Cannabidiol’s Antioxidant Properties in Skin Care Products and Legislative Regulations
Abstract
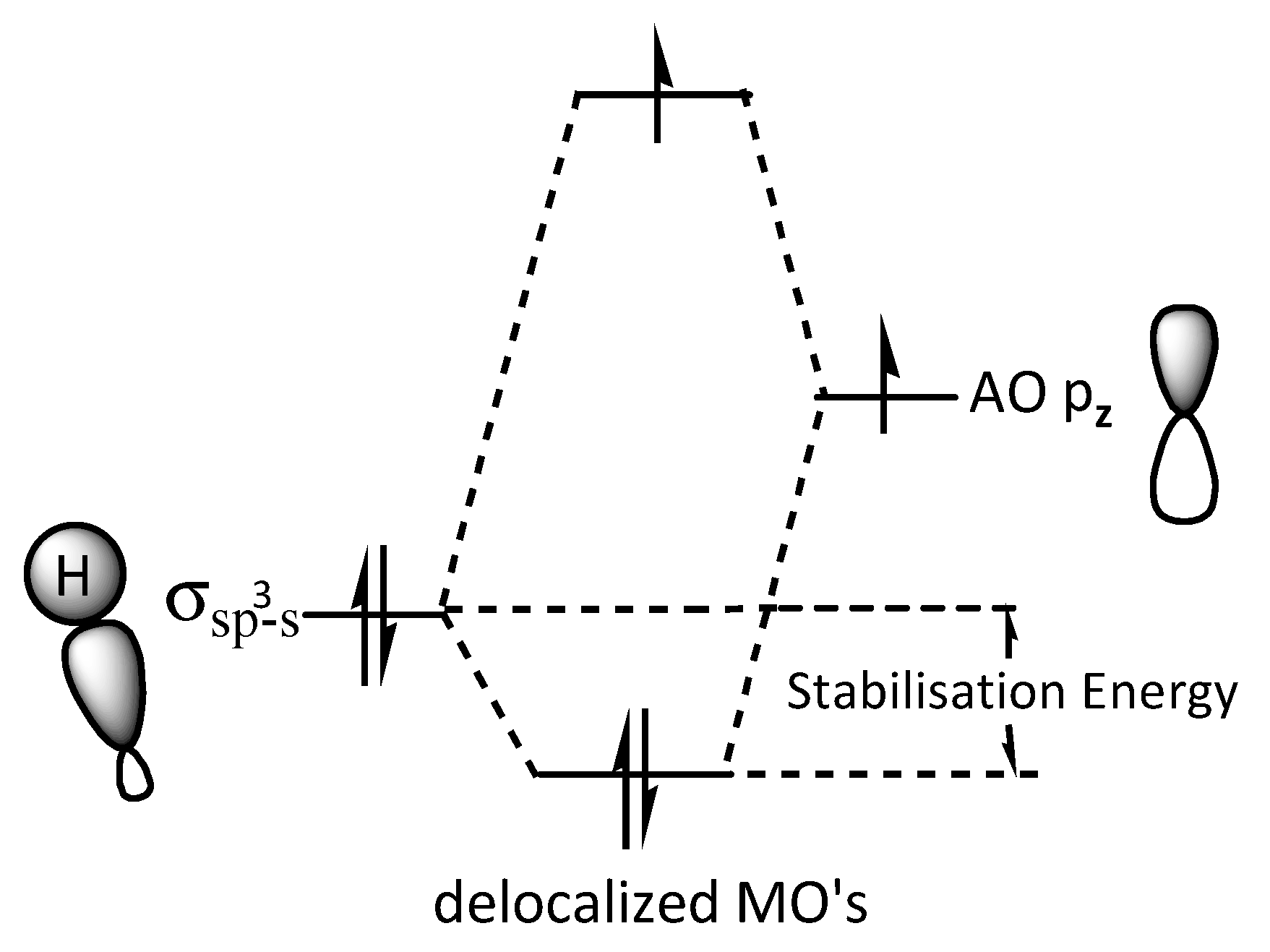
1. Introduction
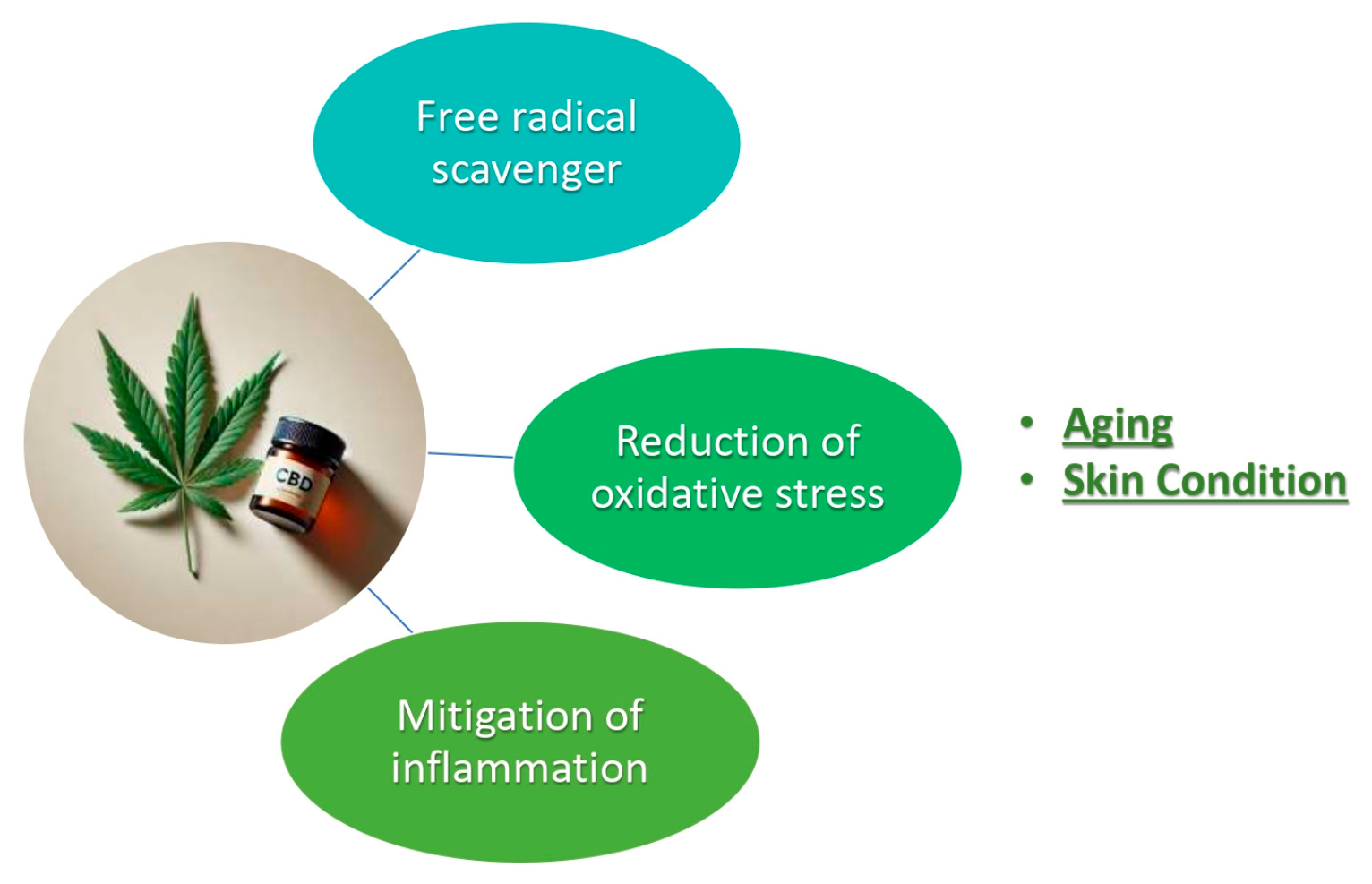
- Anti-inflammatory: CBD’s ability to modulate inflammatory responses makes it suitable for treating skin conditions such as acne, eczema, and psoriasis. It causes a reduction in redness, swelling, and irritation.
- Antioxidant: Antioxidants can protect the skin from damage caused by free radicals and UVA and UVB radiation. This helps in slowing down the aging process and preventing the formation of wrinkles and fine lines.
- Sebum Regulation: CBD can influence the production of sebum, helping in maintaining balanced skin moisture and preventing acne breakouts.
2. Cannabidiol’s Antioxidant and Anti–Inflammatory Properties on Keratinocytes
3. Delivery Systems and CBD
4. Mechanism of CBD’s Transport from Liposomes to the Cell Membrane
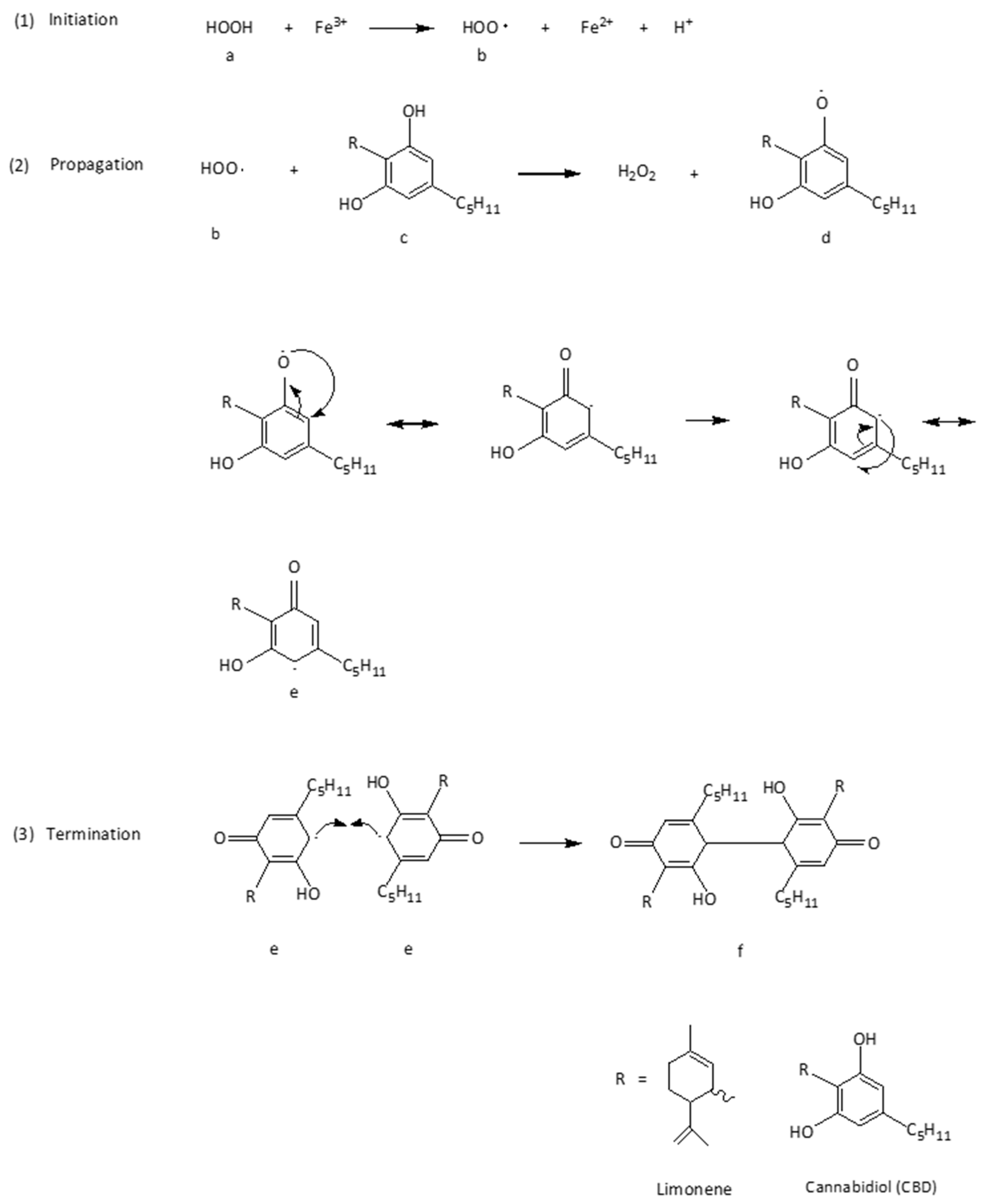
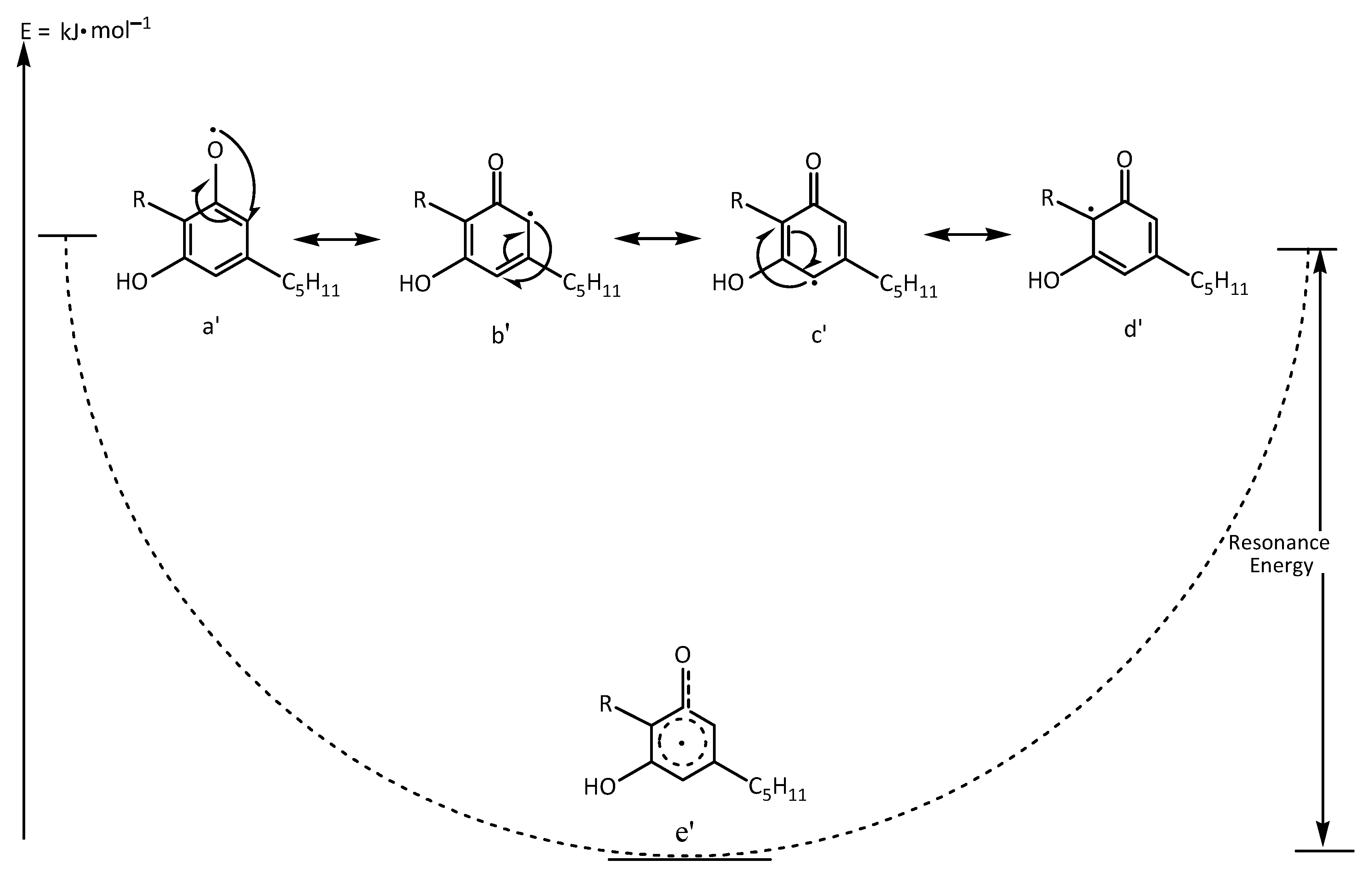
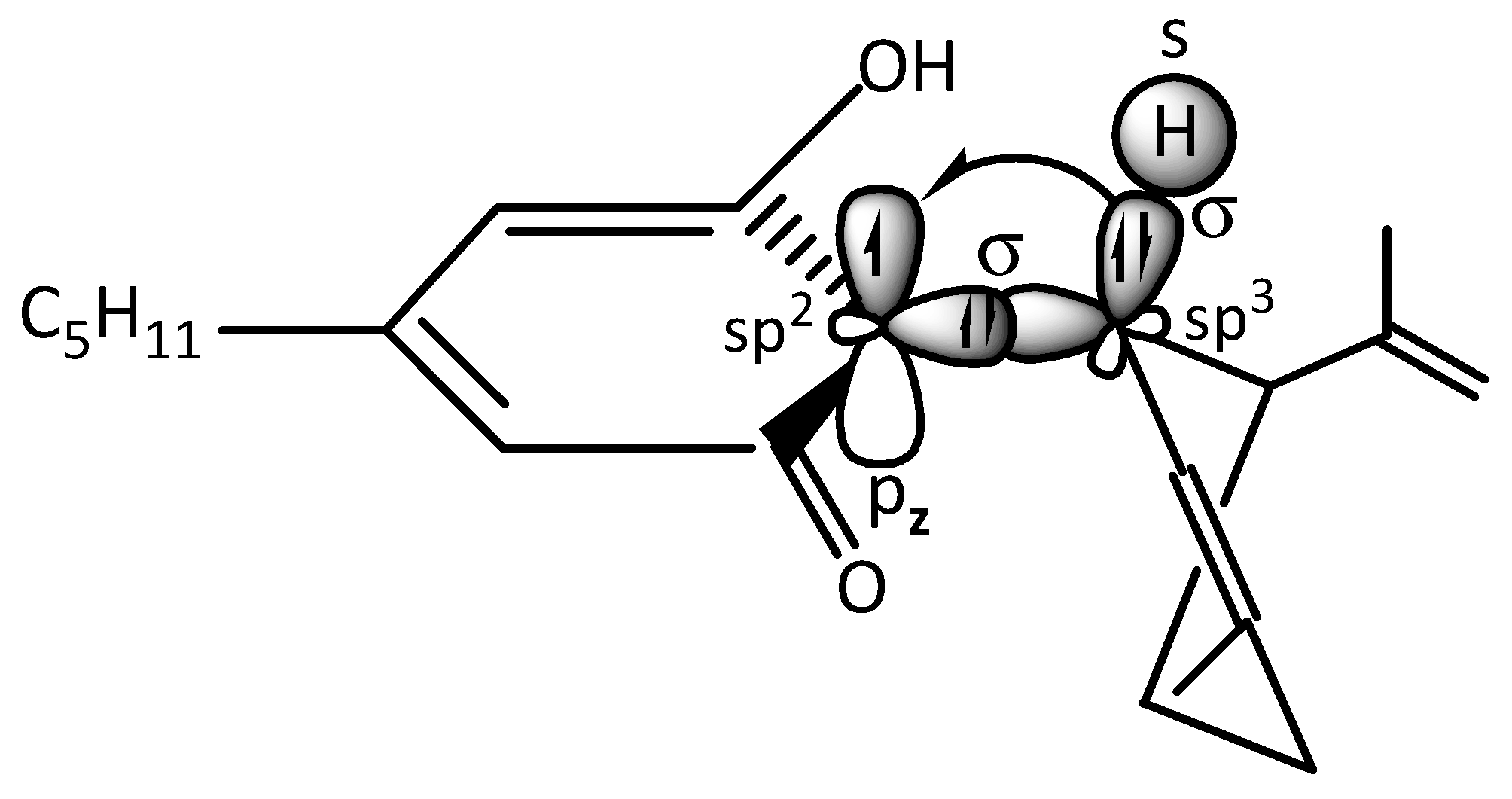
5. Radical Chain Mechanism by the Neutralization of Free Radicals in Keratinocytes by CBD
6. CBD in Cosmetic Industry, Skin Care Products, and Legislative Regulations
- (1)
- CBD Lotion Skin Formula 750mg (CBD: 1.616%, THF: 0.611%, Total Cannabinoids: 1.529%) [52].
- (2)
- Peppermint and Arnica CBD Balm 750 mg (CBD: 1.198%, THC: 0.052%, Total Cannabinoids: 1.323%) [53].
- (3)
- CBD Lotion + Menthol 750 mg (CBD: 1.486%, THF: 0.045%, Total Cannabinoids: 1.571%) [54].
6.1. Legislation of CBD in Different Continents and Countries
6.1.1. North America
6.1.2. Latin America
6.1.3. Stability, pH
6.1.4. Quality Control of CBD in Cosmetics
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | cannabidiol |
| ECS | endocannabinoid system |
| TRP | transient receptor potential |
| SODs | superoxide dismutases |
| CAT | catalases |
| GPx | glutathione peroxidases |
| Prx | peroxyredoxins |
| PUFAs | polyunsaturated fatty acids |
| MDA | malondialdehyde |
| 4–HNE | 4–hydroxynonenal |
| ANA | anandamide |
| TNF–α | tumor necrosis factor |
| LPS | lipopolysaccharides |
| IL | Interleukin |
| NHEK | normal human epidermal keratinocytes |
| NHDF | normal human dermal fibroblasts |
| HMOX1 | hemooxygenase 1 |
| PTX | paclitaxel |
| DOX | doxorubicin |
| DMS | dermal membrane structure |
| SNARE | Soluble NSF Attachment Receptor proteins |
| NSF | N–ethylmaleimide Sensitive Fusion protein |
| DOPE | dioleoylphosphatidylethanolamine |
| ROS | reactive oxygen species |
| Cys | cysteine |
| GSH | Glutathione |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| Keap 1 | Kelch–like ECH–associated protein 1 |
| CAGR | Compound Annual Growth Rate |
| ECDD | Expert Committee on Drug Dependence |
| BHT | butylated hydroxytoluene |
| TRPV4 | transient receptor potential cation channel subfamily V member 4 |
| HU–331 | Cannabidiol hydroxyquinone |
| FDA | Food and Drug Administration |
| EC | European Community |
| WHO | World Health Organization |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| 5-HT1A | serotonin 1A receptor |
References
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The differences in the proteome profile of cannabidiol-treated skin fibroblasts following UVA or UVB irradiation in 2D and 3D cell cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Introductory remarks. In Oxidative Stress; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Rosivaldo, S.; Borges, J.B.J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B.F. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Fischbacher, A.; Von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant function of phytocannabinoids: Molecular basis of their stability and cytoprotective properties under UV-irradiation. Free Radic. Biol. Med. 2021, 164, 258–270. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory properties of cannabidiol, a non-psychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Filipiuc, S.I.; Neagu, A.N.; Uritu, C.M. The skin and natural cannabinoids-topical and transdermal applications. Pharmaceuticals 2023, 16, 1049. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Sermet, S.; Li, J.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes. Toxicology 2021, 464, 153016. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Kim, Y.J.; Kim, M.O.; Kang, M.; Oh, S.W.; Nho, Y.H.; Park, S.-H.; Lee, J. Cannabidiol upregulates melanogenesis through CB1-dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem. Biol. Interact. 2017, 273, 107–114. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.P.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the pathophysiology of skin inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, C.H.; Lin, Y.H.; Wang, C.C.; Jan, T.R. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic. Biol. Med. 2018, 124, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Collaborative power of Nrf2 and PPARγ activators against metabolic and drug-induced oxidative injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Toschi, T.G. Preliminary study: Comparison of antioxidant activity of cannabidiol (CBD) and α-tocopherol added to refined olive and sunflower oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to En-hance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef] [PubMed]
- Uziel, A.; Gelfand, A.; Amsalem, K.; Berman, P.; Lewitus, G.M.; Meiri, D.; Lewitus, D.Y. Full-spectrum cannabis extract microdepots support controlled release of multiple phytocannabinoids for extended therapeutic effect. ACS Appl. Mater. Interfaces 2020, 12, 23707–23716. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, C.; Kondur, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.J.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.; McIlwrath, S.; Stinchcomb, A.; Westlund, K. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Blair, E.; Collins, F. Liposomal cannabidiol delivery: A pilot study. Am. J. Endocannabinoid Med. 2020, 2, 19–21. [Google Scholar]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; do Rosário Domingues, M.; Domingues, P.; Skrzydlewska, E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Szepietowski, J.C.; Szepietowski, T.; Reich, A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: A preliminary study. Acta Dermatovenerol. Croat. 2005, 13, 97–103. [Google Scholar] [PubMed]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and anti-inflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- McCormick, E.; Han, H.; Abdel Azim, S.; Whiting, C.; Bhamidipati, N.; Kiss, A.; Efimova, T.; Berman, B.; Friedman, A. Topical nanoencapsulated cannabidiol cream as an innovative strategy combating UV-A-induced nuclear and mitochondrial DNA injury: A pilot randomized clinical study. J. Am. Acad. Dermatol. 2024, 91, 855–862. [Google Scholar] [CrossRef]
- Knoll, G.; Burger, K.N.; Bron, R.; Meer, G.; Verkleij, A. Fusion of liposomes with the plasma membrane of epithelial cells: Fate of incorporated lipids as followed by freeze fracture and autoradiography of plastic sections. J. Cell Biol. 1988, 107, 2511–2521. [Google Scholar] [CrossRef]
- Damen, J.; Regit, J.; Scherphof, G. Transfer and exchange of phospholipid between small unilamellar liposomes and rat plasma high density lipoproteins: Dependence on cholesterol content and phospholipid composition. Biochim. Biophys. Acta 1981, 665, 538–545. [Google Scholar] [CrossRef]
- Bogaart, G.; Holt, M.G.; Bunt, G.; Riedel, D.; Wouters, F.S.; Jahn, R. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 2010, 17, 358–364. [Google Scholar] [CrossRef]
- Plemper, R.K.; Melikyan, G.B. Membrane fusion. In Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Meng, J.; Wang, J.; Buddenkotte, J.; Buhl, T.; Steinhoff, M. Role of SNAREs in the atopic dermatitis-related cytokine secretion and skin-nerve communication. J. Investig. Dermatol. 2019, 139, 2324–2333. [Google Scholar] [CrossRef]
- Del Río, C.; Millán, E.; García, V.; Appendino, G.; DeMesa, J.; Muñoz, E. The endocannabinoid system of the skin: A potential approach for the treatment of skin disorders. Biochem. Pharmacol. 2018, 157, 122–133. [Google Scholar] [CrossRef]
- Zimmermann, M.; Domke, D.; Schween, M. Effekt und Hyperkonjugation bei Radikalkettenreaktionen. Chemkon 2023, 30, 300–308. [Google Scholar] [CrossRef]
- Alabugin, I.V.; dos Passos Gomes, G.; Abdo, M.A. Hyperconjugation. WIREs Comput. Mol. Sci. 2019, 9, e1389. [Google Scholar] [CrossRef]
- Straits Research. CBD Skin Care Market Size, Share & Trends Analysis Report by Product Type (Oils and Lotions, Moisturizers, Masks & Serums, Cleansers, Others), by Source (Organic, Synthetic), by Distribution Channel (Online, Offline), and by Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025–2033; Straits Research: Pune, India, 2024. [Google Scholar]
- Shah, P.; Holmes, K.; Chibane, F.; Wang, P.; Chagas, P.; Salles, E.; Jones, M.; Palines, P.; Masoumy, M.; Baban, B.; et al. Cutaneous wound healing and the effects of cannabidiol. Int. J. Mol. Sci. 2024, 25, 7137. [Google Scholar] [CrossRef]
- Parikh, A.C.; Jeffery, C.S.; Sandhu, Z.; Brownlee, B.P.; Queimado, L.; Mims, M.M. The effect of cannabinoids on wound healing: A review. Health Sci. Rep. 2024, 7, e1908. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M. Cannabinoids for skin diseases and hair regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Jakus, J.; Portillo, M.; Gvirtz, R.; Ogen-Shtern, N.; Silberstein, E.; Ayzenberg, T.; Rozenblat, S. In vitro, ex vivo, and clinical evaluation of anti-aging gel containing EPA and CBD. J. Cosmet. Dermatol. 2023, 22, 3047–3057. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Ist. Super. Sanita 2020, 56, 285–291. [Google Scholar] [CrossRef]
- Cornbread Hemp. Certficate of Analysis. Available online: https://cdn.shopify.com/s/files/1/0612/5456/6068/files/750mgskin_COA_02052504.PDF?v=1743677384 (accessed on 2 February 2025).
- Cornbread Hemp. Certficate of Analysis. Available online: https://cdn.shopify.com/s/files/1/0612/5456/6068/files/750mgbalm_COA_08152507.pdf?v=1757263856 (accessed on 19 August 2025).
- Cornbread Hemp. Certficate of Analysis. Available online: https://cdn.shopify.com/s/files/1/0612/5456/6068/files/750mgmenthol_COA_07232506.pdf?v=1755509643 (accessed on 24 July 2025).
- Dabrowska, A.; Johnson, R. FDA Regulation of Cannabidiol (CBD) Products, IN FOCUS. Available online: https://www.congress.gov/crs_external_products/IF/PDF/IF11250/IF11250.2.pdf#:~:text=In%20the%20United%20States%2C%20CBD%20is%20marketed%20in,active%20ingredient%20in%20an%20FDA-approved%20pharmaceutical%20drug%2C%20Epidiolex%C2%AE (accessed on 12 June 2019).
- Šikić, J. Worldwide CBD Regulations 2021, Ilesol Pharmaceuticals. Available online: https://ilesol.com/worldwide-cbd-regulations-2021/#latin (accessed on 13 May 2024).
- Rooke, A. Is CBD Legal in Asia for Cosmetics? Clean Beauty Asia. Available online: https://cleanbeautyasia.com/cbd-legal-in-asia (accessed on 9 November 2025).
- The Legal Status of CBD in Different Countries. CBD Store. Available online: https://cbdstor.com/the-legal-status-of-cbd-in-different-countries/ (accessed on 9 October 2025).
- Health Products Regulatory Authority (HPRA). European Commission Consultation on Cannabidiol (CBD) Used in Cosmetic Products. Available online: https://www.drugsandalcohol.ie/40789 (accessed on 6 March 2024).
- LAW No 339 of 29 November 2005 Concerning the Legal Regime of Plants, Narcotic Drugs and Psychotropic Substances, and Narcotic and Psychotropic Preparations. The Official Gazette of Romaia No 1095 of 5 December 2005. Available online: https://ier.gov.ro/wp-content/uploads/legislatie/L-339_2005 (accessed on 5 December 2005).
- Cohen, P.A.; Sharfstein, J. The opportunity of CBD—Reforming the law. N. Engl. J. Med. 2019, 381, 297–299. [Google Scholar] [CrossRef]
- Vlad, R.A.; Hancu, G.; Ciurba, A.; Antonoaea, P.; Redai, E.M.; Todoran, N.; Silasi, O.; Muntean, D.L. Cannabidiol—Therapeutic and legal aspects. Pharmazie 2020, 75, 463–469. [Google Scholar]
- Spinella, A.; de Pinto, M.; Baraldi, C.; Galluzzo, C.; Testoni, S.; Lumetti, F.; Parenti, L.; Guerzoni, S.; Salvarani, C.; Giuggioli, D. Topical Cannabidiol in the Treatment of Digital Ulcers in Patients with Scleroderma: Comparative Analysis and Literature. Rev. Adv. Ski. Wound Care 2023, 36, 18–23. [Google Scholar] [CrossRef]
- Dowd, A.N.; Zamarripa, C.A.; Sholler, D.J.; Cone, E.J.; Murphy, T.P.; ElSohly, M.; ElSohly, K.; Gul, W.; Shahzadi, I.; Mullen, L.D.; et al. Cannabinoid content and label accuracy of various hemp-derived haircare, cosmetic, and edible products available at retail stores and online in the United States. Cannabis Cannabinoid Res. 2024; ahead of print. [Google Scholar] [CrossRef]
- Kirk, D.R.; Akanji, T.; Li, H.; Shen, J.; Allababidi, S.; Seeram, N.P.; Bertin, M.J.; Ma, H. Evaluations of Skin Permeability of Cannabidiol and Its Topical Formulations by Skin Membrane-Based Parallel Artificial Membrane Permeability Assay and Franz Cell Diffusion Assay. Med. Cannabis Cannabinoids 2022, 5, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Schettino, L.; Prieto, M.; Benedé, J.L.; Chisvert, A.; Salvador, A. A Rapid and Sensitive Method for the Determination of Cannabidiol in Cosmetic Products by Liquid Chromatography-Tandem Mass Spectrometry. Cosmetics 2021, 8, 30. [Google Scholar] [CrossRef]
- Vágnerová, M.; Sýkora, D.; Nemeškalová, A.; Kuchař, M. Challenges of cannabidiol determination in emulsified cosmeticsand application of solid-phase extraction followed by HPLC-UV-MS/MS. Anal. Bioanal. Chem. 2025, 417, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
| Study/Reference | Model/Cell Type | Study Design and Treatment | CBD Concentration/Dose | Main Findings/Results | Mechanism/Pathways |
|---|---|---|---|---|---|
| [9] | Human keratinocytes, melanocytes, fibroblasts | UV- or UVB-induced oxidative stress model; pre- and post-treatment with CBD | 1–10 µM (typical range) | CBD reduced ROS generation, restored antioxidant balance, decreased lipid peroxidation, and improved cell viability | Antioxidant defense enhancement, protection of membrane integrity |
| [2] | Normal human epidermal keratinocytes (NHEK); in vivo mouse epidermis | In vitro CBD treatment and topical application in mice | 1–5 µM (cells); 1–2 mg/cm2 (topical) | Upregulation of Nrf2 target genes (HMOX1, NQO1, GCLC); increased keratins 16/17 expression (wound repair) | Activation of Nrf2–ARE pathway; induction of cytoprotective enzymes |
| [3] | Human keratinocytes exposed to UVB and H2O2 | CBD pre-treatment before oxidative challenge | 4–10 µM | CBD maintained redox balance, protected polyunsaturated fatty acids from peroxidation | Membrane stabilization; antioxidant modulation |
| [17] | Sebocytes, keratinocytes (inflammatory model) | LPS-induced inflammation; CBD co-incubation | 5–10 µM | Inhibited TNF-α, IL-1β, and IL-6 expression; normalized lipid production | Anti-inflammatory via TRPV4 and NF-κB pathways |
| [12] | HaCaT keratinocytes (allergic contact dermatitis model) | In vitro cytokine assay with CBD treatment | 1–10 µM | Reduced cytokine release and inflammatory markers | Downregulation of NF-κB signaling |
| [23] | Human keratinocytes (UVB-exposed) | CBD repeated doses vs. control | 2.5–10 µM | Increased GSH levels and GPx/reductase activity; decreased MDA | Enhanced enzymatic antioxidant defense |
| [24] | In vitro (various models, oil-based and aqueous) | Comparative antioxidant capacity assays | Variable | Antioxidant potential higher in oil-based systems; CBD stable under UVA/UVB | Chemical stability; Matrix-dependent antioxidant activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fafaliou, M.; Papadopoulos, A.; Pavlou, P.; Varvaresou, A. Cannabidiol’s Antioxidant Properties in Skin Care Products and Legislative Regulations. Plants 2025, 14, 3521. https://doi.org/10.3390/plants14223521
Fafaliou M, Papadopoulos A, Pavlou P, Varvaresou A. Cannabidiol’s Antioxidant Properties in Skin Care Products and Legislative Regulations. Plants. 2025; 14(22):3521. https://doi.org/10.3390/plants14223521
Chicago/Turabian StyleFafaliou, Maria, Apostolos Papadopoulos, Panagoula Pavlou, and Athanasia Varvaresou. 2025. "Cannabidiol’s Antioxidant Properties in Skin Care Products and Legislative Regulations" Plants 14, no. 22: 3521. https://doi.org/10.3390/plants14223521
APA StyleFafaliou, M., Papadopoulos, A., Pavlou, P., & Varvaresou, A. (2025). Cannabidiol’s Antioxidant Properties in Skin Care Products and Legislative Regulations. Plants, 14(22), 3521. https://doi.org/10.3390/plants14223521








