N-Linked Glycosylation Modulates Golgi-Independent Vacuolar Sorting Mediated by the Plant Specific Insert
Abstract
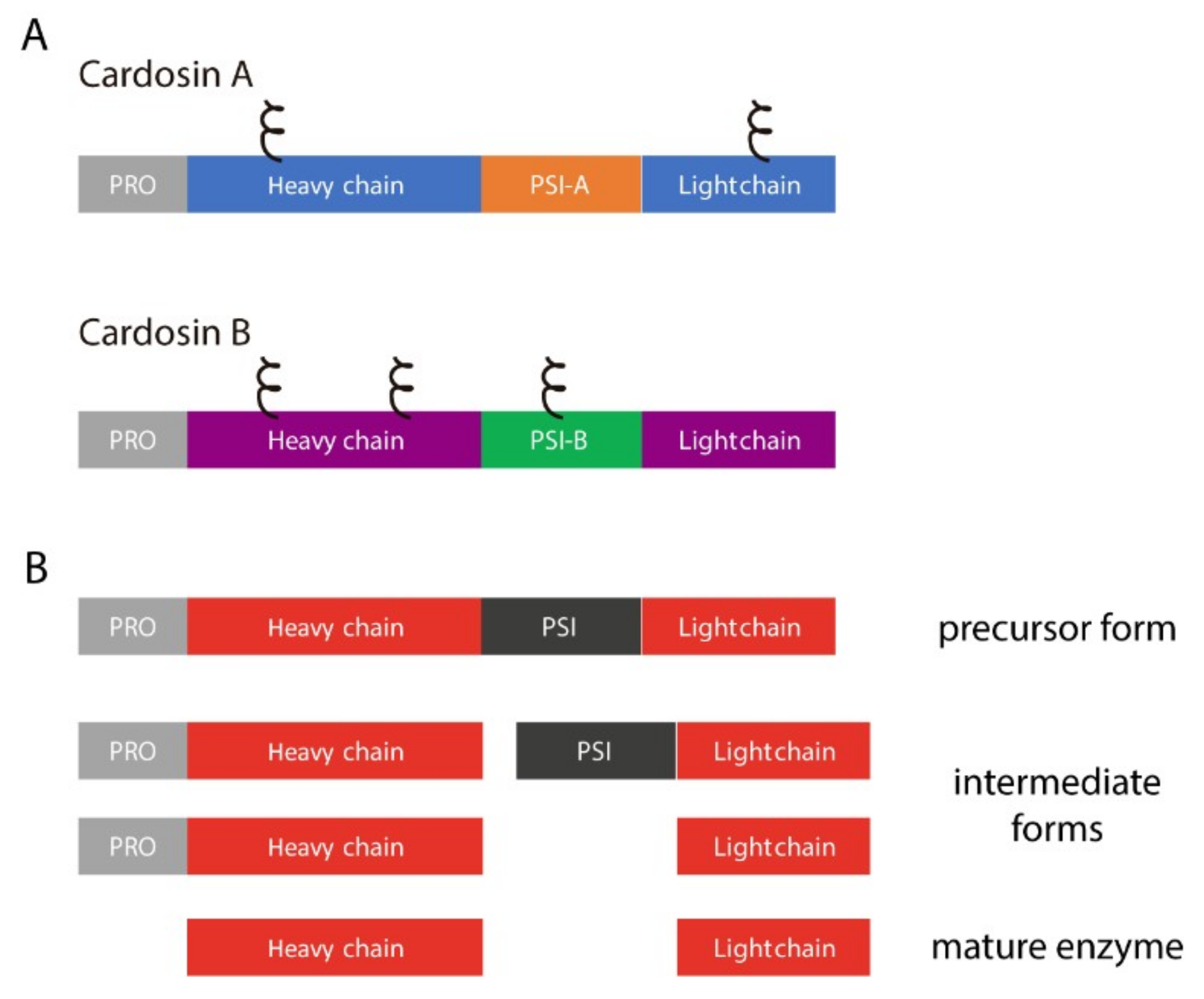
1. Introduction
2. Results
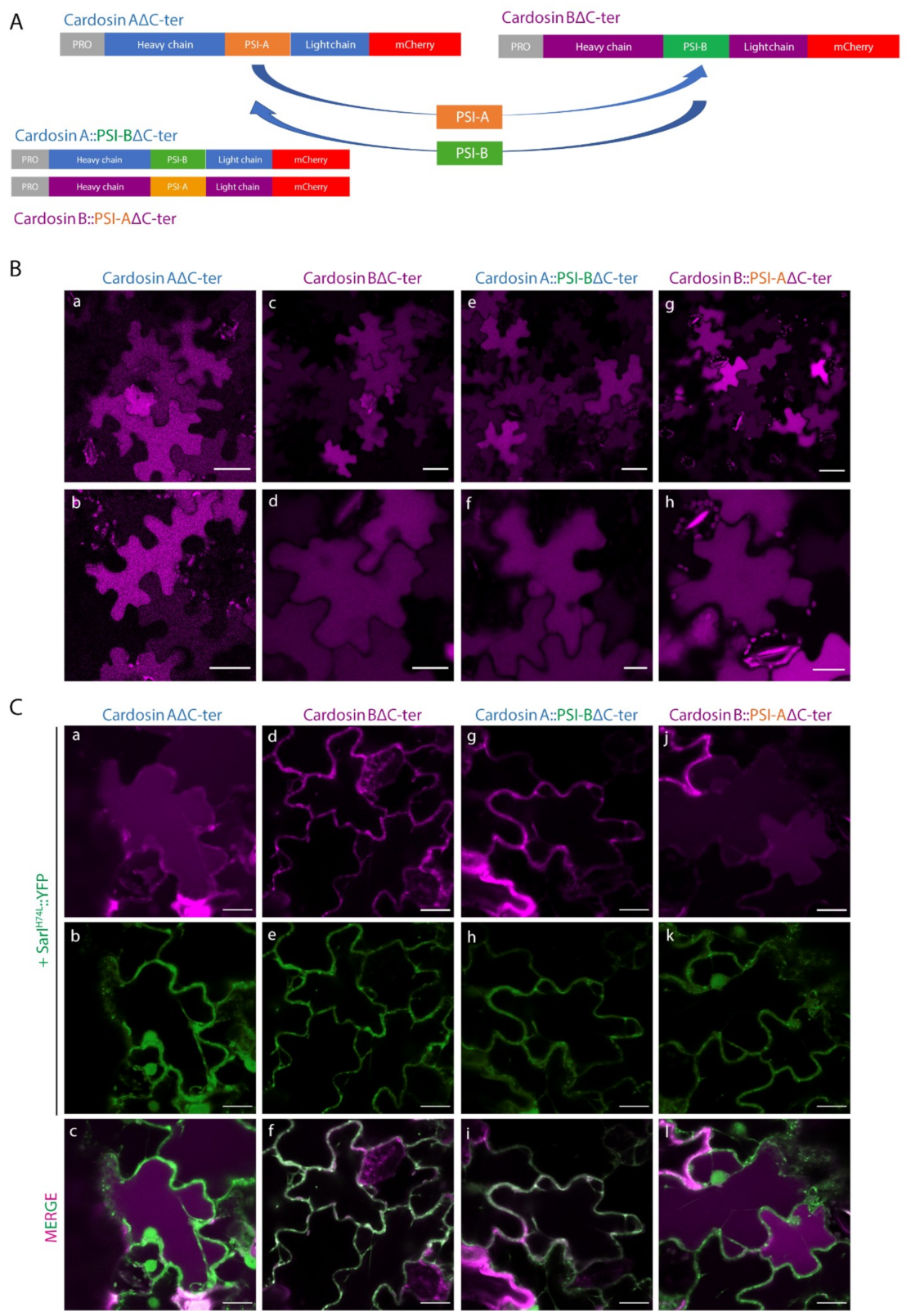
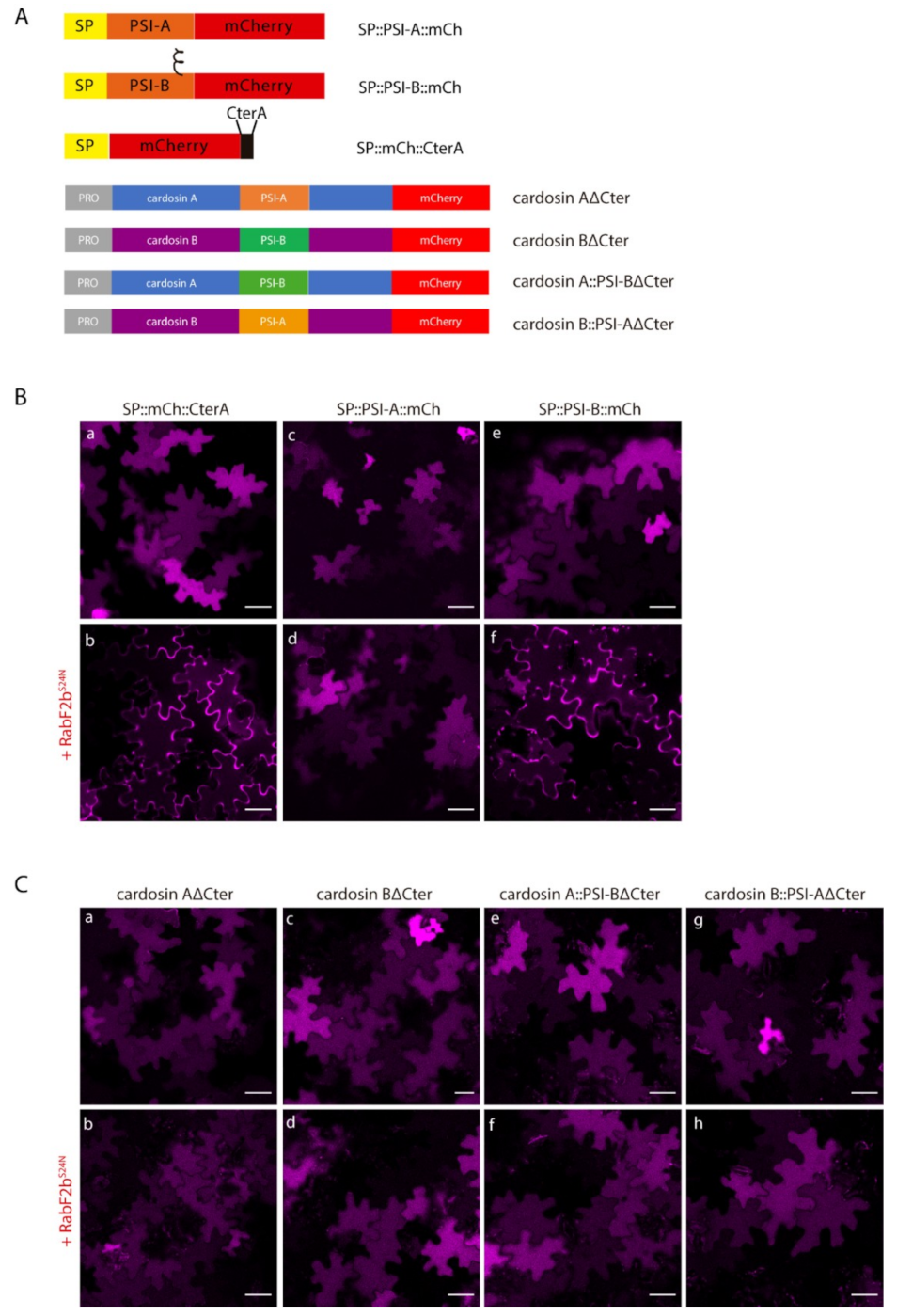
2.1. PSI Domains Maintain Their Sorting Capacity, Independently of the Overall AP Structure
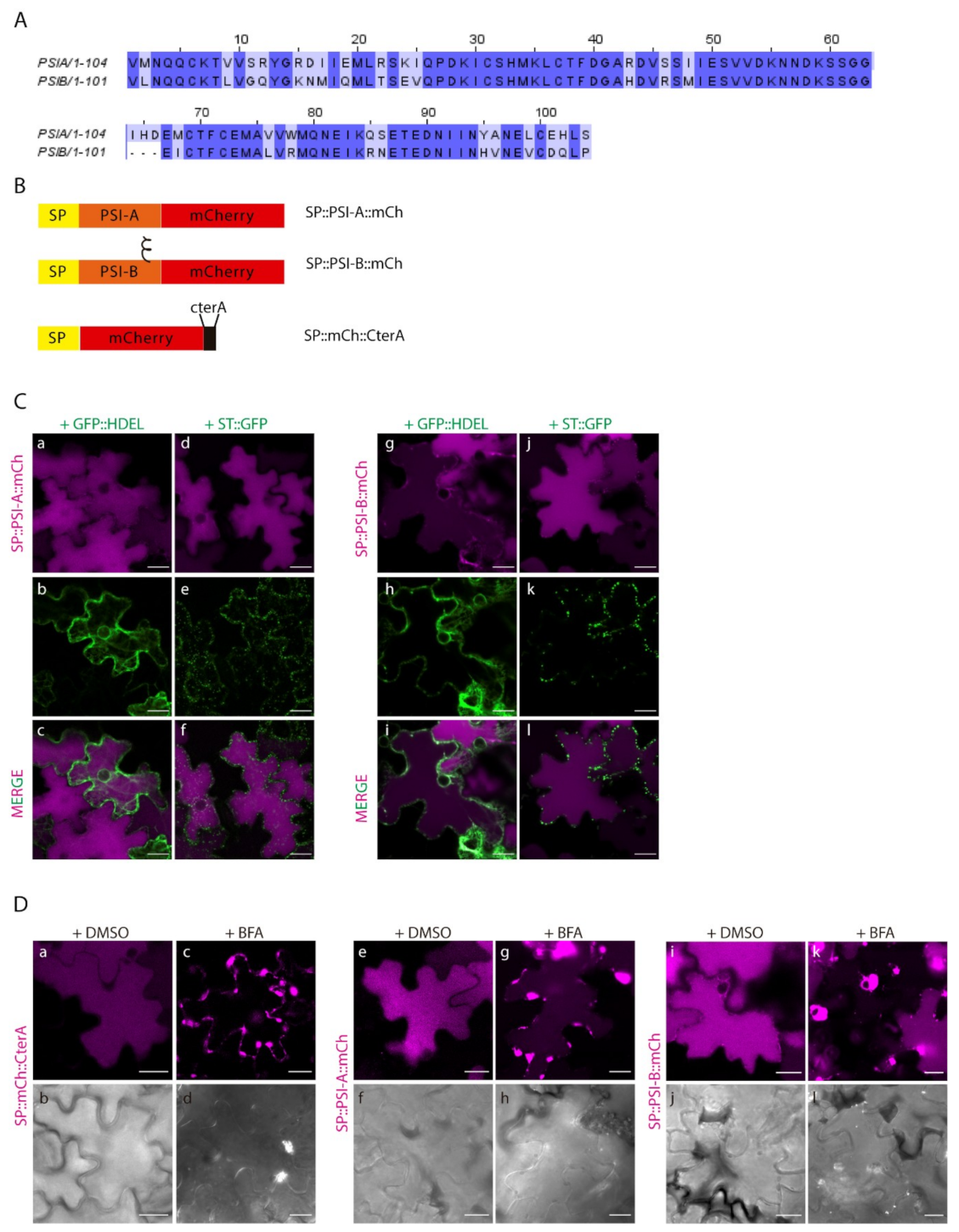
2.2. Different PSIs Mediate Different Sorting Routes to the Vacuole
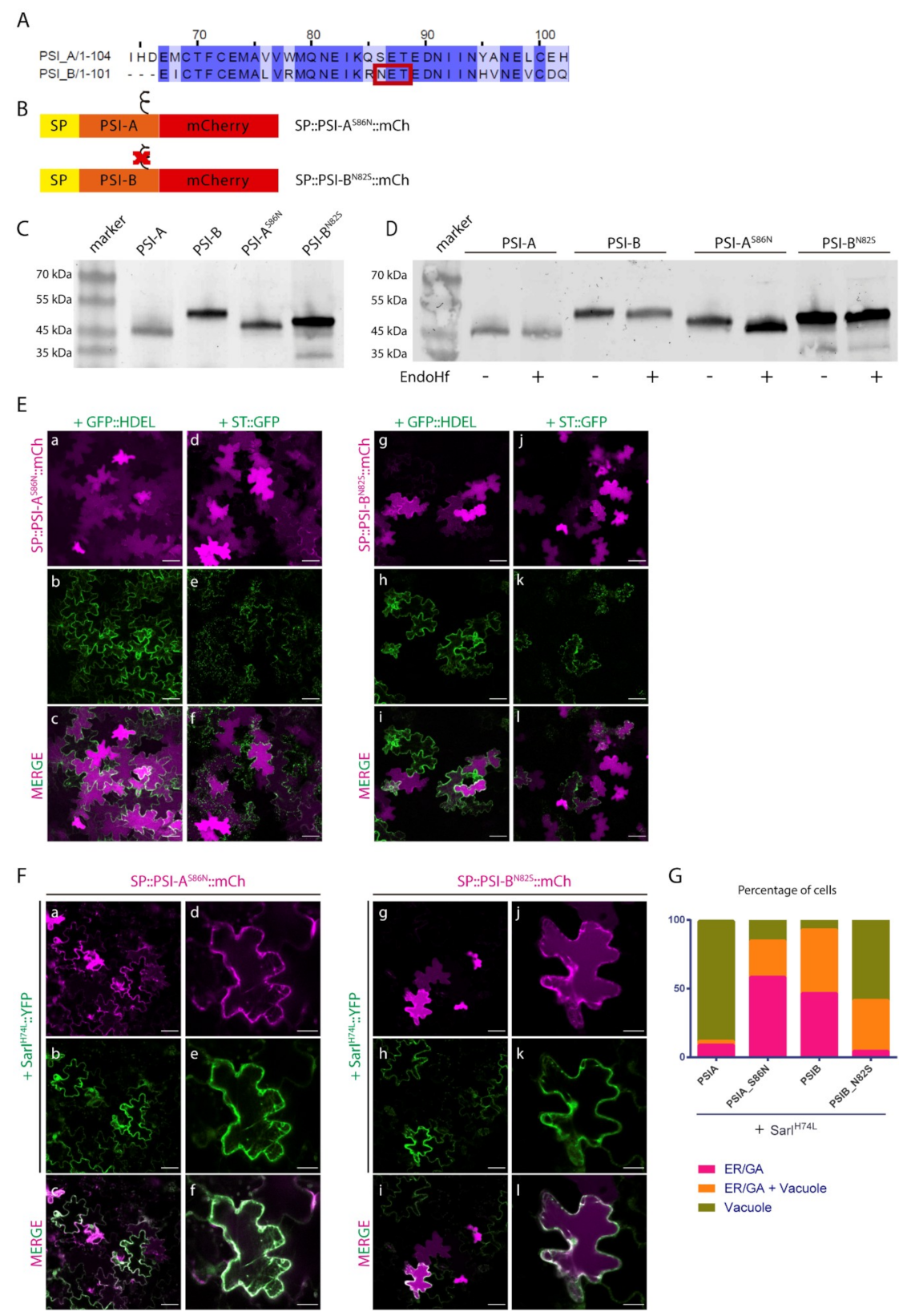
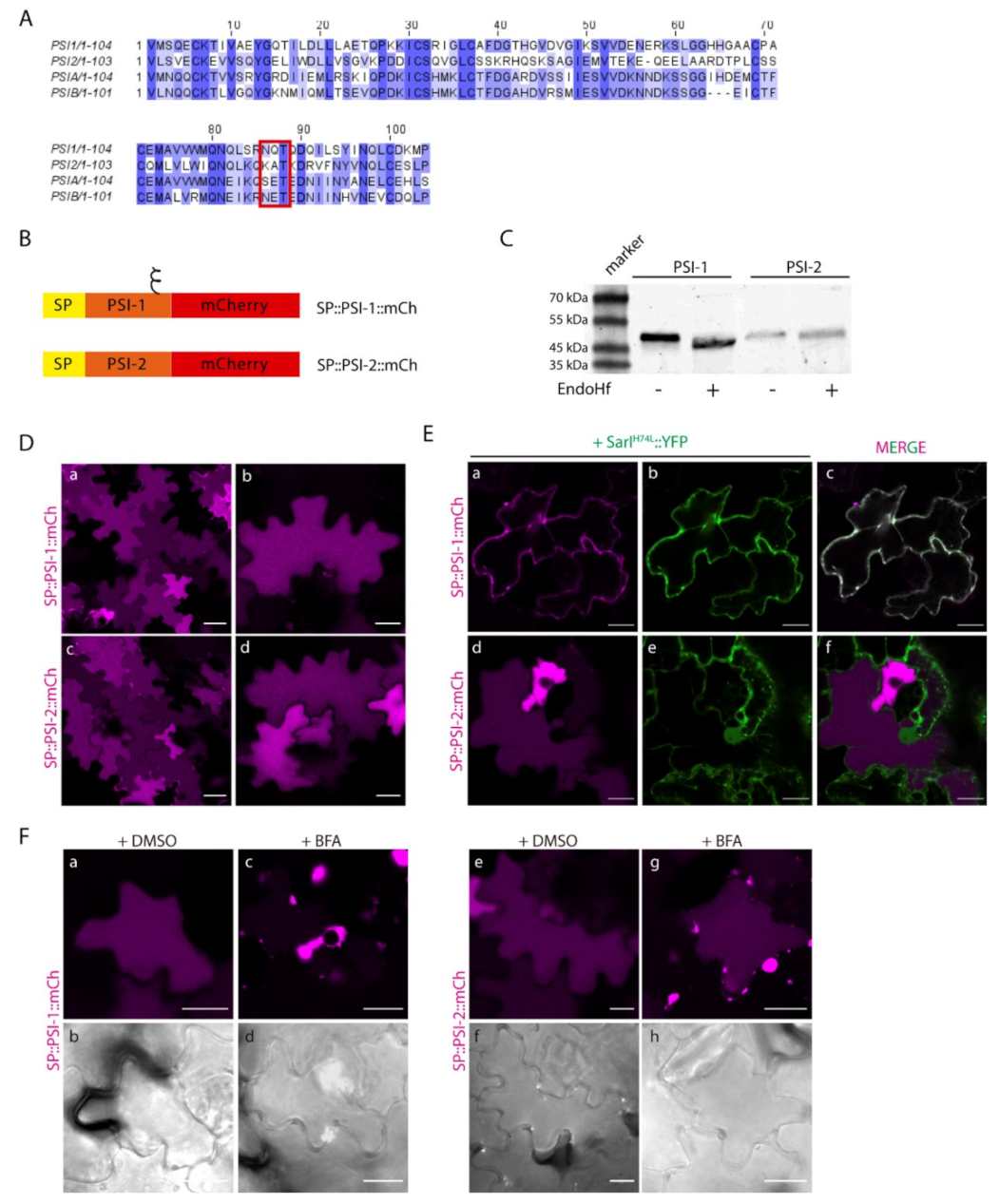
2.3. N-linked Glycosylation Modulates PSI-Mediated Sorting
2.4. Post-Golgi Trafficking of PSI-B Might Be Modulated by Protein Structure
2.5. Proof of Concept: the Soybean PSIs Case
3. Discussion
3.1. A Role for PSIs in Vacuolar Sorting
3.2. A Putative Role for Glycosylation in Sorting?
3.3. Soy PSIs Case–Another Piece of Evidence
3.4. Cardosin B PSI: One VSD, Multiple Pathways?
3.5. PSI-Mediated Sorting: An Unconventional Vacuolar Sorting Mechanism
4. Materials and Methods
4.1. Plasmids and Vectors
4.2. Plant Material
4.3. Transient Expression in N. Tabacum Leaves
4.4. Protoplasts Isolation From N. Tabacum Leaves
4.5. Drug Treatments
4.6. Protein Sample Extraction and Endoglycosidase Assays
4.7. Western Blot
4.8. Confocal Laser Scanning Microscopy-Image Acquisition and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, J.; Mettbach, U.; Menzel, D.; Samaj, J. Molecular dissection of endosomal compartments in plants. Plant Physiol. 2007, 145, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Jiang, L.; Schumacher, K. The endosomal system of plants: Charting new and familiar territories. Plant Physiol. 2008, 147, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Zouhar, J.; Rojo, E. Plant vacuoles: Where did they come from and where are they heading? Curr. Opin. Plant Biol. 2009, 12, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.-M.; Rogers, J.C. Sorting of proteins to vacuoles in plant cells. In Protein Trafficking in Plant Cells; Springer: Dordrecht, The Netherlands, 1998; pp. 127–144. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, S.; Pissarra, J. Delivering of proteins to the plant vacuole—An update. Int. J. Mol. Sci. 2014, 15, 7611–7623. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Hinz, G. Sorting of proteins to storage vacuoles: How many mechanisms? Trends Plant Sci. 2005, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Pompa, A.; De Marchis, F.; Vitale, A.; Arcioni, S.; Bellucci, M. An engineered C-terminal disulfide bond can partially replace the phaseolin vacuolar sorting signal. Plant J. 2010, 61, 782–791. [Google Scholar] [CrossRef]
- Stigliano, E.; Faraco, M.; Neuhaus, J.-M.; Montefusco, A.; Dalessandro, G.; Piro, G.; Di Sansebastiano, G.-P. Two glycosylated vacuolar GFPs are new markers for ER-to-vacuole sorting. Plant Physiol. Biochem. 2013, 73, 337–343. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, S.; Satiat-Jeunemaitre, B.; Pissarra, J. Cardosin A contains two vacuolar sorting signals using different vacuolar routes in tobacco epidermal cells. Plant J. 2013, 76, 87–100. [Google Scholar] [CrossRef]
- De Caroli, M.; Lenucci, M.S.; Di Sansebastiano, G.-P.; Dalessandro, G.; De Lorenzo, G.; Piro, G. Protein trafficking to the cell wall occurs through mechanisms distinguishable from default sorting in tobacco. Plant J. 2011, 65, 295–308. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.-P.; Barozzi, F.; Piro, G.; Denecke, J.; De Lousa, C.M. Trafficking routes to the plant vacuole: Connecting alternative and classical pathways. J. Exp. Bot. 2017, 69, 79–90. [Google Scholar] [CrossRef]
- De Marchis, F.; Bellucci, M.; Pompa, A. Unconventional pathways of secretory plant proteins from the endoplasmic reticulum to the vacuole bypassing the Golgi complex. Plant Signal. Behav. 2013, 8, e25129. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, M.; Veríssimo, P.; Cortes, L.; Samyn, B.; Van Beeumen, J.; Pires, E.; Faro, C. Identification and proteolytic processing of procardosin A. Eur. J. Biochem. 1998, 255, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Pissarra, J.; Veríssimo, P.; Castanheira, P.; Costa, Y.; Pires, E.; Faro, C. Molecular cloning and characterization of cDNA encoding cardosin B, an aspartic proteinase accumulating extracellularly in the transmitting tissue of Cynara cardunculus L. Plant Mol. Biol. 2001, 45, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Soares da Costa, D.; Pereira, S.; de Moura Nogueira, F.; Albuquerque, P.M.; Teixeira, J.; Faro, C.; Pissarra, J. Cardosins in postembryonic development of cardoon: Towards an elucidation of the biological function of plant aspartic proteinases. Protoplasma 2008, 232, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.; Pissarra, J.; Moore, I. Processing and trafficking of a single isoform of the aspartic proteinase cardosin A on the vacuolar pathway. Planta 2008, 227, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.S.; Pereira, S.; Moore, I.; Pissarra, J. Dissecting cardosin B trafficking pathways in heterologous systems. Planta 2010, 232, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Pereira, C.; Soares da Costa, D.; Teixeira, J.; Fidalgo, F.; Pereira, S.; Pissarra, J. Characterization of aspartic proteinases in C. cardunculus L. callus tissue for its prospective transformation. Plant Sci. 2010, 178, 140–146. [Google Scholar] [CrossRef]
- Pissarra, J.; Pereira, C.; Soares da Costa, D.; Figueiredo, R.; Duarte, P.; Teixeira, J.; Pereira, S. From flower to seed germination in Cynara cardunculus: A role for aspartic proteinases. Int. J. Plant Dev. Biol. 2007, 1, 274–281. [Google Scholar]
- Soares, A.; Ribeiro Carlton, S.M.; Simões, I. Atypical and nucellin-like aspartic proteases: Emerging players in plant developmental processes and stress responses. J. Exp. Bot. 2019, 70, 2059–2076. [Google Scholar] [CrossRef]
- Simões, I.; Faro, C. Structure and function of plant aspartic proteinases. Eur. J. Biochem. 2004, 271, 2067–2075. [Google Scholar] [CrossRef]
- Muñoz, F.; Palomares-Jerez, M.F.; Daleo, G.; Villalaín, J.; MG Guevara, M.G. Possible mechanism of structural transformations induced by StAsp-PSI in lipid membranes. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Egas, C.; Lavoura, N.; Resende, R.; Brito RM, M.; Pires, E.; de Lima MC, P.; Faro, C. The saposin-like domain of the plant aspartic proteinase precursor is a potent inducer of vesicle leakage. J. Biol. Chem. 2002, 275, 38190–38196. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.E.; D’ Ippolito, S.; Pepe, A.; Daleo, G.R.; Guevara, M.G. Transgenic expression of plant-specific insert of potato aspartic proteases (StAP-PSI) confers enhanced resistance to Botrytis cinerea in Arabidopsis thaliana. Phytochemistry 2018, 149, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.F.; Mendieta, J.R.; Pagano, M.R.; Paggi, R.A.; Daleo, G.R.; Guevara, M.G. The swaposin-like domain of potato aspartic protease (StAsp-PSI) exerts antimicrobial activity on plant and human pathogens. Peptides 2010, 31, 777–785. [Google Scholar] [CrossRef] [PubMed]
- De Moura, D.C.; Bryksa, B.C.; Yada, R.Y. In silico insights into protein-protein interactions and folding dynamics of the saposin-like domain of Solanum tuberosum aspartic protease. PLoS ONE 2014, 9, e104315. [Google Scholar] [CrossRef] [PubMed]
- Curto, P.; Lufrano, D.; Pinto, C.; Custódio, V.; Gomes, A.; Bakás, L.; Vairo-Cavalli, S.; Faro, C.; Simões, I. Establishing the yeast kluyveromyces lactis as an expression host for production of the saposin-like domain of the aspartic protease cirsin. Appl. Environ. Microbiol. 2014, 80, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, K.; Asakura, T.; Ueda, H.; Tamura, T.; Tamura, K.; Matsumoto, I.; Misaka, T.; Hara-Nishimura, I.; Abe, K. Plant-specific insertions in the soybean aspartic proteinases, soyAP1 and soyAP2, perform different functions of vacuolar targeting. J. Plant Physiol. 2006, 163, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Törmäkangas, K.; Hadlington, J.L.; Pimpl, P.; Hillmer, S.; Brandizzi, F.; Teeri, T.H.; Denecke, J. A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 2007, 13, 2021. [Google Scholar] [CrossRef]
- Boevink, P.; Oparka, K.; Cruz, S.S.; Martin, B.; Betteridge, A.; Hawes, C. Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998, 15, 441–447. [Google Scholar] [CrossRef]
- Saint-Jore, C.M.; Evins, J.; Batoko, H.; Brandizzi, F.; Moore, I.; Hawes, C. Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J. 2002, 29, 661–678. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Snapp, E.L.; Denecke, J.; Lippincott-Schwartz, J.; Hawes, C.; Brandizzi, F. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 2004, 16, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, A.; Hummel, E.; Carvalho, C.M.; Hawes, C. Golgi membrane dynamics after induction of a dominant-negative mutant Sar1 GTPase in tobacco. J. Exp. Bot. 2010, 61, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Satiat-Jeunemaitre, B.; Hawes, C. Redistribution of a Golgi glycoprotein in plant cells treated with Brefeldin A. J. Cell Sci. 1992, 103, 1153–1166. [Google Scholar]
- Hanton, S.L.; Renna, L.; Bortolotti, L.E.; Chatre, L.; Stefano, G.; Brandizzi, F. Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 2005, 17, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Batoko, H.; Zheng, H.Q.; Hawes, C.; Moore, I. A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 2000, 12, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, A.; Carvalho, C.M.; Latijnhouwers, M.; Johansen, J.N.; Stubbs, C.; Botchway, S.; Hawes, C. Fluorescence lifetime imaging of interactions between Golgi tethering factors and small GTPASES in plants. Traffic 2009, 10, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Jung, E.; Brunak, S. “Prediction of N-glycosylation Sites in Human Proteins.” In Preparation. 2004. Available online: http://www.cbs.dtu.dk/services/NetNGlyc/ (accessed on 29 October 2003).
- Terauchi, K.; Nishizawa, N.K.; Matsumoto, I.; Abe, K.; Asakura, T. Characterization of the genes for two soybean aspartic proteinases and analysis of their different tissue-dependent expression. Planta 2004, 218, 947–957. [Google Scholar] [CrossRef]
- Paris, N.; Saint-Jean, B.; Faraco, M.; Krzeszowiec, W.; Dalessandro, G.; Neuhaus, J.-M.; Di Sansebastiano, G.P. Expression of a glycosylated GFP as a bivalent reporter in exocytosis. Plant Cell Rep. 2010, 29, 79–86. [Google Scholar] [CrossRef]
- Rayon, C.; Lerouge, P.; Faye, L. The protein N-glycosylation in plants. J. Exp. Bot. 1998, 49, 1463–1472. [Google Scholar] [CrossRef]
- Wilkins, T.A.; Bednarek, S.Y.; Raikhel, N.V. Role of propeptide glycan in post-translational processing and transport of barley lectin to vacuoles in transgenic tobacco. Plant Cell 1990, 2, 301–313. [Google Scholar] [CrossRef]
- Ramis, C.; Gomord, V.; Lerouge, P.; Faye, L. Deglycosylation is necessary but not sufficient for activation of proconcanavalin A. J. Exp. Bot. 2001, 52, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Raikhel, N.V. What do proteins need to reach different vacuoles? Trends Plant Sci. 1999, 4, 149–155. [Google Scholar] [CrossRef]
- Occhialini, A.; Gouzerh, G.; Di Sansebastiano, G.P.; Neuhaus, J.M. Dimerization of the vacuolar receptors AtRMR1 and -2 from Arabidopsis thaliana contributes to their localization in the trans-Golgi network. Int. J. Mol. Sci. 2016, 17, 1661. [Google Scholar] [CrossRef] [PubMed]
- Pompa, A.; De Marchis, F.; Pallotta, M.T.; Benitez-Alfonso, Y.; Jones, A.; Schipper, K.; Moreau, K.; Žárský, V.; Di Sansebastiano, G.P.; Bellucci, M. Unconventional transport routes of soluble and membrane proteins and their role in developmental biology. Int. J. Mol. Sci. 2017, 18, 703. [Google Scholar] [CrossRef] [PubMed]
- Goring, D.R.; Di Sansebastiano, G.P. Protein and membrane trafficking routes in plants: Conventional or unconventional? J. Exp. Bot. 2017, 69, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kervinen, J.; Tobin, G.J.; Costa, J.; Waugh, D.S.; Wlodawer, A.; Zdanov, A. Crystal structure of plant aspartic proteinase prophytepsin: Inactivation and vacuolar targeting. EMBO J. 1999, 18, 3947–3955. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. Breakthrough Technologies A gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef]
| Construct to Make | Oligonucleotide Fwd | Oligonucleotide Rev | Template DNA |
|---|---|---|---|
| SP::PSI-AS86N::mCherry | GGATGCAAAACGOLGIAATCAAACAAAACGAGACTGAAGATAAC | GTTATCTTCAGTCTCGTTTTGTTTGATTTCGTTTTGCATCC | SP::PSI-A::mCherry |
| SP::PSI-BN92S::mCherry | GCAGAATGAAATCAAACGAAGCGAGACTGAAGATAACATAA | TTATGTTATCTTCAGTCTCGCTTCGTTTGATTTCATTCTGC | SP::PSI-B::mCherry |
| SP::PSI-1::mCherry | ACGTCGACTGTTATGAGCCAAGAATGCAAGACC | TTGTCGACGCACCACCTGCAGCACCACCGCTAGGCATTTTATCGC | SoyAP1 |
| SP::PSI-2::mCherry | ACGTCGACTGTTCTCAGTGTCGAATGTAAGGAAGTC | TTGTCGACGCACCACCACTTGGCAGGCTCTCAC | SoyAP2 |
| PSI-A (for cardosin A::PSI-B::mCherry) | GTCATGAACCAGCAATGCAA | GGATAAGTGTTCACACAACTC | Cardosin A |
| PSI-B (for cardosin B::PSI-A::mCherry) | TTAAACCAACAATGCAAAACATTGG | TTCTGCACTTGAAGTGGGTA | Cardosin B |
| Cardosin A∆PSI::mCherry | ACTTCATCTGAAGAATTACAAG | CCCGTTAGCGCCAATTGCATGATT | Cardosin A |
| Cardosin B∆PSI::mCherry | TCGATAGTAGACTGCAATGG | AACCCCTTTTGCACCAATTG | Cardosin B |
| Cardosin A::mCherry∆c-ter | TCTAGAGCCGCCACCATGGGTACCT | GTCGACGCTAGTAAATTGCCATAATCAAACACTGTG | Cardosin A |
| Cardosin A::PSI-B::mCherry∆c-ter | TCTAGAGCCGCCACCATGGGTACCT | GTCGACGCTAGTAAATTGCCATAATCAAACACTGTG | Cardosin A::PSI-B |
| Cardosin B::mCherry∆c-ter | CATCTAGACTCGAGCCACCATGGGAACCCCAATCAAAGCAAACG | ACGTCGACTTTAACTTGCCATAATCG | Cardosin B |
| Cardosin B::PSI-A::mCherry∆c-ter | CATCTAGACTCGAGCCACCATGGGAACCCCAATCAAAGCAAACG | ACGTCGACTTTAACTTGCCATAATCG | Cardosin B::PSI-A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, V.; Peixoto, B.; Costa, M.; Pereira, S.; Pissarra, J.; Pereira, C. N-Linked Glycosylation Modulates Golgi-Independent Vacuolar Sorting Mediated by the Plant Specific Insert. Plants 2019, 8, 312. https://doi.org/10.3390/plants8090312
Vieira V, Peixoto B, Costa M, Pereira S, Pissarra J, Pereira C. N-Linked Glycosylation Modulates Golgi-Independent Vacuolar Sorting Mediated by the Plant Specific Insert. Plants. 2019; 8(9):312. https://doi.org/10.3390/plants8090312
Chicago/Turabian StyleVieira, Vanessa, Bruno Peixoto, Mónica Costa, Susana Pereira, José Pissarra, and Cláudia Pereira. 2019. "N-Linked Glycosylation Modulates Golgi-Independent Vacuolar Sorting Mediated by the Plant Specific Insert" Plants 8, no. 9: 312. https://doi.org/10.3390/plants8090312
APA StyleVieira, V., Peixoto, B., Costa, M., Pereira, S., Pissarra, J., & Pereira, C. (2019). N-Linked Glycosylation Modulates Golgi-Independent Vacuolar Sorting Mediated by the Plant Specific Insert. Plants, 8(9), 312. https://doi.org/10.3390/plants8090312






