An Overview of Orchidaceae from Brazil: Advances and Shortfalls After 400 Years of Studies
Abstract
1. Introduction

2. Methods: Literature Search Strategy
3. Diversity, Distribution, and Endemism
3.1. Amazon Forest
3.2. Atlantic Forest
3.3. Caatinga
3.4. Cerrado
3.5. Pampas
3.6. Pantanal
4. Taxonomy and Systematics
4.1. Cypripedioideae and Vanilloideae
4.2. Spiranthinae
4.3. Other Orchidoideae
4.4. Epidendreae
4.5. Cymbidieae
4.6. Other Epidendroideae
5. Structural, Genetic, and Ecological Characterization
5.1. Anatomy
5.2. Cytogenetics
5.3. Evolution
5.4. Biogeography
5.5. Conservation
5.6. Phytochemistry
5.7. Reproductive and Pollination Biology
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. The Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 1 February 2025).
- Darwin, C. The Various Contrivances by Which British and Foreign Orchids Are Fertilized by Insects; John Murray: London, UK, 1862. [Google Scholar]
- Dressler, R.L. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Ulloa-Ulloa, C.; Acevedo-Rodríguez, P.; Beck, S.; Belgrano, M.J.; Bernal, R.; Berry, P.E.; Brako, L.; Celis, M.; Davidse, G.; Forzza, R.C.; et al. An integrated assessment of the vascular plant species of the Americas. Science 2017, 358, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- BFG (The Brazil Flora Group). Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon 2022, 71, 178–198. [Google Scholar] [CrossRef]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 1: General Introduction, Apostasioideae, Cypripedioideae; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 2: Orchidoideae (Part One); Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 3: Orchidoideae (Part Two); Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 4: Epidendroideae (Part One); Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 5: Epidendroideae (Part Two); Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. (Eds.) Genera Orchidacearum: Volume 6: Epidendroideae (Part Three); Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Chase, M.W.; Gravendeel, B.; Sulistyo, B.P.; Wati, R.K.; Schuiteman, A. Expansion of the orchid genus Coelogyne (Arethuseae; Epidendroideae) to include Bracisepalum, Bulleyia, Chelonistele, Dendrochilum, Dickasonia, Entomophobia, Geesinkorchis, Gynoglottis, Ischnogyne, Nabaluia, Neogyna, Otochilus, Panisea and Pholidota. Phytotaxa 2021, 510, 94–134. [Google Scholar] [CrossRef]
- Smidt, E.C.; Salazar, G.A.; Mauad, A.V.S.R.; Engels, M.E.; Viruel, J.; Clements, M.; Pérez, I.J.; Chase, M.W. An Indomalesian origin in the Miocene for the diphyletic New World jewel orchids (Goodyerinae, Orchidoideae): Molecular dating and biogeographic analyses document non-monophyly of the Neotropical genera. Bot. J. Lin. Soc. 2021, 197, 322–349. [Google Scholar] [CrossRef]
- Smidt, E.C.; Toscano de Brito, A.L.V.; Mauad, A.V.S.R.; Gutiérrez-Morales, N. An expanded concept of Madisonia including miscellaneous species of Pleurothallidinae (Orchidaceae): Evidence from molecular analysis. Phytotaxa 2021, 505, 71–84. [Google Scholar] [CrossRef]
- Meneguzzo, T.E.C.; Chase, M.W. An enlarged circumscription of Bifrenaria (Orchidaceae: Maxillariiinae). Phytotaxa 2024, 638, 143–154. [Google Scholar] [CrossRef]
- Meneguzzo, T.E.C.; Chase, M.W. An expanded circumscription of Trichocentrum (Orchidaceae: Oncidiinae) to include Grandiphyllum and Saundersia. Phytotaxa 2024, 646, 82–86. [Google Scholar] [CrossRef]
- Baldini, R.M.; Cristofolini, G.; Aedo, C. The extant herbaria from the sixteenth century: A synopsis. Webbia 2022, 77, 23–33. [Google Scholar] [CrossRef]
- Ossembach, C. Precursors of the botanical exploration of South America. Willem Piso 1611–1678 and Georg Marcgrave 1610–1644. Lankesteriana 2017, 17, 61–71. [Google Scholar] [CrossRef][Green Version]
- Marcgrave, G.; Piso, W. Historia Naturalis Brasiliae; Franciscum Hackium (Frans Hacke): Leiden, The Netherlands; Lud. Elzevirium (Lodewijk Elzevir): Amsterdam, The Netherlands, 1648. [Google Scholar] [CrossRef]
- Moulin, D.; Maule, A.F.; Lima, D.A.; Rahn, K.; Pedersen, T.M. O Herbário de Georg Margraff; Fundação Nacional Pró-Memória: Rio de Janeiro, Brazil, 1986. [Google Scholar]
- Pickel, B.J. Flora do Nordeste do Brasil segundo Piso e Marcgrave no Século XVII; EDUFRPE: Recife, Brazil, 2008. [Google Scholar]
- Domingues, A. Notícias do Brasil colonial: A imprensa científica e política a serviço das elites (Portugal, Brasil e Inglaterra). Var. Hist. 2006, 22, 150–174. [Google Scholar] [CrossRef][Green Version]
- Areia, M.L.R.; Miranda, M.A.; Hartman, T. Memória da Amazónia: Alexandre Rodrigues Ferreira e a Viagem Philosophica Pelas Capitanias do Grão-Pará, Rio Negro, Mato Grosso e Cuiabá; Universidade de Coimbra: Coimbra, Portugal, 1991. [Google Scholar]
- Rocha, C.F.D. Naturalistas Viajantes no Brasil: 1783–1888; Andrea Jakobsson Estúdio: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Domingues, A.M.V. No trilho da ‘viagem filosófica’ de Alexandre Rodrigues Ferreira: Uma breve história das suas coleções e sua disseminação. Bol. Mus. Para. Emílio Goeldi. Cienc. Hum. 2021, 16, e20200109. [Google Scholar] [CrossRef]
- Linnaeus, C. Species Plantarum, 2nd ed.; Imprensis Direct. Laurentii Salvii, Holmiae: Stockholm, Sweden, 1763; p. 1352. [Google Scholar]
- Jacquin, N.J. Enumeratio Systematica Plantarum; Theodorum Haak: Leiden, The Netherlands, 1760. [Google Scholar] [CrossRef]
- Paquette, G. Imperial Portugal in the Age of Atlantic Revolutions: The Luso-Brazilian World, c.1770–1850; Cambridge University Press: Cambridge, MA, USA; New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Ossenbach, C. Orchids in the era of Grigory von Langsdorff: Two golden decades in the history of the botanical exploration of Brazil 1813–1830. Lankesteriana 2018, 18, 111–149. [Google Scholar] [CrossRef]
- Urban, I. Index Familiarum. In Flora Brasiliensis; Martius, C.F.P., Eichler, A.W., Urban, I., Eds.; R. Oldenbourg: Munich/Leipzig, Germany, 1906; pp. 239–268. [Google Scholar]
- Lindley, J. Collectanea Botanica; or, Figures and Botanical Illustrations of Rare and Curious Exotic Plants, t27; Richard and Arthur Taylor: London, UK, 1821. [Google Scholar]
- Lindley, J. Collectanea Botanica 33; or, Figures and Botanical Illustrations of Rare and Curious Exotic Plants, t33; Richard and Arthur Taylor: London, UK, 1824. [Google Scholar]
- Martius, C.F.P.; Eschweiler, F.G.; Nees von Esenbeck, C.G.D. Flora Brasiliensis seu Enumeratio Plantarum in Brasilia; J.G. Cottae (Cotta): Stuttgart/Tübingen, Germany, 1833; Volume I, Pars prior. [Google Scholar]
- Urban, I. Vitae Itineraque Collectorum Botanicorum, Notae Collaboratorum Biographicae. In Flora Brasiliensis; Martius, C.F.P., Eichler, A.W., Urban, I., Eds.; R. Oldenbourg: Munich/Leipzig, Germany, 1906; pp. 1–212. [Google Scholar]
- Mori, S.A.; Ferreira, F.C. A distinguished Brazilian botanist, João Barbosa Rodrigues (1842–1909). Brittonia 1987, 39, 73–85. [Google Scholar] [CrossRef]
- Costa, D.L.L.; Curty, M.F.N.S.; Barberena, F.F.V.A. João Barbosa Rodrigues: A compilation of his remarkable trajectory as a scientist and main contributions to orchidology. Rodriguésia 2022, 73, e00222022. [Google Scholar] [CrossRef]
- Sprunger, S.; Cribb, P.; Toscano de Brito, A.L.V. João Barbosa Rodrigues, Iconographie des Orchidées du Brésil; Reinhardt: Basel, Switzerland, 1996; Volumes 1–2. [Google Scholar]
- Cogniaux, A. Orchidaceae. In Flora Brasiliensis; Martius, C.F.P., Eichler, A.W., Urban, I., Eds.; Friedrich Fleischer: Munich/Leipzig, Germany, 1893–1896; pp. 1–672. Volumen 3, Pars 4. tt. 1–133. [Google Scholar]
- Cogniaux, A. Orchidaceae. In Flora Brasiliensis; Martius, C.F.P., Eichler, A.W., Urban, I., Eds.; R. Oldenbourg: Munich/Leipzig, Germany, 1898–1902; pp. 1–664. Volumen 3, Pars 5, tt. 1–119. [Google Scholar]
- Cogniaux, A. Orchidaceae. In Flora Brasiliensis; Martius, C.F.P., Eichler, A.W., Urban, I., Eds.; R. Oldenbourg: Munich/Leipzig, Germany, 1904–1906; pp. 1–604. Volumen 3, Pars 6, tt. 1–120. [Google Scholar] [CrossRef]
- Hoehne, F.C. Orchidaceas. In Flora Brasilica; Hoehne, F.C., Ed.; Secretaria da Agricultura, Indústria e Comércio de São Paulo/“Graphicars” Romiti & Lanzara: São Paulo, Brazil, 1940; pp. 1–254. Volume XII, 1 (1–12). [Google Scholar]
- Hoehne, F.C. Orchidaceas. In Flora Brasilica; Hoehne, F.C., Ed.; Secretaria da Agricultura, Indústria e Comércio de São Paulo/“Graphicars” F. Lanzara: São Paulo, Brazil, 1942; pp. 1–218. Volume XII, 6 (97–114). [Google Scholar]
- Hoehne, F.C. Orchidaceas. In Flora Brasilica; Hoehne, F.C., Ed.; Secretaria da Agricultura, Indústria e Comércio de São Paulo/“Graphicars” F. Lanzara: São Paulo, Brazil, 1945; pp. 1–389. Volume XII, 2 (13–43). [Google Scholar]
- Hoehne, F.C. Orchidaceas. In Flora Brasilica; Hoehne, F.C., Ed.; Instituto de Botânica: São Paulo, Brazil, 1953; pp. 1–397. Volume XII, 7 (115–147). [Google Scholar]
- Hoehne, F.C.; Kuhlmann, J.G. Índice Bibliográfico e Numérico das Plantas Colhidas Pela Comissão Rondon: Ou Comissão de Linhas Telegráficas, Estratégicas de Mato-Grosso ao Amazonas, de 1908 até 1923; São Paulo: Instituto de Botânica: São Paulo, Brazil, 1951. [Google Scholar]
- Pabst, G.F.J.; Dungs, F. Orchidaceae Brasilienses; Band I; Kurt Schmersow: Hildesheim, Germany, 1975. [Google Scholar]
- Pabst, G.F.J.; Dungs, F. Orchidaceae Brasilienses; Band II; Kurt Schmersow: Hildesheim, Germany, 1977. [Google Scholar]
- Barros, F. Notas taxonômicas para espécies brasileiras dos gêneros Epidendrum, Platystele, Pleurothallis e Scaphyglottis (Orchidaceae). Acta Bot. Bras. 1996, 10, 139–151. [Google Scholar] [CrossRef]
- Barros, F.; Vinhos, F.; Rodrigues, V.T.; Barberena, F.F.V.A.; Fraga, C.N. Orchidaceae. In Catálogo de Plantas e Fungos do Brasil; Forzza, R.C., Leitman, P.M., Costa, A., Carvalho, A.A.D., Jr., Peixoto, A.L., Walter, B.M.T., Bicudo, C., Zappi, D., Costa, D.P.D., Lleras, E., Eds.; Andrea Jakobsson/Instituto de Pesquisa Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2010; Volume 2, pp. 1344–1426. [Google Scholar] [CrossRef]
- BFG (The Brazil Flora Group). Growing knowledge: An overview of seed plant diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Delprete, P.G.; Jardim, J.G. Systematics, taxonomy and floristics of Brazilian Rubiaceae: An overview about the current status and future challenges. Rodriguésia 2012, 63, 101–128. [Google Scholar] [CrossRef]
- Goldenberg, R.; Baumgratz, J.F.A.; Souza, M.L.D.R. Taxonomia de Melastomataceae no Brasil: Retrospectiva, perspectivas e chave de identificação para os gêneros. Rodriguésia 2012, 63, 145–161. [Google Scholar] [CrossRef]
- Secco, R.S.; Cordeiro, I.; Senna-Vale, L.D.; Sales, M.F.; Lima, L.R.; Medeiros, D.; Haiad, B.S.; Oliveira, A.S.; Caruzo, M.B.R.; Carneiro-Torres, D.; et al. An overview of recent taxonomic studies on Euphorbiaceae s.l. in Brazil. Rodriguésia 2012, 63, 227–242. [Google Scholar] [CrossRef]
- Alves, M.; Trovó, M.; Forzza, R.C.; Viana, P. Overview of the systematics and diversity of Poales in the Neotropics with emphasis on the Brazilian flora. Rodriguésia 2015, 66, 305–328. [Google Scholar] [CrossRef][Green Version]
- Morim, M.P.; Filardi, F.L.R.; Sartori, A.L.B.; Simon, M.F.; Iganci, J.R.V.; Lewis, G.P.; Lima, H.C.; Lughadha, E.N.; Fernandes, M.F.; Queiroz, L.P.; et al. Leguminosae in Brazil: Biogeography, endemism, and conservation priorities. Braz. J. Bot. 2024, 47, 1245–1271. [Google Scholar] [CrossRef]
- Sobral, M.; Stehmann, J.R. An analysis of new angiosperm species discoveries in Brazil (1990–2006). Taxon 2009, 58, 227–232. [Google Scholar] [CrossRef]
- Endara, L.; Hirtz, A.; Jost, L.; Reynolds, A.; Neubig, K.; Hágsater, E.; Phillip, C.; Simpson, N.; Cornejo, X. Orchidaceae. In Libro rojo de las Plantas Endémicas del Ecuador, 2nd ed.; León-Yánez., S., Valencia., R., Pitman, N., Endara, L., Ulloa-Ulloa, C., Navarrete, H., Eds.; Quito: Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2017; Available online: https://biowebecuador.azurewebsites.net/floraweb/librorojo/ListaEspeciesPorFamilia/500330. (accessed on 12 November 2025).
- Mustaqim, W.A.; Saputra, R.; Al Farishy, D.D.; Tianara, A.; Ahmad, R.P.P.; Kartonegoro, A.; Kartonegoro, A.; Yudistira, Y.R.; Sitepu, B.S.; Randi, A.; et al. Digital Flora of Indonesia. Facilitated by Tumbuhan Asli Nusantara Foundation. Available online: https://www.indonesiaplants.org/angiosperms/orchidaceae/ (accessed on 20 January 2025).
- Valdivieso, P.O.; Viveros, P.; Luer, C.A.; Celis, M.; Hágsater, E.; Blanco, M.; Dueñas, H.C.; Gerlach, G.; van der Berg, C.; Fernández-Concha, G.C.; et al. Orchidaceae. In Catálogo de Plantas y Líquenes de Colombia; Bernal, R., Gradstein, S.R., Celis, M., Eds.; Editorial UNAL: Bogotá, Colombia, 2016; pp. 1727–2026. Volumen II. [Google Scholar]
- Guedes, J.J.M.; Moura, M.R.; Jardim, L.; Diniz-Filho, J.A.F. Global patterns of taxonomic uncertainty and its impacts on biodiversity research. Syst. Biol. 2025, syaf010. [Google Scholar] [CrossRef]
- Pupulin, F.; Bogarín, D.; Karremans, A.P. The Lankester catalogue of Costa Rican Orchidaceae. Lankesteriana 2023, 23, 1–254. [Google Scholar] [CrossRef]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 20 March 2025).
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Laurance, W.F.; Cochrane, M.A.; Bergen, S.; Fearnside, P.M.; Delamônica, P.; Barber, C.; D’angelo, S.; Fernandes, T. The future of the Brazilian Amazon. Science 2001, 291, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Lucena, J.; Monteiro, S.S.N.; Mendonça Júnior, J.P.V.; Costa, J.C.R.; Coutinho, M.M. Transparência na Amazônia: Uma revisão a partir das publicações internacionais. NAU Soc. 2022, 13, 854–870. [Google Scholar]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; Fonseca, G.A.B.; Olivieri, S. Biodiversity Hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Veloso, H.P.; Rangel-Filho, A.L.R.; Lima, J.C.A. Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal; IBGE: Rio de Janeiro, Brazil, 1991. [Google Scholar]
- Cardoso, D.; Särkinen, T.; Alexander, S.; Amorim, A.M.; Bittrich, V.; Celis, M.; Daly, D.C.; Fiaschi, P.; Funk, V.A.; Giacomin, L.L.; et al. Amazon plant diversity revealed by a taxonomically verified species list. Proc. Natl. Acad. Sci. USA 2017, 114, 10695–10700. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Mapa de Biomas do Brasil 1: 5.000.000 (Primeira Aproximação); IBGE/MMA: Brasília, Brazil, 2004. [Google Scholar]
- Stehmann, J.R.; Forzza, R.C.; Salino, A.; Sobral, M.; Costa, D.P.; Kamino, L.H.Y. Plantas da Floresta Atlântica; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brasil, 2009. [Google Scholar]
- Hopkins, M.J.G. Modelling the known and unknown plant biodiversity of the Amazon Basin. J. Biogeogr. 2007, 34, 1400–1411. [Google Scholar] [CrossRef]
- Luz, A.L.S.; Costa, A.A.S.; Moreira, C.R.; Barberena, F.F.V.A. Vascular epiphytes in the Amazon: Main gaps, limitations and perspectives for studies on the subject. Acta Bot. Bras. 2023, 37, e20220311. [Google Scholar] [CrossRef]
- Luz, A.L.S.; Costa, D.L.L.; Pacheco, J.R.V.; Barberena, F.F.V.A. Orchidaceae in the state of Pará, Brazilian Amazon: An updated checklist reveals underestimated species richness. Acta Bot. Bras. 2024, 38, e20240011. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Silva, J.B.F.; Bianchetti, L.B. The Genus Habenaria (Orchidaceae) in the Brazilian Amazon. Rev. Bras. Bot. 2008, 31, 105–134. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Loyola, R.D.; Raia, P.; Mooers, A.O.; Bini, L.M. Darwinian shortfalls in biodiversity conservation. Trends Ecol. Evol. 2013, 28, 689–695. [Google Scholar] [CrossRef]
- Davis, C.C. The herbarium of the future. Trends Ecol. Evol. 2023, 38, 412–423. [Google Scholar] [CrossRef]
- Ranta, P.; Blom, T.; Niemelä, J.; Joensuu, E.; Siitonen, M. The fragmented Atlantic rain forest of Brazil: Size, shape and distribution of forest fragments. Biodivers. Conserv. 1998, 7, 385–403. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Vieira, D.S.L. Frans Post, a paisagem e o exótico: O imaginário do Brasil na cultura da Holanda do século XVII. In Brasil Holandês: História, Memória e Patrimônio Compartilhado; Vieira, H.C., Galvão, N.N.P., Silva, L.D., Eds.; Alameda: São Paulo, Brasil, 2012; pp. 91–122. [Google Scholar]
- Smidt, E.C.; Toscano de Brito, A.L.V.; Martins, A.C.; Royer, C.A.; Whitten, W.M.; Chase, M.W. Phylogenetics, biogeography and character evolution in the Ornithocephalus clade (Orchidaceae, Oncidiinae). Bot. J. Linn. Soc. 2018, 188, 339–354. [Google Scholar] [CrossRef]
- Salazar, G.A.; Batista, J.A.N.; Cabrera, L.I.; van den Berg, C.; Whitten, W.M.; Smidt, E.C.; Buzatto, C.R.; Singer, R.B.; Gerlach, G.; Jiménez-Machorro, R.; et al. Phylogenetic systematics of subtribe Spiranthinae (Orchidaceae: Orchidoideae: Cranichideae) based on nuclear and plastid DNA sequences of a nearly complete generic sample. Bot. J. Linn. Soc. 2018, 186, 273–303. [Google Scholar] [CrossRef]
- Costa, L.P. The historical bridge between the Amazon and the Atlantic Forest of Brazil: A study of molecular phylogeography with small mammals. J. Biogeogr. 2003, 30, 71–86. [Google Scholar] [CrossRef]
- Batalha-Filho, H.; Fjeldså, J.; Fabre, P.H.; Miyaki, C.Y. Connections between the Atlantic and the Amazonian Forest avifaunas represent distinct historical events. J. Ornithol. 2013, 154, 41–50. [Google Scholar] [CrossRef]
- Thomé, M.T.C.; Sequeira, F.; Brusquetti, F.; Carstens, B.; Haddad, C.F.B.; Rodrigues, M.T.; Alexandrino, J. Recurrent connections between Amazon and Atlantic forests shaped diversity in Caatinga four-eyed frogs. J. Biogeogr. 2016, 43, 1045–1056. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Cordeiro, J.M.P.; Felix, L.P.; Lemes, P.; Viruel, J.; Alves, M.; Chase, M.W.; van den Berg, C. The role of Quaternary glaciations in shaping biogeographic patterns in a recently evolved clade of South American epiphytic orchids. Bot. J. Linn. Soc. 2022, 199, 252–266. [Google Scholar] [CrossRef]
- Brieger, F.G. Geographic distribution and phylogeny of orchids. In Proceedings of the 3rd World Orchid Conference, London, UK, 30 May–22 June 1960; pp. 328–333. [Google Scholar]
- Pessoa, E.M.; Cordeiro, J.M.P.; Felix, L.P.; Almeida, E.M.; Costa, L.; Nepomuceno, A.; Souza, G.; Chase, M.W.; Alves, M.; van den Berg, C. Too many species: Morphometrics, molecular phylogenetics and genome structure of a Brazilian species complex in Epidendrum (Laeliinae; Orchidaceae) reveal fewer species than previously thought. Bot. J. Linn. Soc. 2021, 195, 161–188. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Alves, M.; Alves-Araújo, A.; Palma-Silva, C.; Pinheiro, F. Integrating different tools to disentangle species complexes: A case study in Epidendrum (Orchidaceae). Taxon 2012, 61, 721–734. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Maciel, J.R.; Alves, M. Campylocentrum brevifolium (Lindl.) E.M. Pessoa & M. Alves, a neglected and critically endangered orchid from the Atlantic Forest of Brazil. Kew Bull. 2015, 70, 43. [Google Scholar] [CrossRef]
- Fay, M.F. Orchid conservation: How can we meet the challenges in the twenty-first century? Botanical Studies 2018, 59, 16. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Linn. Soc. 2005, 84, 1–54. [Google Scholar] [CrossRef]
- Hartley, S.; Kunin, W.E. Scale dependency of rarity, extinction risk, and conservation priority. Conserv. Biol. 2003, 17, 1559–1570. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and evolution of flowering plants of the Caatinga domain. In Caatinga: The Largest Tropical Dry Forest Region in South America; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 23–63. [Google Scholar]
- Toscano-de-Brito, A.L.V.; Cribb, P. Orquídeas da Chapada Diamantina; Nova Fronteira: São Paulo, Brazil, 2005. [Google Scholar]
- Pessoa, E.M.; Alves, M. Orchidaceae em afloramentos rochosos do estado de Pernambuco, Brasil. Rodriguésia 2014, 65, 717–734. [Google Scholar] [CrossRef]
- Pinheiro, F.; Cozzolino, S.; Draper, D.; Barros, F.; Felix, L.P.; Fay, M.F.; Palma-Silva, C. Rock outcrop orchids reveal the genetic connectivity and diversity of inselbergs of northeastern Brazil. BMC Ecol. Evol. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, C.F.; Borba, E.L.; Resende-Moreira, L.C.; Smidt, E.C.; Knowles, L.L. Geographic isolation alone does not explain divergence of a group of orchid species across Brazil’s campos rupestres sky-islands. Evolution 2023, 77, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.J.; Leducq, J.-B.; Mallet, J. What is speciation? PLoS Genet. 2016, 12, e1005860. [Google Scholar] [CrossRef]
- Fišer, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef]
- Assis, F.N.M.; Souza, B.C.Q.; Medeiros-Neto, E.; Pinheiro, F.; Silva, A.E.B.; Felix, L.P. Karyology of the genus Epidendrum (Orchidaceae: Laeliinae) with emphasis on subgenus Amphiglottium and chromosome number variability in Epidendrum secundum. Bot. J. Linn. Soc. 2013, 172, 329–344. [Google Scholar] [CrossRef]
- Werneck, F.P. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quat. Sci. Rev. 2011, 30, 1630–1648. [Google Scholar] [CrossRef]
- Guillory, W.X.; Magalhães, F.M.; Coelho, F.E.A.; Bonatelli, I.A.S.; Palma-Silva, C.; Moraes, E.M.; Garda, A.A.; Burbrink, F.T.; Gehara, M. Geoclimatic drivers of diversification in the largest arid and semi-arid environment of the Neotropics: Perspectives from phylogeography. Mol. Ecol. 2024, 33, e17431. [Google Scholar] [CrossRef]
- Walter, B.M.T. Fitofisionomias do Bioma Cerrado: Síntese Terminológica e Relações Florísticas. Ph.D. Dissertation, Universidade de Brasília, Brasília, Brazil, 2006. [Google Scholar]
- Haridasan, M. Nutritional adaptations of native plants of the Cerrado biome in acid soils. Braz. J. Plant Physiol. 2008, 20, 183–195. [Google Scholar] [CrossRef]
- Romero-González, G.A.; Batista, J.A.N.; Bianchetti, L.B. A synopsis of the genus Cyrtopodium (Catasetinae: Orchidaceae). Harv. Pap. Bot. 2008, 13, 189–206. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Proite, K.; Bianchetti, L.B. Descriptions and phylogenetic relationships of four new species and a new name of Habenaria (Orchidaceae) from the Cerrado and Campos Rupestres of Brazil. Plant Syst. Evol. 2017, 303, 873–899. [Google Scholar] [CrossRef]
- Giulietti, A.M.; Pirani, J.R. Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In Proceedings of the Workshop on Neotropical Distribution Patterns, Rio de Janeiro, Brazil, 12–16 January 1987; Vanzolini, P.E., Heyer, W.R., Eds.; Academia Brasileira de Ciências: Rio de Janeiro, Brazil, 1988; pp. 39–69. [Google Scholar]
- Jenny, R. Lueckelia, a new genus in the Stanhopeinae. Aust. Orchid. Rev. 1999, 64, 14–16. [Google Scholar]
- Petini-Benelli, A.; Pessoa, E.M. A new non-Andean South American Chysis (Bletiinae-Orchidaceae) with pale flowers. Phytotaxa 2019, 420, 84–88. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Marín-Pérez, L.; Pessoa, E.M.; Alves, M. Lista comentada de Orchidaceae en Uruguay y su distribución en ambientes y eco-regiones. Lankesteriana 2020, 20, 359–394. [Google Scholar] [CrossRef]
- Marín-Pérez, L.; Pessoa, E.M.; Buzatto, C.R.; Alves, M. Spiranthinae (Orchidaceae - Cranichideae) from Uruguay: Taxonomy and distribution. Acta Bot. Bras. 2022, 36, e2021abb0333. [Google Scholar] [CrossRef]
- Nunes da Cunha, C.; Bergier, I.; Tomas, W.M.; DamascenoJúnior, G.A.; Santos, S.A.; Assunçao, V.A.; Sartori, A.L.B.; Pott, A.; Arruda, E.C.; Garcia, A.S.; et al. Classificação dos macrohabitat do Pantanal Brasileiro: Atualização para políticas públicas e manejo de áreas protegidas. Biodiv. Brasil. 2023, 13, 1–26. [Google Scholar] [CrossRef]
- Por, F.D. The Pantanal of Mato Grosso (Brazil); World’s Largest Wetlands; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Barros, F.; Hall, C.F.; Paiva Neto, V.B.; Batista, J.A.N. Check-list das Orchidaceae do Estado de Mato Grosso do Sul, Brasil. Iheringia Sér. Bot. 2018, 73, 287–296. [Google Scholar] [CrossRef]
- Lara, M.; Pessoa, E.M. Synopsis of Epidendrum (Laeliinae) from the state of Mato Grosso, Brazil: Taxonomy and distribution. Lankesteriana 2025, 25, 21–42. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Huang, M.-Z.; Huang, W.-C.; Liu, D.-K.; Zhang, D.; Hu, H.; Downing, J.L.; Liu, Z.-J.; Ma, H. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. J. Integr. Plant. Biol. 2023, 65, 1204–1225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escobar, O.A.; Bogarín, D.; Przelomska, N.A.S.; Ackerman, J.D.; Balbuena, J.A.; Bellot, S.; Bühlmann, R.P.; Cabrera, B.; Cano, J.A.; Charitonidou, M.; et al. The origin and speciation of orchids. New Phytol. 2024, 242, 700–716. [Google Scholar] [CrossRef]
- Pansarin, E.R.; Menezes, E.L.F. A new remarkable Vanilla (Orchidaceae) endemic from Brazilian campos rupestres: Their phylogenetic position and evolutionary relationships among Neotropical congeners. Phytokeys 2023, 227, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, E.M.; Christenhusz, M.J.M. Molecular phylogenetics provides support for the current circumscription of Rodriguezia (Oncidiinae-Orchidaceae) and for a new infrageneric classification of the genus. Plant Syst. Evol. 2024, 310, 46. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Castro, C.; Sambin, A.; Cruz-Lustre, G.; Pansarin, E.R. Clarifying the identity of the Cleistes rosea complex (Orchidaceae) based on integrative taxonomy. Syst. Biodivers. 2023, 21, 2207575. [Google Scholar] [CrossRef]
- Karremans, A.P.; Chinchilla, I.F.; Rojas-Alvarado, G.; Cedeño-Fonseca, M.; Damián, A.; Léotard, G. A reappraisal of Neotropical Vanilla. With a note on taxonomic inflation and the importance of alpha taxonomy in biological studies. Lankesteriana 2020, 20, 395–497. [Google Scholar] [CrossRef]
- Pansarin, E.R. Vanilla lindmaniana and V. palmarum (Orchidaceae) are distinct allopatric species. Plant Ecol. Evol. 2025, 158, 53–62. [Google Scholar] [CrossRef]
- Flanagan, N.S.; Navia-Samboni, A.; Vargas, W.G.; Díaz-Rueda, D.M.; Cruz, J.Y.S.R.; Pansarin, E.; Castaño, A. Some taxonomic clarifications in Vanilla subgenus Xanata (Orchidaceae), and the resurrection of Vanilla calyculata. Kew Bull. 2025, 80, 83–96. [Google Scholar] [CrossRef]
- Pansarin, E.R. Systematics of the Vanilla chamissonis complex (Orchidaceae): A study based on integrative taxonomy. Plant Ecol. Evol. 2025, 158, 260–278. [Google Scholar] [CrossRef]
- Pansarin, E.R.; Miranda, M.R. A new species of Vanilla (Orchidaceae: Vanilloideae) from Brazil. Phytotaxa 2016, 267, 84–88. [Google Scholar] [CrossRef]
- Pansarin, E.R. Taxonomic notes on Vanilleae (Orchidaceae: Vanilloideae): Vanilla dietschiana, a rare south American taxon transferred from Dictyophyllaria. Selbyana 2010, 30, 198–202. Available online: https://www.jstor.org/stable/41760364 (accessed on 12 November 2025).
- Pansarin, E.R. Rediscovery and revalidation of the Brazilian endemic Vanilla schwackeana Hoehne (Orchidaceae): Its distribution and phylogenetic position. Plant Ecol. Evol. 2024, 157, 32–41. [Google Scholar] [CrossRef]
- Pansarin, E.R. Sistemática Filogenética e Biologia Floral de Pogoniinae Sul-Americanas, e Revisão Taxonômica e Análise das Ceras Epicuticulares do Gênero Cleistes Rich. ex Lindl. (Orchidaceae). Ph.D. Dissertation, Universidade Estadual de Campinas, Campinas, Brazil, 2005. [Google Scholar]
- Salazar, G.A.; Batista, J.A.N.; Meneguzzo, T.E.C.; Cabrera, L.I.; Figueroa, C.; Calvillo-Canadell, L.; Vale, A.A.; Jiménez-Machorro, R. Polyphyly of Mesadenus (Orchidaceae, Spiranthinae) and a new genus from the Espinhaço Range, southeastern Brazil. Syst. Bot. 2019, 44, 282–296. [Google Scholar] [CrossRef]
- Borba, E.L.; Salazar, G.A.; Mazzoni-Viveiros, S.; Batista, J.A.N. Phylogenetic position and floral morphology of the Brazilian endemic, monospecific genus Cotylolabium: A sister group for the remaining Spiranthinae (Orchidaceae). Bot. J. Linn. Soc. 2014, 175, 29–46. [Google Scholar] [CrossRef]
- Guimarães, L.R.S.; Salazar, G.; Barros, F. Lectotypifications and taxonomic notes in the Stenorrhynchos clade (Spiranthinae, Orchidaceae). Phytotaxa 2019, 394, 111–117. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Meneguzzo, T.E.C.; Salazar, G.A.; Bianchetti, L.B.; Ramalho, A.J. Phylogenetic placement, taxonomic revision, and a new species of Nothostele (Orchidaceae), an enigmatic genus endemic to the cerrado of central Brazil. Bot. J. Linn. Soc. 2011, 165, 348–363. [Google Scholar] [CrossRef]
- Meneguzzo, T.E.C.; Carvalho, B.M.; Batista, J.A.N.; Gomes, S.M.; Proença, C.E.B. Brachystele guayanensis is a Cyclopogon (Orchidaceae): Notes on its biology and taxonomy. Phytotaxa 2024, 658, 109–119. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Baumgratz, J.F.A.; Meneguzzo, T.E.C. Veyretia is a new synonym of Cyclopogon (Orchidaceae). Phytotaxa 2025, 715, 159–163. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Bianchetti, L.B.; Miranda, Z.J.G. A Revision of Habenaria Section Macroceratitae (Orchidaceae) in Brazil. Brittonia 2006, 58, 10–41. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Bianchetti, L.B.; González-Tamayo, R.; Figueroa, X.M.C.; Cribb, P.J. A Synopsis of New World Habenaria (Orchidaceae) I. Harv. Pap. Bot. 2011, 16, 1–47. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Bianchetti, L.B.; González-Tamayo, R.; Figueroa, X.M.C.; Cribb, P.J. A Synopsis of New World Habenaria (Orchidaceae) II. Harv. Pap. Bot. 2011, 16, 233–273. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Borges, K.S.; Faria, M.W.F.; Proite, K.; Ramalho, A.J.; Salazar, G.A.; van den Berg, C. Molecular phylogenetics of the species-rich genus Habenaria (Orchidaceae) in the New World based on nuclear and plastid DNA sequences. Mol. Phylogenet. Evol. 2013, 67, 95–109. [Google Scholar] [CrossRef]
- Pedron, M.; Buzatto, C.R.; Ramalho, A.J.; Carvalho, B.M.; Radin, J.A.; Singer, R.B.; Batista, J.A.N. Molecular phylogenetics and taxonomic revision of Habenaria section Pentadactylae (Orchidaceae, Orchidinae). Bot. J. Linn. Soc. 2014, 175, 47–73. [Google Scholar] [CrossRef]
- Cruz-Lustre, G.; Castro, C.; Borba, E.L.; Batista, J.A.N. Phylogenetics and taxonomy of Habenaria sect. Micranthae (Orchidaceae), with the description of an overlooked new species from the Espinhaço mountain range, Eastern Brazil. Syst. Biodiv. 2022, 20, 1–20. [Google Scholar] [CrossRef]
- Lau, B.L.; Batista, J.A.N.; Massensini-Junior, A.; Whitten, W.M.; Borba, E.L. Unravelling the Habenaria repens (Orchidaceae) complex in Brazil: A biosystematic and molecular phylogenetic approach. Bot. J. Linn. Soc. 2021, 197, 229–248. [Google Scholar] [CrossRef]
- Singer, R.B.; Buzatto, C.R.; Sanguinetti, A.; Nervo, M.H. Found again: The extremely rare Codonorchis canisioi (Orchidaceae: Codonorchideae) reappears after being missing for 78 years. Plant Syst. Evol. 2018, 304, 1157–1663. [Google Scholar] [CrossRef]
- Engels, M.E. Estudos Taxonômicos da Subtribo Goodyerinae (Orchidaceae: Orchidoideae) No Brasil. Ph.D. Dissertation, Universidade Federal do Paraná, Curitiba, Brazil, 2025. [Google Scholar]
- Engels, M.E.; Barros, F.; Smidt, E.C.S. A subtribo Goodyerinae (Orchidaceae: Orchidoideae) no estado do Paraná, Brasil. Rodriguésia 2016, 67, 917–952. [Google Scholar] [CrossRef]
- Azevedo, C.O.; van den Berg, C.; Barros, F. A Revision of Prescottia (Orchidaceae: Orchidoideae, Cranichideae). Phytotaxa 2014, 178, 233–286. [Google Scholar] [CrossRef]
- Buzatto, C.R.; Sanguinetti, A.; Romero-González, G.A.; van den Berg, C.; Singer, R.B. A taxonomic synopsis of Brazilian Chloraeinae (Orchidaceae: Orchidoideae). Phytotaxa 2014, 158, 1–22. [Google Scholar] [CrossRef]
- Salazar, G.A.; van den Berg, C.; Popovkin, A. Phylogenetic relationships of Discyphus scopulariae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences: Evidence supporting recognition of a new subtribe, Discyphinae. Phytotaxa 2014, 173, 127–139. [Google Scholar] [CrossRef][Green Version]
- Salazar, G.A.; Octaviano-Landa, V.I.; Jiménez-Machorro, R.; Fragoso-Martínez, I.; Clase, T.; Ackerman, J.D. Natural history of the often-misunderstood Govenia utriculata (Orchidaceae): Discovery of a Mexican population upsets West Indies endemism. Phytotaxa 2021, 487, 195–204. [Google Scholar] [CrossRef]
- van den Berg, C.; Higgins, W.E.; Dressler, R.L.; Whitten, W.M.; Soto-Arenas, M.A.; Chase, M.W. A phylogenetic study of Laeliinae (Orchidaceae) based on combined nuclear and plastid DNA sequences. Ann. Bot. 2009, 104, 417–430. [Google Scholar] [CrossRef]
- Vieira, T.L.; Salazar, G.A.; van den Berg, C. Phylogeny of Prosthechea (Laeliinae, Orchidaceae) based on nrITS and plastid DNA sequences: Reassessing the lumper-splitter debate and shedding light on the evolution of this Neotropical genus. Taxon 2024, 73, 142–160. [Google Scholar] [CrossRef]
- Bastos, C.A.; Meneguzzo, T.E.C.; van den Berg, C. A Taxonomic Revision of the Brazilian Species of Encyclia (Orchidaceae: Epidendroideae: Epidendreae). Phytotaxa 2018, 342, 1–84. [Google Scholar] [CrossRef]
- Gonçalves, G.F.; Mauad, A.V.S.R.; Taques, G.; Smidt, E.C.; Barros, F. Molecular and morphological phylogenetic analysis and taxonomic revision of the genus Orleanesia (Laeliinae, Epidendroideae, Orchidaceae). Phytotaxa 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Menini Neto, L.; Forzza, R.C.; van den Berg, C. Taxonomic revision of Pseudolaelia Porto & Brade (Laeliinae, Orchidaceae). Acta Bot. Bras. 2013, 27, 418–435. [Google Scholar] [CrossRef][Green Version]
- Vieira-Silva, T.L. Filogenia Molecular de Prosthechea (Orchidaceae: Laeliinae) e Revisão Taxonômica das Espécies Brasileiras. Ph.D. Dissertation, Universidade Estadual de Feira de Santana, Feira de Santana, Brazil, 2021. [Google Scholar]
- van den Berg, C. Reaching a compromise between conflicting nuclear and plastid phylogenetic trees: A new classification for the genus Cattleya (Epidendreae; Epidendroideae; Orchidaceae). Phytotaxa 2014, 186, 75–86. [Google Scholar] [CrossRef]
- Karremans, A.P. Genera Pleurothallidinarum: An updated phylogenetic overview of Pleurothallidinae. Lankesteriana 2016, 16, 219–241. [Google Scholar] [CrossRef]
- Chiron, G.R.; van den Berg, C. Révision taxinomique du genre Acianthera (Orchidaceae, Pleurothallidinae). Richardiana 2012, 12, 59–77. [Google Scholar]
- Rodrigues, V.T.; Smidt, E.C.; Bolson, M.; Barros, F. Phylogeny of Acianthera sect. Pleurobotryae (Orchidaceae: Pleurothallidinae), an endemic group of the Atlantic Forest. Braz. J. Bot. 2017, 40, 811–817. [Google Scholar] [CrossRef]
- Gutiérrez-Morales, N.G.; Toscano de Brito, A.L.V.; Mauad, A.V.S.R.; Smidt, E.C. Molecular phylogeny and biogeography of Pabstiella (Pleurothallidinae: Orchidaceae) highlight the importance of the Atlantic Rainforest for speciation in the genus. Bot. J. Linn. Soc. 2021, 195, 568–587. [Google Scholar] [CrossRef]
- Rodrigues, V.T.; Smidt, E.C.; Barros, F. Revisão taxonômica de Acianthera sect. Pleurobotryae (Orchidaceae, Pleurothallidinae). Hoehnea 2015, 42, 615–627. [Google Scholar] [CrossRef][Green Version]
- Imig, D.C.; Toscano de Brito, A.L.V.; Smidt, E.C. The genus Dryadella (Orchidaceae, Pleurothallidinae) in Brazil. Rodriguésia 2024, 75, e00442023. [Google Scholar] [CrossRef]
- Melo, M.C.; Borba, E.L. Morphological variability in rupicolous species of the Acianthera prolifera complex (Orchidaceae) occurring in southeastern Brazil. Plant Syst. Evol. 2011, 293, 135–145. [Google Scholar] [CrossRef]
- Borba, E.L.; Semir, J. Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: A multiple population approach. Ann. Bot. 2001, 88, 75–88. [Google Scholar] [CrossRef]
- Borba, E.L.; Felix, J.M.; Semir, J.; Solferini, V.N. Pleurothallis fabiobarrosii, a new Brazilian species: Morphological and genetic data with notes on the taxonomy of Brazilian rupicolous Pleurothallis. Lindleyana 2000, 15, 2–9. [Google Scholar]
- Borba, E.L.; Felix, J.M.; Solferini, V.N.; Semir, J. Fly-pollinated Pleurothallis (Orchidaceae) species have high genetic variability: Evidence from isozyme markers. Amer. J. Bot. 2001, 88, 419–428. [Google Scholar] [CrossRef]
- Borba, E.L.; Semir, J.; Shepherd, G.J. Self-incompatibility, inbreeding depression, and crossing potential in five Brazilian Pleurothallis (Orchidaceae) species. Ann. Bot. 2001, 88, 89–99. [Google Scholar] [CrossRef]
- Borba, E.L.; Trigo, J.R.; Semir, J. Variation of diastereoisomeric pyrrolizidine alkaloids in Pleurothallis (Orchidaceae). Biochem. Syst. Ecol. 2001, 29, 45–52. [Google Scholar] [CrossRef]
- Borba, E.L.; Shepherd, G.J.; van den Berg, C.; Semir, J. Floral and vegetative morphometrics of five Pleurothallis (Orchidaceae) species: Correlation with taxonomy, phylogeny, genetic variability and pollination systems. Ann. Bot. 2002, 90, 1–12. [Google Scholar] [CrossRef][Green Version]
- Hágsater, E.; Soto-Arenas, M.A. Epidendrum. In Genera Orchidacearum, Volume 4: Epidendroideae (Part One); Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 236–251. [Google Scholar]
- Pinheiro, F.; Koehler, S.; Corrêa, A.M.; Salatino, M.L.F.; Salatino, A.; de Barros, F. Phylogenetic relationships and infrageneric classification of Epidendrum subgenus Amphiglottium (Laeliinae, Orchidaceae). Plant Syst. Evol. 2009, 283, 165–177. [Google Scholar] [CrossRef]
- Klein, V.P.; Pessoa, E.M.; Demarchi, L.O.; Sader, M.; Piedade, M.T.F. Encyclia, Epidendrum, or Prosthechea? Clarifying the phylogenetic position of a rare Amazonian orchid (Laeliinae-Epidendroideae-Orchidaceae). Syst. Bot. 2019, 44, 297–309. [Google Scholar] [CrossRef]
- Granados-Mendoza, C.; Jost, M.; Hágsater, E.; Magallón, S.; van den Berg, C.; Lemmon, E.M.; Lemmon, A.R.; Salazar, G.; Wanke, S. Target nuclear and off-target plastid hybrid enrichment data inform a range of evolutionary depths in the orchid genus Epidendrum. Front. Plant Sci. 2020, 10, 1761. [Google Scholar] [CrossRef]
- Forster, W. Estudo Taxonômico das Espécies com Folhas Planas a Conduplicadas do Gênero Octomeria, R.Br. (Orchidaceae). Ph.D. Dissertation, Universidade Estadual de Campinas, Campinas, Brazil, 2007. [Google Scholar]
- Johnson, M.G.; Pokorny, L.; Dodsworth, S.; Botigué, L.R.; Cowan, R.S.; Devault, A.; Eiserhardt, W.L.; Epitawalage, N.; Forest, F.; Kim, J.T.; et al. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Syst. Biol. 2019, 68, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Mauad, A.V.S.R.; Vieira, L.N.; Bolson, M.; Baura, V.A.; Balsanelli, E.; Souza, E.M.; Toscano-de-Brito, A.L.V.; Camargo, E. Complete chloroplast genome of Anathallis obovata (Orchidaceae: Pleurothallidinae). Braz. J. Bot. 2019, 42, 345–352. [Google Scholar] [CrossRef]
- Zavala-Páez, M.; Vieira, L.N.; Baura, V.A.; Balsanelli, E.; Souza, E.M.; Cevallos, M.C.; Chase, M.W.; Smidt, E.C. Comparative plastid genomics of Neotropical Bulbophyllum (Orchidaceae; Epidendroideae). Front. Plant Sci. 2020, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Bogarín, D.; Pérez-Escobar, O.A.; Groenenberg, D.; Holland, S.D.; Karremans, A.P.; Lemmon, E.M.; Lemmon, E.R.; Pupulin, F.; Smets, E.; Gravendeel, B. Anchored hybrid enrichment generated nuclear, plastid and mitochondrial markers resolve the Lepanthes horrida (Orchidaceae: Pleurothallidinae) species complex. Mol. Phyl. Evol. 2018, 129, 27–47. [Google Scholar] [CrossRef]
- Mauad, A.V.S.M.; Vieira, L.N.; Baura, V.A.; Balsanelli, E.; de Souza, E.M.; Chase, M.W.; Smidt, E.C. Plastid phylogenomics of Pleurothallidinae (Orchidaceae): Conservative plastomes, new variable markers, and comparative analyses of plastid, nuclear, and mitochondrial data. PLoS ONE 2021, 16, e0256126. [Google Scholar] [CrossRef]
- Romero-González, G.A.; Fernández-Concha, G.C.; Gerlach, G.; Cetzal-Ix, W. Novelties in the orchid flora of Venezuela VIII. Subtribe Eriopsidinae. Eriopsis. Harv. Pap. Bot. 2015, 20, 101–143. [Google Scholar] [CrossRef]
- Christenson, E.A.; Jenny, R. Archivea. Orchids 1996, 65, 496–499. [Google Scholar]
- Batista, J.A.N.; Mota, A.C.M.; Proite, K.; Bianchetti, L.B.; Romero-González, G.A.; Espinoza, H.M.H.; Salazar, G.A. Molecular phylogenetics of Neotropical Cyanaeorchis (Cymbideae, Epidendroideae, Orchidaceae): Geographical rather than morphological similarities plus a new species. Phytotaxa 2014, 156, 251–272. [Google Scholar] [CrossRef]
- Barros, F.; Lourenço, R.A. Synopsis of the Brazilian Orchid Genus Grobya, with the Description of Two New Species. Bot. J. Linn. Soc. 2004, 145, 119–127. [Google Scholar] [CrossRef]
- Monteiro, S.H.N. Revisão Taxonômica e Filogenia do Gênero Galeandra Lindl. (Orchidaceae: Catasetinae). Ph.D. Dissertation, Universidade Estadual de Feira de Santana, Feira de Santana, Brazil, 2007. [Google Scholar]
- Monteiro, S.H.N.; Silva, M.F.F.; Secco, R.S. O gênero Galeandra (Orchidaceae) na Amazônia Brasileira. Acta Amazon. 2009, 39, 21–34. [Google Scholar] [CrossRef]
- Bochorny, T.; Monteiro, S.H.N.; Smidt, E.C. O gênero Galeandra (Orchidaceae: Catasetinae) no Estado do Paraná, Brasil. Rodriguésia 2015, 66, 221–227. [Google Scholar] [CrossRef][Green Version]
- Santos, T.F.; Brito, A.L.V.T.; Smidt, E.C. Octomeria (Orchidaceae: Pleurothallidinae) no estado do Paraná, Brasil. Rodriguésia 2020, 71, e00752018. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Gottschling, M.; Whitten, W.M.; Salazar, G.; Gerlach, G. Sex and the Catasetinae (Darwin’s favourite orchids). Mol. Phyl. Evol. 2016, 97, 1–10. [Google Scholar] [CrossRef]
- Salazar, G.A.; Cabrera, L.I.; Gerlach, G.; Hágsater, E.; Chase, M.W. Phylogenetic relationships in Mormodes (Orchidaceae, Cymbidieae, Catasetinae) inferred from nuclear and plastid DNA sequences and morphology. Phytotaxa 2016, 263, 18–30. [Google Scholar] [CrossRef]
- Mauad, A.V.S.R.; Petini-Benelli, A.; Izzo, T.J.; Smidt, E.C. Phylogenetic and molecular dating analyses of Catasetum (Orchidaceae) indicate a recent origin and artificial subgeneric groups. Braz. J. Bot. 2022, 45, 1235–1247. [Google Scholar] [CrossRef]
- Hall, C.F. Sistemática Filogenética, Citogenética e Taxonomia de Zygopetalinae (Orchidaceae) com Ênfase em Koellensteinia. Ph.D. Dissertation, . Instituto de Botânica, São Paulo, Brazil, 2015. [Google Scholar]
- Meneguzzo, T.E.C.; Baumgratz, J.F.A.; van den Berg, C. Taxonomic studies in the Aganisia complex (Orchidaceae, Zygopetalinae). Phytotaxa 2015, 238, 1–39. [Google Scholar] [CrossRef]
- Meneguzzo, T.E.C. Taxonomic and nomenclatural notes on Zygopetalinae infraspecies (Orchidaceae). Heringeriana 2020, 14, 157–191. [Google Scholar] [CrossRef]
- Barberena, F.F.V.A. Revisão Taxonômica e Filogenia do Gênero Promenaea Lindl. (Orchidaceae). Ph.D. Dissertation, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Blanco, M.A.; Carnevali, G.; Whitten, W.M.; Singer, R.B.; Koehler, S.; Williams, N.H.; Ojeda, I.; Neubig, K.M.; Endara, L. Generic realignments in Maxillariinae (Orchidaceae). Lankesteriana 2007, 7, 515–537. [Google Scholar] [CrossRef]
- Schuiteman, A.; Chase, M.W. A reappraisal of Maxillaria (Orchidaceae). Phytotaxa 2015, 225, 1–78. [Google Scholar] [CrossRef]
- Koehler, S.; Singer, R.B.; Amaral, M.C.E. Taxonomic revision of the Neotropical genus Christensonella (Maxillariinae, Orchidaceae). Bot. J. Linn. Soc. 2012, 168, 449–472. [Google Scholar] [CrossRef]
- Koehler, S.; Amaral, M.C.E. A taxonomic study of the South American genus Bifrenaria Lindl. (Orchidaceae). Brittonia 2004, 56, 314–345. [Google Scholar] [CrossRef]
- Ormerod, P. A synopsis of the genus Xylobium (Orchidaceae: Maxillareae). Harv. Pap. Bot. 2018, 23, 57–75. [Google Scholar] [CrossRef]
- Koehler, S.; Cabral, J.S.; Whitten, W.M.; Williams, N.H.; Singer, R.B.; Neubig, K.M.; Guerra, M.; Souza, A.P.; Amaral, M.C.E. Molecular phylogeny of the Neotropical genus Christensonella (Orchidaceae, Maxillariinae): Species delimitation and insights into chromosome evolution. Ann. Bot. 2008, 102, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Williams, N.H.; Whitten, W.M.; Amaral, M.C.E. Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spacers (ITS) and chloroplast trnL-trnF region. Int. J. Plant Sci. 2002, 163, 1055–1066. [Google Scholar] [CrossRef]
- Chase, M.W.; Williams, N.H.; Faria, A.D.; Neubig, K.M.; Amaral, M.C.E.; Whitten, W.M. Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): An expanded concept of Gomesa and a new genus Nohawilliamsia. Ann. Bot. 2009, 104, 387–402. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Pessoa, E.; Ferreira, A.W.F.; Meneguzzo, T.E.C.; Viana, P.L. Taxonomic revision of Notylia Lindl. (Orchidaceae: Oncidiinae) from Brazil. Phytotaxa, 2025; in press. [Google Scholar]
- Cetzal-Ix, W.; Carnevali, G.; Romero-González, G. Synopsis of the Trichocentrum clade (Orchidaceae, Oncidiinae). Harv. Pap. Bot. 2016, 21, 141–169. [Google Scholar] [CrossRef]
- Camelo, A.E., Jr.; Ferreira, A.W.C.; Andrade, I.M.; Mayo, S.J.; Nollet, F.; Silva, J.L.; Barros, M.C.; Fraga, E.; Pessoa, E.M. Species delimitation in the Trichocentrum cepula (Oncidiinae, Orchidaceae) complex: A multidisciplinary approach. Syst. Biodiv. 2022, 20, 1–18. [Google Scholar] [CrossRef]
- Royer, C.A.; Toscano de Brito, A.L.V.; Smidt, E.C. O gênero Zygostates (Orchidaceae: Oncidiinae) no estado do Paraná, Brasil. Rodriguésia 2017, 68, 1431–1446. [Google Scholar] [CrossRef][Green Version]
- Royer, C.A.; Toscano de Brito, A.L.V.; Mauad, A.V.S.R.; Smidt, E.C. Phylogenetic position of Centroglossa and Dunstervillea (Ornithocephalus Clade: Oncidiinae: Orchidaceae) based on molecular and morphological data. Syst. Bot. 2022, 47, 927–937. [Google Scholar] [CrossRef]
- Krahl, D.R.P.; Oliveira, M.S.; Schmal, P.; Chiron, G.; Krahl, A.H.; Silva, J.B.F.; Cantuária, P.C. Revealing the true taxonomic status of Catasetum joaquinianum (Orchidaceae: Catasetinae). Phytotaxa 2024, 664, 123–131. [Google Scholar] [CrossRef]
- Romero-González, G.A.; Carnevalli, G. Catasetum ×steyermarkii (Catasetinae: Orchidaceae)—A new putative natural hybrid of Catasetum (Catasetinae, Orchidaceae) from the Venezuelan Guayana. Harv. Pap. Bot. 2024, 29, 1–13. [Google Scholar] [CrossRef]
- Romero-González, G.A.; Jenny, R. Contributions toward a monograph of Catasetum (Catasetinae, Orchidaceae) I: A checklist of species, varieties, and natural hybrids. Harv. Pap. Bot. 1993, 1, 59–84. Available online: https://www.jstor.org/stable/41761485 (accessed on 12 November 2025).
- Mytnik-Ejsmont, J. A Monograph of the Subtribe Polystachyinae Schltr. (Orchidaceae); Uniwersytetu Gdańskiego: Gdańsk, Polska, 2011. [Google Scholar]
- Peraza-Flores, L.N.; Fernández-Concha, G.C.; Romero-González, G.A. Taxonomic notes in American Polystachya (Orchidaceae): The identity of P. foliosa (Hook.) Rchb.f. and the reestablishment of P. caracasana Rchb.f. J. Torrey Bot. Soc. 2011, 138, 366–380. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Alves, M. Taxonomic revision of Campylocentrum (Orchidaceae-Vandeae-Angraecinae): Species with terete leaves. Syst. Bot. 2016, 41, 700–713. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Alves, M. Taxonomical revision of Campylocentrum sect. Dendrophylopsis Cogn. (Orchidaceae-Vandae-Angraecinae). Phytotaxa 2016, 286, 131–152. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Alves, M. Taxonomic revision of Campylocentrum sect. Campylocentrum Cogn. (Orchidaceae, Vandae, Angraecinae) in Brazil. Phytotaxa 2018, 362, 1–20. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Alves, M. Taxonomic revision of Campylocentrum sect. Laevigatum E.M. Pessoa & M.W. Chase (Orchidaceae-Vandeae-Angraecinae). Syst. Bot. 2019, 44, 115–132. [Google Scholar] [CrossRef]
- Pessoa, E.M.; Viruel, J.; Alves, M.; Bogarín, D.; Whitten, W.M.; Chase, M.W. Evolutionary history and systematics of Campylocentrum (Orchidaceae: Vandeae: Angraecinae): A phylogenetic and biogeographical approach. Bot. J. Linn. Soc. 2018, 186, 158–178. [Google Scholar] [CrossRef]
- Smidt, E.C. Filogenia e Revisão Taxonômica de Bulbophyllum Thouars (Orchidaceae) Ocorrentes No Neotrópico. Ph.D. Dissertation, Universidade Estadual de Feira de Santana, Feira de Santana, Brazil, 2007. [Google Scholar]
- Santos, T.F. Revisão da Subtribo Malaxidinae (Orchidaceae) no Brasil e Espécies Novas de Malaxis Sol. ex Sw. Ph.D. Dissertation, Universidade Estadual Paulista, Rio Claro, Brazil, 2024. [Google Scholar]
- Born, M.G.; Maas, P.J.M.; Dressler, R.L.; Westra, L.Y.T. A revision of the saprophytic orchid genera Wullschlaegelia and Uleiorchis. Bot. Jahrbücher Syst. 1999, 121, 45–74. [Google Scholar]
- Ribeiro, P.L.; Borba, E.L.; Smidt, E.C.; Lambert, S.M.; Selbach-Schnadelbach, A.; van den Berg, C. Genetic and morphological variation in the Bulbophyllum exaltatum (Orchidaceae) complex occurring in the Brazilian ‘‘Campos rupestres’’: Implications for taxonomy and biogeography. Plant Syst. Evol. 2008, 270, 109–137. [Google Scholar] [CrossRef]
- Pinheiro, F.; Barros, F.; Palma-Silva, C.; Meyer, D.; Fay, M.F.; Suzuki, R.M.; Lexer, C.; Cozzolino, S. Hybridization and introgression across different ploidy levels in the Neotropical orchids Epidendrum fulgens and E. puniceoluteum (Orchidaceae). Mol. Ecol. 2010, 19, 3981–3994. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.G.; Vieira, F.A.; Felix, L.P.; Molina, W.F. Negligence in the Atlantic forest, northern Brazil: A case study of an endangered orchid. Biodivers. Conserv. 2017, 26, 1047–1063. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes: General Biology and Related Biota; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Zotz, G.; Winkler, U. Aerial roots of epiphytic orchids: The velamen radicum and its role in water and nutrient uptake. Oecologia 2013, 171, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, Y.P.S. (Ed.) High-Tech and Micropropagation. Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Arditti, J.; Abdul Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Leroux, G.; Barabé, D.; Vieth, J. Morphogenesis of the protocorm of Cypripedium acaule (Orchidaceae). Plant Syst. Evol. 1997, 205, 53–72. [Google Scholar] [CrossRef]
- Pereira, M.C.; Rocha, D.I.; Veloso, T.G.R.; Pereira, O.l.; Francino, D.M.T.; Meira, R.M.S.A.; Kasuya, M.C.M. Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Bot. Bras. 2015, 29, 567–574. [Google Scholar] [CrossRef]
- Sousa, K.C.I.; de Araújo, L.G.; Silva, C.S.; Carvalho, J.C.B.; Sibov, S.T.; Gonçalve, L.A.; Pereira, M.C.; Gonçalves, F.J.; Filippi, M.C.C. Seed germination and development of orchid seedlings (Cyrtopodium saintlegerianum) with fungi. Rodriguésia 2019, 70, e02302016. [Google Scholar] [CrossRef]
- dos Santos Anjos, J.; Stefanello, C.A.; Vieira, L.d.N.; Polesi, L.G.; Guerra, M.P.; Fraga, H.P. de F. The Cytokinin 6 benzylaminopurine improves the formation and development of Dryadella zebrina (Orchidaceae) in vitro shoots. Bras. J. Bot. 2021, 44, 811–819. [Google Scholar] [CrossRef]
- Fritsche, Y.; Pinheiro, M.V.M.; Guerra, M.P. Light quality and natural ventilation have different effects on protocorm development and plantlet growth stages of the in vitro propagation of Epidendrum fulgens (Orchidaceae). S. Afr. J. Bot. 2022, 146, 864–874. [Google Scholar] [CrossRef]
- Kerbauy, G.B.; Estelita, M.E.M. Formation of protocorm-like bodies from sliced root apexes of Clowesia warscewiczii. Rev. Bras. Fis. Veg. 1996, 8, 157–159. [Google Scholar]
- Mayer, J.L.S.; Stancato, G.C.; Appezzato-Da-Glória, B. Direct regeneration of protocorm-like bodies (PLBs) from leaf apices of Oncidium flexuosum Sims (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 103, 411–416. [Google Scholar] [CrossRef]
- Picolotto, D.R.N.; de Paiva Neto, V.B.; de Barros, F.; Padilha, D.R.C.; da Cruz, A.C.F.; Otoni, W.C. Micropropagation of Cyrtopodium paludicolum (Orchidaceae) from root tip explants. Crop Breed. Appl. Plant Biotechnol. 2017, 17, 191–197. [Google Scholar] [CrossRef][Green Version]
- Andriolli, B.V.; Corredor-Prado, J.P.; Pescador, R.; Montoya-Serrano, F.S.; Dal Vesco, L.L.; Suzuki, R.M. Morpho-Anatomy of in vitro germination and cryopreservation of the orchid Cattleya crispa (Orchidaceae). Rev. Biol. Trop. 2023, 71, e52338. [Google Scholar] [CrossRef]
- Bidartondo, M.I. The evolutionary ecology of myco-heterotrophy. New Phytol. 2005, 167, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Merckx, V.S.F.T.; Freudenstein, J.V.; Kissling, J.; Christenhusz, M.J.M.; Stotler, R.E.; Crandall-Stotler, B.; Wickett, N.; Rudall, P.J.; Maas-van de Kamer, H.; Maas, P.J.M. Taxonomy and classification. In Mycoheterotrophy: The Biology of Plants Living on Fungi; Merckx, V.S.F.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 19–101. [Google Scholar]
- Pansarin, E.R.; Salatino, A.; Salatino, M.L.F. Phylogeny of South American Pogonieae (Orchidaceae, Vanilloideae) based on sequences of nuclear ribosomal (ITS) and chloroplast (psaB, rbcL, rps16, and trnL-F) DNA with emphasis on Cleistes and discussion of biogeographic implications. Org. Divers. Evol. 2008, 8, 171–181. [Google Scholar] [CrossRef]
- Pansarin, E.R. Recent advances on evolution of pollination systems and reproductive biology of Vanilloideae (Orchidaceae). Lankesteriana 2016, 16, 255–267. [Google Scholar] [CrossRef]
- Alves, M.F.; Pinheiro, F.; Niedzwiedzki, M.P.; Mayer, J.L.S. First Record of Ategmic Ovules in Orchidaceae Offers New Insights Into Mycoheterotrophic Plants. Front. Plant Sci. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Alves, M.F.; Pinheiro, P.; de Toni, K.L.G.; Baumgratz, J.F.A. Anatomical features of pollinia and caudicle in Epidendrum (Orchidaceae; Epidendroideae). Braz. J. Bot. 2024, 47, 219–228. [Google Scholar] [CrossRef]
- Sisti, L.S.; Flores-Borges, D.N.A.; de Andrade, S.A.L.; Koehler, S.; Bonatelli, M.L.; Mayer, J.L.S. The role of non-mycorrhizal fungi in germination of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. Front. Plant Sci. 2019, 29, 1589. [Google Scholar] [CrossRef] [PubMed]
- Sisti, L.S.; Pena-Passos, M.; Mayer, J.L.S. Isolation, characterization, and total DNA extraction to identify endophytic fungi in Mycoheterotrophic plants. J. Vis. Exp. 2023, 195, e65135. [Google Scholar] [CrossRef]
- Pena-Passos, M.; Sisti, L.S.; Mayer, J.L.S. Microscopy techniques for interpreting fungal colonization in Mycoheterotrophic plants tissues and symbiotic germination of seeds. J. Vis. Exp. 2022, 183, e63777. [Google Scholar] [CrossRef]
- Pereira, O.L.; Kasuya, M.C.M.; Rollemberg, C.L.; Chaer, G.M. Isolamento e identificação de fungos micorrízicos rizoctonióides associados a três espécies de orquídeas epífitas neotropicais no Brasil. Rev. Bras. Cienc. Solo 2005, 29, 191–197. [Google Scholar] [CrossRef][Green Version]
- Pereira, M.C.; Pereira, O.L.; Costa, M.D.; Rocha, R.B.; Kasuya, M.C.M. Diversidade de fungos micorrízicos Epulorhiza spp. isolados de Epidendrum secundum (Orchidaceae). Rev. Bras. Cienc. Solo 2009, 33, 1387–1397. [Google Scholar] [CrossRef]
- Massana, R.; del Campo, J.; Sieracki, M.E.; Audic, S.; Logares, R. Exploring the uncultured microeukaryote majority in the oceans: Reevaluation of ribogroups within stramenopiles. Int. Soc. Microb. Ecol. J. 2014, 8, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Obiol, A.; Giner, C.R.; Sánchez, P.; Duarte, C.M.; Acinas, S.G.; Massana, R. (2024). How marine are Marine Stramenopiles (MAST)? A cross-system evaluation. FEMS Microbiol. Ecol. 2024, 100, fiae130. [Google Scholar] [CrossRef]
- Oliveira, E.M.; Mayer, E.; Lovato, P.E.; Guerra, M.P.; Gris, T.; Almeida, A.N.; Mayer, J.L.S. Intracellular cyanobacteria and velamen microorganisms reveal orchid roots as hotspots for microbial communities. Nat. Commun. 2025; in press. [Google Scholar]
- Álvarez, C.; Pérez-Llano, Y.; Rodríguez-Carres, M.; Batista-García, R.A. Quantitative proteomics at early stages of the symbiotic interaction between Oryza sativa and Nostoc punctiforme reveals novel proteins involved in the symbiotic crosstalk. Plant Cell. Physiol. 2022, 63, 1433–1445. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Botina, S.G.; Netrusov, A.I. Cyanobacterial root associations of leafless epiphytic orchids. Microorganisms 2022, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, A.; Abdelkhalek, A.; Sadowsky, M.J. Recent changes to the classification of symbiotic, nitrogen-fixing, legume-associating bacteria: A review. Symbiosis 2017, 71, 91–109. [Google Scholar] [CrossRef]
- Deepthi, A.S.; Ray, J.G. Algal associates and the evidence of cyanobacterial nitrogen fixation in the velamen roots of epiphytic orchids. Global Ecol. Cons. 2020, 22, e00946. [Google Scholar] [CrossRef]
- Deepthi, A.S.; Ray, J.G. Endophytic diversity of hanging velamen roots in the epiphytic orchid Acampe praemorsa. Plant Ecol. Div. 2018, 11, 649–661. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Carmello-Guerreiro, S.M.; Appezzato-da-Glória, B. Anatomical development of the pericarp and seed of Oncidium flexuosum Sims (Orchidaceae). Flora 2011, 206, 601–609. [Google Scholar] [CrossRef]
- Duarte, M.O.; Oliveira, D.M.T.; Borba, E.L. Ontogenesis of ovary and fruit of Acianthera johannensis (Pleurothallidinae, Orchidaceae) reveals a particular female embryology. Flora 2019, 259, 151462. [Google Scholar] [CrossRef]
- Bento, J.P.S.P.; Pinheiro, F.; Mayer, J.S. From flower to fruit: The origin of the trilocular ovary and fruit development in Phragmipedium longifolium (Warsz. & Rchb.f.) Rolfe (Orchidaceae: Cypripedioideae). Plant Biol. 2025; in press. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Scopece, G.; Lumaga, M.R.B.; Coiro, M.; Pinheiro, F.; Cozzolino, S. Ecological and phylogenetic constraints determine the stage of anthetic ovule development in orchids. Am. J. Bot. 2021, 108, 2405–2415. [Google Scholar] [CrossRef]
- Leitão, C.A.E.; Cortelazzo, A.L. Structure and histochemistry of the stigmatic and transmitting tissues of Rodriguezia venusta (Orchidaceae) during flower development. Austr. J. Bot. 2010, 58, 233–240. [Google Scholar] [CrossRef]
- Costa, G.V.; Alves, M.F.; Duarte, M.O.; Caetano, A.P.S.; Koehler, S.; Mayer, J.L.S. Apomixis beyond trees in the Brazilian savanna: New insights from the orchid Zygopetalum mackayi. AoB Plants 2024, 16, plae037. [Google Scholar] [CrossRef]
- Teixeira, S.P.; Borba, E.L.; Semir, J. Lip anatomy and its implications for the pollination mechanisms of Bulbophyllum species (Orchidaceae). Ann. Bot. 2004, 93, 499–505. [Google Scholar] [CrossRef][Green Version]
- Nunes, C.E.P.; Amorim, F.W.; Mayer, J.L.S.; Sazima, M. (2015). Pollination ecology of two species of Elleanthus (Orchidaceae): Novel mechanisms and underlying adaptations to hummingbird pollination. Plant Biol. 2015, 18, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.O.; Oliveira, D.M.T.; Borba, E.L. Two self-incompatibility sites occur simultaneously in the same Acianthera species (Orchidaceae, Pleurothallidinae). Plants 2020, 9, 1758. [Google Scholar] [CrossRef]
- Ricci, N.A.P.; Bento, J.P.S.P.; Mayer, J.L.S.; Singer, R.B.; Koehler, S. Gametophytic self-incompatibility in Maxillariinae orchids. Protoplasma 2024, 261, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Rodrigues, G.; Barros, F.; Davis, A.R.; Cardoso-Gustavson, P. Floral glands in myophilous and sapromyophilous species of Pleurothallidinae (Epidendroideae, Orchidaceae) osmophores, nectaries, and a unique sticky gland. Protoplasma 2021, 258, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G. The genus Coryanthes: A paradigm in ecology. Lankesteriana 2011, 11, 253–264. [Google Scholar] [CrossRef]
- Casique, J.V.; Soares, M.V.B.; Silva, E.F.; Kikuchi, T.Y.; Andrade, E.H.D.A.; Mastroberti, A.A. Coryanthes macrantha (Orchidaceae: Stanhopeinae) and their floral and extrafloral secretory structures: An anatomical and phytochemical approach. Plants 2022, 14, plac039. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Cardoso-Gustavson, P.; Appezzato-da-Glória, B. Colleters in monocots: New record for Orchidaceae. Flora 2011, 206, 185–190. [Google Scholar] [CrossRef]
- Cassola, F.; Nunes, C.E.P.; Lusa, M.G.; Garcia, V.L.; Mayer, J.L.S. Deep in the jelly: Histochemical and functional aspects of mucilage-secreting floral colleters in the orchids Elleanthus brasiliensis and E. crinipes. Front. Plant Sci. 2019, 10, 518. [Google Scholar] [CrossRef]
- Bonfante, N.O.; de Camargo Smidt, E.; Bona, C. Evolution of vegetative morphoanatomical characters in Pabstiella (Pleurothallidinae: Orchidaceae). Flora 2024, 317, 152529. [Google Scholar] [CrossRef]
- de Siqueira Pieczak, F.; de Camargo Smidt, E.; Engels, M.E.; de Paula Machado, R.G.; Bona, C. Floral micromorphology and anatomical diversity in Microchilus (Orchidaceae: Goodyerinae). Flora 2022, 290, 152045. [Google Scholar] [CrossRef]
- Santos, I.S.; Silva, M.J.D. Anatomy and histochemistry of the vegetative system of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a potential medicinal species. Plants 2023, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.M.; Boff, L.; de Almeida Caputti Araújo, G.; Silva, S.M. Ecological inferences in Orchidaceae species from the Brazilian subtropical Atlantic Forest based on morphological and functional anatomical traits. Flora 2024, 317, 152558. [Google Scholar] [CrossRef]
- Blanco, G.D.; Hanazaki, N.; Rodrigues, A.C. Anatomical study of Orchidaceae epiphytic species occurring in indigenous territory in the Parque Estadual da Serra do Tabuleiro (P.E.S.T.), Santa Catarina, Brazil. Rodriguésia 2021, 72, e02052019. [Google Scholar] [CrossRef]
- Lima, J.F.; Oliveira, D.C.D.; Kuster, V.C.; Moreira, A.S.F.P. Aerial and terrestrial root habits influence the composition of the cell walls of Vanilla phaeantha (Orchidaceae). Protoplasma 2025, 262, 87–98. [Google Scholar] [CrossRef]
- Moreira, A.S.F.P.; Borba, E.L.; Lemos-Filho, J.P. Testing arbitrary classes of light in a physiognomically heterogeneous area of ‘campo rupestre’ vegetation. An. Acad. Bras. Ciênc. 2013, 85, 635–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreira, A.S.F.P.; Borba, E.L.; Oliveira, D.C.; Isaias, R.M.S.; Ducatti, C.; Lemos-Filho, J.P. Intermediate C 3 -CAM metabolism in Bulbophyllum involutum: A species with limited leaf morphological variation in relation to light. S. Afr. J. Bot. 2017, 113, 40–46. [Google Scholar] [CrossRef]
- Blumenschein, A. Estudos Citológicos na Família Orchidaceae. Ph.D. Dissertation, Universidade de São Paulo, Piracicaba, Brazil, 1957. [Google Scholar]
- Blumenschein, A. Número de cromossomas de algumas espécies de orquídeas. Publ. Cien. Inst. Gen. 1960, 1, 45–50. [Google Scholar]
- Blumenschein, A.; Paker, I.U. Número de Cromossomas nas Maxillariinae (Orchidaceae). Ciência Cult. 1963, 15, 255. [Google Scholar]
- Felix, L.P.; Guerra, M. Cytogenetic studies on species of Habenaria (Orchidoideae: Orchidaceae) occurring in the northeast of Brazil. Lindleyana 1998, 13, 224–230. [Google Scholar]
- Felix, L.P.; Guerra, M. Chromosome analysis in Psygmorchis pusilla (L.) Dodson & Dressier: The smallest chromosome number known in Orchidaceae. Caryologia 1999, 52, 165–168. [Google Scholar] [CrossRef]
- Felix, L.P.; Guerra, M. Cytogenetics and cytotaxonomy of some Brazilian species of Cymbidioid orchids. Genet. Mol. Biol. 2000, 23, 957–978. [Google Scholar] [CrossRef]
- Felix, L.P.; Guerra, M. Basic chromosome numbers of terrestrial orchids. Plant Syst. Evol. 2005, 254, 131–148. [Google Scholar] [CrossRef]
- Felix, L.P.; Guerra, M. Variation in chromosome number and the basic number of subfamily Epidendroideae (Orchidaceae). Bot. J. Linn. Soc. 2010, 163, 234–278. [Google Scholar] [CrossRef]
- Cabral, J.S.; Felix, L.P.; Guerra, M. Heterochromatin diversity and its co-localization with 5S and 45S rDNA sites in chromosomes of four Maxillaria species (Orchidaceae). Gen. Mol. Biol. 2006, 29, 659–664. [Google Scholar] [CrossRef]
- Moraes, A.P.; Leitch, I.J.; Leitch, A.R. Chromosome studies in Orchidaceae: Karyotype divergence in Neotropical genera in subtribe Maxillariinae. Bot. J. Linn. Soc. 2012, 170, 29–39. [Google Scholar] [CrossRef]
- Moraes, A.P.; Chinaglia, M.; Palma-Silva, C.; Pinheiro, F. Interploidy hybridization in sympatric zones: The formation of Epidendrum fulgens× E. puniceoluteum hybrids (Epidendroideae, Orchidaceae). Ecol. Evol. 2013, 3, 3824–3837. [Google Scholar] [CrossRef]
- Moraes, A.P.; Simões, A.O.; Alayon, D.I.O.; Barros, F.; Forni-Martins, E.R. Detecting mechanisms of karyotype evolution in Heterotaxis (Orchidaceae). PLoS ONE 2016, 11, e0165960. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.P.; Koehler, S.; Cabral, J.S.; Gomes, S.S.L.; Viccini, L.F.; Barros, F.; Felix, L.P.; Guerra, M.; Forni-Martins, E.R. Karyotype diversity and genome size variation in Neotropical Maxillariinae orchids. Plant Biol. 2017, 19, 298–308. [Google Scholar] [CrossRef]
- Querino, B.C.; Ferraz, M.E.; Mata-Sucre, Y.; Souza, G.; Felix, L.P. Cytomolecular diversity of the subtribe Laeliinae (Epidendroidae, Orchidaceae) suggests no relationship between genome size and heterochromatin abundance. Plant Syst. Evol. 2020, 306, 1–15. [Google Scholar] [CrossRef]
- Nollet, F.; Medeiros Neto, E.; Cordeiro, J.M.; Buril, M.T.; Chase, M.W.; Felix, L.P. Chromosome numbers and heterochromatin variation in introgressed and non-introgressed populations of Epidendrum (Orchidaceae: Epidendroideae): Interspecific transfers of heterochromatin lead to divergent variable karyotypes in the parental populations. Bot. J. Linn. Soc. 2022, 199, 694–705. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Chase, M.W.; Hágsater, E.; Almeida, E.M.; Costa, L.; Souza, G.; Felix, L.P. Chromosome number, heterochromatin, and genome size support recent polyploid origin of the Epidendrum nocturnum group and reveal a new species (Laeliinae, Orchidaceae). Botany 2022, 100, 409–421. [Google Scholar] [CrossRef]
- Oliveira, I.G.; Moraes, A.P.; Almeida, E.M.; Assis, F.N.M.; Cabral, J.S.; De Barros, F.; Felix, L.P. Chromosomal evolution in Pleurothallidinae (Orchidaceae: Epidendroideae) with an emphasis on the genus Acianthera: Chromosome numbers and heterochromatin. Bot. J. Linn. Soc. 2015, 178, 102–120. [Google Scholar] [CrossRef]
- Baranow, P.; Rojek, J.; Dudek, M.; Szlachetko, D.; Bohdanowicz, J.; Kapusta, M.; Jedrzejczyk, I.; Rewers, M.; Moraes, A.P. Chromosome Number and Genome Size Evolution in Brasolia and Sobralia (Sobralieae, Orchidaceae). Int. J. Mol. Sci. 2022, 23, 3948. [Google Scholar] [CrossRef]
- Pinheiro, F.; Cardoso-Gustavson, P.; Suzuki, R.M.; Abrao, M.C.R.; Guimaraes, L.R.; Draper, D.; Moraes, A.P. Strong postzygotic isolation prevents introgression between two hybridizing Neotropical orchids, Epidendrum denticulatum and E. fulgens. Evol. Ecol. 2015, 29, 229–248. [Google Scholar] [CrossRef]
- Arida, B.L.; Scopece, G.; Machado, R.M.; Moraes, A.P.; Forni-Martins, E.; Pinheiro, F. Reproductive Barriers and Fertility of Two Neotropical Orchid Species and Their Natural Hybrid. Evol. Ecol. 2021, 35, 41–64. [Google Scholar] [CrossRef]
- Moraes, A.P.; Engel, T.B.; Forni-Martins, E.R.; Barros, F.; Felix, L.P.; Cabral, J.S. Are chromosome number and genome size associated with habit and environmental niche variables? Insights from the Neotropical orchids. Ann. Bot. 2022, 130, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Via, S. Natural selection in action during speciation. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. 1), 9939–9946. [Google Scholar] [CrossRef]
- Scopece, G.; Lexer, C.; Widmer, A.; Cozzolino, S. Polymorphism of postmating reproductive isolation within plant species. Taxon 2010, 59, 1367–1374. [Google Scholar] [CrossRef]
- Pinheiro, F.; Dantas-Queiroz, M.V.; Palma-Silva, C. Plant species complexes as models to understand speciation and evolution: A review of South American studies. Crit. Rev. Plant Sci. 2018, 37, 54–80. [Google Scholar] [CrossRef]
- Edmands, S. Does parental divergence predict reproductive compatibility? Trends Ecol. Evol. 2002, 17, 520–527. [Google Scholar] [CrossRef]
- Yardeni, G.; Tessler, N.; Imbert, E.; Sapir, Y. Reproductive isolation between populations of Iris atropurpurea is associated with ecological differentiation. Ann. Bot. 2016, 118, 971–982. [Google Scholar] [CrossRef]
- Bicalho, H.D. Contribuição à Sistemática do Gênero Catasetum L.C.Rich (Orchidaceae). Ph.D. Dissertation, Universidade de São Paulo, Piracicaba, Brazil, 1960. [Google Scholar]
- Vencovsky, R. Aplicação de Alguns Métodos Estatísticos à Sistemática. Ph.D. Dissertation, Universidade de São Paulo, Piracicaba, Brazil, 1960. [Google Scholar]
- Martins, P.S. Estudo da Variação Intra-Específica no Gênero Miltonia Ldl. (Orchidaceae–Oncidieae). Ph.D. dissertation, Universidade de São Paulo, Piracicaba, Brazil, 1970. [Google Scholar]
- Chacur, F. Análise da variação e da Taxonomia no Gênero Brassavola R.Br. (Orchidaceae–Epidendroideae). Ph.D. Dissertation, Universidade de São Paulo, Ribeirão Preto, Brazil, 1973. [Google Scholar]
- Illg, R.D. Aspectos Evolutivos em Algumas Maxillárias Brasileiras. Ph.D. Dissertation, Universidade Estadual de Campinas, Campinas, Brazil, 1975. [Google Scholar]
- Stort, M.N.S. Estudos em Híbridos F1 Artificiais de Orquídeas (Orchidaceae) com vistas à esterilidade. Ph.D. Dissertation, University of São Paulo, Piracicaba, Brazil, 1970. [Google Scholar]
- Alves, M.F.; Pinheiro, F.; Nunes, C.E.P.; Vettorazzi, R.G.; Pansarin, E.R.; Sazima, M.; Pereira, R.A.S. Reproductive development and genetic structure of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. BMC Plant Biol. 2021, 21, 307. [Google Scholar] [CrossRef]
- Batista, J.A.N.; Cruz-Lustre, G.; Vale, A.A.; Bianchetti, L.B. Checklist and Molecular Phylogenetics Reveal Three Taxonomic Novelties in Habenaria (Orchidaceae, Orchidoideae). Eur. J. Taxon. 2023, 891, 51–86. [Google Scholar] [CrossRef]
- Borba, E.L.; Funch, R.R.; Ribeiro, P.L.; Smidt, E.C.; Silva-Pereira, V. Demography, and genetic and morphological variability of the endangered Sophronitis sincorana (Orchidaceae) in the Chapada Diamantina, Brazil. Plant Syst. Evol. 2007, 267, 129–146. [Google Scholar] [CrossRef]
- Cruz, D.T.; Selbach-Schnadelbach, A.; Lambert, S.M.; Ribeiro, P.L.; Borba, E.L. Genetic and morphological variability in Cattleya elongata Barb. Rodr. (Orchidaceae), endemic to the campo rupestre vegetation in northeastern Brazil. Plant Syst. Evol. 2011, 294, 87–98. [Google Scholar] [CrossRef]
- Leles, B.; Chaves, A.V.; Russo, P.; Batista, J.A.; Lovato, M.B. Genetic structure is associated with phenotypic divergence in floral traits and reproductive investment in a high-altitude orchid from the Iron Quadrangle, southeastern Brazil. PLoS ONE 2015, 10, e0120645. [Google Scholar] [CrossRef]
- Rodrigues, J.F.; van den Berg, C.; Abreu, A.G.; Novello, M.; Veasey, E.A.; Oliveira, G.C.; Koehler, S. Species delimitation of Cattleya coccinea and C. mantiqueirae (Orchidaceae): Insights from phylogenetic and population genetics analyses. Plant Syst. Evol. 2015, 301, 1345–1359. [Google Scholar] [CrossRef]
- Leal, B.S.; Chaves, C.J.; Koehler, S.; Borba, E.L. When hybrids are not hybrids: A case study of a putative hybrid zone between Cattleya coccinea and C. brevipedunculata (Orchidaceae). Bot. J. Linn. Soc. 2016, 181, 621–639. [Google Scholar] [CrossRef]
- Gomes, P.C.L.; Smidt, E.C.; Fraga, C.N.; Silva-Pereira, V. High genetic variability is preserved in relict populations of Cattleya lobata (Orchidaceae) in the Atlantic Rainforests inselbergs. Braz. J. Bot. 2018, 41, 185–195. [Google Scholar] [CrossRef]
- Nazareno, A.G.; Neto, L.M.; Buzatti, R.S.; Berg, C.V.; Forzza, R.C. Four raised to one equals one: A genetic approach to the Pseudolaelia vellozicola complex does not follow a math rule. Ecol. Evol. 2020, 10, 4562–4569. [Google Scholar] [CrossRef]
- Azevedo, C.O.; Borba, E.L.; van den Berg, C. Evidence of natural hybridization and introgression in Bulbophyllum involutum Borba, Semir & F. Barros and B. weddellii (Lindl.) Rchb. f. (Orchidaceae) in the Chapada Diamantina, Brazil, by using allozyme markers. Braz. J. Bot. 2006, 29, 415–421. [Google Scholar] [CrossRef]
- Azevedo, M.T.A.; Borba, E.L.; Semir, J.; Solferini, V.N. High genetic variability in Neotropical myophilous orchids. Bot. J. Linn. Soc. 2007, 153, 33–40. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Borba, E.L. Variation in self-incompatibility and interspecific compatibility in a lineage of the mostly self-compatible genus Bulbophyllum (B. sect. Micranthae–Orchidaceae). Plant Syst. Evol. 2023, 309, 9. [Google Scholar] [CrossRef]
- Barbosa, A.R.; Silva-Pereira, V.; Borba, E.L. High Genetic variability in self-incompatible myophilous Octomeria (Orchidaceae, Pleurothallidinae) Species. Bras. J. Bot. 2013, 36, 179–187. [Google Scholar] [CrossRef]
- Borba, E.L.; Funch, R.R.; Ribeiro, P.L.; Smidt, E.C.; Silva-Pereira, V. Demografia, variabilidade genética e morfológica e conservação de Cattleya tenuis (Orchidaceae), espécie ameaçada de extinção da Chapada Diamantina. Sitientibus Série Ciênc. Biológicas 2007, 7, 211–222. [Google Scholar]
- Pinheiro, F.; Cozzolino, S. Epidendrum (Orchidaceae) as a model system for ecological and evolutionary studies in the Neotropics. Taxon 2013, 62, 77–88. [Google Scholar] [CrossRef]
- Pinheiro, F.; de Melo e Gouveia, Z.; Manuel, T.; Cozzolino, S.; Cafasso, D.; Cardoso-Gustavson, P.; Suzuki, R.M.; PalmaSilva, C. Strong but permeable barriers to gene exchange between sister species of Epidendrum. Am. J. Bot. 2016, 103, 1472–1482. [Google Scholar] [CrossRef]
- Sujii, P.S.; Cozzolino, S.; Pinheiro, F. Hybridization and geographic distribution shapes the spatial genetic structure of two co-occurring orchid species. Heredity 2019, 123, 458–469. [Google Scholar] [CrossRef]
- Pinheiro, F.; Cozzolino, S.; de Barros, F.; Gouveia, T.M.Z.M.; Suzuki, R.M.; Fay, M.F.; Palma-Silva, C. Phylogeographic structure and outbreeding depression reveal early stages of reproductive isolation in the Neotropical orchid Epidendrum denticulatum. Evolution 2013, 67, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.; Veiga, G.S.; Chaves, C.J.N.; da Costa Cacossi, T.; da Silva, C.P. Reproductive barriers and genetic differentiation between continental and island populations of Epidendrum fulgens (Orchidaceae). Plant Syst. Evol. 2021, 307, 2407. [Google Scholar] [CrossRef]
- Arida, B.L.; Izquierdo, J.V.; Teixeira, M.C.; Turchetto, C.; Benitez-Vieyra, S.; Pinheiro, F. Different but not isolated: Absence of reproductive barriers and strong floral divergence between ecotypes of Epidendrum fulgens (Orchidaceae). Bot. J. Linn. Soc. 2025, 208, 313–324. [Google Scholar] [CrossRef]
- Leal, B.S.; Brandão, M.M.; Palma-Silva, C.; Pinheiro, F. Differential gene expression reveals mechanisms related to habitat divergence between hybridizing orchids from the Neotropical coastal plains. BMC Plant Biol. 2020, 20, 554. [Google Scholar] [CrossRef]
- Lima, T.M.; Silva, S.F.; Ribeiro, R.V.; Sánchez-Vilas, J.; Pinheiro, F. Short-term salt spray reveals high salt tolerance in a neotropical orchid species. Theor. Exp. Plant. Physiol. 2023, 35, 355–362. [Google Scholar] [CrossRef]
- Lima, T.M.; Silva, S.F.; Ribeiro, R.V.; Sánchez-Vilas, J.; Pinheiro, F. Salt tolerance in a neotropical orchid in the absence of local adaptation to salt spray. Am. J. Bot. 2024, 111, e16373. [Google Scholar] [CrossRef] [PubMed]
- Mattos, J.S.; Pinheiro, F.; Luize, B.G.; Chaves, C.J.N.; Lima, T.M.; Palma-Silva, C.; Leal, B.S.S. The relative role of climate and biotic interactions in shaping the range limits of a neotropical orchid. J. Biogeogr. 2023, 50, 1315–1328. [Google Scholar] [CrossRef]
- Lima, T.M.; Silva, S.F.; Sánchez-Vilas, J.; Júnior, W.L.S.; Mayer, J.L.S.; Ribeiro, R.V.; Pinheiro, F. Phenotypic plasticity rather than ecotypic differentiation explains the broad realized niche of a Neotropical orchid species. Plant Biol. 2024, 26, 989–997. [Google Scholar] [CrossRef]
- Arida, B.L.; Pinheiro, F.; Laccetti, L.; Camargo, M.G.G.; Freitas, A.V.L.; Scopece, G. The consequences of flower colour polymorphism on the reproductive success of a neotropical deceptive orchid. Plant Biol. 2025, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.S.; Vidal, J.D.; Neves, C.S.; Zorzatto, C.; Campacci, T.V.S.; Lima, A.K.; Koehler, S.; Viccini, L.F. Genome size and climate segregation suggest distinct colonization histories of an orchid species from Neotropical high-elevation rocky complexes. Biol. J. Linn. Soc. 2018, 124, 456–465. [Google Scholar] [CrossRef]
- Dufresne, F.; Stift, M.; Vergilino, R.; Mable, B.K. Recent progress and challenges in population genetics of polyploid organisms: An overview of current state of the art molecular and statistical tools. Mol. Ecol. 2014, 23, 40–69. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Liu, S.; van Tienderen, P.H. The analysis of polyploid genetic data. J. Hered. 2018, 109, 283–296. [Google Scholar] [CrossRef]
- Rowan, J.S.; Twyford, A.D.; Pennington, R.T. Hybridization: A ‘double-edged sword’ for Neotropical plant diversity. Bot. J. Linn. Soc. 2022, 199, 331–356. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Bogarín, D.; Schley, R.; Bateman, R.M.; Gerlach, G.; Harpke, D.; Brassac, J.; Fernández-Mazuecos, M.; Dodsworth, S.; Hagsater, E.; et al. Resolving relationships in an exceedingly young Neotropical orchid lineage using genotyping-by-sequencing data. Mol. Phylogenet. Evol. 2020, 144, 106672. [Google Scholar] [CrossRef] [PubMed]
- van der Niet, T. Paucity of natural history data impedes phylogenetic analyses of pollinator-driven evolution. New Phytol. 2021, 229, 1201–1205. [Google Scholar] [CrossRef]
- van der Pijl, L.; Dodson, C.H. Orchid Flowers: Their Pollination and Evolution; University of Miami Press: Coral Gables, FL, USA, 1969. [Google Scholar]
- Brieger, F.G. Dispersão e migração na evolução das orquídeas americanas. Publ. Cien. Inst. Gen. 1961, 2, 69–82. [Google Scholar]
- Chiron, G.R. Riqueza e endemismo de espécies de Baptistonia (Orchidaceae), no Brasil. Hoehnea 2009, 36, 459–477. [Google Scholar] [CrossRef]
- Menini-Neto, L.; Forzza, R.C. Biogeography and conservation status assessment of Pseudolaelia (Orchidaceae). Bot. J. Linn. Soc. 2012, 171, 191–200. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Doucette, A.; Giraldo, G.; McDaniel, J.; Clements, M.A.; et al. Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. J. Biogeogr. 2016, 43, 1905–1916. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Chase, M.W. Biogeographical patterns of plants in the Neotropics—Dispersal rather than plate tectonics is most explanatory. Bot. J. Linn. Soc. 2013, 171, 277–286. [Google Scholar] [CrossRef]
- Smidt, E.C.; Borba, E.L.; Gravendeel, B.; Fischer, G.A.; van den Berg, C. Molecular phylogeny of the Neotropical sections of Bulbophyllum (Orchidaceae) using nuclear and plastid markers. Mol. Phylogenet. Evol. 2011, 59, 318–330. [Google Scholar]
- Smidt, E.C.; Silva-Pereira, V.; Borba, E.L.; van den Berg, C. Richness, distribution and important areas to preserve Bulbophyllum in the Neotropics. Lankesteriana 2007, 7, 107–113. [Google Scholar] [CrossRef][Green Version]
- Mauad, A.V.S.R.; Imig, D.C.; Da Silva-Pereira, V.; Toscano De Brito, A.L.V.; De Camargo Smidt, E. Biogeographical evidence suggests a Panama–Northwest South American origin for Dryadella (Orchidaceae: Pleurothallidinae) and two independent lineages of Atlantic Rainforest endemics. Bot. J. Linn. Soc. 2025, boaf057. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Gottschling, M.; Chomicki, G.; Condamine, F.L.; Kligard, B.B.; Pansarin, E.; Gerlach, G. Andean mountain building did not preclude dispersal of lowland epiphytic orchids in the Neotropics. Sci. Rep. 2017, 7, 4919. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Bochorny, T.; Pérez-Escobar, A.O.; Chomicki, G.; Monteiro, S.H.N.; Smidt, E.C. From tree tops to the ground: Reversals to terrestrial habit in Galeandra orchids (Epidendroideae: Catasetinae). Mol. Phylogenet. Evol. 2018, 127, 952–960. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN red list for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef]
- Nic-Lughadha, E.M.; Staggemeier, V.G.; Vasconcelos, T.N.C.; Walker, B.E.; Canteiro, C.; Lucas, E.J. Harnessing the potential of integrated systematics for conservation of taxonomically complex, megadiverse plant groups. Conserv. Biol. 2019, 33, 511–522. [Google Scholar] [CrossRef]
- CNCFlora. Centro Nacional de Conservação da Flora. 2025. Available online: https://cncflora.jbrj.gov.br/ (accessed on 30 May 2025).
- Martinelli, G.; Moraes, M.A. Livro Vermelho da Flora do Brasil; Andrea Jakobsson: Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Machnicki-Reis, M.; Engels, M.E.; Petini-Benelli, A.; Smidt, E.C. O gênero Catasetum Rich. ex Kunth (Orchidaceae, Catasetinae) no Estado do Paraná, Brasil. Hoehnea 2015, 42, 185–194. [Google Scholar] [CrossRef][Green Version]
- Santos, I.S.; Silva, M.J. O gênero Galeandra Lindl. (Orchidaceae, Epidendroideae) no Distrito Federal e no Estado de Goiás, Brasil. Hoehnea 2020, 47, e462020. [Google Scholar] [CrossRef]
- Cintra, M.C.S.; Lemes, P.; Alvarado, S.T.; Pessoa, E.M. Filling the gap to avoid extinction: Conservation status of Brazilian species of Epidendrum L. (Orchidaceae). J. Nat. Conserv. 2023, 71, 126328. [Google Scholar] [CrossRef]
- Siqueira, C.E.; Zanin, A.; Menini Neto, L. Orchidaceae in Santa Catarina: Update, geographic distribution and conservation. Check List 2014, 10, 1452–1478. [Google Scholar] [CrossRef]
- Hagsater, E.; Dumont, V.; Pridgeon, A.M. Orchids: Status Survey and Conservation Action Plan; IUCN: Cambridge, UK, 1996; Volume 28. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Marquet, P.A.; Enquist, B.J.; Midgley, G.; Foden, W.; Svenning, J.C. 30% land conservation and climate action reduces tropical extinction risk by more than 50%. Ecography 2020, 43, 943–953. [Google Scholar] [CrossRef]
- Amaral, S.; Metzger, J.P.; Rosa, M.; Adorno, B.V.; Gonçalves, G.C.; Pinto, L.F.G. Alarming patterns of mature forest loss in the Brazilian Atlantic Forest. Nat. Sustain. 2025, 8, 256–264. [Google Scholar] [CrossRef]
- Souza, G.F.; Ferreira, M.C.; Munhoz, C.B.R. Decrease in species richness and diversity, and shrub encroachment in Cerrado grasslands: A 20 years study. Appl. Veg. Sci. 2022, 25, e12668. [Google Scholar] [CrossRef]
- Zotarelli, H.G.; Molina, J.M.; Ribeiro, J.E.; Sofia, S.H. A commensal network of epiphytic orchids and host trees in an Atlantic Forest remnant: A case study revealing the important role of large trees in the network structure. Austral Ecol. 2019, 44, 114–125. [Google Scholar] [CrossRef]
- Lowe, A.J.; Boshier, D.; Ward, M.; Bacles, C.F.E.; Navarro, C. Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 2005, 95, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.D.; Reiter, N.; Peakall, R. Orchid conservation: From theory to practice. Ann. Bot. 2020, 126, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Zandoná, L.R.; Catharino, E.L.M. Orchidaceae do Parque Estadual da Cantareira e sua conservação. Rev. Inst. Flor. 2015, 27, 83–101. [Google Scholar] [CrossRef]
- Endres Júnior, D.; Sasamori, M.H.; Silveira, T.; Schmitt, J.L.; Droste, A. Reintrodução de Cattleya intermedia Graham (Orchidaceae) em borda e interior de um fragmento de Floresta Estacional Semidecidual no sul do Brasil. Rev. Bras. Biociênc. 2015, 13, 33–40. Available online: https://seer.ufrgs.br/index.php/rbrasbioci/article/view/114793. (accessed on 12 November 2025).
- Fajardo, C.G.; Vieira, F.A.; Molina, W.F. Interspecific genetic analysis of orchids in Brazil using molecular markers. Plant Syst. Evol. 2014, 300, 1825–1832. [Google Scholar] [CrossRef]
- Fajardo, C.G.; Vieira, F.A.; Molina, W.F. Conservação genética de populações naturais: Uma revisão para Orchidaceae. Biota Amaz. 2016, 6, 108–118. [Google Scholar] [CrossRef]
- Diniz, M.F.; Gonçalves, T.V.; Brito, D. Last of the green: Identifying priority sites to prevent plant extinctions in Brazil. Oryx 2017, 51, 131–136. [Google Scholar] [CrossRef]
- Oliveira, J.; Moraes, M.C.; Custódio, C.C.; Machado-Neto, N.B. In vitro development and acclimatization of Cyrtopodium aliciae L. Linden & Rolfe, an endemic species of the Chapada Diamantina. Ciênc. Rural 2023, 53, e20210599. [Google Scholar] [CrossRef]
- Seaton, P.; Zettler, L.W. Saving Orchids: Stories of Species Survival in a Changing World. Kew and The University of Chicago Press: Royal Botanic Gardens, UK, 2025. [Google Scholar]
- Thomann, M.; Imbert, E.; Devaux, C.; Cheptou, P.-O. Flowering plants under global pollinator decline. Trends Plant Sci. 2013, 18, 353–359. [Google Scholar] [CrossRef]
- Yudaputra, A.; Munawaroh, E.; Usmadi, D.; Purnomo, D.W.; Astuti, I.P.; Puspitaningtyas, D.M.; Handayani, T.; Garvita, R.V.; Aprilianti, P.; Wawangningrum, H.; et al. Vulnerability of lowland and upland orchids in their spatial response to climate change and land cover change. Ecol. Inform. 2024, 80, 102534. [Google Scholar] [CrossRef]
- Bond, W.J. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos. Trans. R. Soc. B London. Ser. B Biol. Sci. 1994, 344, 83–90. [Google Scholar] [CrossRef]
- Petanidou, T.; Kallimanis, A.S.; Sgardelis, S.P.; Mazaris, A.D.; Pantis, J.D.; Waser, N.M. Variable flowering phenology and pollinator use in a community suggest future phenological mismatch. Acta Oecol. 2014, 59, 104–111. [Google Scholar] [CrossRef]
- Kolanowska, M.; Rewicz, A.; Baranow, P. Ecological niche modeling of the pantropical orchid Polystachya concreta (Orchidaceae) and its response to climate change. Sci. Rep. 2020, 10, 14801. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant–pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef] [PubMed]
- Silva, U.F.; Borba, E.L.; Semir, J.; Marsaioli, A.J. A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry. 1999, 50, 3–34. [Google Scholar] [CrossRef]
- Cardoso, P.R. Desenvolvimento Floral em Espécies de Pleurothallidinae (Orchidaceae) Com Ênfase nas Estruturas Secretoras. Ph.D. Dissertation, Instituto de Botânica, São Paulo, Brazil, 2014. [Google Scholar]
- Oliveira, M.S.; Oliveira, M.S.; Nascimento, L.D.; Pessoa, E.; Aguiar, A.E.H.; Viana, P.L. First comprehensive report on the chemical composition of the floral perfume of Notylia (Orchidaceae). Nat. Prod. Res. 2025, 1, 1–7. [Google Scholar] [CrossRef]
- Vogel, S. The Role of Scent Glands in Pollination. English translation of Vogel S. (1963) Duftdrüsen im Dienste der Bestäubung. Über Bau und Funktion des Osmophoren; Renner, S., Ed.; Smithsonian Institution Libraries: Washington, DC, USA, 1990. [Google Scholar]
- Williams, N.H.; Whitten, W.M. Orchid floral fragrances and male Euglossini bees: Methods and advances in the last sesquidecade. Biol. Bull. 1983, 164, 355–395. [Google Scholar] [CrossRef]
- Curry, K.J.; McDowell, L.M.; Judd, W.S.; Stern, W.L. Osmophores, floral features, and systematics of Stanhopea (Orchidaceae). Amer. J. Bot. 1991, 78, 610–623. [Google Scholar] [CrossRef]
- Nunes, C.E.P.; Gerlach, G.; Bandeira, K.D.O.; Gobbo-Neto, L.; Pansarin, E.R.; Sazima, M. Intriguing chemical similarity: Floral scents of Catasetum cernuum and Gongora bufonia suggest convergent evolution to a unique pollination niche. Flora 2017, 232, 207–216. [Google Scholar] [CrossRef]
- Sydney, N.V.; Gonçalves, R.B.; Faria, L.R. Padrões espaciais na distribuição de abelhas Euglossina (Hymenoptera, Apidae) da região Neotropical. Pap. Avulsos Zool. 2010, 50, 667–679. [Google Scholar] [CrossRef]
- Müller, F. Ueber einige Fälle von Dimorphismus bei Blüthen. Bot. Zeitung 1868, 26, 257–260. [Google Scholar]
- Müller, F. Ueber Befruchtungserscheinungen (Eschscholtzia, Faramea, Epidendrum, Scorzonera). Bot. Ztg. 1869, 27, 224–226. [Google Scholar]
- Vogel, S. Organography of the Flowers of Ophrydeae of the Cape Province, with Remarks on the Problem of Coaptation. Part I. Disinae and Satyriinae. Akad. Wiss. Anz. Math.-Nat. Kl. 1959, 6, 27–401. [Google Scholar]
- Vogel, S. Parfümsammelnde Bienen als Bestäuber von Orchidaceen und Gloxinia. Österr. Bot. Z. 1966, 113, 302–361. Available online: https://www.jstor.org/stable/43337485 (accessed on 12 November 2025). [CrossRef]
- Carvalho, R.; Machado, I.C. Pollination of Catasetum macrocarpum (Orchidaceae) by Eulaema bombiformis (Euglossini). Lindleyana 2002, 17, 85–90. [Google Scholar] [CrossRef][Green Version]
- Carvalho, R.; Machado, I.C. Rodriguezia bahiensis Rchb. f.: Biologia floral, polinizadores e primeiro registro de polinização por moscas Acroceridae em Orchidaceae. Braz. J. Bot. 2006, 29, 461–470. [Google Scholar] [CrossRef]
- Milet-Pinheiro, P.; Navarro, D.M.A.F.; Dötterl, S.; Carvalho, A.T.; Pinto, C.E.; Ayasse, M.; Schlindwein, C. (2015). Pollination biology in the dioecious orchid Catasetum uncatum: How does floral scent influence the behaviour of pollinators? Phytochemistry 2015, 116, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Milet-Pinheiro, P.; Gerlach, G. Biology of the Neotropical orchid genus Catasetum: A historical review on floral scent chemistry and pollinators. Perspect. Plant Ecol. Evol. Syst. 2017, 27, 23–34. [Google Scholar] [CrossRef]
- Albuquerque, N.S.L.; Milet-Pinheiro, P.; Cruz, D.D.; Navarro, D.M.A.F.; Machado, I.C. Pollination of the strongly scented Sarcoglottis acaulis (Orchidaceae) by male orchid bees: Nectar as resource instead of perfume. Plant Biol. 2021, 23, 719–727. [Google Scholar] [CrossRef]
- Albuquerque, N.S.L.; Milet-Pinheiro, P.; Cruz, D.D.; Pimentel, G.M.; Souza, A.C.; Carneiro, A.M.; Machado, I.C. Phenology, Abundance and Efficiency of Pollinators Drive the Reproductive Success of Sarcoglottis acaulis (Orchidaceae) at the Atlantic Forest. Acta Bot. Bras. 2022, 36, e2021abb0121. [Google Scholar] [CrossRef]
- Liu, J.W.; Milet-Pinheiro, P.; Gerlach, G.; Ayasse, M.; Nunes, C.E.P.; Alves-dos-Santos, I.; Ramírez, S.R. Macroevolution of floral scent chemistry across radiations of male euglossine bee-pollinated plants. Evolution 2024, 78, 98–110. [Google Scholar] [CrossRef]
- Stort, M.N.S. Influência do número de polínias sobre a fertilidade em cruzamentos de orquídeas. Ciênc. Cult. 1969, 21, 238–239. [Google Scholar]
- Stort, M.N.S. Estudos em híbridos F1 artificiais de orquídeas. Ciênc. Cult. 1972, 24, 847–851. [Google Scholar]
- Stort, M.N.S. Desarrollo del óvulo, posterior a la polinización en Cyrtopodium cardiochilum Lindl. Orquideología 1972, 7, 22–28. [Google Scholar]
- Stort, M.N.S. Ovule development after pollination in Eulophidium orchids. Amer. Orch. Soc. Bull. 1972, 41, 23–28. [Google Scholar]
- Stort, M.N.S. Cruzamentos artificiais entre plantas diplóides e tetraplóides de Cattleya bicolor Lindl. Ciênc. Cult. 1976, 28, 1208–1211. [Google Scholar]
- Stort, M.N.S. Autopolinização e polinização cruzada em algumas espécies do gênero Cattleya (Orchidaceae). Ciênc. Cult. 1979, 32, 1080–1083. [Google Scholar]
- Stort, M.N.S. Sterility barriers of some artificial F1 orchid hybrids: Male sterility. I. Microsporogensis and pollen germination. Amer. J. Bot. 1984, 71, 309–318. [Google Scholar] [CrossRef]
- Stort, M.N.S. (1986) Fertilidade de cruzamentos e relação filogenética entre algumas espécies do gênero Cattleya Lindl. (Orchidaceae). Rev. Bras. Bot. 1986, 9, 69–74. [Google Scholar]
- Coleta, M.H.D.; Stort, M.N.S. Estudo da possível influência do comprimento do órgão reprodutor feminino como barreira de isolamento reprodutivo em orquídeas. Ciênc. Cult. 1976, 28, 936–939. [Google Scholar]
- Pansarin, E.R.; Amaral, M.C.E. Reproductive biology and pollination of southeastern Brazilian Stanhopea Frost ex Hook. (Orchidaceae). Flora 2009, 204, 238–249. [Google Scholar] [CrossRef]
- Caballero-Villalobos, L.; Silva-Arias, G.A.; Buzatto, C.R.; Nervo, M.H.; Singer, R.B. Generalized food-deceptive pollination in four Cattleya (Orchidaceae: Laeliinae) species from Southern Brazil. Flora 2017, 234, 195–206. [Google Scholar] [CrossRef]
- Milet-Pinheiro, P.; Silva, J.B.F.; Navarro, D.M.A.F.; Machado, I.C.S.; Gerlach, G. Notes on pollination ecology and floral scent chemistry of the rare neotropical orchid Catasetum galeritum Rchb.f. Plant Spec. Biol. 2018, 33, 158–163. [Google Scholar] [CrossRef]
- Amano, T.; Lamming, J.D.L.; Sutherland, W.J. Spatial gaps in global biodiversity information and the role of citizen science. BioScience 2016, 66, 393–400. [Google Scholar] [CrossRef]
- Croce, A.; Nazzaro, R. An atlas of orchids distribution in the Campania region (Italy), a citizen science project for the most charming plant family. Ital. Bot. 2017, 4, 15–32. [Google Scholar] [CrossRef][Green Version]
- Bloom, E.H.; Crowder, D.W. Promoting data collection in pollinator citizen science projects. Citizen Sci. Theory Pract. 2020, 5, 3. [Google Scholar] [CrossRef]
- Bitonto, F.F.; Costantino, R.; Barberis, M.; Bogo, G.; Birtele, D.; Cangelmi, G.; Dal Cin, M.; Devalez, J.; Lenzi, L.; Magagnoli, S.; et al. LIFE4 Pollinators’ platform: How citizen science can help monitoring plants and ollinators. AoB Plants 2025, 17, plaf023. [Google Scholar] [CrossRef]
- Singer, R.B.; Sazima, M. The pollination mechanism of three sympatric Prescottia (Orchidaceae: Prescottinae) species in southeastern Brazil. Ann. Bot. 2001, 88, 999–1005. [Google Scholar] [CrossRef]
- Pansarin, L.M.; Pansarin, E.R.; Gerlach, G.; Sazima, M. The natural history of Cirrhaea and the pollination system of Stanhopeinae (Orchidaceae). Int. J. Plant Sci. 2018, 179, 436–449. [Google Scholar] [CrossRef]
- Brandt, K.; Machado, I.C.; Navarro, D.M.A.F.; Dötterl, S.; Ayasse, M.; Milet-Pinheiro, P. Sexual dimorphism in floral scents of the neotropical orchid Catasetum arietinum and its possible ecological and evolutionary significance. AoB Plants 2020, 12, 4. [Google Scholar] [CrossRef]
- Singer, R.B.; Cocucci, A.A. Pollination mechanism in southern Brazilian orchids which are exclusively or mainly pollinated by halictid bees. Plant Syst. Evol. 1999, 217, 101–117. [Google Scholar] [CrossRef]
- Borba, E.L.; Barbosa, A.R.; Melo, M.C.; Gontijo, S.L.; Oliveira, H.O. Mating systems in the Pleurothallidinae (Orchidaceae): Evolutionary and systematic implications. Lankesteriana 2011, 11, 207–221. [Google Scholar] [CrossRef][Green Version]
- Borba, E.L.; Shepherd, G.J.; Semir, J. Reproductive systems and crossing potential in three species of Bulbophyllum (Orchidaceae) occurring in Brazilian ‘campo rupestre’ vegetation. Plant Syst. Evol. 1999, 217, 205–214. [Google Scholar] [CrossRef]

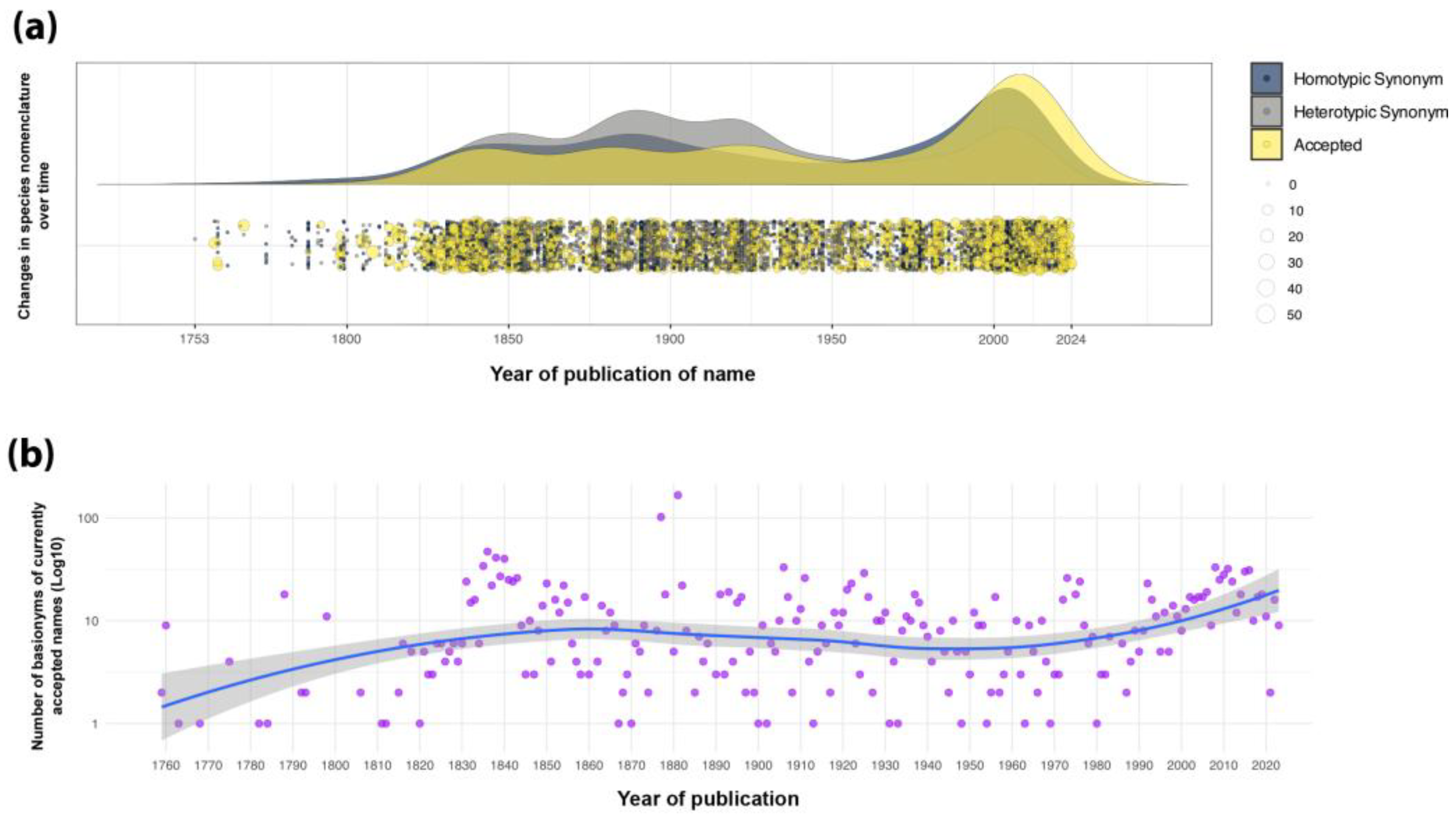

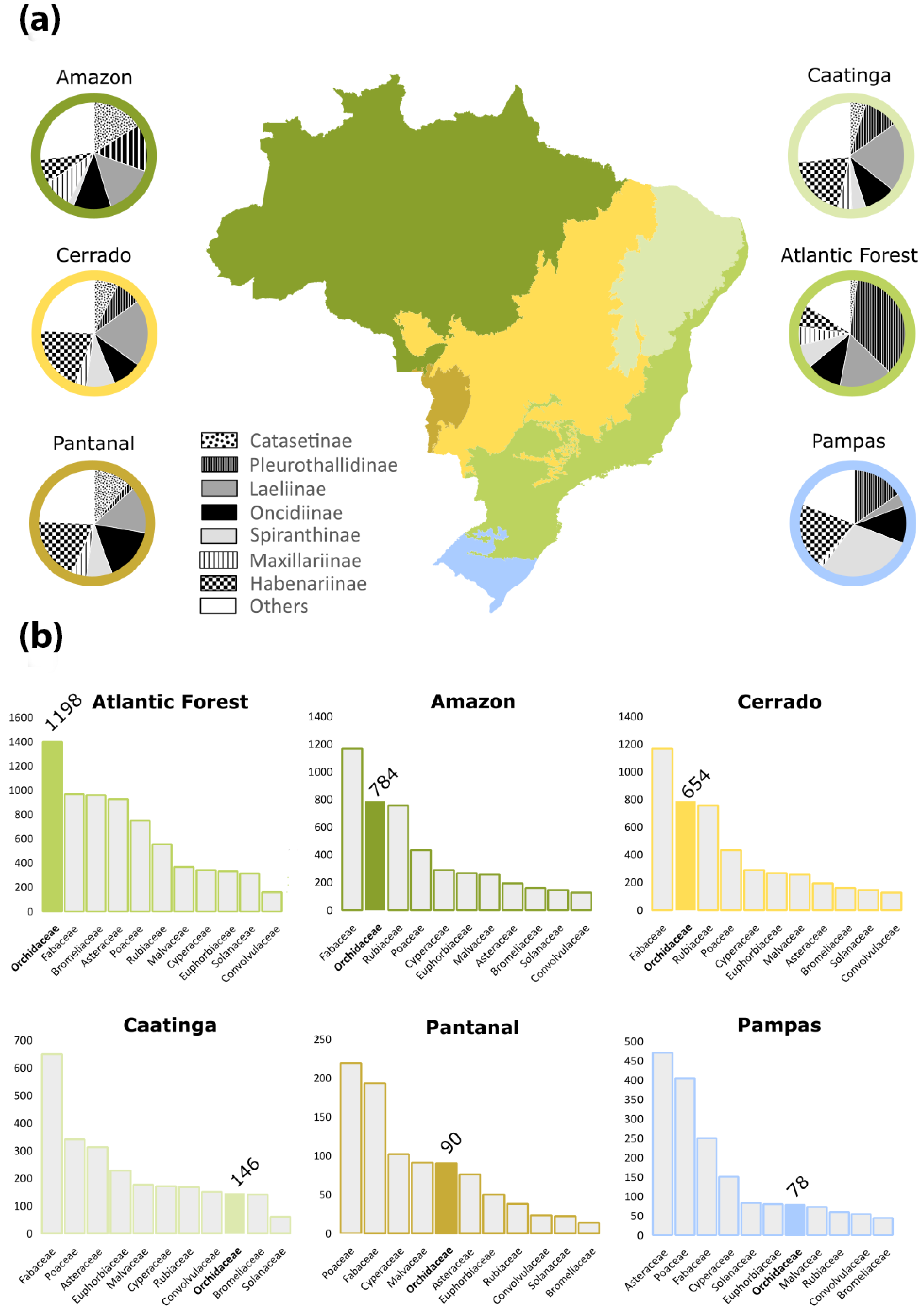
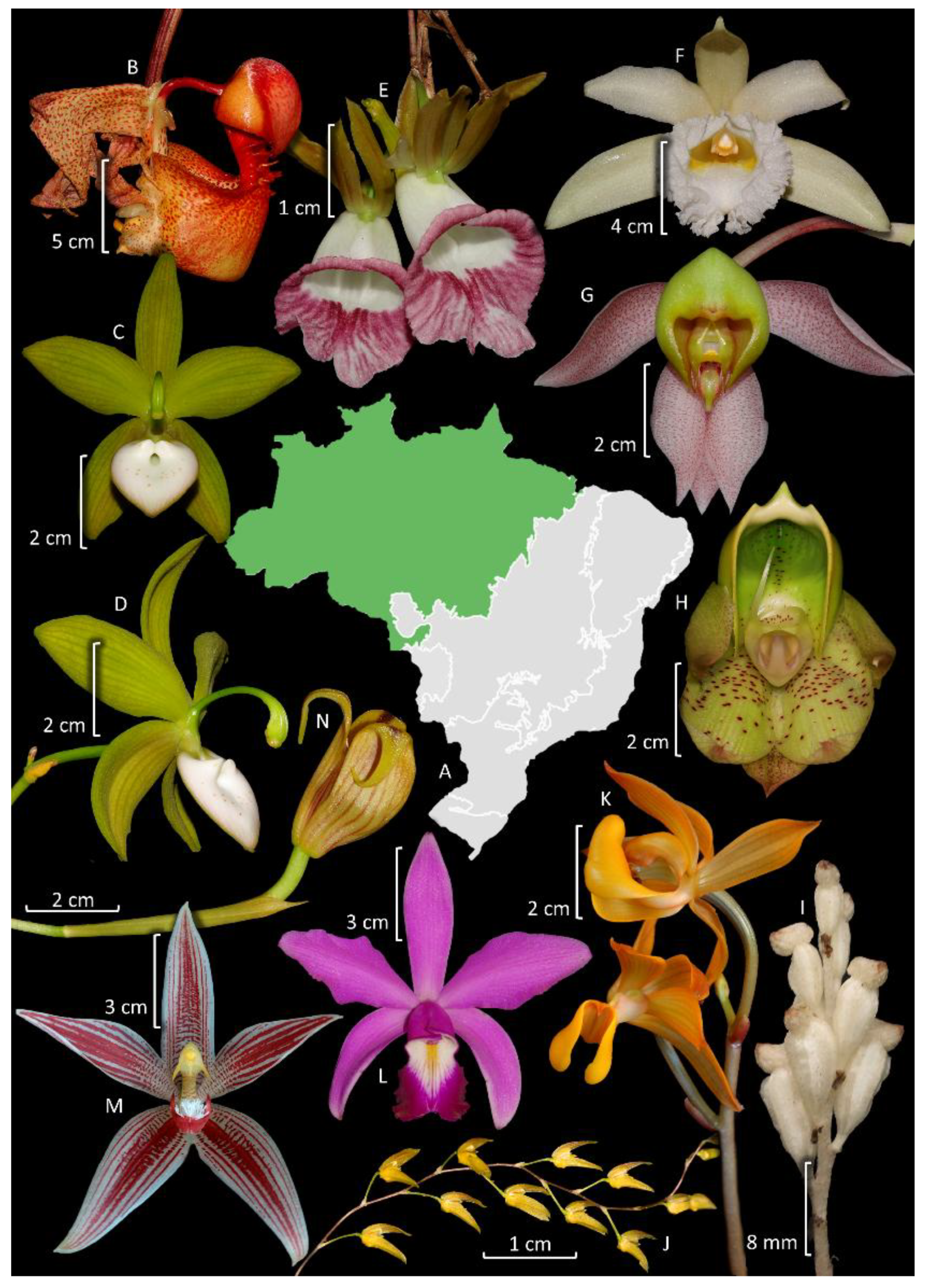
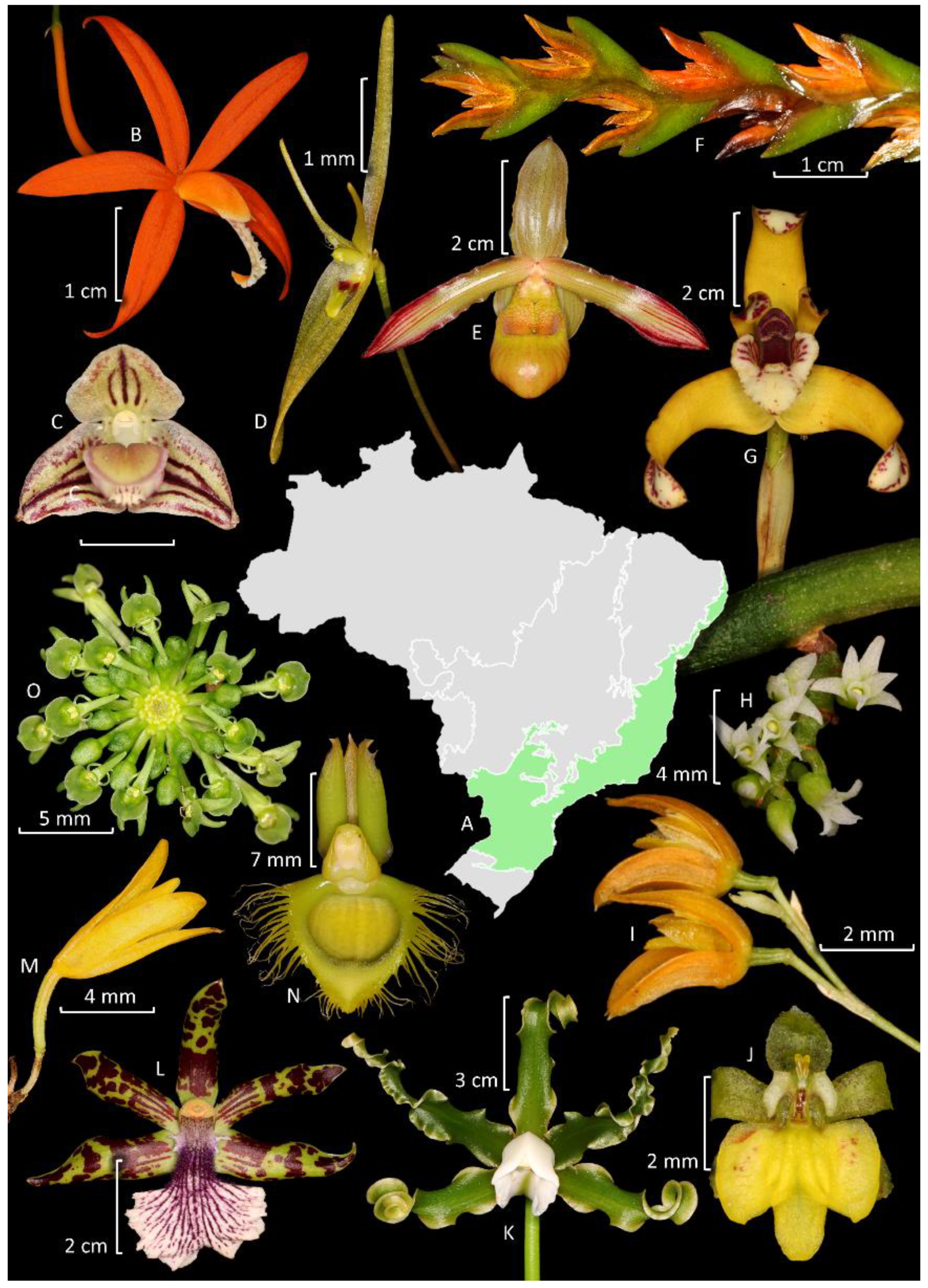

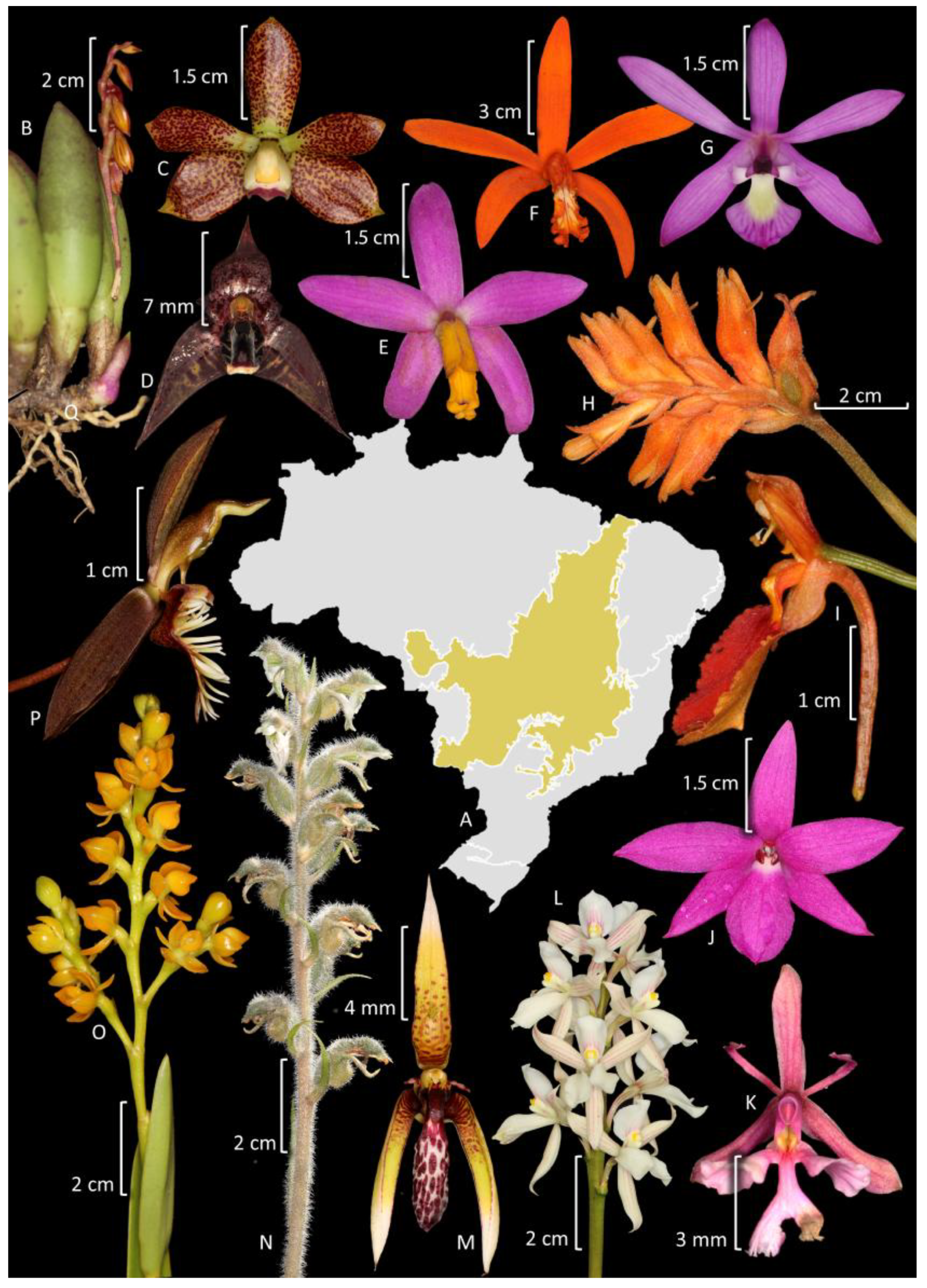
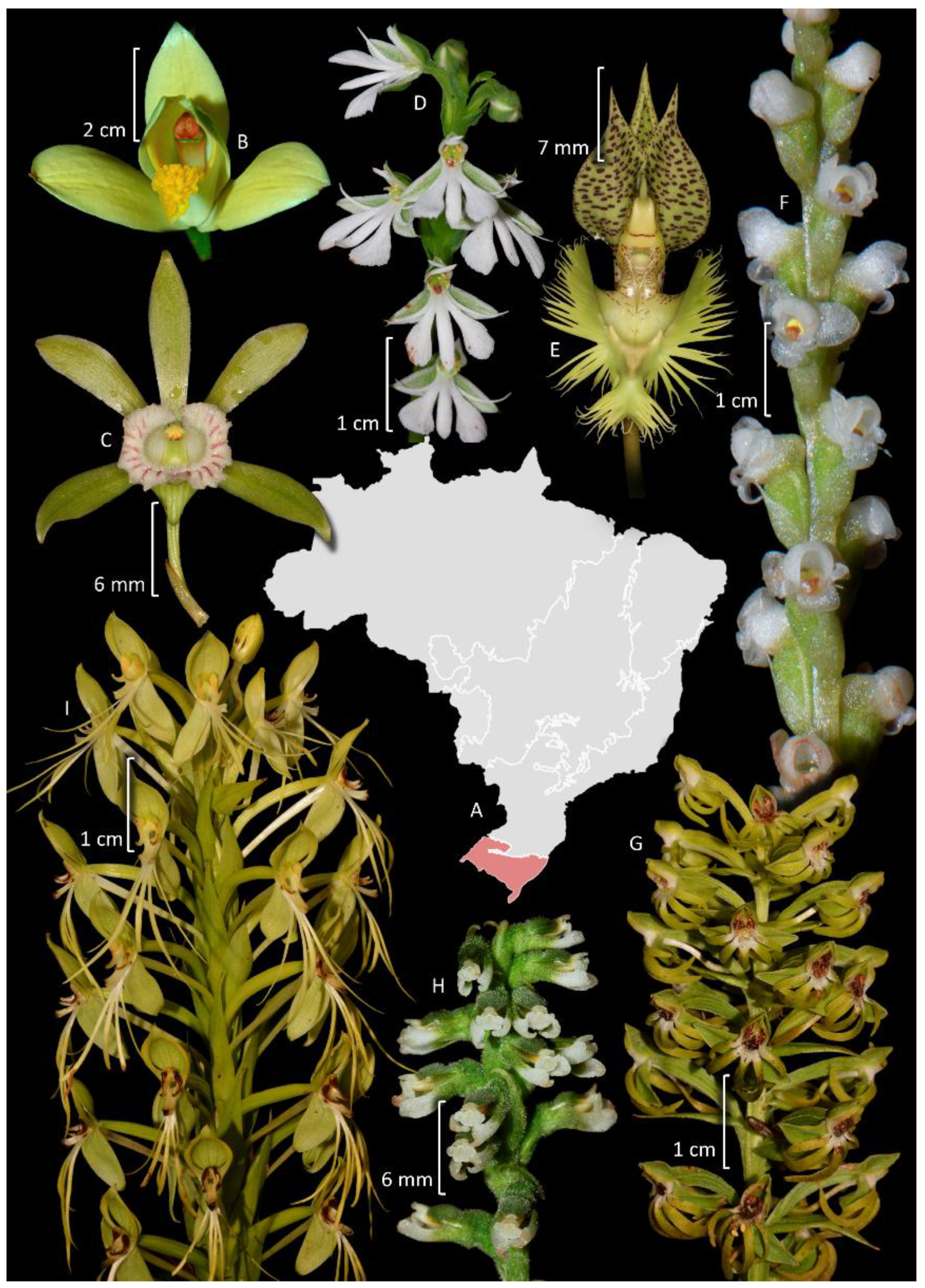
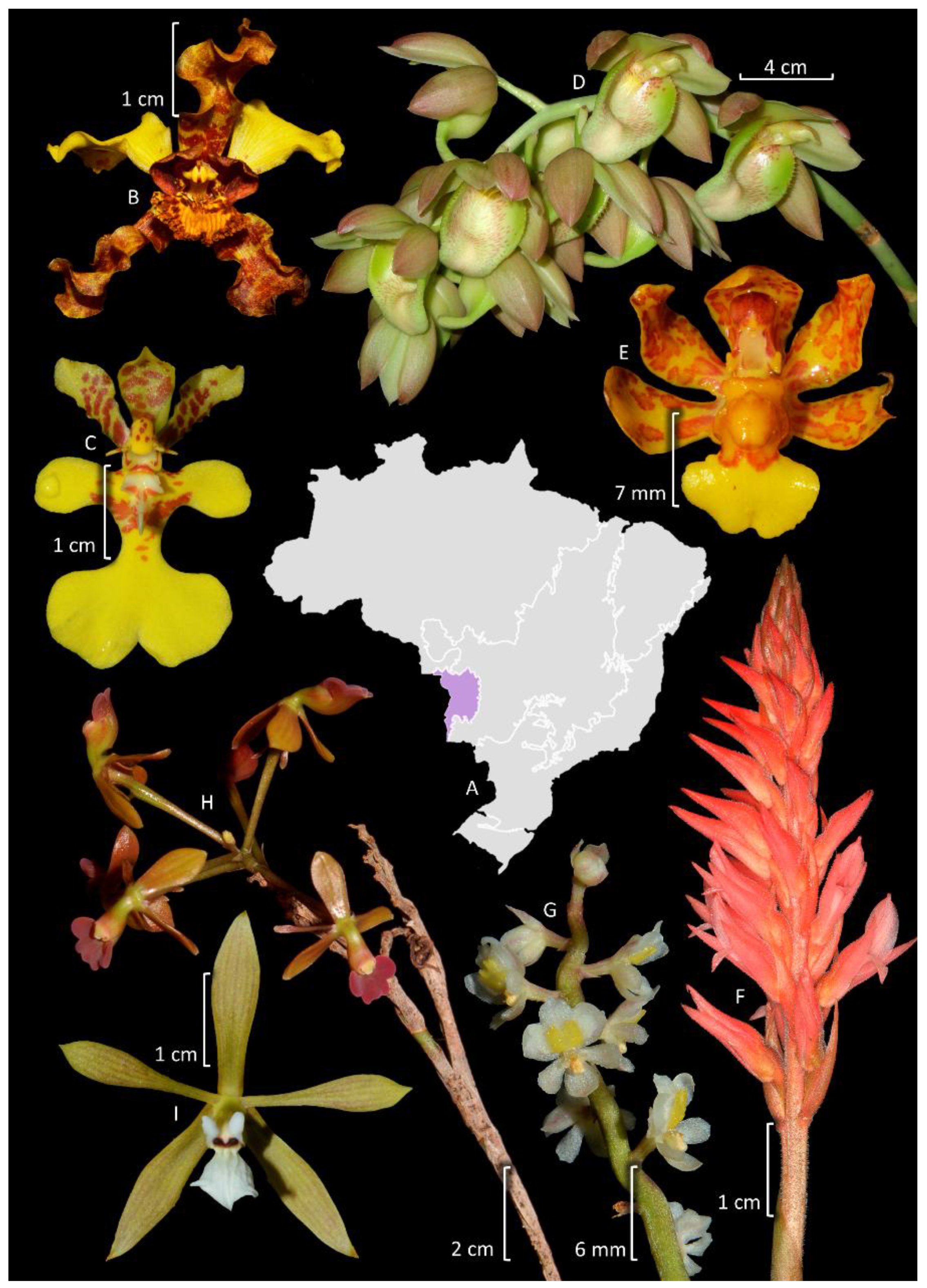
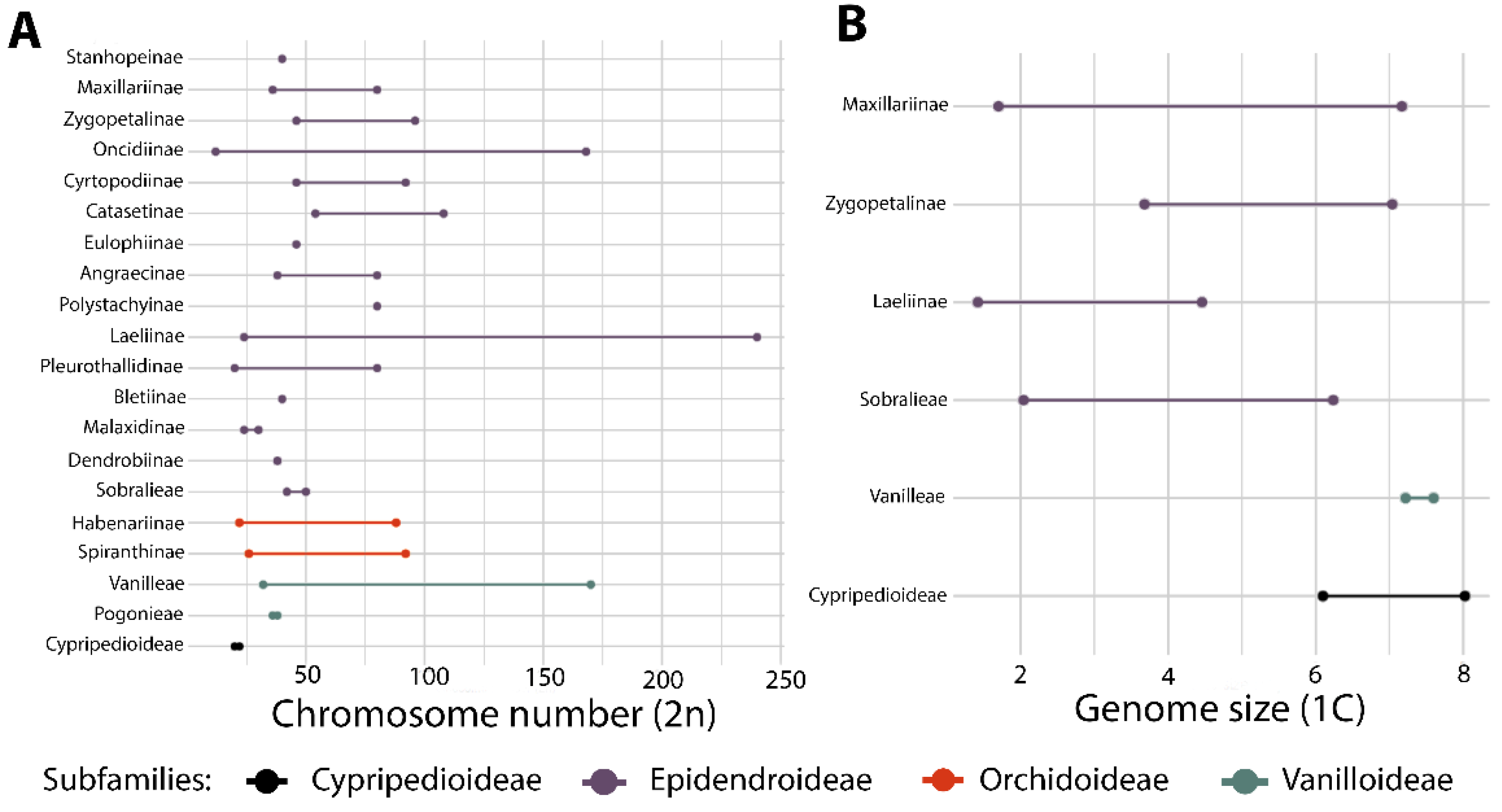
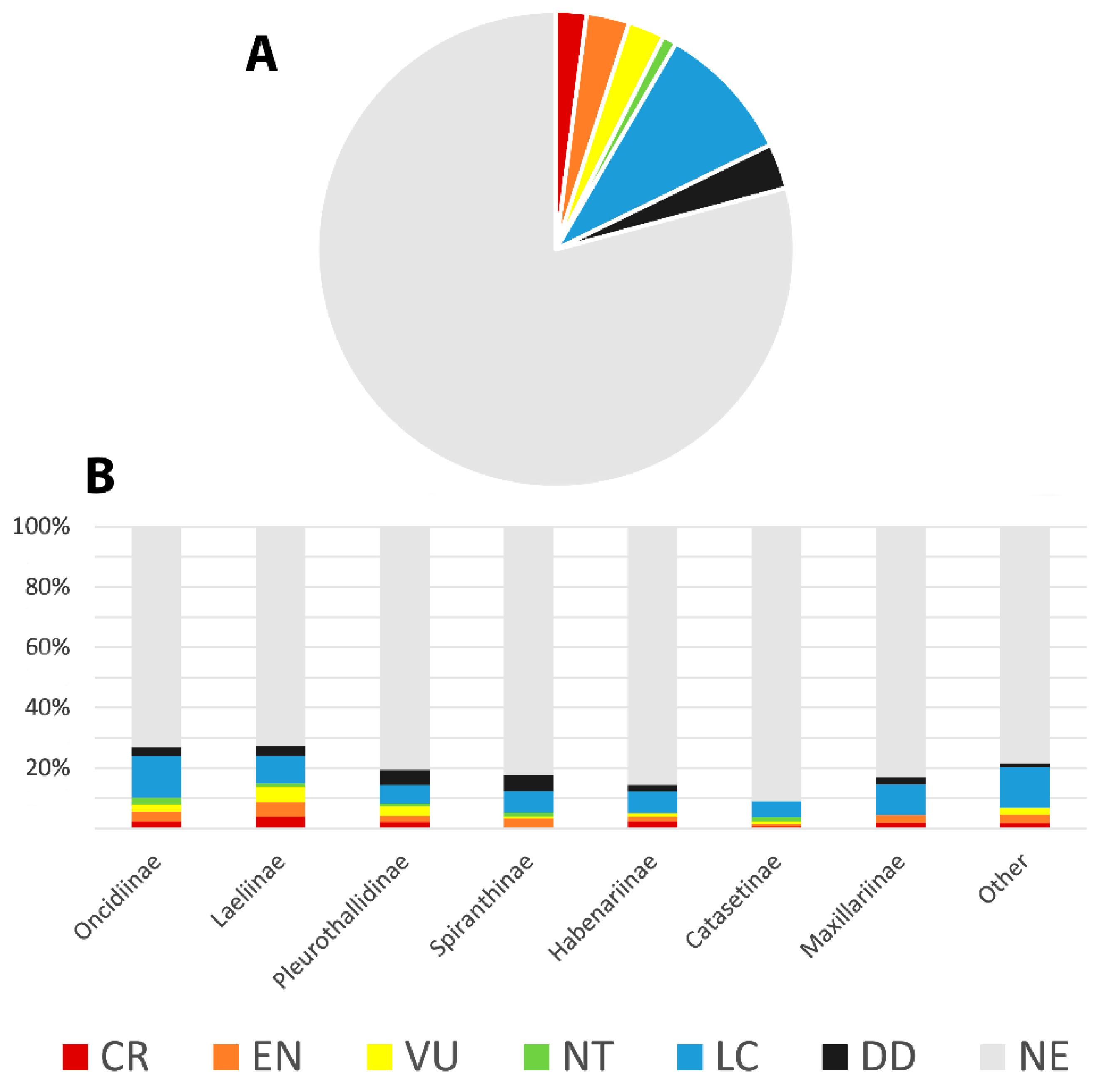
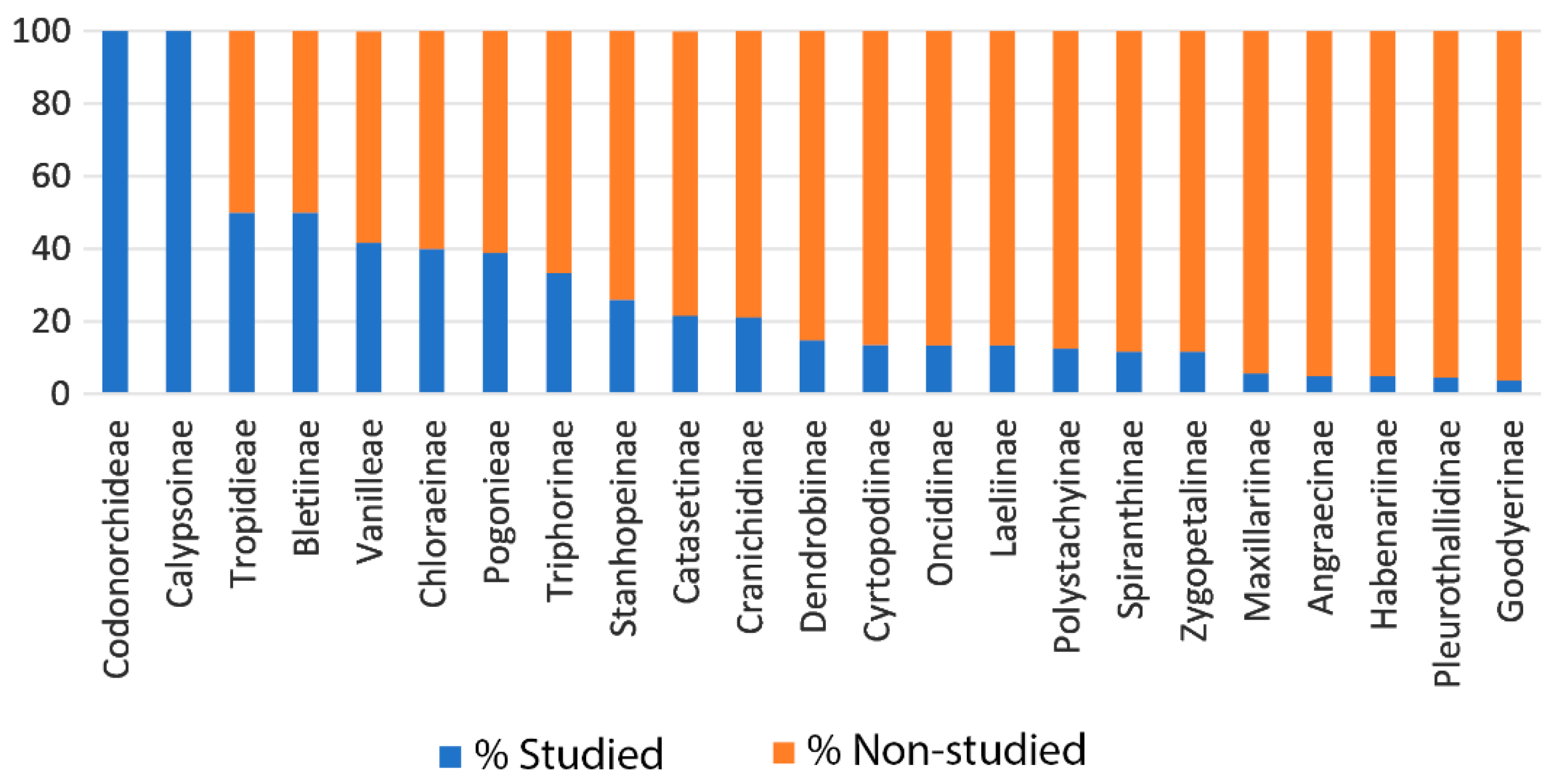
| Subfamilies | Tribes | Subtribes | |||
|---|---|---|---|---|---|
| Total (Endemic) | Total (Endemic) | Total (Endemic) | |||
| Cypripedioideae | 11 (4) | N/A | - | N/A | - |
| Orchidoideae | 387 (230) | Codonorchideae | 1 (1) | N/A | |
| Cranichideae | 206 (112) | Cranichidinae | 19 (13) | ||
| Goodyerinae | 27 (9) | ||||
| Spiranthinae | 154 (90) | ||||
| Chloraeinae | 5 (0) | ||||
| Discyphinae | 1 (0) | ||||
| Orchideae | 180 (117) | Habenariinae | 180 (117) | ||
| Vanilloideae | 54 (25) | Pogonieae | 18 (11) | N/A | - |
| Vanilleae | 36 (14) | N/A | - | ||
| Epidendroideae | 2063 (1281) | Cymbidieae | 818 (440) | Catasetinae | 186 (127) |
| Coeliopsidinae | 4 (1) | ||||
| Cyrtopodiinae | 37 (19) | ||||
| Eriopsidinae | 2 (0) | ||||
| Eulophiinae | 3 (0) | ||||
| Maxillariinae | 159 (71) | ||||
| Oncidiinae | 283 (163) | ||||
| Stanhopeinae | 73 (33) | ||||
| Zygopetalinae | 71 (26) | ||||
| Epidendreae | 1044 (748) | Bletiinae | 2 (1) | ||
| Pleurothallidinae | 642 (484) | ||||
| Calypsoinae | 1 (0) | ||||
| Ponerinae | 2 (1) | ||||
| Laeliinae | 397 (262) | ||||
| Gastrodieae | 3 (2) | N/A | - | ||
| Malaxideae | 94 (49) | Dendrobiinae | 81 (41) | ||
| Malaxidinae | 13 (8) | ||||
| Neottieae | 8 (4) | N/A | - | ||
| Sobralieae | 33 (8) | N/A | - | ||
| Triphoreae | 9 (4) | Triphorinae | 9 (4) | ||
| Tropidieae | 2 (0) | N/A | - | ||
| Vandeae | 48 (26) | Angraecinae | 40 (24) | ||
| Polystachynae | 8 (2) | ||||
| Wullschlaegelieae | 2 (0) | N/A | |||
| Xerorchideae | 2 (0) | N/A | - | ||
| Total | 2515 (1540) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, E.M.; Araújo, A.M.; Barberena, F.F.V.A.; Batista, J.A.N.; Benelli, A.P.; Bento, J.S.P.; Borba, E.L.; Camelo-Júnior, A.E.; Cantuária, P.C.; Cavalcanti, L.W.; et al. An Overview of Orchidaceae from Brazil: Advances and Shortfalls After 400 Years of Studies. Plants 2025, 14, 3520. https://doi.org/10.3390/plants14223520
Pessoa EM, Araújo AM, Barberena FFVA, Batista JAN, Benelli AP, Bento JSP, Borba EL, Camelo-Júnior AE, Cantuária PC, Cavalcanti LW, et al. An Overview of Orchidaceae from Brazil: Advances and Shortfalls After 400 Years of Studies. Plants. 2025; 14(22):3520. https://doi.org/10.3390/plants14223520
Chicago/Turabian StylePessoa, Edlley M., Adriane M. Araújo, Felipe F. V. A. Barberena, João A. N. Batista, Adarilda P. Benelli, João S. P. Bento, Eduardo L. Borba, Antônio Edmilson Camelo-Júnior, Patrick C. Cantuária, Letícia W. Cavalcanti, and et al. 2025. "An Overview of Orchidaceae from Brazil: Advances and Shortfalls After 400 Years of Studies" Plants 14, no. 22: 3520. https://doi.org/10.3390/plants14223520
APA StylePessoa, E. M., Araújo, A. M., Barberena, F. F. V. A., Batista, J. A. N., Benelli, A. P., Bento, J. S. P., Borba, E. L., Camelo-Júnior, A. E., Cantuária, P. C., Cavalcanti, L. W., Cintra, M. C. S., Engels, M., Feitoza, L. H. J., Felix, L. P., Ferreira, A. W. C., F. Fiorini, C., Guimarães, L. R. S., Klein, V. P., Koch, A. K., ... van den Berg, C. (2025). An Overview of Orchidaceae from Brazil: Advances and Shortfalls After 400 Years of Studies. Plants, 14(22), 3520. https://doi.org/10.3390/plants14223520








