Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis
Abstract
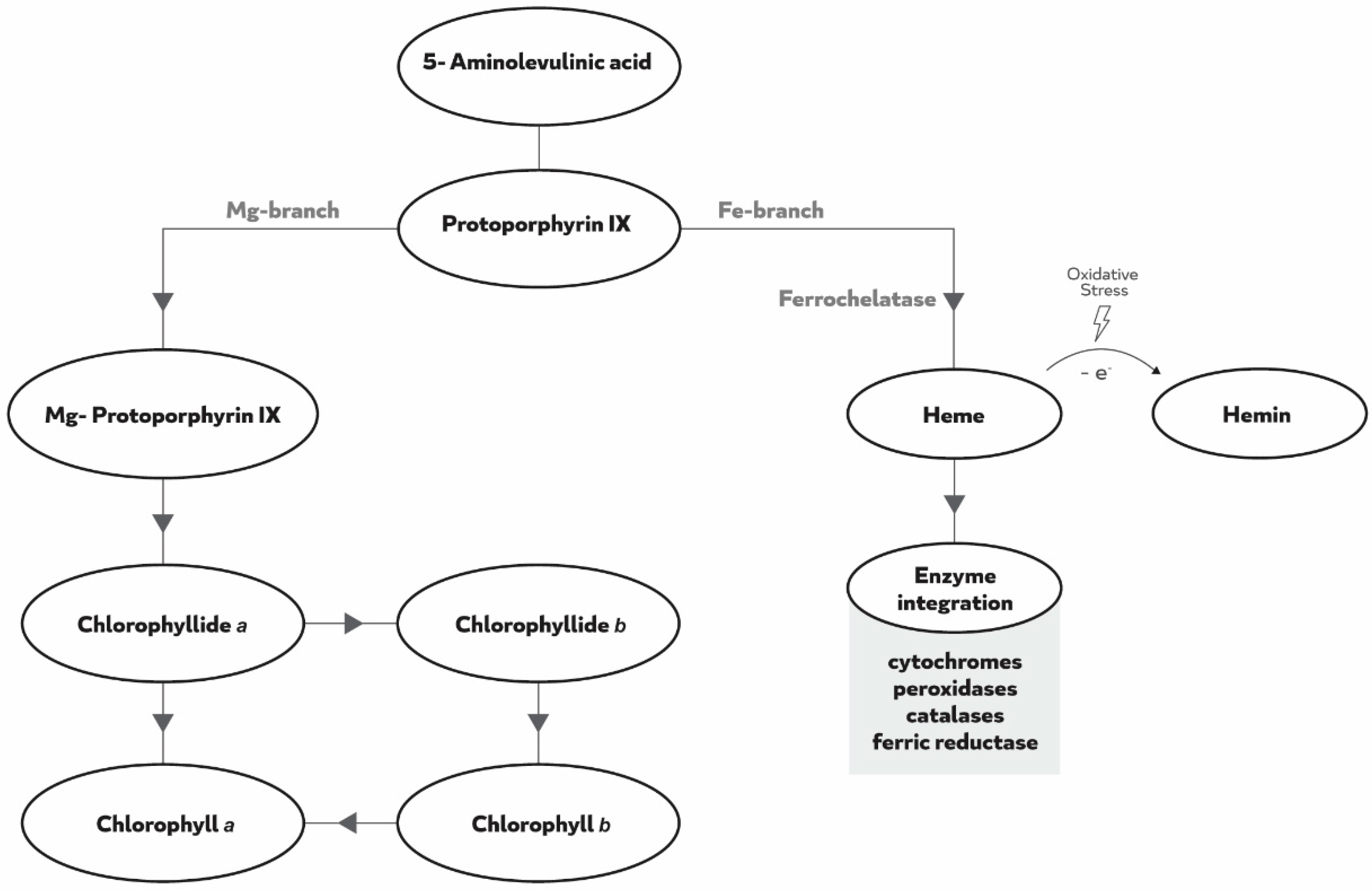
1. Introduction
2. Results
2.1. Growth and Chlorosis Evaluation
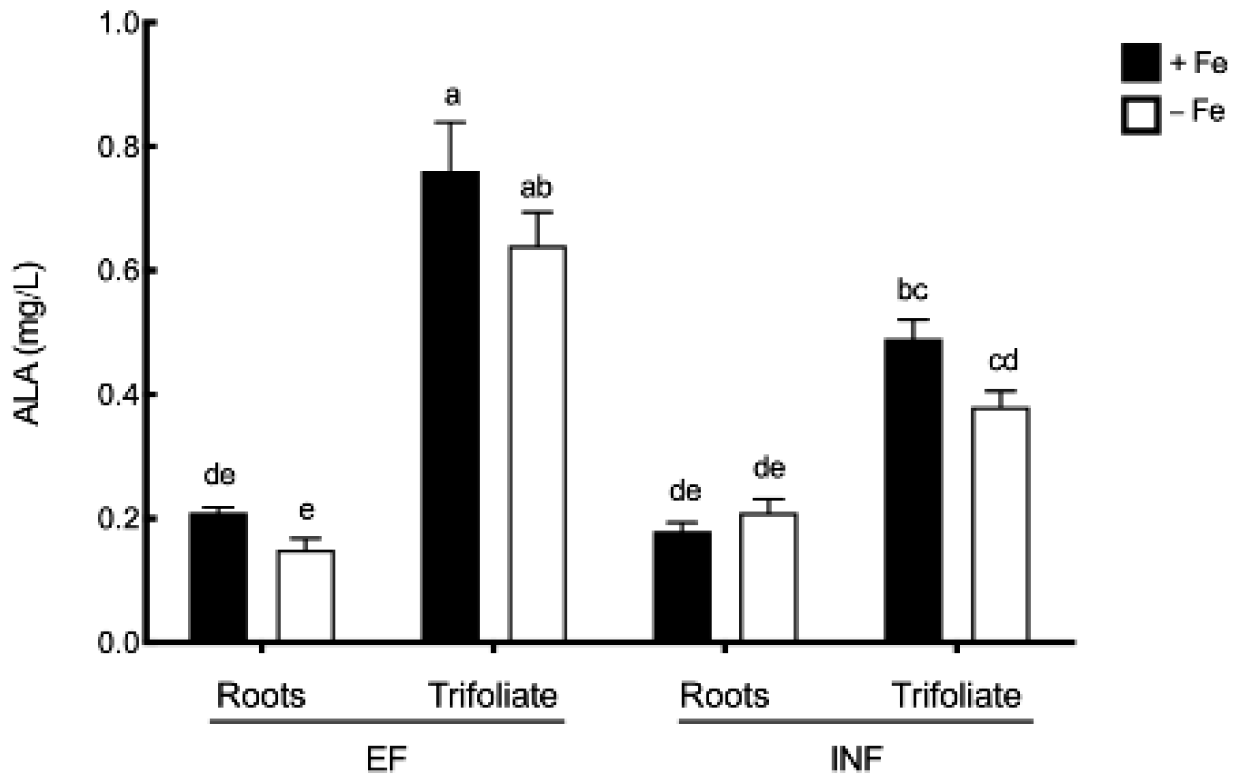
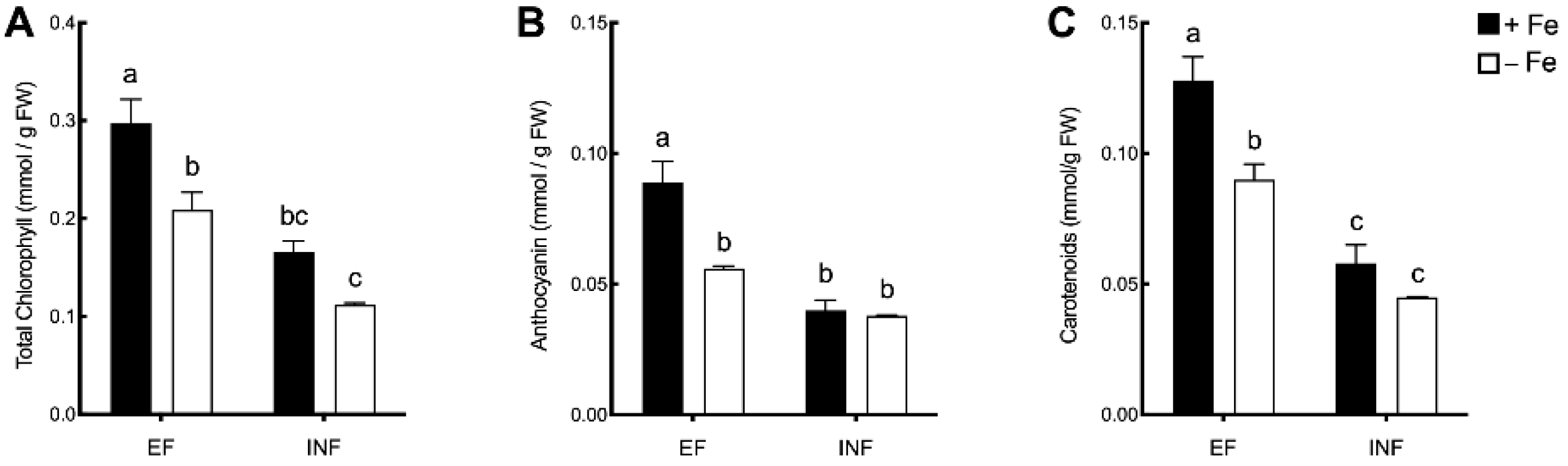
2.2. ALA and Photosynthetic Pigments Evaluation
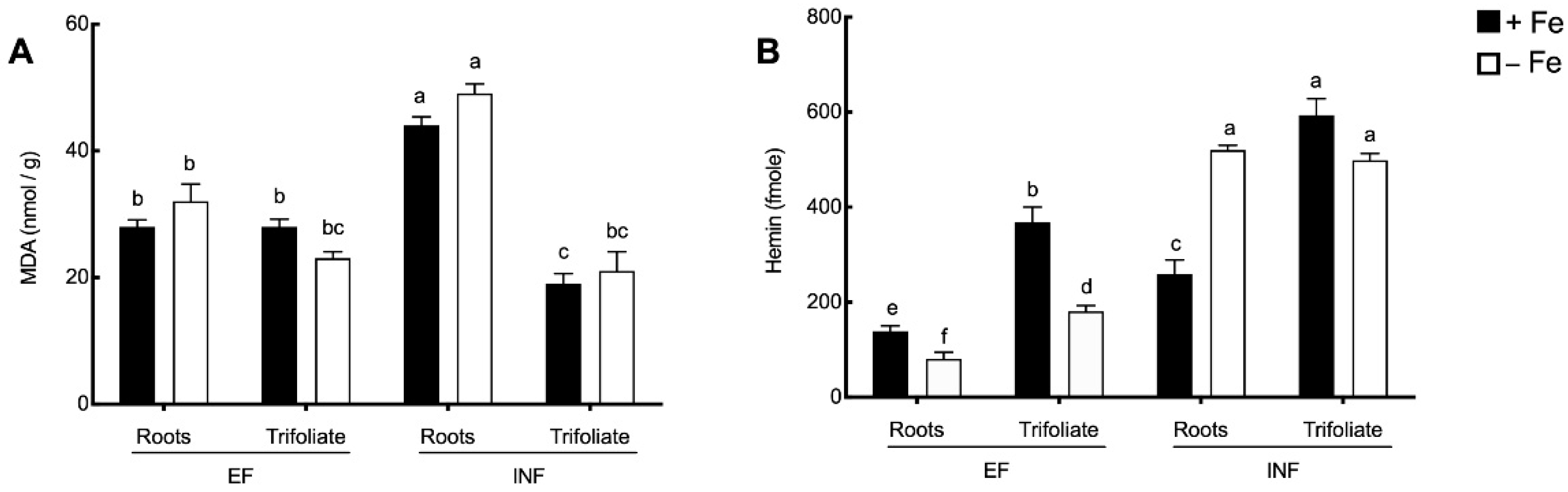
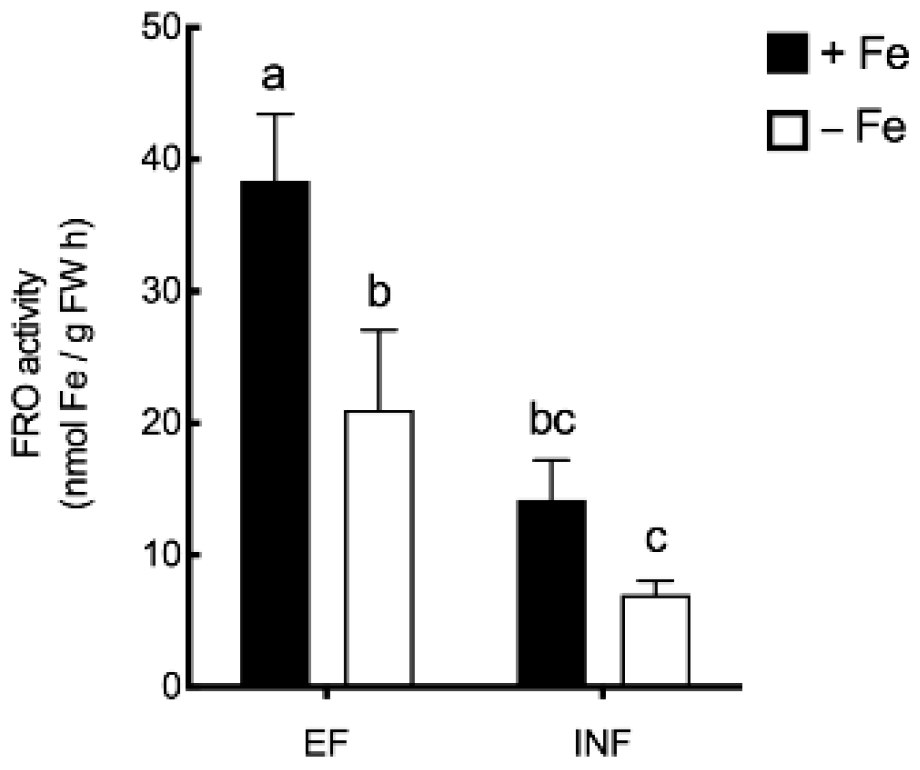
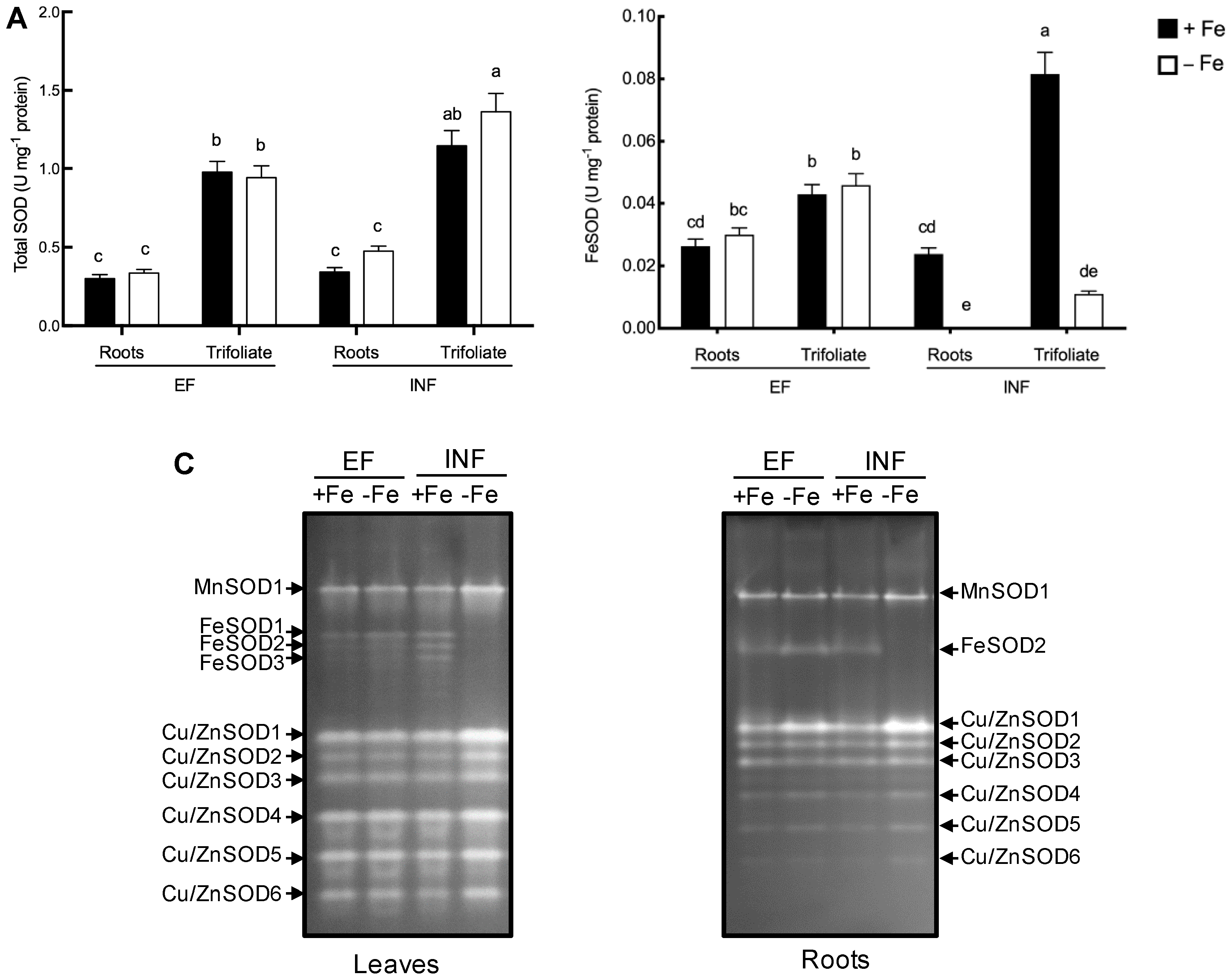
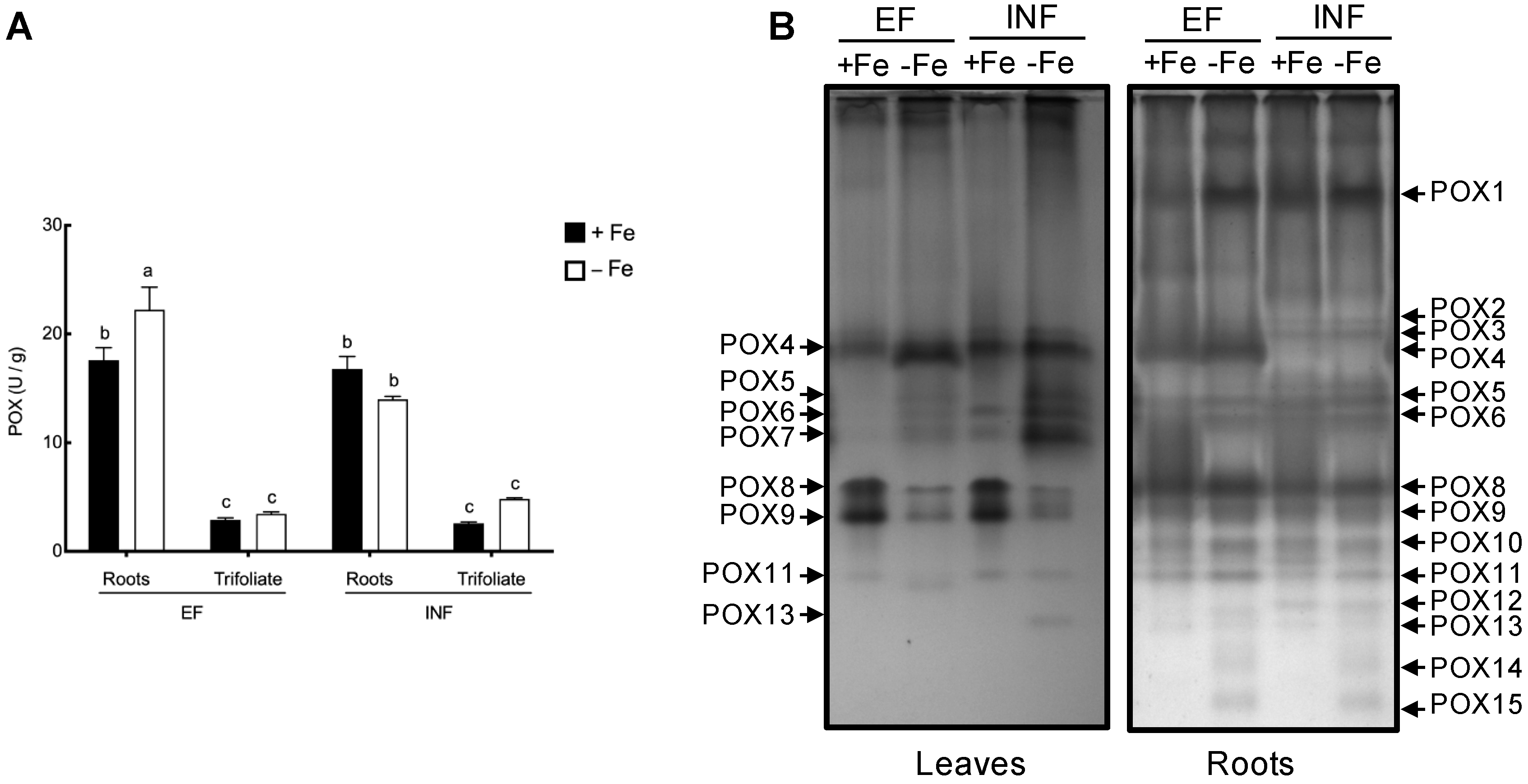
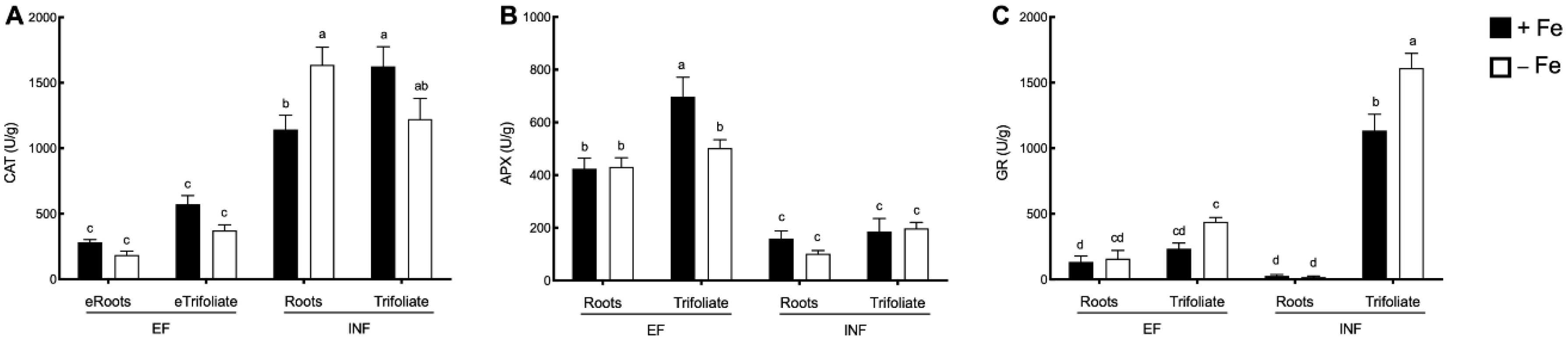
2.3. Oxidative Stress Evaluation
2.4. Enzymatic Activity
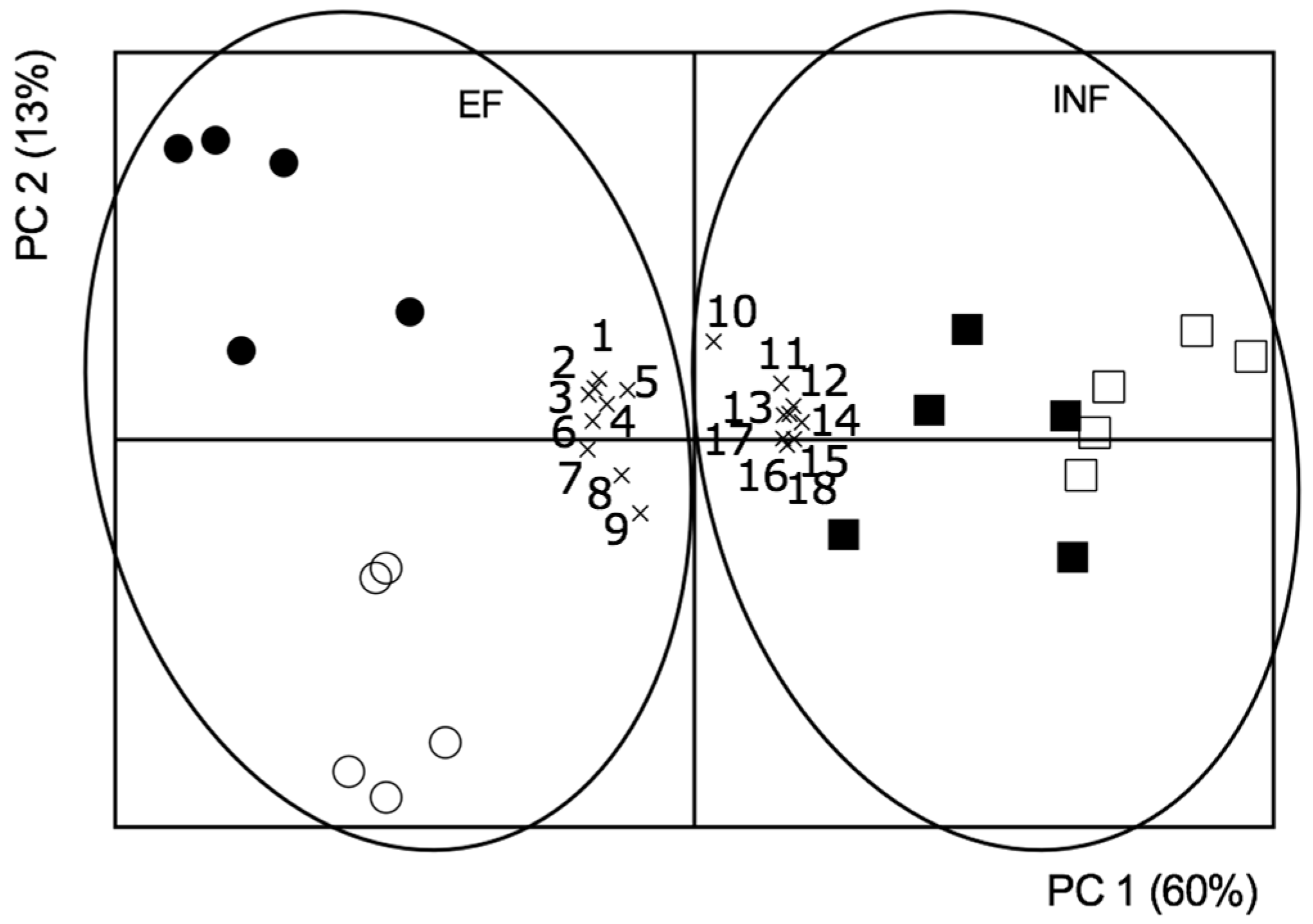
2.5. Principal Component Analysis
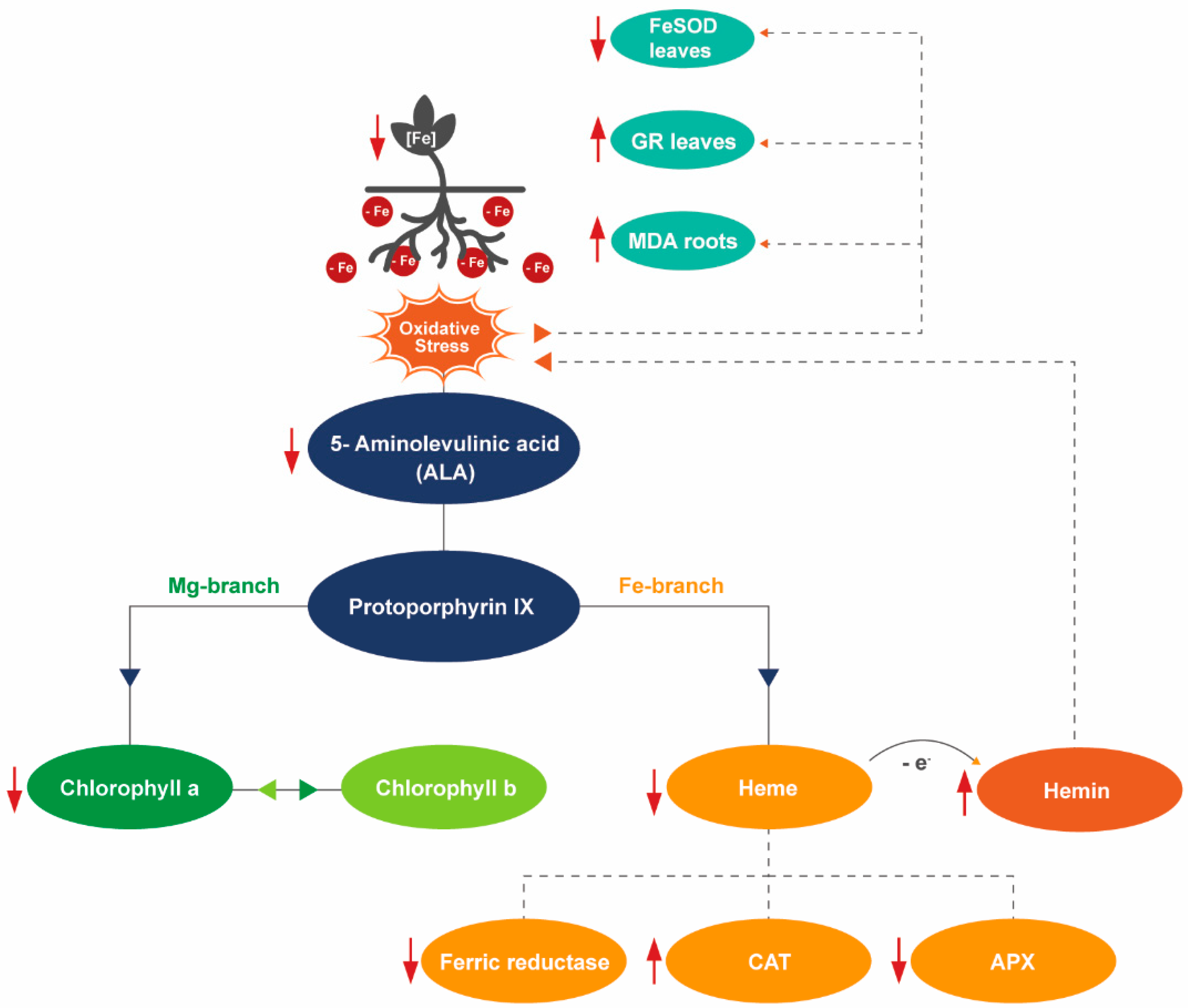
3. Discussion
4. Methods
4.1. Plant Material and Growth Conditions
4.2. Fe Determination by ICP-OES
4.3. ALA, Hemin, and Photosynthetic Pigments Evaluation
4.4. Lipid Peroxidation
4.5. Enzymatic Activity
4.5.1. Root Iron Reductase Activity
4.5.2. SOD and POX Activity
4.5.3. CAT and APX Activity
4.5.4. GR Activity
4.5.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#search/soybeans (accessed on 12 September 2019).
- Prasad, P.V.V. Plant nutrition: iron Chlorosis. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Elsevier: London, UK, 2003; pp. 649–656. [Google Scholar]
- Hansen, N.C.; Jolley, V.D.; Naeve, S.L.; Goos, R.J. Iron deficiency of soybean in the North Central U.S. an associated soil properties. Soil Sci. Plant Nutr. 2004, 50, 983–987. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Nelson, R.T.; Grant, D.; Schmutz, J.; Grimwood, J.; Cannon, S.; Vance, C.P.; Graham, M.A.; Shoemaker, R.C. Integrating microarray analysis and the soybean genome to understand the soybeans iron deficiency response. BMC Genomics 2009, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Jolley, V.D. Plant metabolic responses to iron-deficiency stress. Bioscience. 1989, 39, 546–551. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Grusak, M.A. Morpho-physiological parameters affecting iron deficiency chlorosis in soybean (Glycine max L.). Plant Soil 2014, 374, 161–172. [Google Scholar] [CrossRef]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Jeong, J.; Connolly, E.L. Iron uptake mechanisms in plants: Functions of the FRO family of ferric reductases. Plant Sci. 2009, 176, 709–714. [Google Scholar] [CrossRef]
- Mauzerall, D.; Granick, S. The occurrence and determination of S-aminolevulinic acid and porphobilinogen in urine. J. Biol. Chem. 1956, 219, 435–446. [Google Scholar] [PubMed]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.M. Tetrapyrrole Signaling in Plants. Front. Plant Sci. 2016, 7, 1586. [Google Scholar] [CrossRef] [PubMed]
- Brzezowski, P.; Richter, A.S.; Grimm, B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim. Biophys. Acta 2015, 1847, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Müllebner, A.; Moldzio, R.; Redl, H.; Kozlov, A.V.; Duvigneau, J.C. Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin. Biomolecules 2015, 5, 679–701. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Chen, W.; Zhu, J.; Peng, Y.Y. Enhancement of nitrite on heme-induced oxidative reactions: A potential toxicological implication. Toxicol. Vitr. 2012, 26, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, T.; Zhai, L.; Li, D.; Zhang, X.; Xu, X.; Ma, H.; Wang, Y.; Han, Z. Reactive oxygen species function to mediate the Fe deficiency response in an Fe-efficient apple genotype: an early response mechanism for enhancing reactive oxygen production. Front. Plant Sci. 2016, 7, 1726. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jing, Y.; Chen, K.; Song, L.; Chen, F.; Zhang, L. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J. Plant Physiol. 2007, 164, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Le, C.T.T.; Brumbarova, T.; Ivanov, R.; Stoof, C.; Weber, E.; Mohrbacher, J.; Fink-Straube, C.; Bauer, P. Zinc finger of arabidopsis thaliana12 (ZAT12) interacts with FER-like iron deficiency-induced transcription factor (FIT) linking iron deficiency and oxidative stress responses. Plant Physiol. 2016, 170, 540–557. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Env. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Busch, A.W.U.; Montgomery, B.L. Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol. 2015, 4, 260–271. [Google Scholar] [CrossRef]
- Spirt, S.; Lutter, K.; Stahl, W. Carotenoids in Photooxidative Stress. Curr. Nutr. Food Sci. 2010, 6, 36–43. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.S.; Lee, C.B. The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J. Plant Physiol. 2001, 158, 737–745. [Google Scholar] [CrossRef]
- Shi, Q.; Ding, F.; Wang, X.; Wei, M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol. Biochem. 2007, 45, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Env. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Asensio, A.C.; Gil-Monreal, M.; Pires, L.; Gogorcena, Y.; Aparicio-Tejo, P.M.; Moran, J.F. Two Fe-superoxide dismutase families respond differently to stress and senescence in legumes. J. Plant Physiol. 2012, 169, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Van Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral. Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: New York, USA, 2012; pp. 191–248. [Google Scholar]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, L.; Bartoli, C.G.; Lamattina, L. Glutathione and ascorbic acid protect Arabidopsis plants against detrimental effects of iron deficiency. J. Exp. Bot. 2013, 64, 3169–3178. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Bashir, K.; Nagasaka, S.; Itai, R.N.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol. Biol. 2007, 65, 277–284. [Google Scholar] [CrossRef]
- Carvalho, S.M.P.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- Roriz, M.; Carvalho, S.M.; Vasconcelos, M.W. High relative air humidity influences mineral accumulation and growth in iron deficient soybean plants. Front. Plant Sci. 2014, 5, 726. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.S.; Roriz, M.; Susana, S.M.; Vasconcelos, M.W. Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.). Front. Plant Sci. 2015, 6, 325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, W.; Qiu, Q.; Meng, F.; Zhang, M.; Rao, D.; Wang, Z.; Yan, X. Physiological regulation associated with differential tolerance to iron deficiency in soybean. Crop Sci. 2018, 57, 1349–1359. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Yang, Z.M. Co-expression analysis reveals a group of genes potentially involved in regulation of plant response to iron-deficiency. Gene 2015, 554, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mbonankira, J.E.; Coq, S.; Vromman, D.; Lutts, S.; Nizigiyimana, A.; Bertin, P. Catalase and ascorbate peroxidase activities are not directly involved in the silicon-mediated alleviation of ferrous iron toxicity in rice. J. Plant Nutr. Soil Sci. 2015, 178, 477–485. [Google Scholar] [CrossRef]
- Crisp, R.J.; Pollington, A.; Galea, C.; Jaron, S.; Yamaguchi-Iwai, Y.; Kaplan, J. Inhibition of heme biosynthesis prevents transcription of iron uptake genes in yeast. J. Biol. Chem. 2003, 278, 45499–45506. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Gokul, A.; Carelse, M.F.; Jobe, T.O.; Long, T.A.; Keyster, M. Keep talking: crosstalk between iron and sulfur networks fine-tunes growth and development to promote survival under iron limitation. J. Exp. Bot. 2019, 70, 4197–4210. [Google Scholar] [CrossRef]
- Alvarez-Fernández, A.; Díaz-Benito, P.; Abadía, A.; López-Millán, A.F.; Abadía, J. Metal species involved in long distance metal transport in plants. Front. Plant Sci. 2014, 5, 105. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Organization of chlorophyll biosynthesis and insertion of chlorophyll into the chlorophyll-binding proteins in chloroplasts. Photosynth. Res. 2015, 126, 189–202. [Google Scholar] [CrossRef]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New Physiological Effects of 5-Aminolevulinic Acid in Plants: The Increase of Photosynthesis, Chlorophyll Content, and Plant Growth. Biosci. Biotec. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chang, Z.; Sun, L.; Yu, J.; Huang, B. Physiological and Metabolic Effects of 5-Aminolevulinic Acid for Mitigating Salinity Stress in Creeping Bentgrass. PLoS ONE 2014, 9, e116283. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Peter, E.; Pörs, Y.; Lorenzen, S.; Grimm, B.; Czarnecki, O. Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol. 2010, 51, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. The role of metals in production and scavenging of reactive oxygen species in photosystem II. Plant Cell Physiol. 2014, 55, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Caramanico, L.; Rustioni, L.; De Lorenzis, G. Iron deficiency stimulates anthocyanin accumulation in grapevine apical leaves. Plant Physiol. Biochem. 2017, 119, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Elkhouni, A.; Rabhi, M.; Ivanov, A.G.; Krol, M.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Huner, N.P.A. Structural and functional integrity of Sulla carnosa photosynthetic apparatus under iron deficiency conditions. Plant Biol. 2018, 20, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: a review. Plant Growth Reg. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinshi, F.; Florito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharm. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Guidi, L.; Pardossi, A.; Tattini, M.; Gould, K.S. Photoprotection by foliar anthocyanins mitigates effects of boron toxicity in sweet basil (Ocimum basilicum). Planta 2014, 240, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Matthus, E.; Wilkins, K.A.; Swarbreck, S.M.; Doddrell, N.H.; Doccula, F.G.; Costa, A.; Davies, J.M. Phosphate starvation alters abiotic-stress-induced cytosolic free calcium increases in roots. Plant Physiol. 2019, 179, 1754–1767. [Google Scholar] [CrossRef]
- Santos, C.S.; Carvalho, S.M.; Leite, A.; Moniz, T.; Roriz, M.; Rangel, A.O.; Rangel, M.; Vasconcelos, M.W. Effect of tris(3-hydroxy-4-pyridinonate) iron(III) complexes on iron uptake and storage in soybean (Glycine max L.). Plant Physiol. Biochem. 2016, 106, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Meng, L.; Zhang, Y.; Mao, P.; Tian, X.; Li, S.; Zhang, L. Antioxidative systems, metal ion homeostasis and cadmium distribution in Iris lactea exposed to cadmium stress. Ecotoxicol. Env. Saf. 2017, 139, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Membrane lipid peroxidation and its conflict of interest: the two faces of oxidative stress. Curr. Sci. 2014, 107, 1811–1823. [Google Scholar]
- Schmid, N.B.; Giehl, R.F.H.; Döll, S.; Mock, H.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6’-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Wang, Y.W.; Tsednee, M.; Karunakaran, K.; Yeh, K.C. Glutathione plays an essential role in nitric oxide-mediated iron-deficiency signaling and iron-deficiency tolerance in Arabidopsis. Plant J. 2015, 84, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, S.; Lu, Q.; Yang, Z.; Wen, X.; Zhang, L.; Lu, C. Characterization of photosystem II in transgenic tobacco plants with decreased iron superoxide dismutase. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; McInturf, S.A.; Stein, R.J. Rosette iron deficiency transcript and microRNA profiling reveals links between copper and iron homeostasis in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 5903–5918. [Google Scholar] [CrossRef]
- Myouga, F.; Hosoda, C.; Umezawa, T.; Iizumi, H.; Kuromori, T.; Motohashi, R.; Shinozak, K. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 2008, 20, 3148–3162. [Google Scholar] [CrossRef]
- Jelali, N.; Bonnini, S.; Dell’Orto, M.; Abdelly, C.; Gharsalli, M.; Zocchi, G. Root antioxidant responses of two Pisum sativum cultivars to direct and induced Fe deficiency. Plant Biol. 2014, 16, 607–614. [Google Scholar] [CrossRef]
- Donnini, S.; Dell’Orto, M.; Zocchi, G. Oxidative stress responses and root lignification induced by Fe deficiency conditions in pear and quince genotypes. Tree Physiol. 2011, 31, 102–113. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Valle, E.M.; Federico, M.L.; Gómez, L.D.; Melchiorre, M.N.; Paleo, A.D.; Carrillo, N.; Acevedo, A. Status of antioxidant metabolites and enzymes in a catalase-deficient mutant of barley (Hordeum vulgare L.). Plant Sci. 2002, 162, 363–371. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakajima, H. Chapter Twenty – Creation of a thermally tolerant peroxidase. Methods Enzym. 2016, 580, 455–470. [Google Scholar]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Shaheen, R. Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant. Physiol. 2007, 19, 149–161. [Google Scholar] [CrossRef]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium tolerance is associated with the root-driven coordination of cadmium sequestration, iron regulation, and ROS scavenging in rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef]
- Lok, H.C.; Suryo Tahmanto, Y.; Hawkins, C.L.; Kalinowski, D.S.; Morrow, C.S.; Townsend, A.J.; Ponka, P.; Richardson, D.R. Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes. J. Biol. Chem. 2012, 287, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Turella, P.; Pedersen, J.Z.; Caccuri, A.M.; Maria, F.; Mastroberardino, P.; Bello, M.L.; Federici, G.; Ricci, G. Glutathione transferase superfamily behaves like storage proteins for dinitrosyl-diglutathionyl-iron complex in heterogeneous systems. J. Biol. Chem. 2003, 278, 42294–42299. [Google Scholar] [CrossRef]
- Germplasm Resources Information Network. Available online: http://www.ars-grin.gov/ (accessed on 12 September 2019).
- Vasconcelos, M.; Eckert, H.; Arahana, V.; Graefficient, G.; Grusak, M.A.; Clemente, T. Molecular and phenotypic characterization of transgenic soybean expressing the Arabisopsis ferric chelate reductase gene, FRO2. Planta 2006, 224, 1116–1128. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Env. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Li, H. Principles and techniques of plants physiological biochemical experimental; Higher education Press: Beijing, China, 2000. [Google Scholar]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signaling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012, 182, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V. Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol. Biochem. 2004, 42, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1983; pp. 273–286. [Google Scholar]
- United States Environmental Protection Agency. Plant peroxidase activity determination. SOP#: 2035. 1994. Available online: https://clu-in.org/download/ert/2035-R00.pdf (accessed on 28 November 1994).
- Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Polyamines as protectores against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci. 2001, 161, 481–488. [Google Scholar] [CrossRef]
- Rakotomalala, R. TANAGRA: un logiciel gratuit pour l’enseignement et la recherche. In Proceedings of the Extraction et gestion des connaissances (EGC’2005), Actes des cinquièmes journées Extraction et Gestion des Connaissances, Paris, France, 18–21 January 2005; Volume RNTI-E-3, pp. 697–702. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.S.; Ozgur, R.; Uzilday, B.; Turkan, I.; Roriz, M.; Rangel, A.O.S.S.; Carvalho, S.M.P.; Vasconcelos, M.W. Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis. Plants 2019, 8, 348. https://doi.org/10.3390/plants8090348
Santos CS, Ozgur R, Uzilday B, Turkan I, Roriz M, Rangel AOSS, Carvalho SMP, Vasconcelos MW. Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis. Plants. 2019; 8(9):348. https://doi.org/10.3390/plants8090348
Chicago/Turabian StyleSantos, Carla S., Rengin Ozgur, Baris Uzilday, Ismail Turkan, Mariana Roriz, António O.S.S. Rangel, Susana M.P. Carvalho, and Marta W. Vasconcelos. 2019. "Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis" Plants 8, no. 9: 348. https://doi.org/10.3390/plants8090348
APA StyleSantos, C. S., Ozgur, R., Uzilday, B., Turkan, I., Roriz, M., Rangel, A. O. S. S., Carvalho, S. M. P., & Vasconcelos, M. W. (2019). Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis. Plants, 8(9), 348. https://doi.org/10.3390/plants8090348







