
| β-bourbonene | [18,23] |
| β-elemene | [19,23] |
| β-caryophyllene | [18,19,20,23] |
| β-selinene | [18,22,23,24,26] |
| γ-gurjunene | [18,23] |
| γ-cadinene | [18,22] |
| δ-cadinene | [18,23,25] |
| Diterpenoids | |
| 1-(E)-8-isopropyl-1,5-dimethyl-nona-4,8-dienyl-4-methyl-2,3-dioxa-bicyclo(2, 2, 2)oct-5-ene, iso-1-(E)-8-isopropyl-1,5-dimethyl-nona-4,8-dienyl-4-methyl- 2,3-dioxa-bicyclo(2, 2, 2)oct-5-ene | [1,32] |
| vulgarol A, vulgarol B | [18] |
| Phenylpropanoids | |
| methyleugenol | [27] |
| estragole | [27] |
| Chemical Group | Compound | References |
|---|---|---|
| Sesquiterpenoid lactones | absintholide | [9,16] |
| absinthin | [1,9,15,16,21,31] | |
| anabsin, ketopepenolid-A, β-santonin | [16] | |
| anabsinthin | [16,21,31] | |
| arabsin, ketopelenolide, santonin related lactones | [21] | |
| artabin | [16,21] | |
| artabsin | [15,16,21] | |
| artenolide, deacetyloglobicin, isoabsinthin, parishine B and C | [9] | |
| germacranolide, hydroxypelenolide | [34] | |
| caruifolin D | [41] | |
| matricin | [9,16] | |
| Bitter principles | 24-zeta-ethylcholesta-7,22-dien-3-β-ol, artamaridin, artamaridinin, artamarin, artamarinin, quebrachitol | [21] |
| Azulenes | 3,6-dihydrochamazulene | [26] |
| 7-ethyl-1,4-dimethylazulene | [19] | |
| 7-ethyl-5,6-dihydro-1,4-dimethylazulene | [16] | |
| azulene | [1,21] | |
| chamazulene | [18,21,22,23,26,28] | |
| dihydrochamazulene isomer | [16] | |
| prochamazulenogen | [21] | |
| Flavonoids | quercetin-3-rutinoside | [36] |
| 5,6,32′,5′-tetramethoxy 7,4′-hydroxyflavone | [21,42] | |
| 5-hydroxy-3,3′,4′,6,7-pentamethoxyflavone, glycosides of quercetin | [21] | |
| apigenin, quercetin dihydrate, flavone, kaempferol, catechin, myristin, naryngenin | [22] | |
| artemetin | [1,21,34] | |
| Artemisia bis-isoflavonyl dirhamnoside, Artemisia isoflavonyl glucosyl diester | [1] | |
| casticin | [34] | |
| quercetin | [16] | |
| rutoside | [16,21] | |
| Chalcones | cardamonin | [38,39] |
| Coumarins | herniarin | [27] |
| coumarin | [22] | |
| Phenolic acids | 1′,3′-O-dicaffeoylquinic acid, 1′,5′-O-dicaffeoylquinic acid, 3′,5′-O-dicaffeoylquinic acid, 4′,5′-O-dicaffeoylquinic acid, 5′-O-caffeoylquinic acid | [37] |
| chlorogenic acid | [16,21,36,37] | |
| ferulic acid | [22,31] | |
| gallic acid | [22,35] | |
| caffeic acid | [16,21,22,31,35] | |
| coumaric acid, salicylic acid | [16] | |
| p-coumaric acid, rosmarinic acid, tannic acid | [22] | |
| syringic acid, vanillic acid | [16,22] | |
| Organic acids | succinic acid, malic acid, (E)-cinnamic acid | [22,31] |
| Fatty acids | 9- hydroxy-(E)-10,12-octadecadienoic acid, 13- hydroxy-(E), (E)-9, 11-octadcadienoic acid, epoxyoleic acid, linoleic acid, oleic acid, palmitic acid, stearic acid | [1] |
| dodecanoic acid | [18] | |
| Sterols | 3,11-dimethyldodecan-1,7-dioic acid-1-β-D-glucopyranosyl-6′- octadec-9′′-enoate, lanost-24-en-3β-ol-11-one-28-oic acid-21,23 α-olide-3β-D-glucopyranosyl-2′-dihydrocaffeoate-6′- decanoate | [40] |
| Fatty acid glycosides | ethyl linoleate, methyl linoleate, ethyl palmitate, methyl palmitate | [23] |
| Tannins | nd * | [16,21,22,31] |
| Lignans | nd | [16,21] |
| Carotenoids | nd | [16,21] |
| Resinous substances | nd | [31] |
| Polysaccharides | nd | [43] |
| Other compounds | (5Z)-2,6-dimethylocta-5,7-diene-2,3-diol | [19] |
| (Z)-2,6-dimethylocta-5,7-diene-2,3-diol | [24,26] | |
| (Z)-jasmone, 2-ethyl-4-methyl-1,3-pentadienylbenzene, 3-octanol, bicyclo[2.2.1]-hept-2-en-7-ol, (E)-3-hexenyl butyrate, (Z)-3-hexenyl butyrate, benzeneacetaldehyde, fraganol, 3,7-dimethyl-2-metyl propanoic acid | [18] | |
| 1H-benzocycloheptene, 4-hexen-1-ol, benzenemethanol, benzene, 1-butanol, en-in-dicycloether, (E)-photonerol, | [25] | |
| (E)-nuciferyl 2-methylpropanoate, albene, (E)-nuciferyl butanoate, hexanal, (Z)-nuciferyl propanoate | [23] | |
| trimethoxybezoic acid | [1] | |
| (E)-3-hexenyl butyrate | [19,26] | |
| nuciferol butanoate, nuciferol propionate | [21] | |
| silica | [31] | |
| stigmast-5,22-dien-3β-ol-21-oic acid-3β-glucopyranosyl-2′- octadec-9′′-enoate, tricosan-14-on-1,4-olide-5-eicos-9′-enoate | [40] |
| Activity | Mechanism of Action | References |
|---|---|---|
| Stimulating digestion |
| [72] |
| Stimulating appetite |
| [73] |
| [74] | |
| Anthelmintic |
| [75] |
| [19,76] | |
| [77] | |
| [78] | |
| Antiprotozoal |
| [79] |
| [80] | |
| [81] | |
| [82] | |
| [83] | |
| [24,34] | |
| [26] | |
| [84] | |
| [85] | |
| AntibacterialAntifungal |
| [20] |
| [86] | |
| [87] | |
| [24] | |
| [18] | |
| [22] | |
| [37] | |
| [88] | |
| Anti-ulcer |
| [89] |
| Hepatoprotective |
| [90] |
| [91] | |
| [36] | |
| Anti-inflammatory |
| [25,92] |
| [42] | |
| [38] | |
| [39] | |
| [93] | |
| Immuno-stimulating |
| [94] |
| [43] | |
| Cytotoxic |
| [95] |
| [37] | |
| Analgesic |
| [92] |
| [25] | |
| Neuroprotective |
| [96] |
| [97] | |
| [41] | |
| Antidepressant |
| [98] |
| Procognitive |
| [99] |
| Neurotrophic |
| [100] |
| Stabilizing cell membranes |
| [101] |
| Antioxidant |
| [35] |
| [102] | |
| [98] | |
| [34] | |
| [22] | |
| [20] | |
| [103] |
| CosIng Data | Description | Functions |
|---|---|---|
| Artemisia absinthium extract |
| skin conditioning |
| Artemisia absinthium herb extract |
| perfuming |
| Artemisia absinthium oil |
| antimicrobial |
| Artemisia absinthium herb oil |
| perfuming |
| Lactobacillus/Artemisia absinthium leaf extract ferment filtrate |
| skin conditioning |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. https://doi.org/10.3390/plants9091063
Szopa A, Pajor J, Klin P, Rzepiela A, Elansary HO, Al-Mana FA, Mattar MA, Ekiert H. Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants. 2020; 9(9):1063. https://doi.org/10.3390/plants9091063
Chicago/Turabian StyleSzopa, Agnieszka, Joanna Pajor, Paweł Klin, Agnieszka Rzepiela, Hosam O. Elansary, Fahed A. Al-Mana, Mohamed A. Mattar, and Halina Ekiert. 2020. "Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses" Plants 9, no. 9: 1063. https://doi.org/10.3390/plants9091063
APA StyleSzopa, A., Pajor, J., Klin, P., Rzepiela, A., Elansary, H. O., Al-Mana, F. A., Mattar, M. A., & Ekiert, H. (2020). Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants, 9(9), 1063. https://doi.org/10.3390/plants9091063








