Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus
Abstract
1. Introduction
2. Results
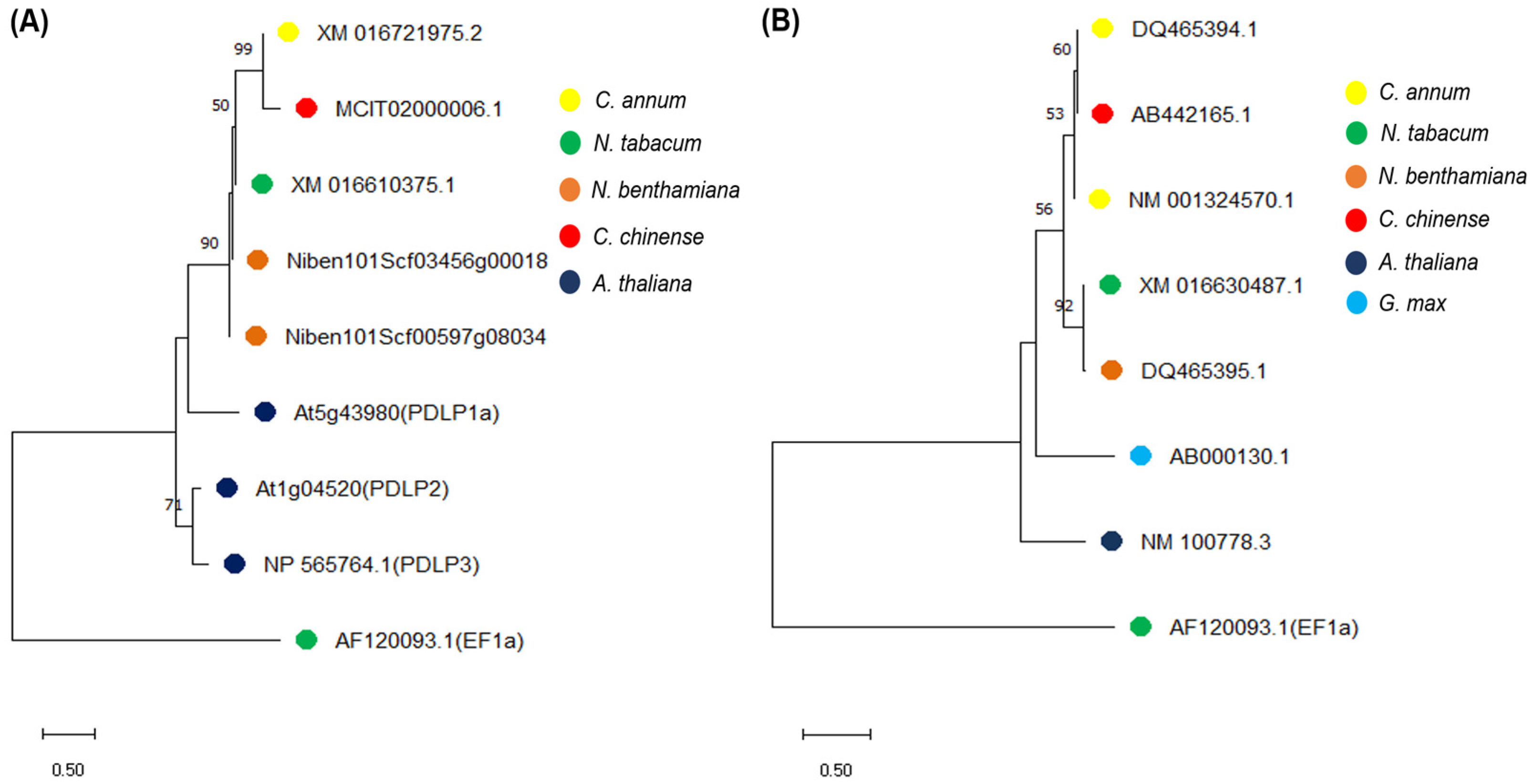
2.1. Homology Analysis of PDLP and SRC2 Genes in the Nicotiana and Capsicum Species
2.2. Relative Expression of PDLP and SRC2 Genes During CMV Infection in N. benthamiana
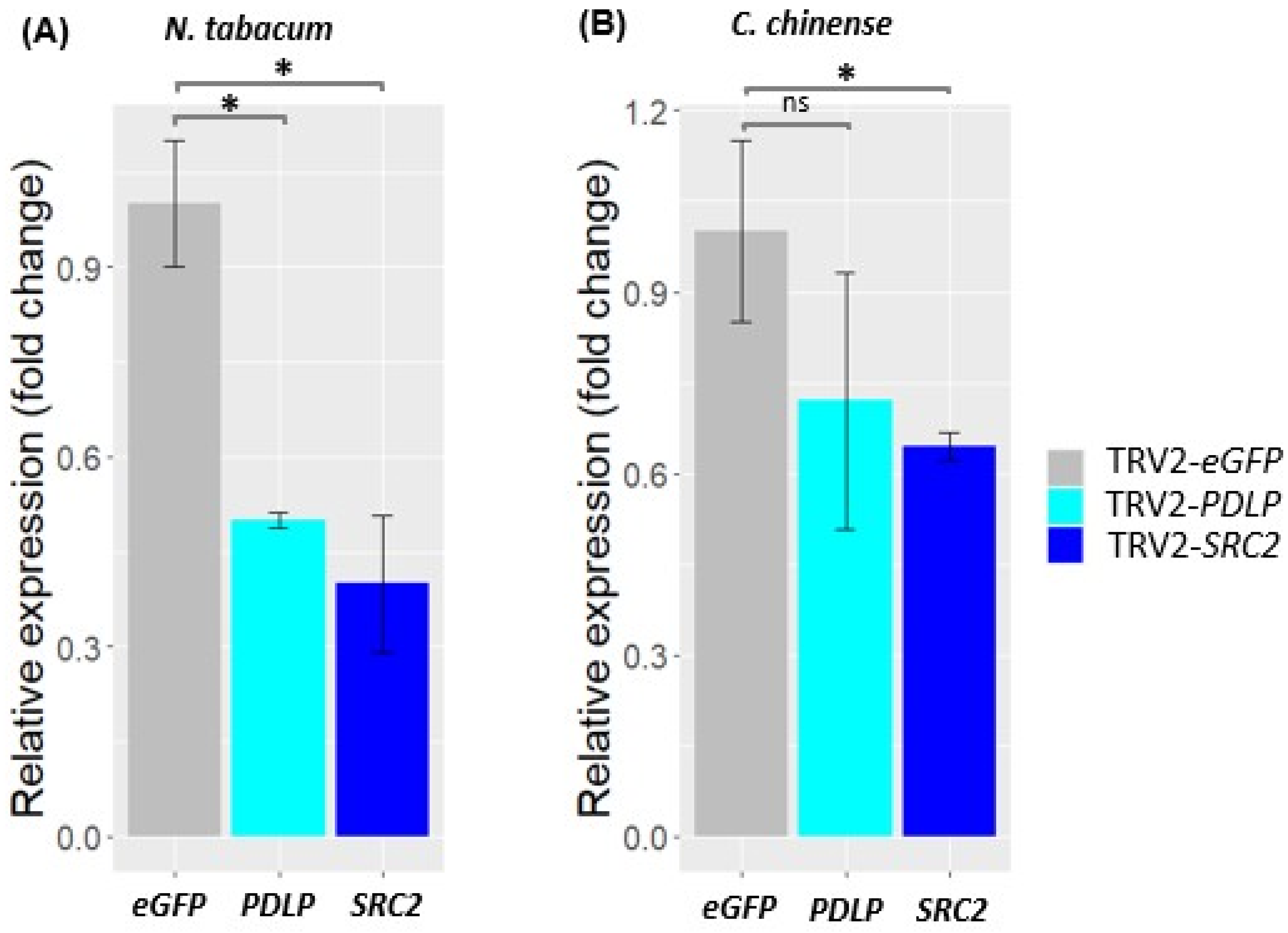
2.3. VIGS of PDLP and SRC2 Genes in Tobacco and Bhut Jolokia Pepper
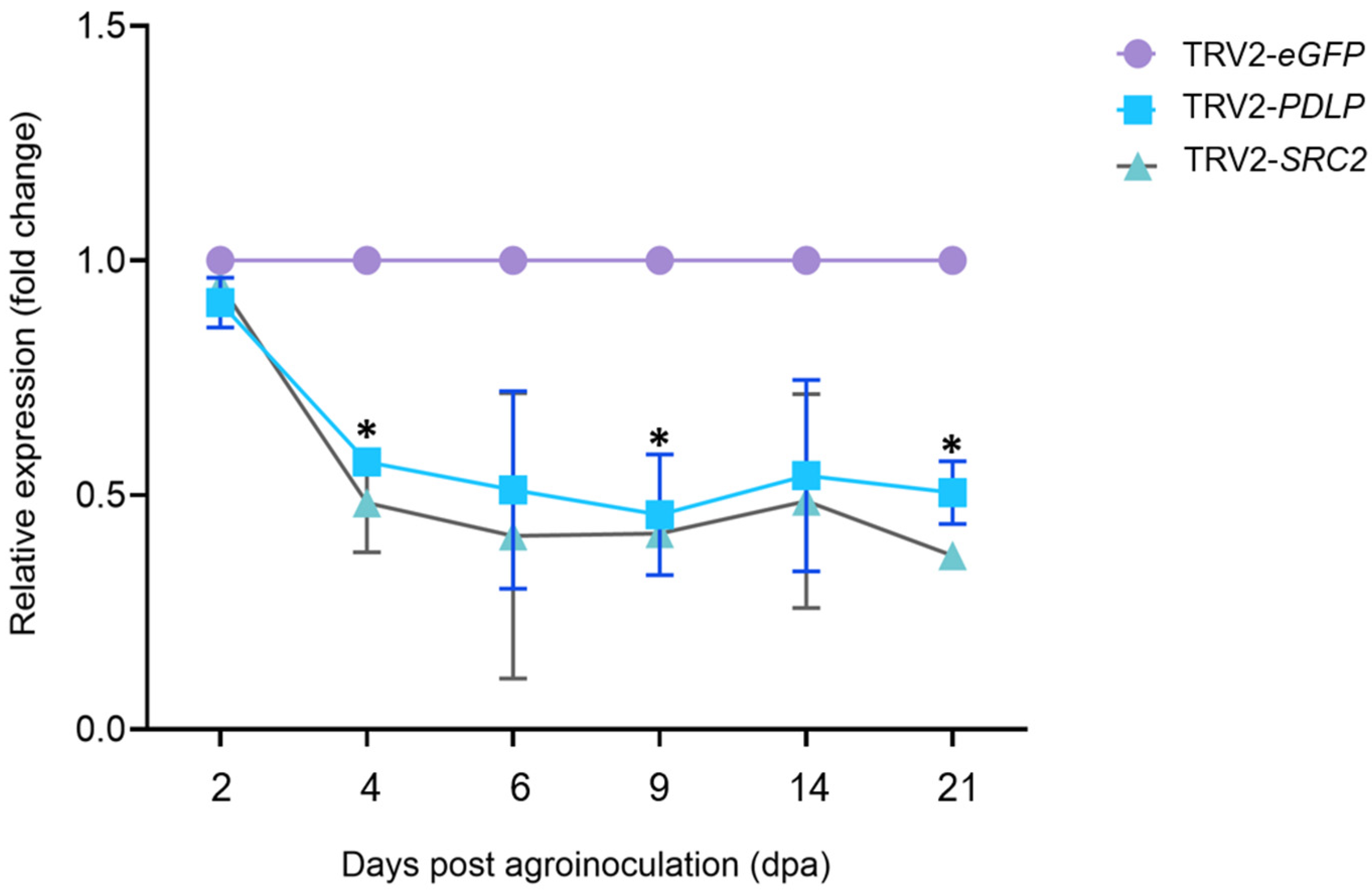
2.3.1. Designing of VIGS Constructs and Silencing of PDLP and SRC2 Genes in N. benthamiana, N. tabacum, and C. chinense
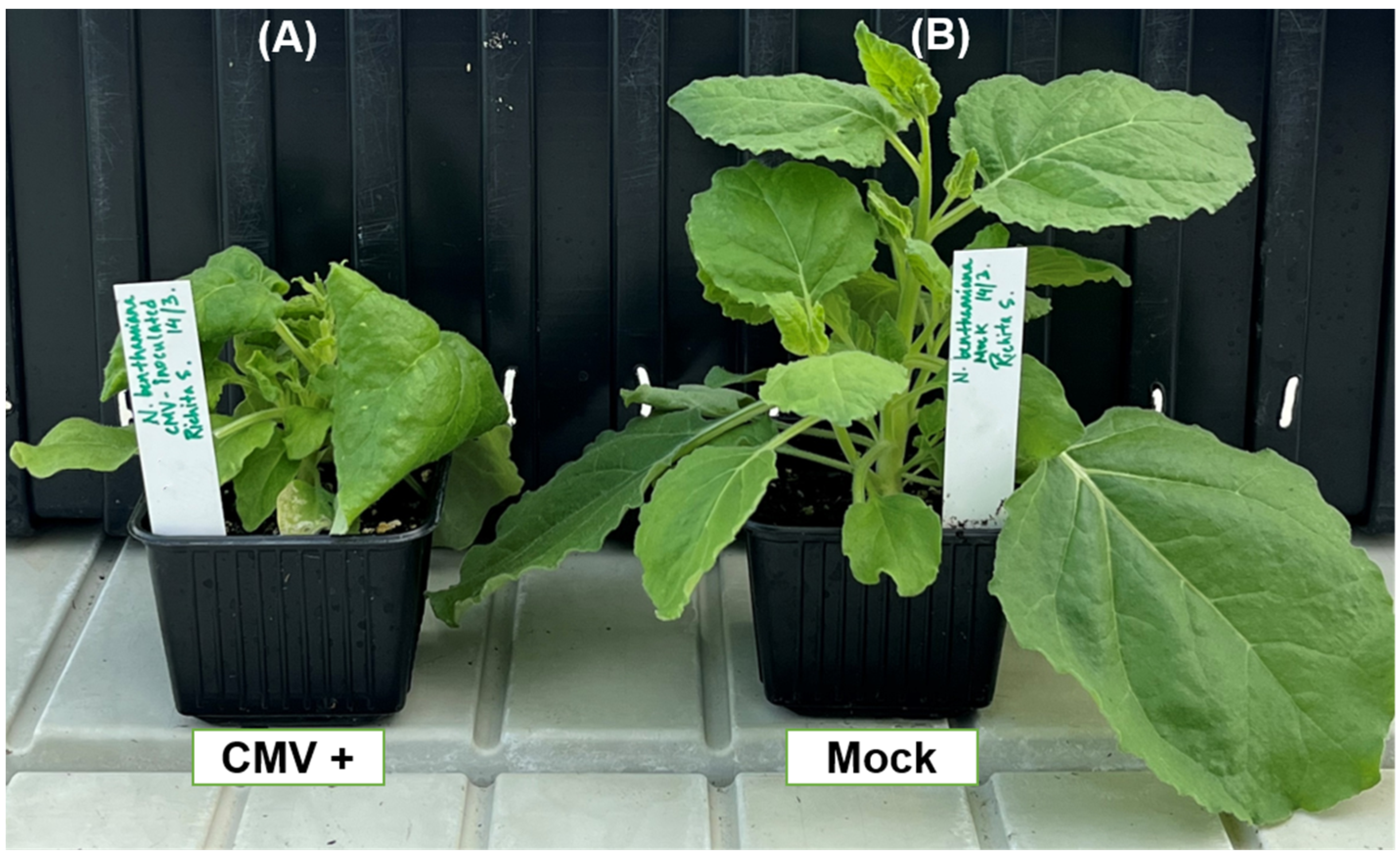
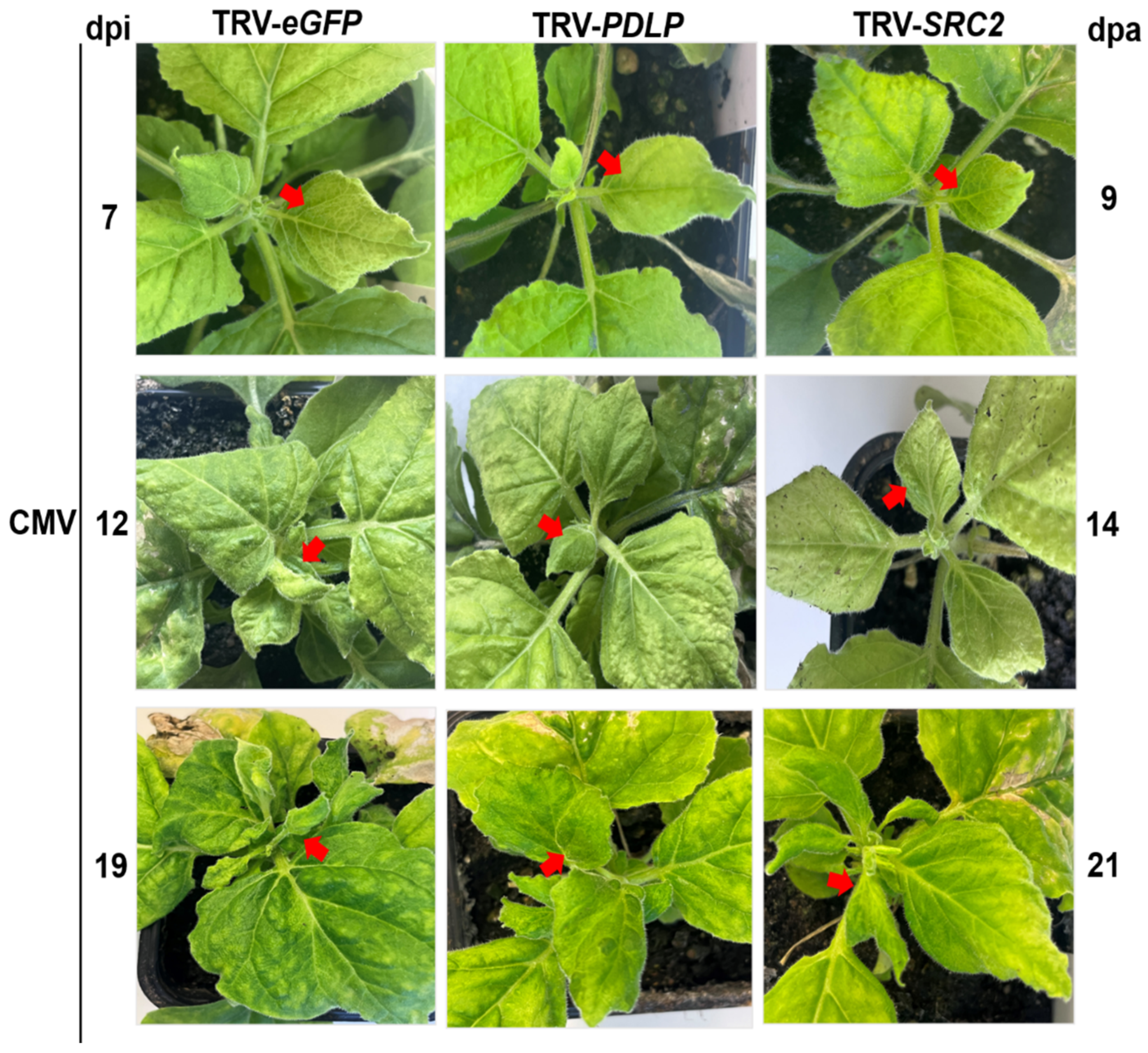
2.3.2. Disease Development in PDLP- and SRC2-Silenced N. benthamiana Plants Upon CMV Infection
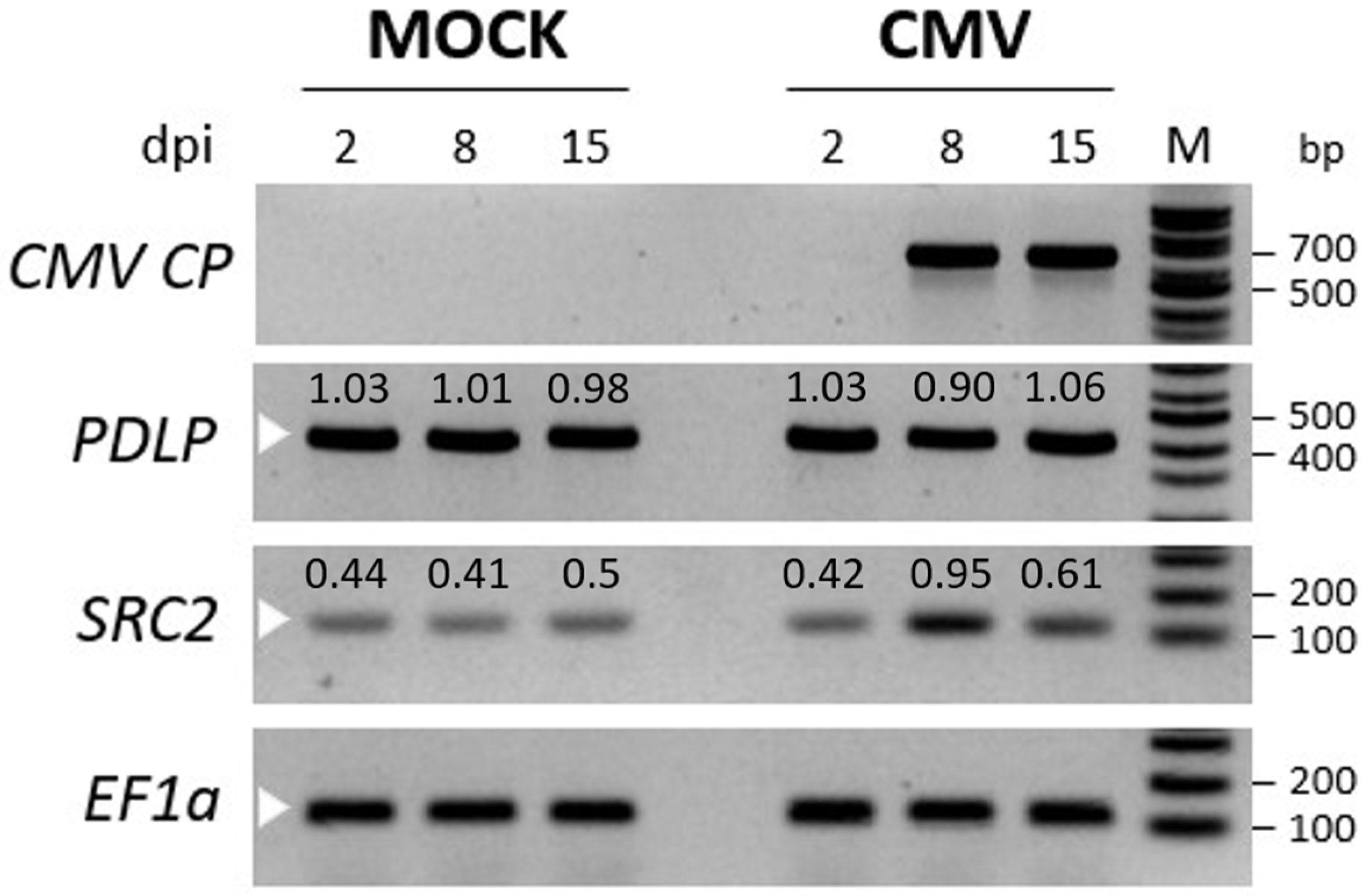
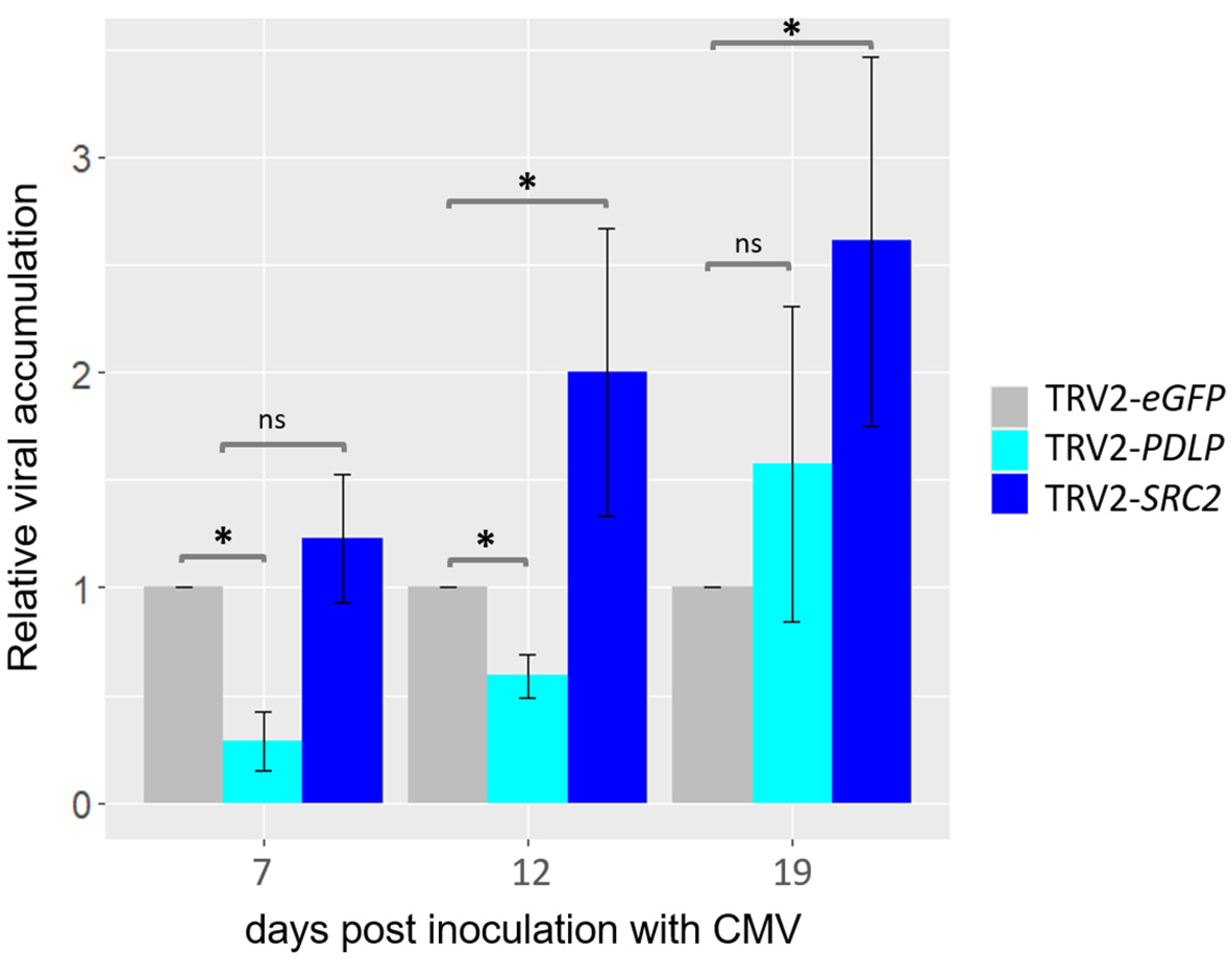
2.3.3. Relative Quantification of CMV Titer in PDLP- and SRC2-Silenced N. benthamiana Plants
2.3.4. Callose Deposition in the PDLP- and SRC2-Silenced N. benthamiana Plants Upon CMV Infection
2.3.5. Accumulation of Reactive Oxygen Species (ROS) in the PDLP- and SRC2-Silenced N. benthamiana Plants Upon CMV Infection
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Development of TRV-Based Recombinant Constructs for VIGS
| Name of Primer | Sequence (5′ to 3′) * | Product Size (bp) | Target Species | Function |
|---|---|---|---|---|
| Nt_PDLP-INF-378F Nt_PDLP-INF-760R | GCCTCCATGGGGATCGTGTACAAAGGCTGTGCTAA GCTCGGTACCGGATCGTCTCAAGCCTATCACTAAAC | 413 | N. tabacum N. benthamiana | Cloning of PDLP gene fragment to pTRV2 |
| Nt_SRC2-INF-125F Nt_SRC2-INF-488R | GCCTCCATGGGGATCCATTAGATATCAAAGTTATTGC GCTCGGTACCGGATCGGTTTTCCGCTCTCTGTTAT | 394 | N. tabacum N. benthamiana | Cloning of SRC2 gene fragment to pTRV2 |
| eGFP-INF-286F eGFP-INF-646R | GCCTCCATGGGGATCGAGCGCACCATCTTCTTCAA GCTCGGTACCGGATCGCTTCT CGTTGGGGTCTTTG | 391 | - | Cloning of eGFP gene fragment to pTRV2 |
| TRV2-1530F TRV2-1809R | GTTTTTATGTTCAGGCGGTTC TCAAGATCAGTCGAGAAT GTCA | 280 | E. coli A. tumefaciens agroinfiltrated plant species | Colony PCR, Detection of TRV2 |
| TRV1-257F TRV1-491R | GCTGAGCAGAGGAGTCATTTC ACCCATGAACCATGTTTTTGT | 235 | E. coli A. tumefaciens agroinfiltrated plant species | Detection of TRV1 |
| Nb_PDLP-RT-F Nb_PDLP-RT-R | GGGACTGTGTGAACTGTGTG ACTCCTAACCCCACACCAAC | 210 | N. tabacum N. benthamiana | Gene expression analysis for PDLP |
| Nb_SRC2-RT-F Nb_SRC2-RT-R | ATCCACCCGTACAACAACCT AGCAGCCATCTCTCCAACAT | 164 | N. tabacum N. benthamiana | Gene expression analysis for SRC2 |
| CMV_CP_RT-F CMV_CP_RT-R | GAAGCTTGTTTCGCGCATTC TCCGGATGCTGCATACTGAT | 175 | CMV | Gene expression analysis for CMV CP |
| Nb_EF1a-F Nb_EF1a-R | AGCTTTACCTCCCAAGTCATC AGAACGCCTGTCAATCTTGG | 116 | N. tabacum N. benthamiana | Gene expression analysis for EF1a [47] |
4.3. Transformation and Mobilization of VIGS Constructs into Agrobacterium Tumefaciens (GV3101 Strain)
4.4. Agrobacterium-Mediated Transient Expression and Virus Induced Gene Silencing
4.5. Mechanical Inoculation of CMV in Agroinfiltrated N. benthamiana Plants
4.6. PCR and RT-qPCR Analysis
4.7. Histochemical Analysis
4.7.1. Staining for Callose Deposition in Leaf Tissues
4.7.2. Staining for H2O2 Detection in Leaf Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, D.; Kumar, R.; Hyun, T.K.; Kim, J.-Y. Cell-to-Cell Movement of Viruses via Plasmodesmat. J. Plant Res. 2015, 128, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schoelz, J.E.; Harries, P.A.; Nelson, R.S. Intracellular Transport of Plant Viruses: Finding the Door out of the Cell. Mol. Plant 2011, 4, 813–831. [Google Scholar] [CrossRef]
- Lucas, W.J. Plant Viral Movement Proteins: Agents for Cell-to-Cell Trafficking of Viral Genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef]
- Heinlein, M. Plasmodesmata: Channels for Viruses on the Move. Methods Mol. Biol. 2015, 1217, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Amari, K.; Boutant, E.; Hofmann, C.; Schmitt-Keichinger, C.; Fernandez-Calvino, L.; Didier, P.; Lerich, A.; Mutterer, J.; Thomas, C.L.; Heinlein, M.; et al. A Family of Plasmodesmal Proteins with Receptor-like Properties for Plant Viral Movement Proteins. PLoS Pathog. 2010, 6, e1001119. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, C.; Hofmann, C. Tubule-Guided Movement of Plant Viruses. Plant Cell Monogr. 2007, 7, 64–83. [Google Scholar] [CrossRef]
- Chen, M.-H.; Tian, G.-W.; Gafni, Y.; Citovsky, V. Effects of Calreticulin on Viral Cell-to-Cell Movement. Plant Physiol. 2005, 138, 1866–1876. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Taoka, K.; Yoo, B.-C.; Ben-Nissan, G.; Kim, D.-J.; Lucas, W.J. Plasmodesmal-Associated Protein Kinase in Tobacco and Arabidopsis Recognizes a Subset of Non-Cell-Autonomous Proteins. Plant Cell 2005, 17, 2817–2831. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Bayer, E.; Lafarge, D.; Cluzet, S.; German Retana, S.; Boubekeur, T.; Leborgne-Castel, N.; Carde, J.-P.; Lherminier, J.; Noirot, E.; et al. Remorin, a Solanaceae Protein Resident in Membrane Rafts and Plasmodesmata, Impairs Potato Virus X Movement. Plant Cell 2009, 21, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.; Wang, X.; Toscano-Morales, R.; Lin, J.; Lucas, W. Plasmodesmal Endoplasmic Reticulum Proteins Regulate Intercellular Trafficking of Cucumber Mosaic Virus in Arabidopsis. J. Exp. Bot. 2023, 74, 4401–4414. [Google Scholar] [CrossRef]
- Tilsner, J.; Kriechbaumer, V. Reticulons 3 and 6 Interact with Viral Movement Proteins. Mol. Plant Pathol. 2022, 23, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Sagi, G.; Gera, A.; Epel, B.L. The Constitutive Expression of Arabidopsis Plasmodesmal-Associated Class 1 Reversibly Glycosylated Polypeptide Impairs Plant Development and Virus Spread. J. Exp. Bot. 2010, 61, 131–142. [Google Scholar] [CrossRef]
- Ueki, S.; Spektor, R.; Natale, D.M.; Citovsky, V. ANK, a Host Cytoplasmic Receptor for the Tobacco Mosaic Virus Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata. PLoS Pathog. 2010, 6, e1001201. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Shimada-Beltran, H.; Levy, A.; Zheng, J.Y.; Javia, P.A.; Lazarowitz, S.G. The Arabidopsis Synaptotagmin SYTA Regulates the Cell-to-Cell Movement of Diverse Plant Viruses. Front. Plant Sci. 2014, 5, 584. [Google Scholar] [CrossRef]
- den Hollander, P.W.; Kieper, S.N.; Borst, J.W.; van Lent, J.W.M. The Role of Plasmodesma-Located Proteins in Tubule-Guided Virus Transport Is Limited to the Plasmodesmata. Arch. Virol. 2016, 161, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Angel, C.A.; Lutz, L.; Leisner, S.M.; Nelson, R.S.; Schoelz, J.E. Association of the P6 Protein of Cauliflower Mosaic Virus with Plasmodesmata and Plasmodesmal Proteins. Plant Physiol. 2014, 166, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, S.Y.; Choi, D.; Ryu, C.M.; Park, J.M. Molecular Characterization of a Pepper C2 Domain-Containing SRC2 Protein Implicated in Resistance against Host and Non-Host Pathogens and Abiotic Stresses. Planta 2008, 227, 1169–1179. [Google Scholar] [CrossRef]
- Roossinck, M.J. Evolutionary History of Cucumber Mosaic Virus Deduced by Phylogenetic Analyses. J. Virol. 2002, 76, 3382–3387. [Google Scholar] [CrossRef]
- Edwardson, J.R.; Christie, R.G. CRC Handbook of Viruses Infecting Legumes; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Ashwathappa, K.V.; Krishna Reddy, M.; Venkataravanappa, V.; Madhavi Reddy, K.; Hemachandra Reddy, P.; Lakshminarayana Reddy, C.N. Genome Characterization and Host Range Studies of Cucumber Mosaic Virus Belonging to the Subgroup IB Infecting Chilli in India and Screening of Chilli Genotypes for Identification of Resistance. VirusDisease 2021, 32, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Cucumber Mosaic Virus; a Model for RNA Virus Evolution. Mol. Plant Pathol. 2001, 2, 59–63. [Google Scholar] [CrossRef]
- Sáray, R.; Fábián, A.; Palkovics, L.; Salánki, K. The 28 Ser Amino Acid of Cucumber Mosaic Virus Movement Protein Has a Role in Symptom Formation and Plasmodesmata Localization. Viruses 2021, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Llave, C.; Díaz-Ruíz, J.R. RNA Interference as a New Biotechnological Tool for the Control of Virus Diseases in Plants. Virus Res. 2004, 102, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Yellina, A.L.; Orashakova, S.; Becker, A. Virus-Induced Gene Silencing (VIGS) in Plants: An Overview of Target Species and the Virus-Derived Vector Systems. Methods Mol. Biol. 2013, 975, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Kumar, M.; Mysore, K.S. Tobacco Rattle Virus–Based Virus-Induced Gene Silencing in Nicotiana Benthamiana. Nat. Protoc. 2014, 9, 1549–1562. [Google Scholar] [CrossRef]
- Purkayastha, A.; Dasgupta, I. Virus-Induced Gene Silencing: A Versatile Tool for Discovery of Gene Functions in Plants. Plant Physiol. Biochem. 2009, 47, 967–976. [Google Scholar] [CrossRef]
- Becker, A.; Lange, M. VIGS--Genomics Goes Functional. Trends Plant Sci. 2010, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Hao, M.; Tian, B.; Cao, G.; Wei, F.; Xie, Z. A Methodological Advance of Tobacco Rattle Virus-Induced Gene Silencing for Functional Genomics in Plants. Front. Plant Sci. 2021, 12, 671091. [Google Scholar] [CrossRef]
- Kumagai, M.H.; Donson, J.; Della-Cioppa, G.; Harvey, D.; Hanley, K.; Grill, L.K. Cytoplasmic Inhibition of Carotenoid Biosynthesis with Virus-Derived RNA. Proc. Natl. Acad. Sci. USA 1995, 92, 1679–1683. [Google Scholar] [CrossRef]
- Ratcliff, F.; Martin-Hernandez, A.M.; Baulcombe, D.C. Technical Advance. Tobacco Rattle Virus as a Vector for Analysis of Gene Function by Silencing. Plant J. 2001, 25, 237–245. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-Induced Gene Silencing in Tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.F.; Scott, J.W.; Edwards, J.D.; Bai, Y. The Tomato Yellow Leaf Curl Virus Resistance Genes Ty-1 and Ty-3 Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases. PLOS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Padmanabhan, M.; Dinesh-Kumar, S.P. Virus-Induced Gene Silencing in Nicotiana Benthamiana and Other Plant Species. Methods Mol. Biol. 2011, 678, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, D.; Zhu, B.; Yan, H.; Shen, X.; Zuo, J.; Zhu, Y.; Luo, Y. Virus-Induced Gene Silencing in Eggplant (Solanum Melongena). J. Integr. Plant Biol. 2012, 54, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Reagan, B.C.; Burch-Smith, T.M. Viruses Reveal the Secrets of Plasmodesmal Cell Biology. Mol. Plant-Microbe Interact. 2020, 33, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Shimosaka, E. CDNA Sequence Analysis and Expression of Two Cold-Regulated Genes in Soybean. Plant Sci. 1997, 123, 93–104. [Google Scholar] [CrossRef]
- Caillaud, M.-C.; Wirthmueller, L.; Sklenar, J.; Findlay, K.; Piquerez, S.J.M.; Jones, A.M.E.; Robatzek, S.; Jones, J.D.G.; Faulkner, C. The Plasmodesmal Protein PDLP1 Localises to Haustoria-Associated Membranes during Downy Mildew Infection and Regulates Callose Deposition. PLOS Pathog. 2014, 10, e1004496. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Shi, L.; Yang, S.; Shen, L.; Yu, H.; Wang, R.; Wen, J.; Tang, Q.; Hussain, A.; et al. SGT1 Is Required in PcINF1/SRC2-1 Induced Pepper Defense Response by Interacting with SRC2-1. Sci. Rep. 2016, 6, 21651. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Chen, Q.-F.; Chye, M.-L. Arabidopsis Thaliana Acyl-CoA-Binding Protein ACBP6 Interacts with Plasmodesmata-Located Protein PDLP8. Plant Signal. Behav. 2017, 12, e1359365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative Stress and Antioxidative Responses in Plant–Virus Interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wang, X.; Cui, W.; Sager, R.; Modla, S.; Czymmek, K.; Zybaliov, B.; Van Wijk, K.; Zhang, C.; Lu, H.; et al. A Plasmodesmata-Localized Protein Mediates Crosstalk between Cell-to-Cell Communication and Innate Immunity in Arabidopsis. Plant Cell 2011, 23, 3353–3373. [Google Scholar] [CrossRef]
- Lim, G.-H.; Shine, M.B.; de Lorenzo, L.; Yu, K.; Cui, W.; Navarre, D.; Hunt, A.G.; Lee, J.-Y.; Kachroo, A.; Kachroo, P. Plasmodesmata Localizing Proteins Regulate Transport and Signaling during Systemic Acquired Immunity in Plants. Cell Host Microbe 2016, 19, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Carella, P.; Isaacs, M.; Cameron, R.K. Plasmodesmata-Located Protein Overexpression Negatively Impacts the Manifestation of Systemic Acquired Resistance and the Long-Distance Movement of DEFECTIVE IN INDUCED RESISTANCE1 in Arabidopsis. Plant Biol. 2015, 17, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, Y.; Fang, X.; Gou, H.; Sun, R.; Xuan, H.; Wang, H.; Zhao, J.; Xing, H.; Guo, N. Overexpression of GmSRC2 Confers Resistance to Phytophthora Sojae in Soybean. Preprint 2023. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lazarowitz, S.G. Arabidopsis Synaptotagmin SYTA Regulates Endocytosis and Virus Movement Protein Cell-to-Cell Transport. Proc. Natl. Acad. Sci. USA 2010, 107, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Kawamura, Y.; Minami, A.; Uemura, M. Calcium-Dependent Freezing Tolerance in Arabidopsis Involves Membrane Resealing via Synaptotagmin SYT1. Plant Cell 2008, 20, 3389–3404. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Han, C.; Yu, J.; Li, D.; Zhang, Y. Validation of Reference Genes for Gene Expression Studies in Virus-Infected Nicotiana Benthamiana Using Quantitative Real-Time PCR. PLoS ONE 2012, 7, e46451. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Transformation of Agrobacterium Using Electroporation. CSH Protoc. 2006, 2006. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Schenk, S.T.; Schikora, A. Staining of Callose Depositions in Root and Leaf Tissues. Bio-protocol 2015, 5, e1429. [Google Scholar] [CrossRef]
- Bach-Pages, M.; Preston, G.M. Methods to Quantify Biotic-Induced Stress in Plants. Methods Mol. Biol. 2018, 1734, 241–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikia, R.; Kaldis, A.; Spetz, C.J.; Borah, B.K.; Voloudakis, A. Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus. Plants 2025, 14, 495. https://doi.org/10.3390/plants14030495
Saikia R, Kaldis A, Spetz CJ, Borah BK, Voloudakis A. Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus. Plants. 2025; 14(3):495. https://doi.org/10.3390/plants14030495
Chicago/Turabian StyleSaikia, Richita, Athanasios Kaldis, Carl Jonas Spetz, Basanta Kumar Borah, and Andreas Voloudakis. 2025. "Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus" Plants 14, no. 3: 495. https://doi.org/10.3390/plants14030495
APA StyleSaikia, R., Kaldis, A., Spetz, C. J., Borah, B. K., & Voloudakis, A. (2025). Silencing of Putative Plasmodesmata-Associated Genes PDLP and SRC2 Reveals Their Differential Involvement during Plant Infection with Cucumber Mosaic Virus. Plants, 14(3), 495. https://doi.org/10.3390/plants14030495








