Assessing the Effect of Diesel Fuel on the Seed Viability and Germination of Medicago sativa Using the Event-Time Model
Abstract
1. Introduction
2. Results
2.1. Effect of Diesel Fuel on Germination
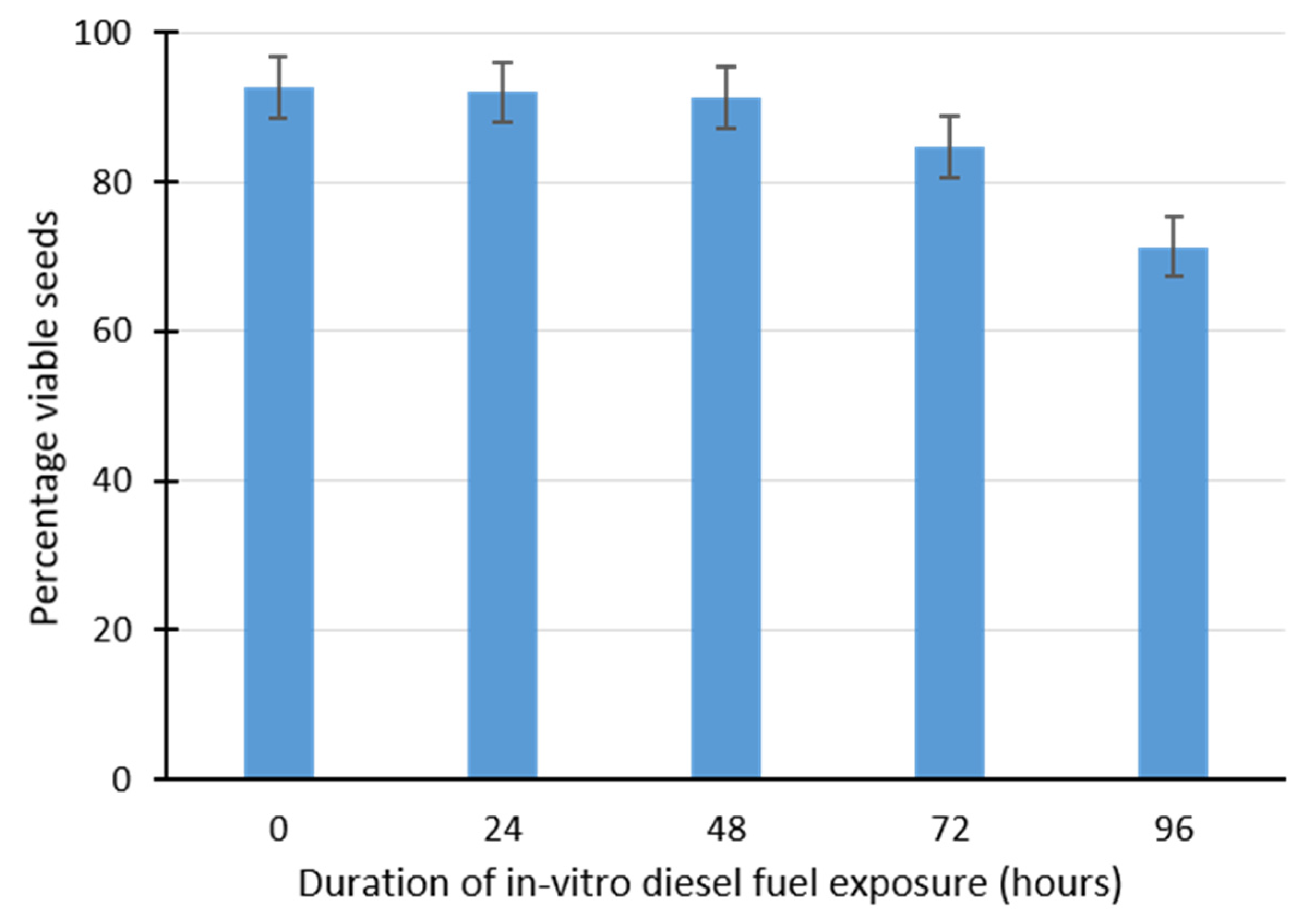
2.2. Effect of Diesel Fuel Exposure on Seed Viability Using Triphenyltetrazolium Chloride
3. Discussion
4. Materials and Methods
4.1. Soil Preparation
4.2. Germination as Grouped Time-to-Event Data
4.3. Seed Viability Test
4.4. Statistical Analysis: Event-Time Model
4.5. Statistical Analysis: Viability Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, T.A.; Guthrie, E.A.; Walton, B.T. Bioremediation in the rhizosphere. Environ. Sci. Technol. 1993, 27, 2630–2636. [Google Scholar] [CrossRef]
- Corgié, S.C.; Joner, E.J.; Leyval, C. Rhizospheric degradation of phenanthrene is a function of proximity to roots. Plant Soil 2003, 257, 143–150. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.R. Remediation of contaminated soils with green plants: An overview. In Vitro Cell. Dev. Biol. Plant 1993, 29, 207–212. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.R.; Huang, J.W. Phytoremediation of contaminated soils. Trends Biotechnol. 1995, 13, 393–397. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Kirk, J.L.; Klirnomos, J.N.; Lee, H.; Trevors, J.T. Phytotoxicity assay to assess plant species for phytoremediation of petroleum-contaminated soil. Bioremediation J. 2002, 6, 57–63. [Google Scholar] [CrossRef]
- Chouychai, W.; Thongkukiatkul, A.; Upatham, S.; Lee, H.; Pokethitiyook, P.; Kruatrachue, M. Phytotoxicity assay of crop plants to phenanthrene and pyrene contaminants in acidic soil. Environ. Toxicol. 2007, 22, 597–604. [Google Scholar] [CrossRef]
- Kaimi, E.; Mukaidani, T.; Tamaki, M. Screening of Twelve Plant Species for Phytoremediation of Petroleum Hydrocarbon-Contaminated Soil. Plant Prod. Sci. 2007, 10, 211–218. [Google Scholar] [CrossRef]
- USEPA. Introduction to Phytoremediation; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Chekol, T.; Vough, L.R. A study of the use of Alfalfa (Medicago sativa L.) for the phytoremediation of organic contaminants in soil. Remediat. J. 2001, 11, 89–101. [Google Scholar] [CrossRef]
- Sun, M.; Fu, D.; Teng, Y.; Shen, Y.; Luo, Y.; Li, Z.; Christie, P. In situ phytoremediation of PAH-contaminated soil by intercropping alfalfa (Medicago sativa L.) with tall fescue (Festuca arundinacea Schreb.) and associated soil microbial activity. J. Soils Sediments 2011, 11, 980–989. [Google Scholar] [CrossRef]
- Hamdi, H.; Benzarti, S.; Aoyama, I.; Jedidi, N. Rehabilitation of degraded soils containing aged PAHs based on phytoremediation with alfalfa (Medicago sativa L.). Int. Biodeterior. Biodegrad. 2012, 67, 40–47. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Turkovskaya, O. Comparison of the phytoremediation potentials of Medicago falcata L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 3117–3130. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Sanz, D.; Redondo-Nieto, M.; Guirado, M.; Pindado Jiménez, O.; Millán, R.; Martin, M.; Rivilla, R. Metagenomic insights into the bacterial functions of a diesel-degrading consortium for the rhizoremediation of diesel-polluted soil. Genes 2019, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O.; George, S.C. Ethanol-blended petroleum fuels: Implications of co-solvency for phytotechnologies. RSC Adv. 2020, 10, 6473–6481. [Google Scholar] [CrossRef]
- Fenlon, J.S. Chapter 7: Time to event analysis in the agricultural sciences. In Risk Assessment with Time to Event Models; Crane, M., Newman, M.C., Chapman, P.F., Fenlon, J., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2002; pp. 103–119. [Google Scholar]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Ritz, C. Toward a unified approach to dose–response modeling in ecotoxicology. Environ. Toxicol. Chem. 2010, 29, 220–229. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. The effect of diesel fuel on common vetch (Vicia sativa L.) plants. Environ. Geochem. Health 2003, 25, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Oyaizu, H. Evaluation of the phytoremediation potential of four plant species for dibenzofuran-contaminated soil. J. Hazard. Mater. 2009, 168, 760–764. [Google Scholar] [CrossRef]
- Palmroth, M.R.T.; Pichtel, J.; Puhakka, J.A. Phytoremediation of subarctic soil contaminated with diesel fuel. Bioresour. Technol. 2002, 84, 221–228. [Google Scholar] [CrossRef]
- Jagtap, S.S.; Woo, S.M.; Kim, T.-S.; Dhiman, S.S.; Kim, D.; Lee, J.-K. Phytoremediation of diesel-contaminated soil and saccharification of the resulting biomass. Fuel 2014, 116, 292–298. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Influence of diesel fuel on seed germination. Environ. Pollut. 2002, 120, 363–370. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Thomson, P.C.; Jacobs, B.C.; Brain, P.; Butler, R.C.; Turner, H.; Mitakda, B. Fitting and comparing seed germination models with a focus on the inverse normal distribution. Aust. N. Z. J. Stat. 2004, 46, 349–366. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.; Yndgaard, F.; Fredlund, K.; Steinrücken, G. Modelling flowering of plants using time-to-event methods. Eur. J. Agron. 2010, 32, 155–161. [Google Scholar] [CrossRef]
- Ahmed, M.; George, S.C. Changes in the molecular composition of crude oils during their preparation for GC and GC–MS analyses. Org. Geochem. 2004, 35, 137–155. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H.J. Effect of diesel fuel on growth of selected plant species. Environ. Geochem. Health 1999, 21, 353–357. [Google Scholar] [CrossRef]
- Bossert, I.; Bartha, R. The fate of petroleum in soil ecosystems. In Petroleum Microbiology; Atlas, R.M., Ed.; Macmillan Publishing Company: New York, NY, USA, 1984; pp. 435–473. [Google Scholar]
- Rogers, H.B.; Beyrouty, C.A.; Nichols, T.D.; Wolf, D.C.; Reynolds, C.M. Selection of cold-tolerant plants for growth in soils contaminated with organics. J. Soil Contam. 1996, 5, 171–186. [Google Scholar] [CrossRef]
- Van Waes, J.M.; Debergh, P.C. Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some Western European orchids. Physiol. Plant. 1986, 66, 435–442. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Dantigny, P.; Tchobanov, I.; Bensoussan, M.; Zwietering, M.H. Modeling the Effect of Ethanol Vapor on the Germination Time of Penicillium chrysogenum. J. Food Prot. 2005, 68, 1203–1207. [Google Scholar] [CrossRef]
- Keshtkar, E.; Kordbacheh, F.; Mesgaran, M.B.; Mashhadi, H.R.; Alizadeh, H.M. Effects of the sowing depth and temperature on the seedling emergence and early growth of wild barley (Hordeum spontaneum) and wheat. Weed Biol. Manag. 2009, 9, 10–19. [Google Scholar] [CrossRef]
- Shafii, B.; Price, W.J. Estimation of cardinal temperatures in germination data analysis. J. Agric. Biol. Environ. Stat. 2001, 6, 356–366. [Google Scholar] [CrossRef]
- Jensen, S.M.; Andreasen, C.; Streibig, J.C.; Keshtkar, E.; Ritz, C. A note on the analysis of germination data from complex experimental designs. Seed Sci. Res. 2017, 27, 321–327. [Google Scholar] [CrossRef]
- Hose, G.C.; Symington, K.; Lott, M.J.; Lategan, M.J. The toxicity of arsenic (III), chromium (VI) and zinc to groundwater copepods. Environ. Sci. Pollut. Res. 2016, 23, 18704–18713. [Google Scholar] [CrossRef] [PubMed]


| Concentration of Diesel Fuel in Soils (g/kg) | b (Slope at t50) | d (Upper Limit) | t50 (h) |
|---|---|---|---|
| 0 (control) | −6.16 (0.61) | 0.92 (0.03) | 90.6 (2.78) |
| 5 | −7.41 (0.74) | 0.91 (0.03) | 106.6 (2.74) |
| 10 | −8.26 (0.82) | 0.91 (0.03) | 114.2 (2.67) |
| 20 | −11.12 (1.18) | 0.84 (0.04) | 135.9 (2.47) |
| 30 | −11.87 (1.38) | 0.70 (0.05) | 136.0 (2.54) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eze, M.O.; Hose, G.C.; George, S.C. Assessing the Effect of Diesel Fuel on the Seed Viability and Germination of Medicago sativa Using the Event-Time Model. Plants 2020, 9, 1062. https://doi.org/10.3390/plants9091062
Eze MO, Hose GC, George SC. Assessing the Effect of Diesel Fuel on the Seed Viability and Germination of Medicago sativa Using the Event-Time Model. Plants. 2020; 9(9):1062. https://doi.org/10.3390/plants9091062
Chicago/Turabian StyleEze, Michael O., Grant C. Hose, and Simon C. George. 2020. "Assessing the Effect of Diesel Fuel on the Seed Viability and Germination of Medicago sativa Using the Event-Time Model" Plants 9, no. 9: 1062. https://doi.org/10.3390/plants9091062
APA StyleEze, M. O., Hose, G. C., & George, S. C. (2020). Assessing the Effect of Diesel Fuel on the Seed Viability and Germination of Medicago sativa Using the Event-Time Model. Plants, 9(9), 1062. https://doi.org/10.3390/plants9091062







