Phenological, Physiological, and Ultrastructural Analyses of ‘Green Islands’ on Senescent Leaves of Norway Maple (Acer platanoides L.)
Abstract
1. Introduction
2. Results
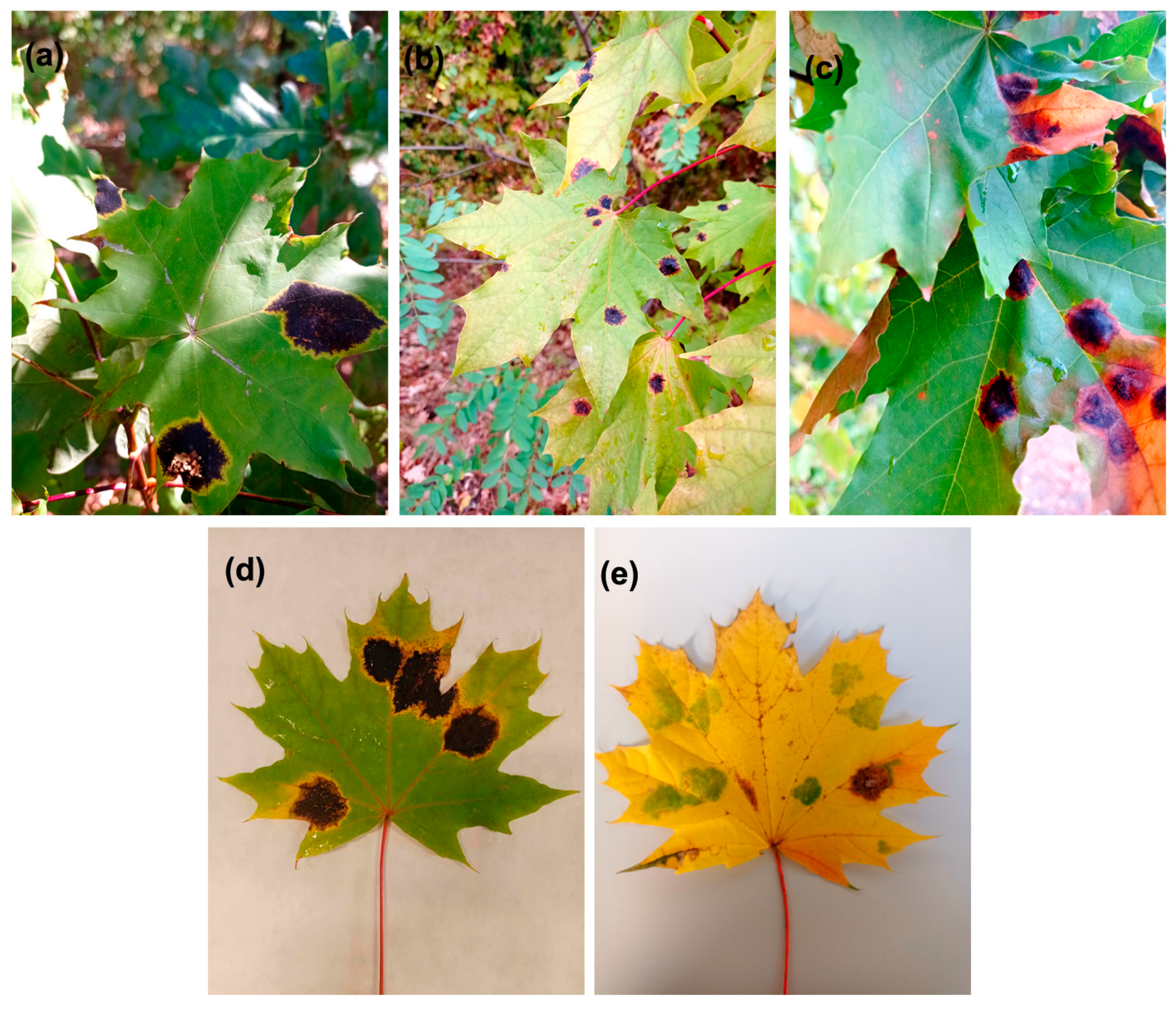
2.1. ‘Green Island’ Symptoms
2.2. Non-Destructive Assessment of Physiological Parameters
2.3. Chlorophyll and Carotenoid Contents
2.4. Analysis of Photosynthetic Parameters
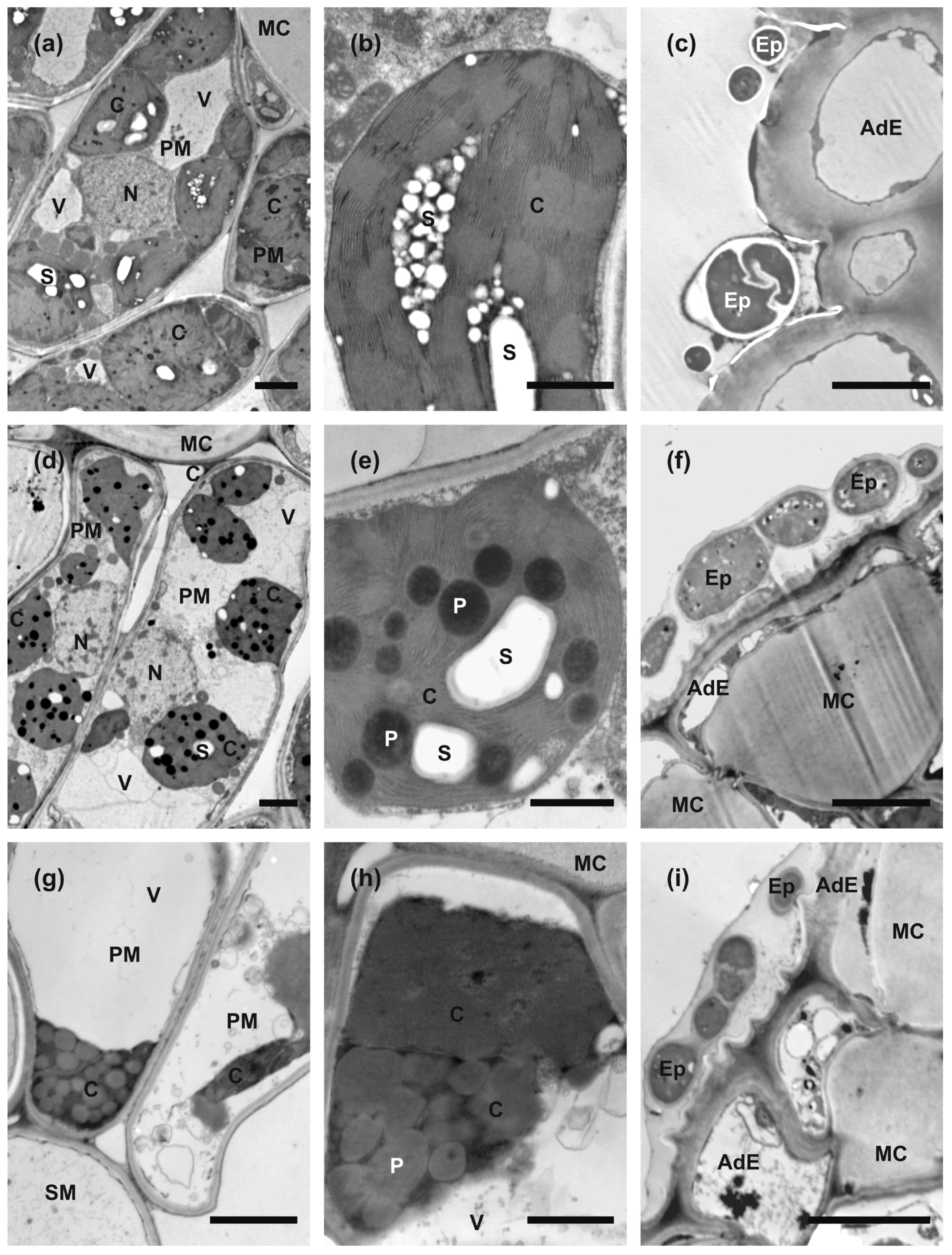
2.5. Anatomy and Ultrastructure of Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Non-Destructive Evaluation of Physiological Parameters
4.3. Chlorophyll and Carotenoid Content
4.4. Analysis of Photosynthesis
4.5. Microscopic Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Akhmetov, A.; Ianbaev, R.; Boronnikova, S.; Yanbaev, Y.; Gabitova, A.; Kulagin, A. Norway maple (Acer platanoides) and pedunculate oak (Quercus robur) demonstrate different patterns of genetic variation within and among populations on the eastern border of distribution ranges. J. For. Sci. 2021, 67, 522–532. [Google Scholar] [CrossRef]
- Caudullo, G.; de Rigo, D. Acer platanoides in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., De Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Joint Research Centre, Publications Office of the European Union, European Commission: Luxembourg, 2016; pp. 54–55. Available online: https://data.europa.eu/doi/10.2788/4251 (accessed on 23 November 2024).
- Fang, W.; Wang, X.Z. Impact of invasion of Acer platanoides on canopy structure and understory seedling growth in a hardwood forest in North America. Trees-Struct. Func. 2011, 25, 455–464. [Google Scholar] [CrossRef]
- Fang, W.; Wang, X.Z. A field experimental study on the impact of Acer platanoides, an urban tree invader, on forest ecosystem processes in North America. Ecol. Process. 2020, 9, 9. [Google Scholar] [CrossRef]
- Mitchell, A.F. A Field Guide to the Trees of Britain and Northern Europe, 1st ed.; Collins: London, UK, 1974. [Google Scholar]
- Kerr, G.; Niles, J. Growth and provenance of Norway maple (Acer platanoides) in lowland Britain. Forestry 1998, 71, 219–224. [Google Scholar] [CrossRef]
- Gilman, E.F.; Watson, D.G. Acer platanoides—Norway Maple. Fact Sheet ST-28; University of Florida: Gainesville, FL, USA, 1993; Available online: https://hort.ifas.ufl.edu/trees/ACEPLAA.pdf (accessed on 12 December 2024).
- Praciak, A.; Pasiecznik, N.; Sheil, D.; Van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, G.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI: Oxfordshire, UK, 2013. [Google Scholar]
- Bosco, C.; de Rigo, D.; Dewitte, O.; Poesen, J.; Panagos, P. Modelling soil erosion at European scale: Towards harmonization and reproducibility. Nat. Hazards Earth Syst. Sci. 2015, 15, 225–245. [Google Scholar] [CrossRef]
- Stokes, A.; Norris, J.E.; van Beek, L.P.H.; Bogaard, T.; Cammeraat, E.; Mickovski, S.B.; Jenner, A.; Di Iorio, A.; Fourcaud, T. How Vegetation Reinforces Soil on Slopes. In Slope Stability and Erosion Control: Ecotechnological Solutions; Norris, J.E., Stokes, A., Mickovski, S.B., Cammeraat, E., van Beek, L.P.H., Achim, A., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Walters, D.R.; McRoberts, N.; Fitt, B.D. Are green islands red herrings? Significance of green islands in plant interactions with pathogens and pests. Biol. Rev. 2008, 83, 79–102. [Google Scholar] [CrossRef]
- Le Cocq, K.; Gurr, S.J.; Hirsch, P.R.; Mauchline, T.H. Exploitation of endophytes for sustainable agricultural intensification. Mol. Plant Pathol. 2016, 18, 469–473. [Google Scholar] [CrossRef]
- van Overbeek, L.S.; Saikkonen, K. Impact of bacterial–fungal interactions on the colonization of the endosphere. Trends Plant Sci. 2016, 21, 230–242. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Wemheuer, F.; Wemheuer, B.; Daniel, R.; Vidal, S. Deciphering bacterial and fungal endophyte communities in leaves of two maple trees with green islands. Sci. Rep. 2019, 9, 14183. [Google Scholar] [CrossRef]
- Moore, C.J.; Sutherland, P.W.; Forster, R.L.S.; Gardner, R.C.; MacDiarmid, R.M. Dark green islands in plant virus infection are the result of posttranscriptional gene silencing. Mol. Plant-Microbe Interact. 2001, 14, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; McRoberts, N. Plants and biotrophs: A pivotal role for cytokinins? Trends Plant Sci. 2006, 11, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Humbeck, K.; Hause, G.; Deising, H.B.; Wirsel, S.G.R. The hemibiotroph Colletotrichum graminicola locally induces photosynthetically active green islands but globally accelerates senescence on aging maize leaves. Mol. Plant-Microbe Interact. 2009, 23, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Gutzwiller, F.; Dedeine, F.; Kaiser, W.; Giron, D.; Lopez-Vaamonde, C. Correlation between the green-island phenotype and Wolbachia infections during the evolutionary diversification of Gracillariidae leaf-mining moths. Ecol. Evol. 2015, 5, 4049–4062. [Google Scholar] [CrossRef]
- Angra-Sharma, R.; Sharma, D. Cytokinins in pathogenesis and disease resistance of Pyrenophora teres-barley and Dreschslera maydis-maize interactions during early stages of infection. Mycopathologia 2000, 148, 87–95. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Sobczak, M.; Skoczowski, A.; Oliwa, J.; Michlewska, S.; Gapinska, M.; Ciereszko, I.; Kononowicz, A.K. The Effect of Photoperiod on Necrosis Development, Photosynthetic Efficiency and ‘Green Islands’ Formation in Brassica juncea Infected with Alternaria brassicicola. Int. J. Mol. Sci. 2021, 22, 8435. [Google Scholar] [CrossRef]
- Ashby, A.M. Biotrophy and the cytokinin conundrum. Physiol. Mol. Plant Pathol. 2000, 57, 147–158. [Google Scholar] [CrossRef]
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.J.; Dicke, M. Cytokinins as key regulators in plant–microbe–insect interactions: Connecting plant growth and defence. Funct. Ecol. 2013, 27, 599–609. [Google Scholar] [CrossRef]
- Naseem, M.; Wölfling, M.; Dandekar, T. Cytokinins for immunity beyond growth, galls and green islands. Trends Plant Sci. 2014, 19, 481–484. [Google Scholar] [CrossRef]
- Bushnell, W.R. Symptom development in mildewed and rusted tissue. In The Dynamic Role of Molecular Constituents in Plant-Parasite Interactions; Mirocha, C.J., Uritani, I., Eds.; Bruce Publishing Company: St. Paul, MN, USA, 1967; pp. 21–39. [Google Scholar]
- Kaiser, W.; Huguet, E.; Casas, J.; Commin, C.; Giron, D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. R. Soc. B Biol. Sci. 2010, 277, 2311–2319. [Google Scholar] [CrossRef]
- Pińskwar, I.; Choryński, A.; Kundzewicz, Z.W. Severe Drought in the Spring of 2020 in Poland—More of the Same? Agronomy 2020, 10, 1646. [Google Scholar] [CrossRef]
- Hejduk, L.; Kaznowska, E.; Wasilewicz, M.; Hejduk, A. Hydrological Droughts in the Białowieża Primeval Forest, Poland, in the Years 1951–2020. Forests 2021, 12, 1744. [Google Scholar] [CrossRef]
- Skoczowski, A.; Rut, G.; Oliwa, J.; Kornaś, A. Sporulation modifies the photosynthetic activity of sporotrophophyll leaves of Platycerium bifurcatum. Photosynthetica 2020, 58, 488–496. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J. Chloroplasts at the Crossroad of Photosynthesis, Pathogen Infection and Plant Defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Shang, J.; Xi, D.-H.; Yuan, S.; Xu, F.; Xu, M.-Y.; Qi, H.-L.; Huang, Q.-R.; Wen, L.; Lin, H.-H.; Wang, S.-D. Difference of Physiological Characters in Dark Green Islands and Yellow Leaf Tissue of Cucumber mosaic Virus (CMV)-Infected Nicotiana tabacum Leaves. Z. Naturforsch. C 2010, 65, 73–78. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Wielanek, M.; Morkunas, I.; Ciereszko, I.; Kononowicz, A.K. Leaf position-dependent effect of Alternaria brassicicola development on host cell death, photosynthesis and secondary metabolites in Brassica juncea. Physiol. Plant. 2020, 168, 601–616. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Lee, D.W.; Gould, K.S. Anthocyanins in leaves and other vegetative organs: An introduction. Adv. Bot. Res. 2002, 3, 1–16. [Google Scholar] [CrossRef]
- Verhoeven, A.; Southwick, C.; Miller, E.; Blood, M.; Thibodeau, A. Do red and yellow autumn leaves make use of different photoprotective strategies during autumn senescence? Physiol. Plant. 2024, 176, e14327. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; López-Pozo, M.; Artetxe, U.; Becerril, J.M.; Hernández, A.; García-Plazaola, J.I.; Esteban, R. Carotenoids and their derivatives: A “Swiss Army knife-like” multifunctional tool for fine-tuning plant-environment interactions. Environ. Exp. Bot. 2023, 207, 105229. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta—Bioenerg. 2011, 1807, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, Germany, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Abdel-Fattah, G.M.; Baka, Z.A. Changes in chlorophyll, polyamines and chloroplast ultrastructure of Puccinia striiformis induced ‘green islands’ on detached leaves of Triticum aestivum. Plant Physiol. Biochem. 2000, 38, 613–620. [Google Scholar] [CrossRef]
- Pereira, W.E.; de Siqueira, D.L.; Martínez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J. Plant Physiol. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Itoh, S.; Govindjee. Global spectral–kinetic analysis of room temperature chlorophyll a fluorescence from light-harvesting antenna mutants of barley. Philos. Trans. R. Soc. B 2000, 355, 1371–1384. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and Epiphytic Phyllosphere Fungal Communities Are Shaped by Different Environmental Factors in a Mediterranean Ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Change Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of Nitrogen Nutrition and Water Deficit on Net Photosynthetic Rate and Chlorophyll Fluorescence in Winter Wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Crespo-Martínez, S.; Sobczak, M.; Różańska, E.; Forneck, A.; Griesser, M. The role of the secondary phloem during the development of the grapevine Berry Shrivel ripening disorder. Micron 2019, 116, 36–45. [Google Scholar] [CrossRef]


| 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|
| Percentage of ‘green’ island area | 23.99 a | 15.60 b | 26.17 a | 19.49 ab |
| SD | 9.62 | 4.61 | 6.43 | 4.42 |
| Year | Leaf Variant | Chl (µg/cm2) | Flav Index | Anth Index | NBI |
|---|---|---|---|---|---|
| 2019 | ‘Green island’ Yellow area | 11.34 ± 5.40 a | 1.06 ± 0.22 a | 0.27 ± 0.07 a | 11.09 ± 5.60 a |
| 3.35 ± 1.96 b | 1.25 ± 0.20 b | 0.38 ± 0.05 b | 2.74 ± 1.68 b | ||
| 2020 | Summer leaves | 35.91 ± 2.63 c | 0.90 ± 0.06 c | 0.00 | 40.10 ± 3.77 c |
| ‘Green island’ Yellow area | 15.85 ± 5.25 d | 1.02 ± 0.22 a | 0.24 ± 0.05 a | 16.50 ± 7.37 d | |
| 2.47 ± 1.33 b | 1.24 ± 0.16 b | 0.38 ± 0.05 b | 2.02 ± 1.10 b | ||
| 2021 | Summer leaves | 34.15 ± 3.62 c | 0.80 ± 0.14 c | 0.00 | 43.99 ± 9.71 e |
| ‘Green island’ Yellow area | 12.75 ± 2.29 a | 0.96 ± 0.16 a | 0.63 ± 0.05 c | 13.38 ± 2.27 a | |
| 1.99 ± 0.97 b | 1.06 ± 0.21 a | 0.88 ± 0.06 d | 1.89 ± 0.78 b |
| Parameters | Summer Leaves | Senescent Leaves | |
|---|---|---|---|
| (μg mg−1 F.W.) | ‘Green Islands’ | Yellow Area | |
| Chla | 1.933 ± 0.10 a | 1.395 ± 0.27 b | 0.268 ± 0.09 c |
| Chlb | 1.156 ± 0.19 a | 0.814 ± 0.14 b | 0.272 ± 0.08 c |
| Chla:b | 1.709 ± 0.27 a | 1.709 ± 0.08 a | 1.00 ± 0.22 b |
| Total Chl | 3.09 ± 0.24 a | 2.209 ± 0.42 b | 0.540 ± 0.15 c |
| Car | 0.190 ± 0.66 a | 0.400 ± 0.06 b | 0.431 ± 0.07 b |
| Total Chl:Car | 18.627 ± 8.63 a | 5.636 ± 1.40 b | 1.201 ± 0.37 b |
| Parameters | Summer Leaves | Senescent Leaves | |
|---|---|---|---|
| ‘Green Islands’ | Yellow Area | ||
| Measured parameters and basic JIP-test parameters | |||
| Fo | 866.7 ± 143.78 a | 397.1 ± 125.22 b | 561.74 ± 226.7 c |
| Fm | 34,039 ± 1447 a | 27,345 ± 5723 b | 4452 ± 2818 c |
| Fv | 26,032 ± 1232 a | 18,997 ± 4913 b | 1702 ± 1453 c |
| Fv/Fm | 0.765 ± 0.012 a | 0.688 ± 0.07 b | 0.340 ± 0.128 c |
| Fv/Fo | 3.261 ± 0.215 a | 2.349 ± 0.641 b | 0.581 ± 0.374 c |
| Vj | 0.380 ± 0.032 a | 0.733 ± 0.032 b | 0.730 ± 0.102 b |
| Vi | 0.929 ± 0.013 a | 0.919 ± 0.023 a | 0.827 ± 0.053 b |
| PI abs | 4.268 ± 1.065 a | 0.283 ± 0.151 b | 0.029 ± 0.031 c |
| Specific energy fluxes expressed per active RC of PSII | |||
| ABS/RC | 1.294 ± 0.125 a | 3.500 ± 0.875 b | 10.392 ± 5.032 c |
| DIo/RC | 0.306 ± 0.043 a | 1.151 ± 0.623 b | 7.389 ± 5.038 c |
| TRo/RC | 0.988 ± 0.084 a | 2.349 ± 0.291 b | 3.003 ± 0.286 c |
| ETo/RC | 0.611 ± 0.039 a | 0.621 ± 0.067 a | 0.813 ± 0.315 b |
| REo/RC | 0.070 ± 0.012 a | 0.194 ± 0.075 b | 0.523 ± 0.167 c |
| Quantum yields parameters | |||
| ϕ(Po) | 0.765 ± 0.012 a | 0.688 ± 0.074 b | 0.340 ± 0.128 c |
| ψ(Eo) | 0.620 ± 0.032 a | 0.267 ± 0.032 b | 0.270 ± 0.102 b |
| ϕ(Eo) | 0.474 ± 0.029 a | 0.185 ± 0.036 b | 0.088 ± 0.041 c |
| Δ(Ro) | 0.114 ± 0.019 a | 0.308 ± 0.107 b | 0.668 ± 0.181 c |
| ϕ(Ro) | 0.054 ± 0.010 a | 0.054 ± 0.010 a | 0.056 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macioszek, V.K.; Chalamońska, K.; Oliwa, J.; Staszak, A.M.; Sobczak, M. Phenological, Physiological, and Ultrastructural Analyses of ‘Green Islands’ on Senescent Leaves of Norway Maple (Acer platanoides L.). Plants 2025, 14, 909. https://doi.org/10.3390/plants14060909
Macioszek VK, Chalamońska K, Oliwa J, Staszak AM, Sobczak M. Phenological, Physiological, and Ultrastructural Analyses of ‘Green Islands’ on Senescent Leaves of Norway Maple (Acer platanoides L.). Plants. 2025; 14(6):909. https://doi.org/10.3390/plants14060909
Chicago/Turabian StyleMacioszek, Violetta Katarzyna, Kamila Chalamońska, Jakub Oliwa, Aleksandra Maria Staszak, and Mirosław Sobczak. 2025. "Phenological, Physiological, and Ultrastructural Analyses of ‘Green Islands’ on Senescent Leaves of Norway Maple (Acer platanoides L.)" Plants 14, no. 6: 909. https://doi.org/10.3390/plants14060909
APA StyleMacioszek, V. K., Chalamońska, K., Oliwa, J., Staszak, A. M., & Sobczak, M. (2025). Phenological, Physiological, and Ultrastructural Analyses of ‘Green Islands’ on Senescent Leaves of Norway Maple (Acer platanoides L.). Plants, 14(6), 909. https://doi.org/10.3390/plants14060909







