Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose
Abstract
1. Introduction
2. Results
2.1. Colletotrichum spp. Virulence
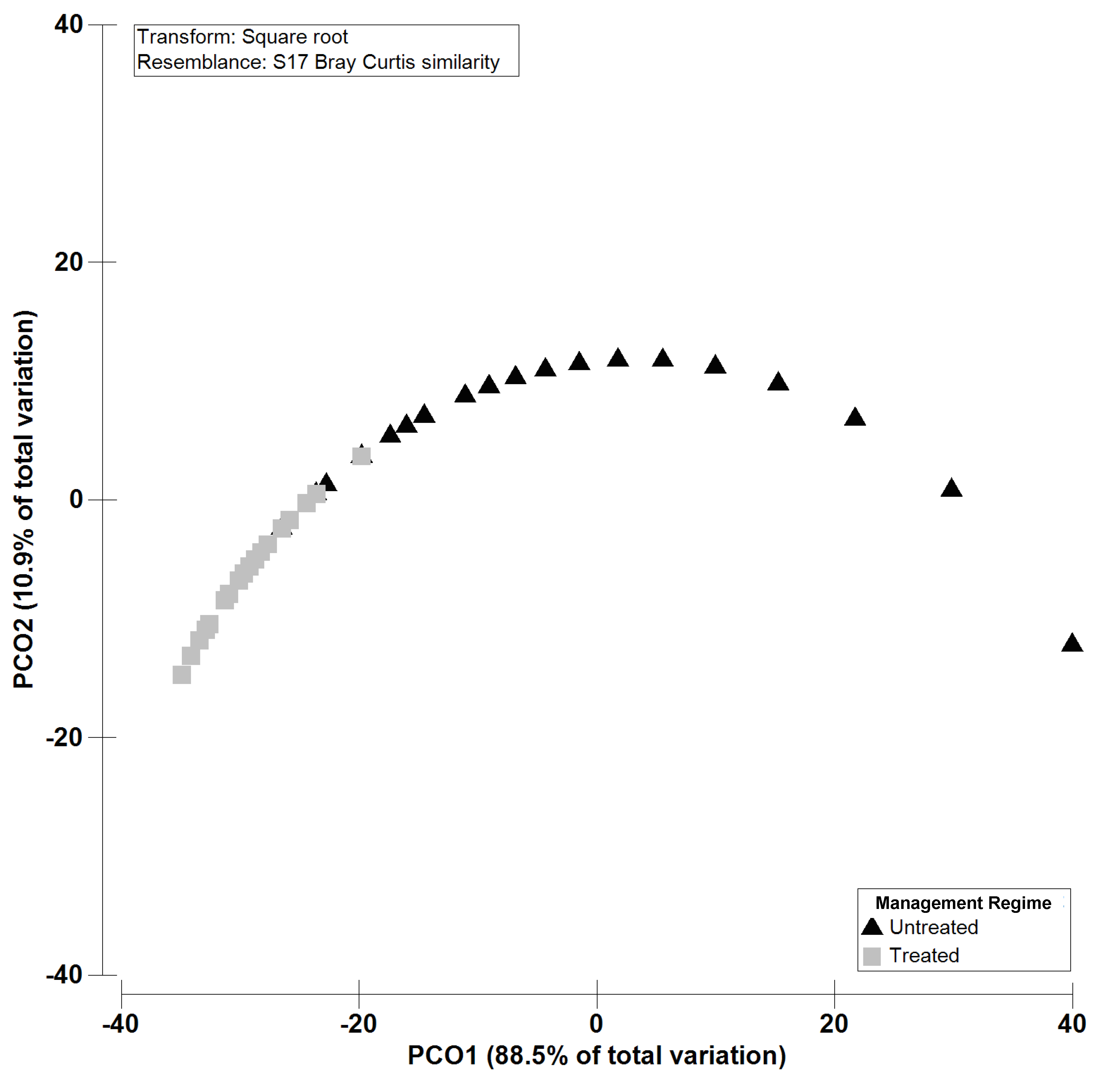
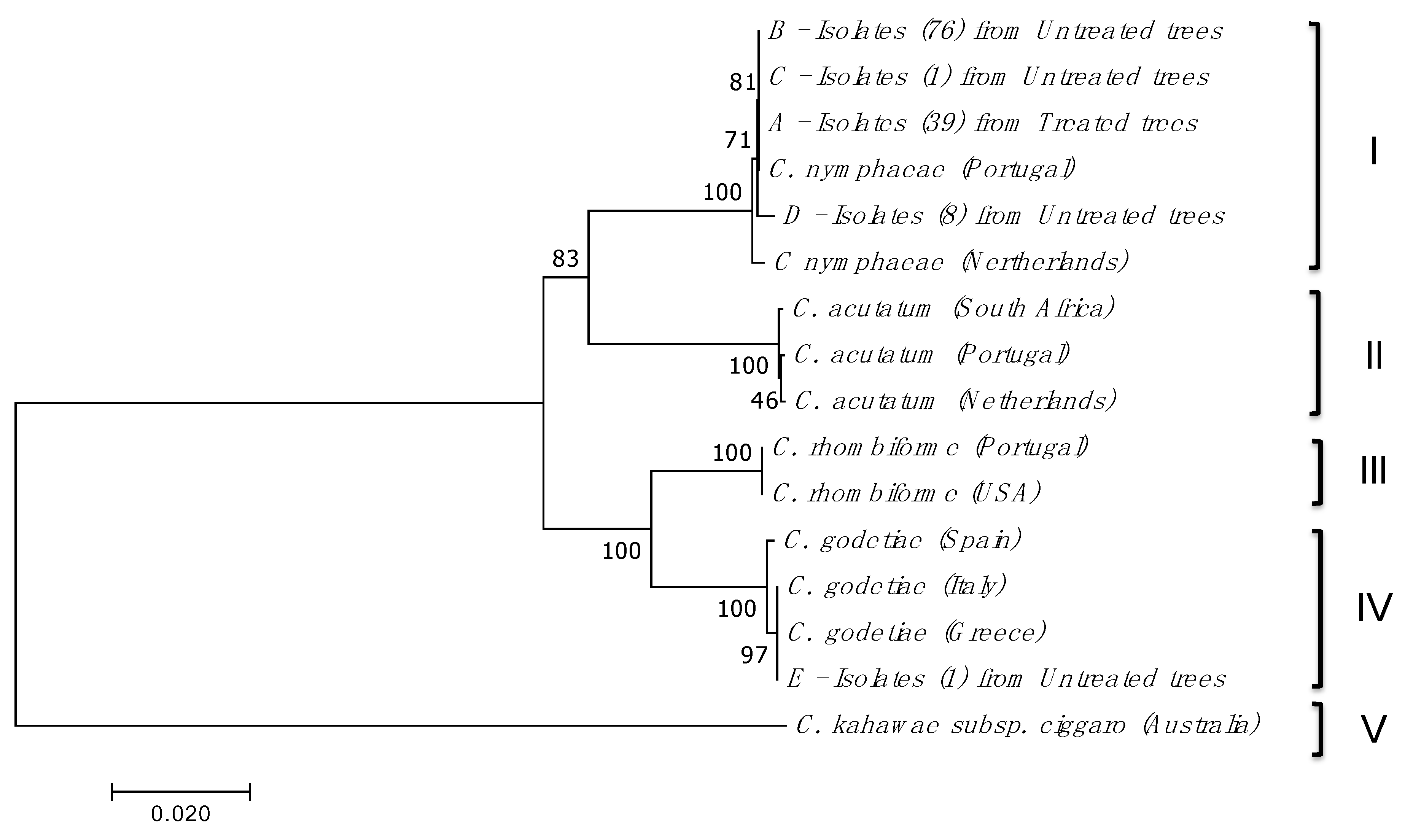
2.2. Isolation, Amplification and Identification of Colletotrichum spp.
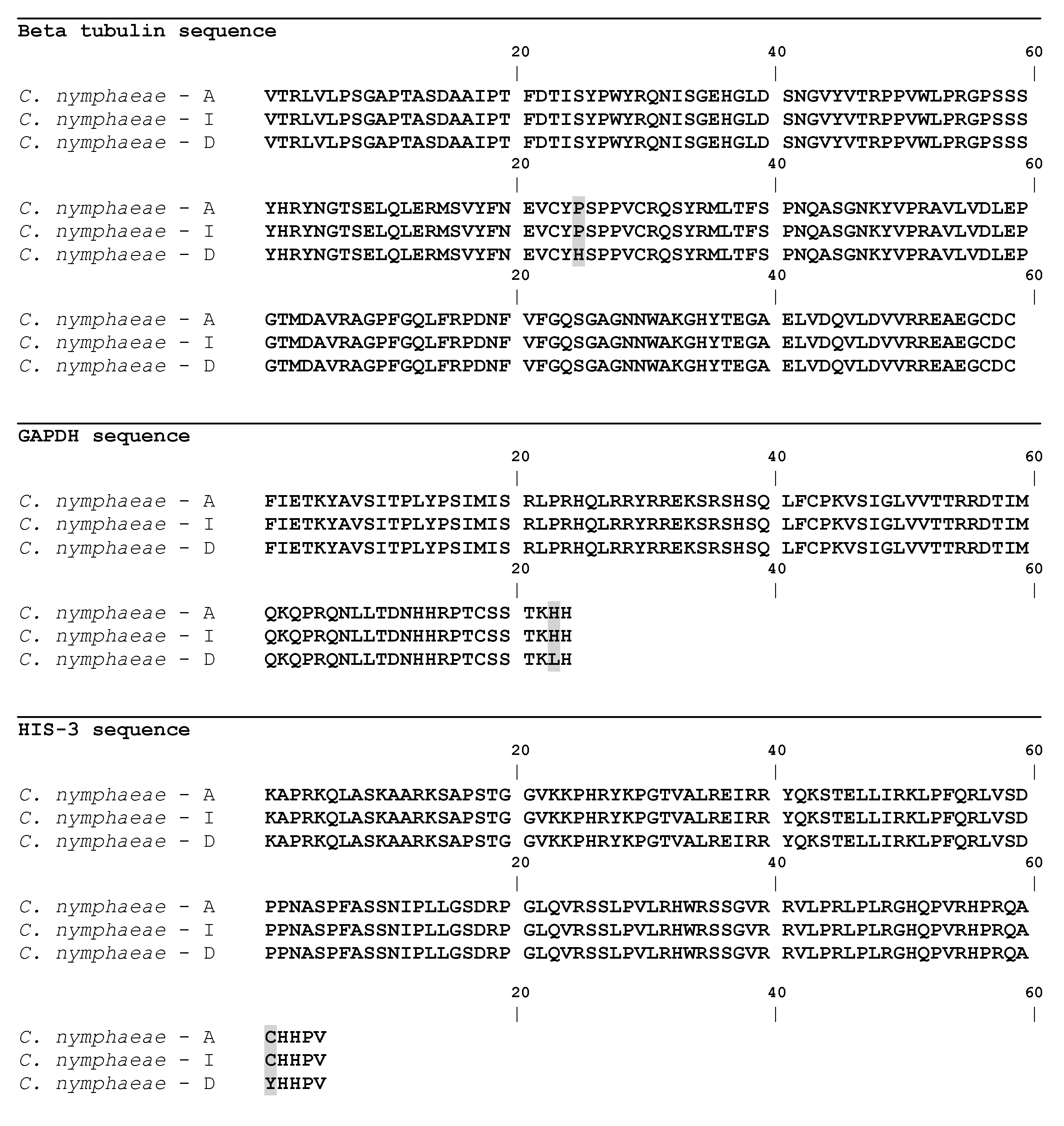
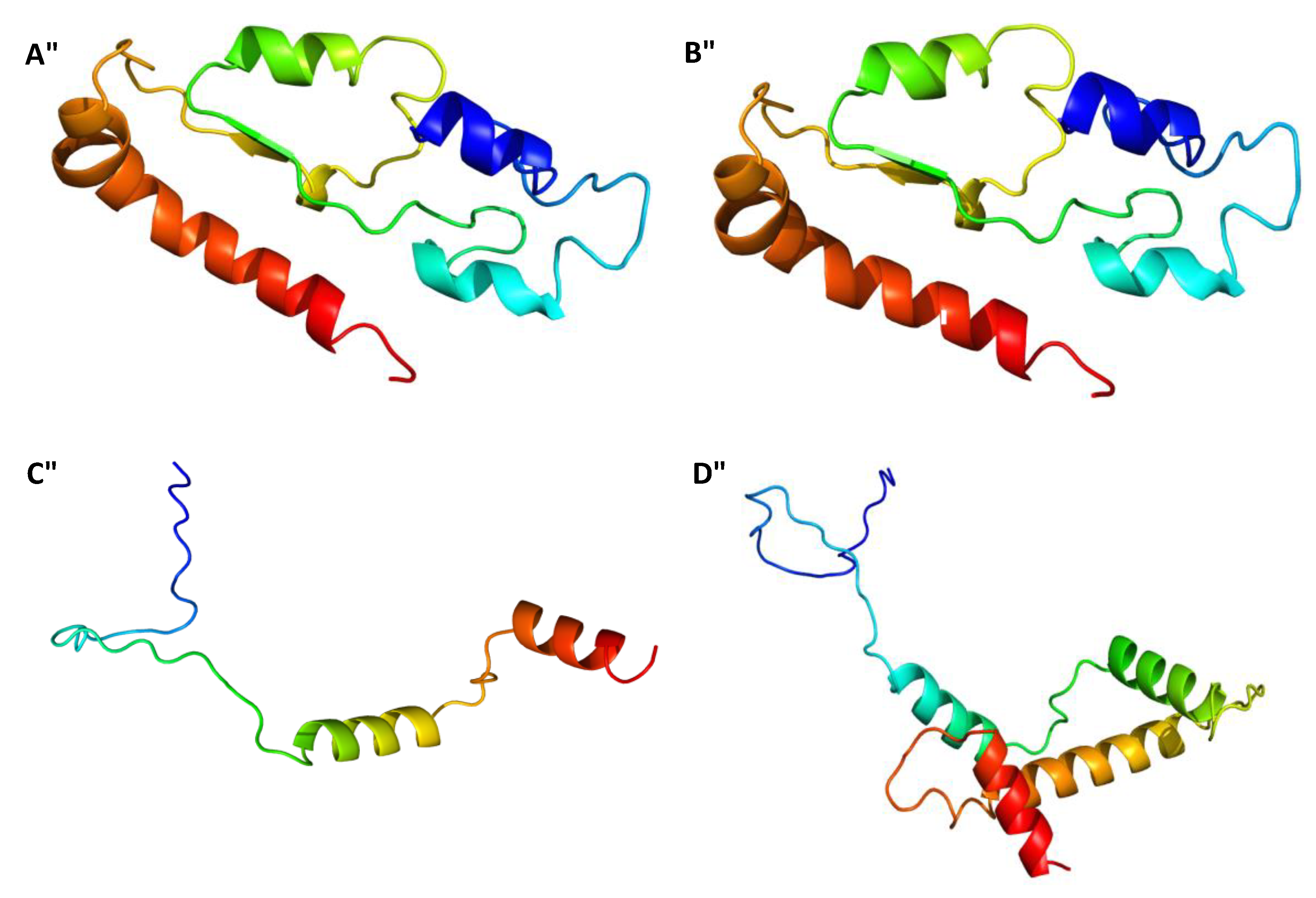
2.3. Gene Sequences Analysis and Characterisation of the Predicted Proteins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Area and Sample Collection
5.2. Isolation of Colletotrichum spp.
5.3. DNA Extraction and Identification of Colletotrichum spp.
5.4. Statistical Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- IOC. Available online: http://www.Internationaloliveoil.Org/ (accessed on 4 June 2018).
- Grieco, F.; Alkowni, R.; Saponari, M.; Pantaleo, V.; Savino, V.; Martelli, G.P. Molecular Detection of Olive-Infecting Viruses. In Proceedings of the Fourth International Symposium on Olive Growing, Valenzano, Italy, 25–30 September 2000; pp. 737–740. [Google Scholar]
- Barranco, D.; Rallo, L. Las Variedades de Olivo Cultivadas em Andalucia; Junta de Andalucia, Ministério de Agricultura: Seville, Spain, 1984.
- Graniti, A.; Frisullo, S.; Pennisi, A.; Magnano di San Lio, G. Infections of glomerella cingulata on olive in italy. Eppo Bull. 1993, 23, 457–465. [Google Scholar] [CrossRef]
- Talhinhas, P.; Sreenivasaprasad, S.; Neves-Martins, J.; Oliveira, H. Molecular and phenotypic analyses reveal the association of diverse colletotrichum acutatum groups and a low level of c. Gloeosporioides with olive anthracnose. Appl. Environ. Microbiol. 2005, 71, 2987–2998. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Bouhmidi, K.; Trapero, A. Influence of fruit maturity, cultivar susceptibility, and inoculation method on infection of olive fruit by colletotrichum acutatum. Plant Dis. 2008, 92, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.; Faedda, O.; Sinatra, R.; Agosteo, F.; Schena, G.; Frisullo, L.; di San, S.M.; Lio, G. Olive anthracnose. J. Plant Pathol. 2012, 94, 29–44. [Google Scholar]
- Mosca, S.; Li Destri Nicosia, M.; Cacciola, S.; Schena, L. Molecular analysis of colletotrichum species in the carposphere and phyllosphere of olive. PLoS ONE 2014, 9, e114031. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Gonçalves, E.; Sreenivasaprasad, S.; Oliveira, H. Virulence diversity of anthracnose pathogens (colletotrichum acutatum and c. Gloeosporioides species complexes) on eight olive cultivars commonly grown in portugal. Eur. J. Plant Pathol. 2015, 142, 73–83. [Google Scholar] [CrossRef]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Xaviér, C.; Roca, L.; Romero, J.; Moreda, W.; Trapero, A. La antracnosis del olivo y su efecto en la calidad del aceite. Grasas Y Aceites 2014, 65, e028. [Google Scholar] [CrossRef]
- Moral, J.; Trapero, A. Assessing the susceptibility of olive cultivars to anthracnose caused by colletotrichum acutatum. Plant Dis. 2009, 93, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Neves-Martins, J.; Oliveira, H.; Sreenivasaprasad, S. The distinctive population structure of colletotrichum species associated with olive anthracnose in the algarve region of portugal reflects a host–pathogen diversity hot spot. Fems Microbiol. Lett. 2009, 296, 31–38. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.; Campos, M.; Rei, F.; Félix, M. Diversity of colletotrichum species associated with olive anthracnose and new perspectives on controlling the disease in portugal. Agronomy 2018, 8, 301. [Google Scholar] [CrossRef]
- Martelli, G. A first contribution to the knowledge of the biology of gloeosporium olivarum. Phytopathol. Mediterr. 1960, 1, 101–112. [Google Scholar]
- Trapero, A.; Blanco-López, M. Enfermedades. In El Cultivo de Olivo; Barranco, D., Escobar, R.F., Rallo, L., Eds.; Junta de Andalucía/Mundi-Prensa: Madrid, Spain, 2008; pp. 57–614. [Google Scholar]
- Gang, G.H.; Cho, H.J.; Kim, H.S.; Kwack, Y.B.; Kwak, Y.S. Analysis of fungicide sensitivity and genetic diversity among colletotrichum species in sweet persimmon. Plant Pathol. J. 2015, 31, 115–122. [Google Scholar] [CrossRef]
- Keinath, A.P.; Zitter, T.A. Resistance to benomyl and thiophanate-methyl in didymella bryoniae from south carolina and new york. Plant Dis. 1998, 82, 479–484. [Google Scholar] [CrossRef]
- Bernstein, B.; Zehhr, E.I.; Dean, R.A.; Shabi, E. Characteristics of colletotrichum from peach, apple, pecan, and other hosts. Plant Dis. 1995, 79, 478–482. [Google Scholar] [CrossRef]
- Young, J.R.; Tomaso-Peterson, M.; Tredway, L.P.; de la Cerda, K. Occurrence and molecular identification of azoxystrobin-resistant colletotrichum cereale isolates from golf course putting greens in the southern united states. Plant Dis. 2010, 94, 751–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shrestha, S.K.; Cochran, A.; Mengistu, A.; Lamour, K.; Castro-Rocha, A.; Young-Kelly, H. Genetic diversity, qoi fungicide resistance, and mating type distribution of cercospora sojina—Implications for the disease dynamics of frogeye leaf spot on soybean. PLoS ONE 2017, 12, e0177220. [Google Scholar] [CrossRef] [PubMed]
- Ghini, R.; Kimati, H. Resistência de Fungos a Fungicidas; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2000; 78p. [Google Scholar]
- Ramdial, H.; De Abreu, K.; Rampersad, S.N. Fungicide sensitivity among isolates of colletotrichum truncatum and fusarium incarnatum-equiseti species complex infecting bell pepper in trinidad. Plant Pathol. J. 2017, 33, 118–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanders, G.M.; Korsten, L. Survey of fungicide resistance of colletotrichum gloeosporioides from different avocado production areas. S. Afr. Avocado Grow. Assoc. Yearb. 1999, 22, 35–38. [Google Scholar]
- Tozze, J.H.J.; Mello, M.B.A.; Massola, J.N.S. Caracterização morfológica e fisiológica de isolados de colletotrichum sp. causadores de antracnose em solanáceas. Summa Phytopathol. 2006, 32, 77–79. [Google Scholar]
- Rodrigues, M.B.C.; Andreote, F.D.; Spòsito, M.B.; Vildoso, C.I.A.; Araujo, W.L.; Kleiner, A.A.P. Resistência a benzimidazóis por guignardia citricarpa. Pesqui. Agropecuária Bras. 2007, 42, 323–327. [Google Scholar] [CrossRef]
- Avila-Adame, C.; Köller, W. Characterization of spontaneous mutants of magnaporthe grisea expressing stable resistance to the qo-inhibiting fungicide azoxystrobin. Curr. Genet. 2003, 42, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.J.; Grabke, A.; Dowling, M.; Holstein, H.; Schnabel, G. Resistance in colletotrichum siamense from peach and blueberry to thiophanate-methyl and azoxystrobin. Plant Dis. 2015, 99, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Vela-Corcía, D.; Romero, D.; de Vicente, A.; Pérez-García, A. Analysis of β-tubulin-carbendazim interaction reveals that binding site for mbc fungicides does not include residues involved in fungicide resistance. Sci. Rep. 2018, 8, 7161. [Google Scholar] [CrossRef] [PubMed]
- Peres, N.A.R.; Souza, N.L.; Peever, T.L.; Timmer, L.W. Benomyl sensitivity of isolates of colletotrichum acutatum and c. Gloeosporioides from citrus. Plant Dis. 2004, 88, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Abdelnabby, H.; Xiao, Y. Novel point mutations inb-tubulingene for carbendazim resistance maintaining nematode pathogenicity of paecilomyces lilacinus. Eur. J. Plant Pathol. 2015, 143, 57–68. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.; Woudenberg, J.; Crous, P. The colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Agosteo, G.; Magnano di San Lio, G.; Cacciola, S.; Frisullo, S. Characterisation of the causal agent of olive anthracnose in southern italy. Acta Hortic. 2002, 586, 713–716. [Google Scholar] [CrossRef]
- Gomes, S.; Martins-Lopes, P.; Lopes, L.; Guedes-Pinto, H. Assessing genetic diversity in olea europaea l. using issr and ssr markers. Plant Mol. Biol. Report. 2009, 123, 82–89. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.; Campos, M.; Rei, F.; Félix, M. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Gomes, S.; Bacelar, E.; Martins-Lopes, P.; Carvalho, T.; Guedes-Pinto, H. Infection process of olive fruits by colletotrichum acutatum and the protective role of the cuticle and epidermis. J. Agric. Sci. 2012, 4, 101–110. [Google Scholar] [CrossRef]
- Freeman, S.; Horowitz, S.; Sharon, A. Pathogenic and nonpathogenic lifestyles in colletotrichum acutatum from strawberry and other plants. Phytopathology 2001, 91, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Sagasta, E. Estudios básicos sobre gloeosporium olivarum alm. (deuteromiceto melanconial). Bol. Patol. Veg. Entomol. Agric. 1968, 30, 31–135. [Google Scholar]
- Hayes, L.E.; Sackett, K.E.; Anderson, N.P.; Flowers, M.D.; Mundt, C.C. Evidence of selection for fungicide resistance in zymoseptoria tritici populations on wheat in western oregon. Plant Dis. 2015, 100, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Cools, H.J.; Fraaije, B.A. Update on mechanisms of azole resistance in mycosphaerella graminicola and implications for future control. Pest Manag. Sci. 2013, 69, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control septoria leaf blotch in wheat. Mol. Plant Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms influencing the evolution of resistance to qo inhibitor fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Chattaoui, M.; Raya, M.; Bouri, M.; Moral, J.; Perez-Rodriguez, M.; Trapero, A.; Msallem, M.; Rhouma, A. Characterization of a colletotrichum population causing anthracnose disease on olive in northern tunisia. J. Appl. Microbiol. 2016, 120, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Faedda, R.; Agosteo, G.; Schena, L.; Mosca, S.; Frisullo, S.; Maganano Di San Lio, G.; Cacciola, S. Colletotrichum clavatum sp. Nov. Identified as the causal agent of olive anthracnose in italy. Phytopathol. Mediterr. 2011, 50, 283–302. [Google Scholar]
- Eto, K.; Tsubamoto, Y.; Terauchi, Y.; Sugiyama, T.; Kishimoto, T.; Takahashi, N.; Yamauchi, N.; Kubota, N.; Murayama, S.; Aizawa, T.; et al. Role of nadh shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 1999, 283, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Pahlman, I.L.; Ansell, R.; Rigoulet, M.; Adler, L.; Gustafsson, L. The importance of the glycerol 3-phosphate shuttle during aerobic growth of saccharomyces cerevisiae. Yeast 1998, 4, 347–357. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Báo, S.N.; Andreotti, P.F.; de Faria, F.P.; Felipe, M.S.S.; dos Santos Feitosa, L.; Mendes-Giannini, M.J.S.; de Almeida Soares, C.M. Glyceraldehyde- 3-phosphate dehydrogenase of paracoccidioides brasiliensis is a cell sur- face protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 2006, 74, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shen, W.; Dauk, M.; Wang, F.; Selvaraj, G.; Zou, J. Targeted gene disruption of glycerol-3-phosphate dehydrogenase in colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. J. Biol. Chem. 2004, 279, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.D.; O’Neil, J.D.; Woehrling, E.K.; Ndunge, O.B.A.; Hill, E.J.; Menache, A.; Reiss, C.J. A preliminary investigation into the impact of a pesticide combination on human neuronal and glial cell lines in vitro. PLoS ONE 2012, 7, e42768. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W.; Butters, J.A.; Barker, H.; Hall, L. Fungal β-tubulin, expressed as fusion protein, binds benzimidazole and phenylcarbamate fungicides. Antimicrob. Agents Chemother. 1998, 42, 2171–2173. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Laniel, M.A. Histones and histone modifications. Curr. Biol. 2004, 14, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Rivera, C.; Gurard-Levin, Z.A.; Almouzni, G.; Loyola, A. Histone lysine methylation and chromatin replication. Biochim. Et Biophys. Acta 2014, 1839, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Ji, T.; Sun, X.; Huang, H.; Zhang, H.; Lu, X.; Wu, L.; Huo, R.; Wu, H.; Gao, X. Histone h3 lysine 9 methyltransferase fvdim5 regulates fungal development, pathogenicity and osmotic stress responses in fusarium verticillioides. Fems Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Mari, M.; Chierici, E.; Pondrelli, M.; Bertolini, P.; Pratella, G.C. Studies on thiabendazole resistance of penicillium expansum of pears: Pathogenic fitness and genetic characterization. Plant Pathol. 2003, 52, 362–370. [Google Scholar] [CrossRef]
- Banno, S.; Fukumori, F.; Ichiishi, A.; Okada, K.; Uekusa, H.; Kimura, M.; Fujimura, M. Genotyping of benzimidazole-resistant and dicarboximide- resistant mutations in botrytis cinerea using real-time polymerase chain reaction assays. Phytopathology 2008, 98, 397–404. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Schnabel, G. Field strains of monilinia fructicola resistant to both mbc and dmi fungicides isolated from stone fruit orchards in the eastern united states. Plant Dis. 2013, 97, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Felts, D.; Michailides, T.J. Resistance to azoxystrobin in alternaria isolates from pistachio in california. Pestic. Biochem. Physiol. 2003, 77, 66–74. [Google Scholar] [CrossRef]
- Moreira, A.J.A. Epidemiologia da Antracnose Foliar da Cebola, Causada por Colletotrichum Gloeosporioides; Federal University of Viçosa: Viçosa, Brazil, 2000. [Google Scholar]
- Haddad, F.; Maffia, L.A.; Mizubuti, E.S.G. Avaliação de fungicidas para o controle de colletotrichum gloeosporioides em cebola. Fitopatol. Bras. 2003, 28, 435–437. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; Félix, M.R. Fungal endophytic communities associated to phyllospheres of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In Pcr Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rdna its2 types within a monophyletic lineage of the fungus fusarium are nonorthologous. Mol. Phylogenet Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Guerber, J.; Liu, B.; Correll, J.; Johnston, P. Characterization of diversity in colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtdna and intron rflps, and mating compatibility. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risede, J.M.; Hywel-Jones, N.L. Calonectria species and their cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 40, 415–430. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. Signalp 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Identification and analysis tools on the expasy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Kelly, L.A.; Mezulis, S.; Yates, C.; Wass, M.; Sternberg, M. The phyre2 web portal for protein modelling, prediction, and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.K.; Brenner, S.E.; Chandonia, J.M. Scope: Structural classification of proteins—extended, integrating scop and astral data and classification of new structures. Nucleic Acids Res. 2014, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova a+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.; Green, R. Statistical design and analysis for a biological effects study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
| Orchard | Treated | Untreated | ||
|---|---|---|---|---|
| Infected Trees | Infected Fruits | Infected Trees | Infected Fruits | |
| Nº of Trees | Mean ± SE | Nº of Trees | Mean ± SE | |
| Orchard 1 | 1 | 7.0 ± 7.0 | 3 | 2.4 ± 1.4 |
| Orchard 2 | 1 | 8.0 ± 8.0 | 4 | 3.2 ± 1.7 |
| Orchard 3 | 1 | 5.8 ± 5.8 | 4 | 2.2 ± 0.7 |
| Orchard 4 | 2 | 13.0 ± 8.3 | 4 | 1.4 ± 0.7 |
| Orchard 5 | 2 | 14.6 ± 9.0 | 3 | 2.0 ± 0.9 |
| Orchard 6 | 1 | 6.2 ± 6.2 | 5 | 3.8 ± 1.2 |
| Orchard 7 | 1 | 8.2 ± 8.2 | 5 | 13.6 ± 3.3 |
| Orchard 8 | 2 | 20.0 ± 12.2 | 4 | 9.0 ± 2.8 |
| Orchard 9 | 2 | 11.0 ± 7.0 | 3 | 5.6 ± 2.8 |
| Orchard 10 | 2 | 12.2 ± 7.5 | 5 | 9.8 ± 1.9 |
| Orchard 11 | 2 | 10.0 ± 6.2 | 5 | 3.0 ± 1.1 |
| Orchard 12 | 2 | 18.6 ± 11.4 | 5 | 2.6 ± 0.5 |
| Orchard 13 | 2 | 12.4 ± 7.8 | 5 | 8.6 ± 4.1 |
| Orchard 14 | 2 | 8.0 ± 4.9 | 4 | 1.2 ± 0.4 |
| Orchard 15 | 4 | 24.4 ± 6.3 | 5 | 6.4 ± 3.5 |
| Orchard 16 | 2 | 11.6 ± 7.1 | 3 | 4.2 ± 2.9 |
| Orchard 17 | 2 | 11.0 ± 7.0 | 5 | 12.6 ± 2.7 |
| Orchard 18 | 2 | 19.2 ± 11.8 | 3 | 1.4 ± 0.7 |
| Orchard 19 | 2 | 10.4 ± 6.4 | 3 | 3.4 ± 1.4 |
| Orchard 20 | 2 | 14.6 ± 9.0 | 5 | 8.2 ± 2.1 |
| Orchard 21 | 2 | 14.6 ± 9.1 | 3 | 1.2 ± 0.6 |
| Samples | Source | Degrees of Freedom | Sum of Squares | Mean Squares | Pseudo-F | Unique Perms | p (Perm) |
|---|---|---|---|---|---|---|---|
| Infected fruits | Treated versus Untreated | 1 | 51773 | 51773 | 139.17 | 9942 | 0.0001 |
| Residual | 123 | 45757 | 372.01 | ||||
| Total | 124 | 97530 | |||||
| Infected trees | Treated versus Untreated | 1 | 4167 | 4167 | 77.073 | 638 | 0.0001 |
| Residual | 40 | 2162.6 | 54.065 | ||||
| Total | 41 | 6329.6 |
| Species | Host | Country | GenBank Accession Number | ||||
|---|---|---|---|---|---|---|---|
| TUB2 | GAPDH | ACT | CHS-1 | HIS-3 | |||
| C. nymphaeae | Olea europaea | Portugal | JQ949852 | JQ948531 | JQ949522 | JQ948862 | JQ949192 |
| C. nymphaeae | Anemone sp. | The Netherlands | JQ949876 | JQ948555 | JQ949546 | JQ948886 | JQ949216 |
| C. godetiae | Olea europaea | Greece | JQ950066 | JQ948746 | JQ949736 | JQ949076 | JQ949406 |
| C. godetiae | Olea europaea | Italy | JQ950064 | JQ948744 | JQ949734 | JQ949074 | JQ949404 |
| C. godetiae | Fragaria x ananassa | Spain | JQ950075 | JQ948755 | JQ949745 | JQ949085 | JQ949415 |
| C. acutatum | Olea europaea | South Africa | JQ950014 | JQ948694 | JQ949684 | JQ949024 | JQ949354 |
| C. acutatum | Olea europaea | Portugal | JQ950015 | JQ948695 | JQ949685 | JQ949025 | JQ949355 |
| C. acutatum | Anemone sp. | The Netherlands | JQ950017 | JQ948697 | JQ949687 | JQ949027 | JQ949357 |
| C. rhombiforme | Olea europaea | Portugal | JQ950108 | JQ948788 | JQ949778 | JQ949118 | JQ949448 |
| C. rhombiforme | V. macrocarpum | USA | JQ950109 | JQ948789 | JQ949779 | JQ949119 | JQ949449 |
| C. kahawae subsp. ciggaro | Olea europaea | Australia | JX010434 | JX009966 | JX009523 | JX009800 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Gomes, L.; Nobre, T.; Rei, F.; Félix, M.d.R. Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose. Plants 2019, 8, 311. https://doi.org/10.3390/plants8090311
Materatski P, Varanda C, Carvalho T, Dias AB, Campos MD, Gomes L, Nobre T, Rei F, Félix MdR. Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose. Plants. 2019; 8(9):311. https://doi.org/10.3390/plants8090311
Chicago/Turabian StyleMateratski, Patrick, Carla Varanda, Teresa Carvalho, António Bento Dias, Maria Doroteia Campos, Luis Gomes, Tânia Nobre, Fernando Rei, and Maria do Rosário Félix. 2019. "Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose" Plants 8, no. 9: 311. https://doi.org/10.3390/plants8090311
APA StyleMateratski, P., Varanda, C., Carvalho, T., Dias, A. B., Campos, M. D., Gomes, L., Nobre, T., Rei, F., & Félix, M. d. R. (2019). Effect of Long-Term Fungicide Applications on Virulence and Diversity of Colletotrichum spp. Associated to Olive Anthracnose. Plants, 8(9), 311. https://doi.org/10.3390/plants8090311







