Molecular Evolution of Cu Transporters and Transcription Factors in Plant Response to Copper Stress
Abstract
1. Introduction
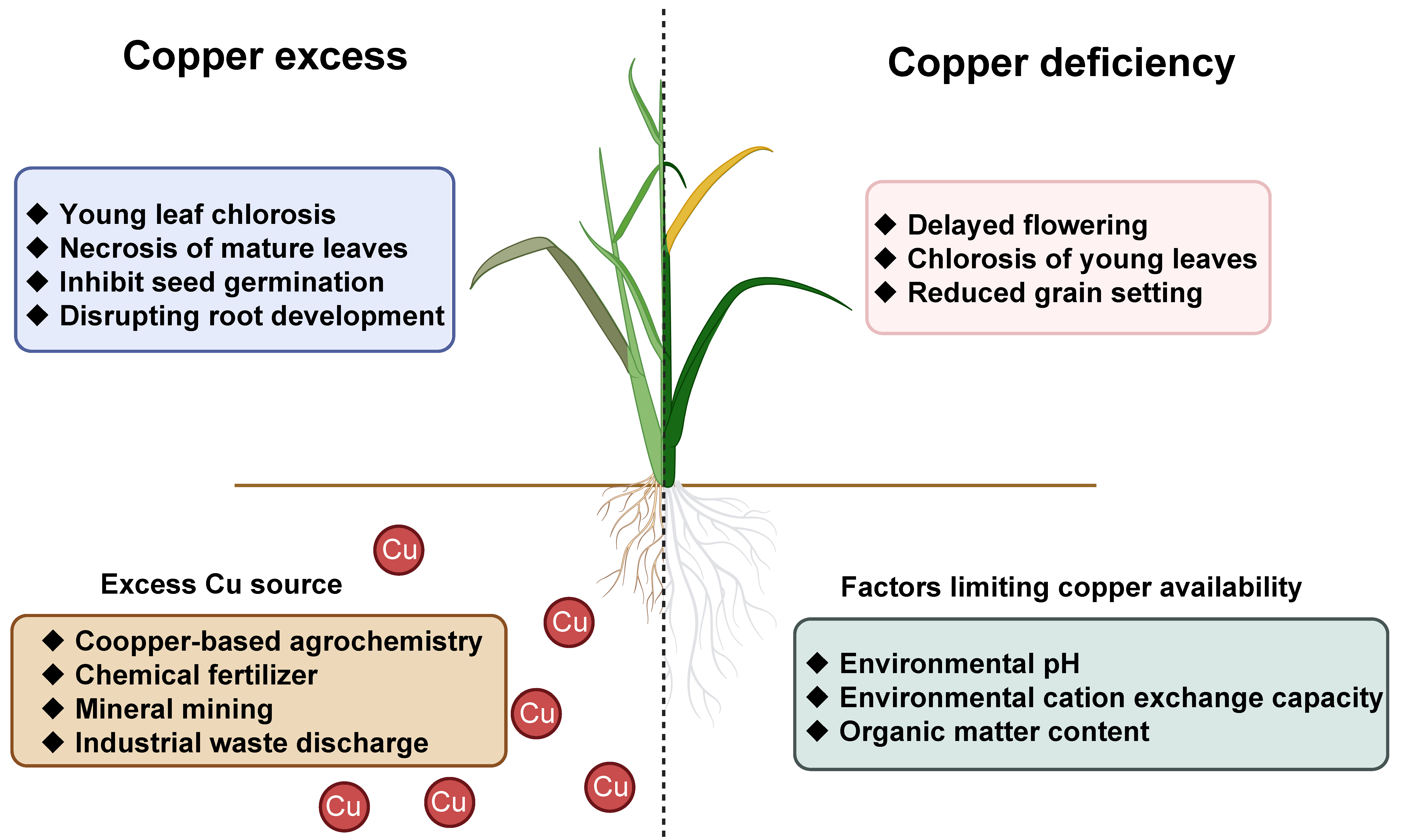
2. Soil Copper Contamination: Sources, Ecological Risks, and Crop Toxicity
3. CTR/COPT Copper Transporters: Structural Conservation and Functional Diversification
3.1. Functional Diversification of CTR/COPT Copper Transporters Across Green Plants and Yeast
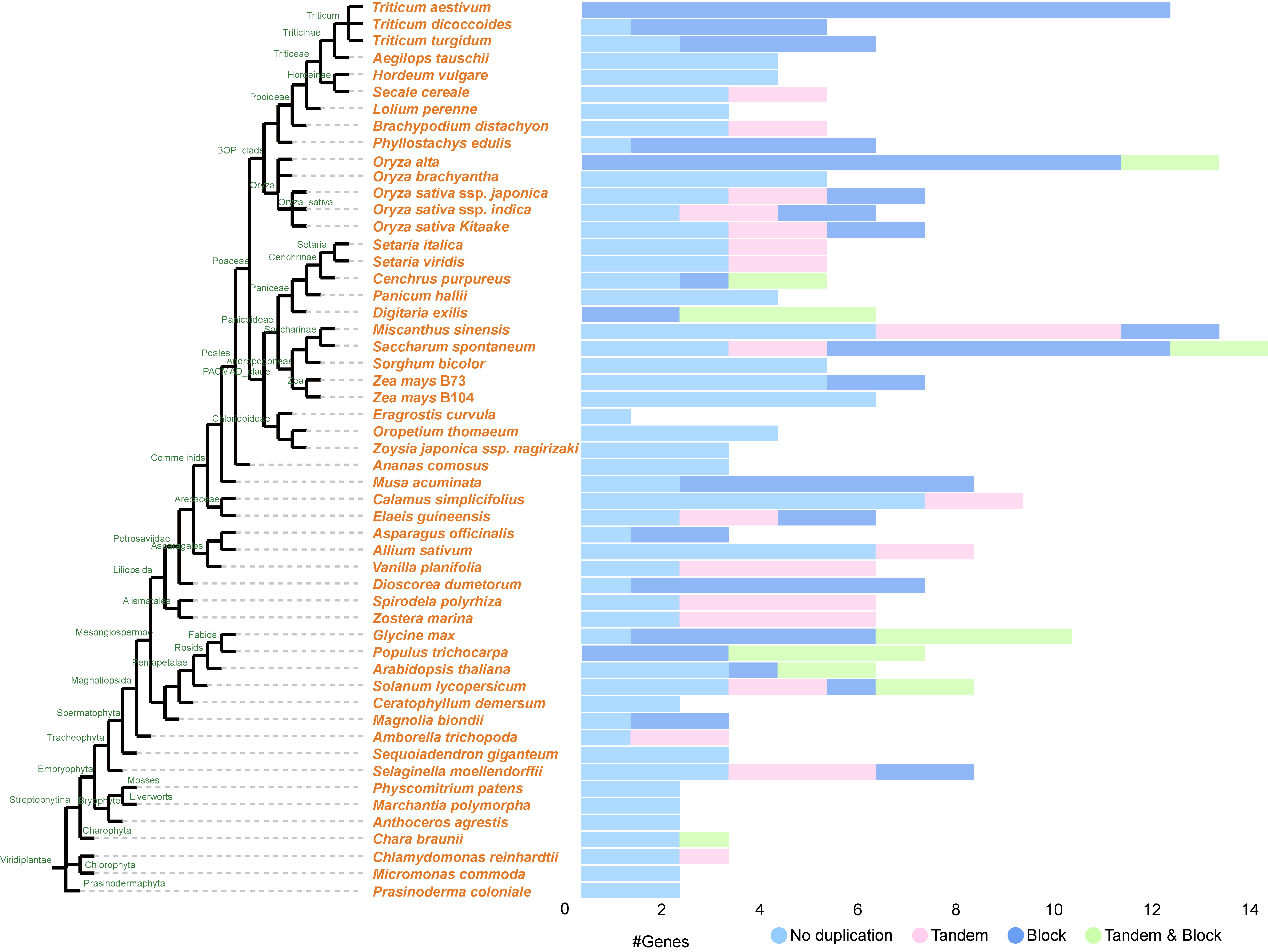
3.2. Evolutionary Conservation of COPT Copper Transporters Across Green Plants
4. Expression Analysis of COPT Genes in Diverse Plants
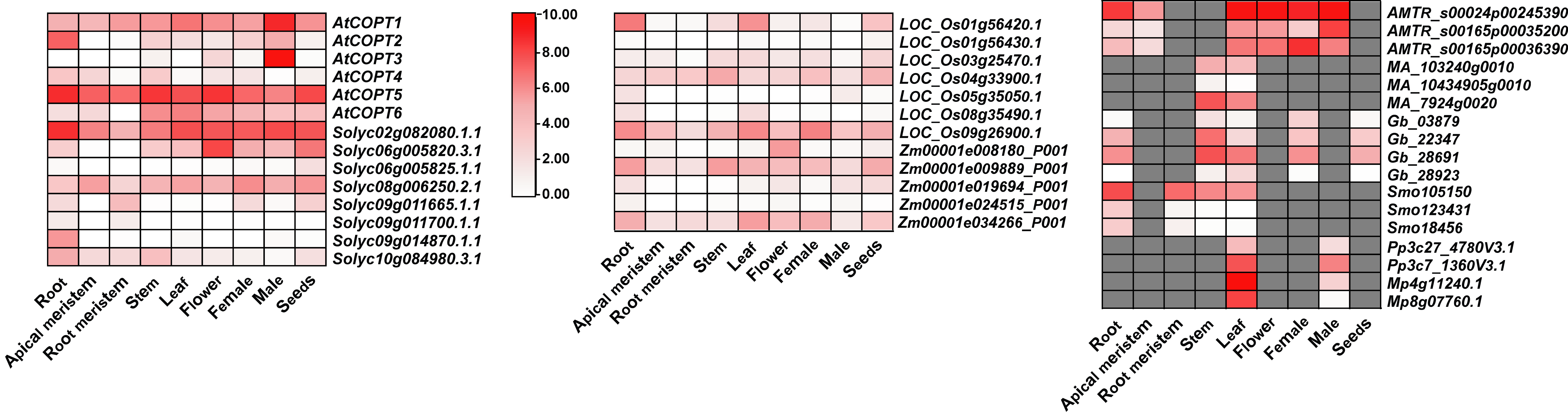
4.1. Tissue-Specific Gene Expression Analysis of COPT Genes
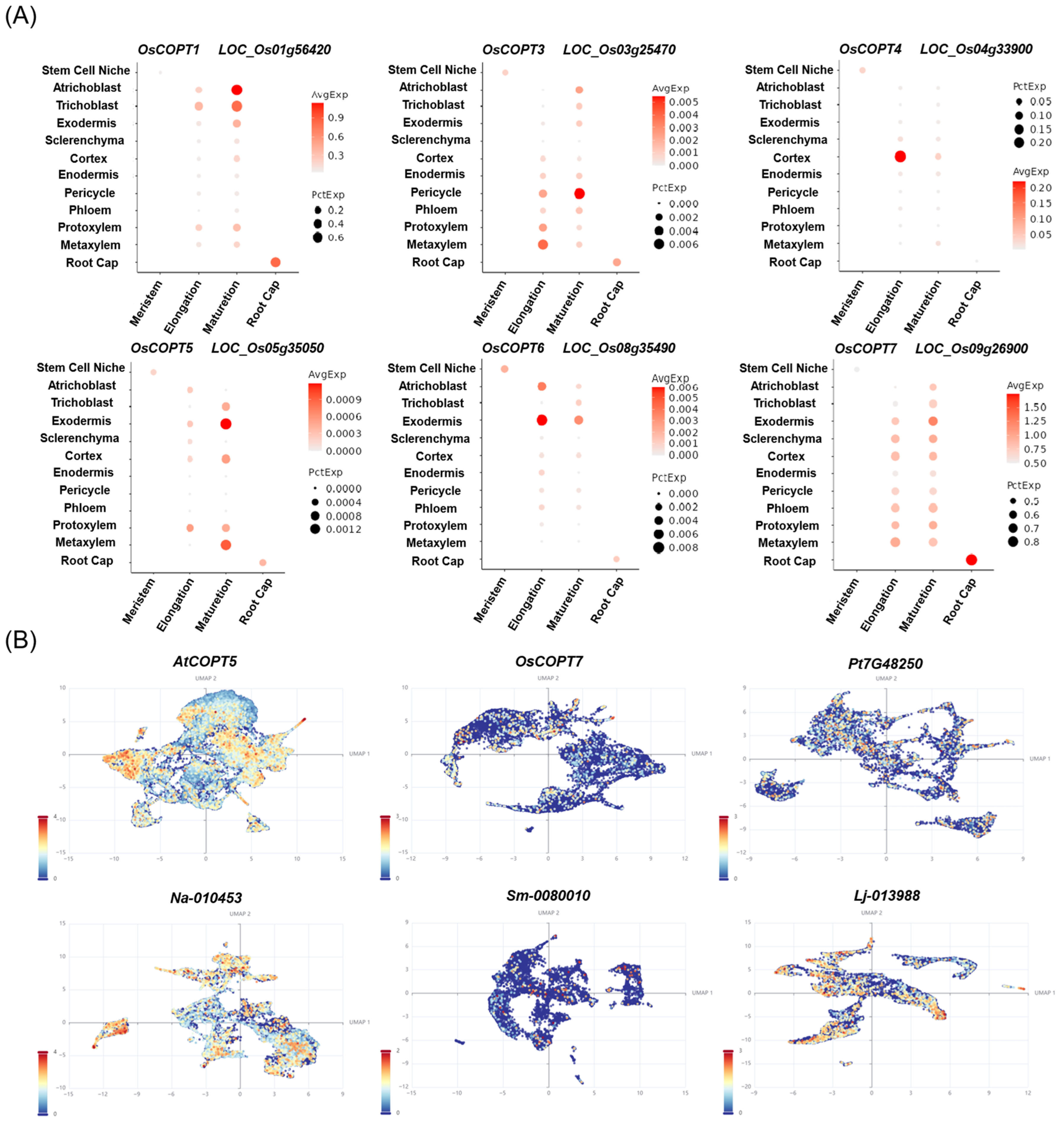
4.2. Single-Cell Expression Analysis of COPT Genes
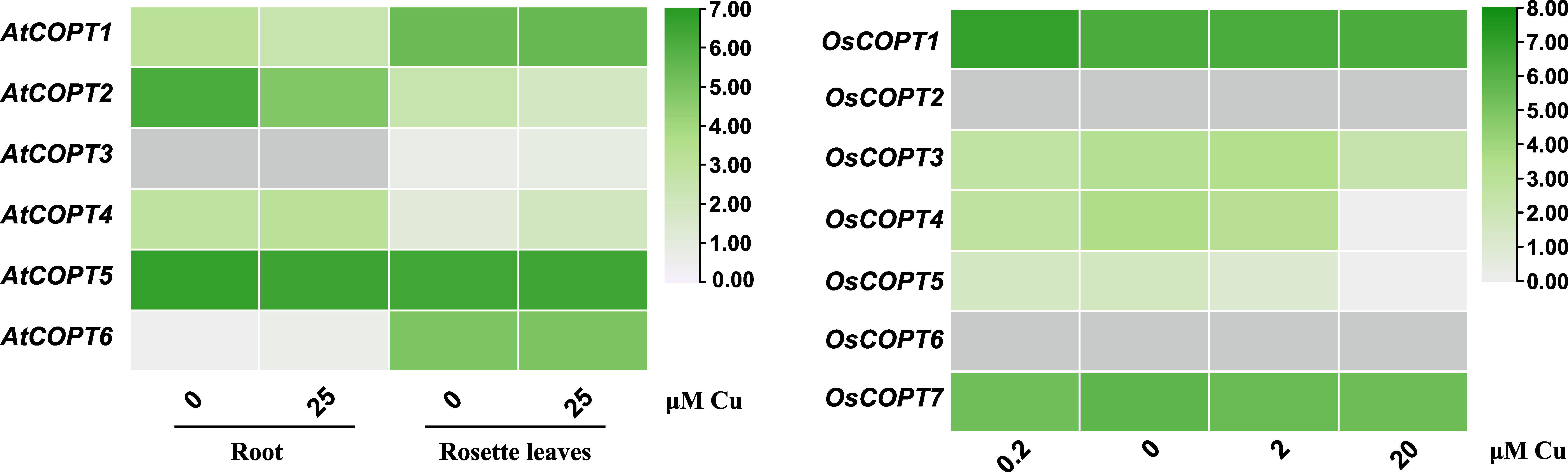
4.3. Gene Expression Analysis of COPT Genes in a Eudicot and a Monocot Under Conditions of Cu Excess or Deficiency
5. Cu Transport Systems and Cu Chaperone Proteins in Plants
6. Plant Cu Detoxification and Tolerance Mechanisms
6.1. Root Exudates
6.2. Isolation and Compartmentalization
6.3. Metal Efflux
6.4. Antioxidant Enzymes
7. Transcription Factors in Response to Cu Stress in Plants
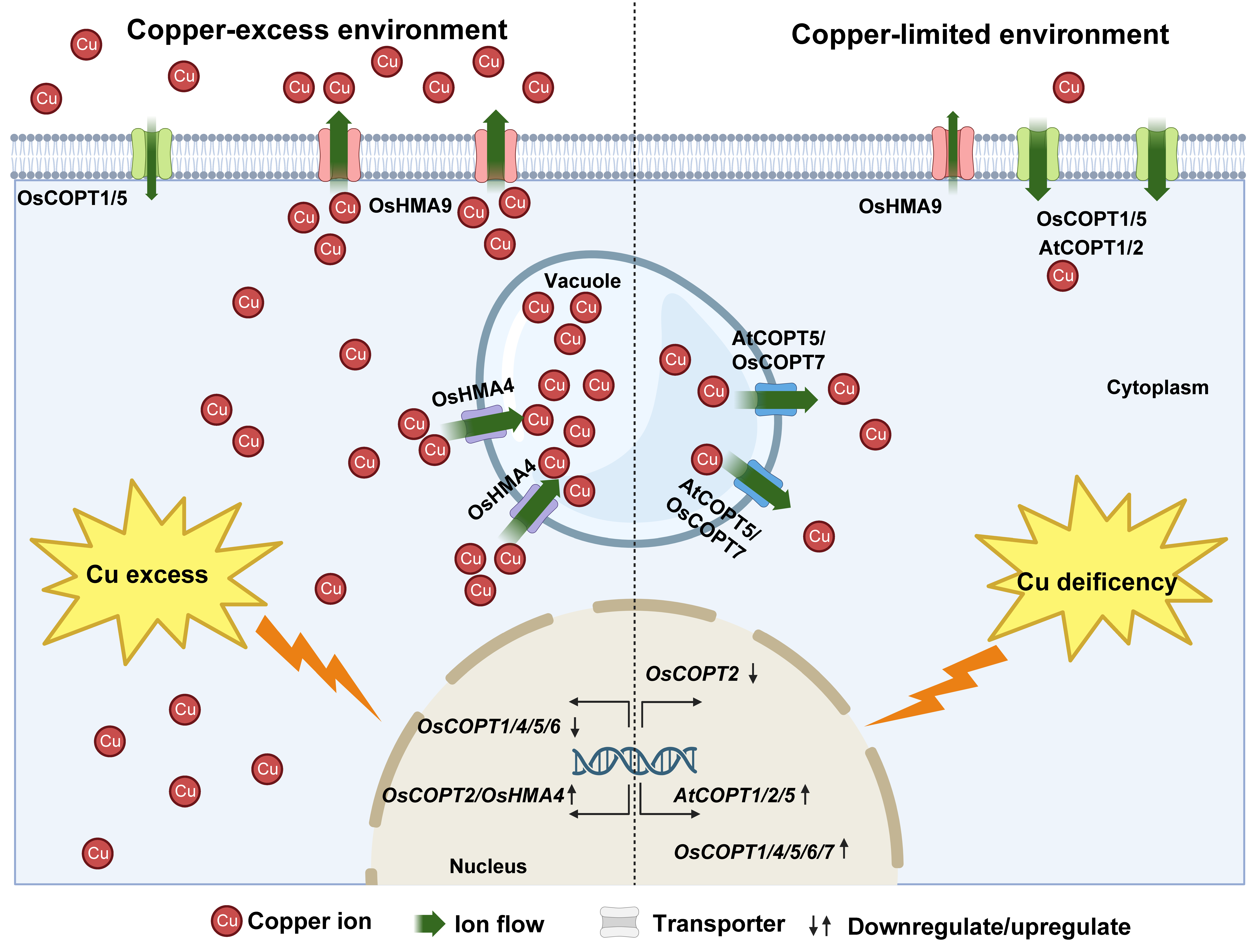
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. FPB 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chia, J.C.; Sheng, H.; Jung, H.I.; Zavodna, T.O.; Zhang, L.; Huang, R.; Jiao, C.; Craft, E.J.; Fei, Z.; et al. Arabidopsis Pollen Fertility Requires the Transcription Factors CITF1 and SPL7 That Regulate Copper Delivery to Anthers and Jasmonic Acid Synthesis. Plant Cell 2017, 29, 3012–3029. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Broadley, M.R. Marschner’s Mineral Nutrition of Higher Plants; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yuan, M.; Chu, Z.; Li, X.; Xu, C.; Wang, S. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 2010, 22, 3164–3176. [Google Scholar] [CrossRef]
- Wang, R.-X.; Wang, Z.-H.; Sun, Y.-D.; Wang, L.-L.; Li, M.; Liu, Y.-T.; Zhang, H.-M.; Jing, P.-W.; Shi, Q.-F.; Yu, Y.-H. Molecular mechanism of plant response to copper stress: A review. Environ. Exp. Bot. 2024, 218, 105590. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Rahmati Ishka, M.; Vatamaniuk, O.K. Copper deficiency alters shoot architecture and reduces fertility of both gynoecium and androecium in Arabidopsis thaliana. Plant Direct 2020, 4, e00288. [Google Scholar] [CrossRef]
- Li, X.Y.; Lin, M.L.; Lu, F.; Zhou, X.; Xiong, X.; Chen, L.S.; Huang, Z.R. Physiological and Ultrastructural Responses to Excessive-Copper-Induced Toxicity in Two Differentially Copper Tolerant Citrus Species. Plants 2023, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Daughety, M.M.; DeLoughery, T.G. Unusual Anemias. Med. Clin. N. Am. 2017, 101, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Llanos, R.M.; Mercer, J.F.B. The molecular basis of copper homeostasis copper-related disorders. DNA Cell Biol. 2002, 21, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.C.; Vatamaniuk, O.K. Shall we talk? New details in crosstalk between copper and iron homeostasis uncovered in Arabidopsis thaliana. New Phytol. 2024, 242, 832–835. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, T.; Guo, Y.; Wang, M.; Brachhold, K.; Chu, C.; Hanson, A.; Kumar, S.; Lin, R.; Long, W.; et al. 100 essential questions for the future of agriculture. Mod. Agric. 2023, 1, 4–12. [Google Scholar] [CrossRef]
- Kou, C.; Song, F.; Li, D.; Xu, H.; Zhang, S.; Yang, W.; Shi, W.; Gao, Z. A necessary considering factor for crop resistance: Precise regulation and effective utilization of beneficial microorganisms. New Crops 2024, 1, 100023. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Li, H.; Huang, Y.; Ding, J.; Liu, C.; Wang, N.; Lan, H.; Zhang, S. Identification and functional characterization of the ZmCOPT copper transporter family in maize. PLoS ONE 2018, 13, e0199081. [Google Scholar] [CrossRef]
- Romero, P.; Gabrielli, A.; Sampedro, R.; Perea-García, A.; Puig, S.; Lafuente, M.T. Identification and molecular characterization of the high-affinity copper transporters family in Solanum lycopersicum. Int. J. Biol. Macromol. 2021, 192, 600–610. [Google Scholar] [CrossRef]
- Guo, L.; Li, T.; Zhang, B.; Yan, K.; Meng, J.; Chang, M.; Hou, L. Family Identification and Functional Study of Copper Transporter Genes in Pleurotus ostreatus. Int. J. Mol. Sci. 2024, 25, 12154. [Google Scholar] [CrossRef]
- Hussain, Q.; Ye, T.; Li, S.; Nkoh, J.N.; Zhou, Q.; Shang, C. Genome-Wide Identification and Expression Analysis of the Copper Transporter (COPT/Ctr) Gene Family in Kandelia obovata, a Typical Mangrove Plant. Int. J. Mol. Sci. 2023, 24, 15579. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, N.; Jin, X.; Min, X.; Ma, Y.; Liu, W. Molecular characterization of the COPT/Ctr-type copper transporter family under heavy metal stress in alfalfa. Int. J. Biol. Macromol. 2021, 181, 644–652. [Google Scholar] [CrossRef]
- Escaray, F.J.; Antonelli, C.J.; Copello, G.J.; Puig, S.; Peñarrubia, L.; Ruiz, O.A.; Perea-García, A. Characterization of the Copper Transporters from Lotus spp. and Their Involvement under Flooding Conditions. Int. J. Mol. Sci. 2019, 20, 3136. [Google Scholar] [CrossRef]
- Rosas-Santiago, P.; Zechinelli Pérez, K.; Gómez Méndez, M.F.; Vera López Portillo, F.; Ruiz Salas, J.L.; Cordoba Martínez, E.; Acosta Maspon, A.; Pantoja, O. A differential subcellular localization of two copper transporters from the COPT family suggests distinct roles in copper homeostasis in Physcomitrium patens. Plant Physiol. Biochem. 2021, 167, 459–469. [Google Scholar] [CrossRef]
- Guan, M.Y.; Cao, Z.; Xia, Y.C.; Xv, P.; Lin, X.Y.; Chen, M.X. OsCOPT7 is involved in copper accumulation and transport through xylem. J. Hazard. Mater. 2024, 477, 135245. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.F.; Zhang, Z.H.; Huang, X.Y.; Zhao, F.J. OsCOPT7 is a copper exporter at the tonoplast and endoplasmic reticulum and controls Cu translocation to the shoots and grain of rice. Plant Cell Environ. 2024, 47, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, H.; Hu, B.; Zhang, J.; Qin, Y.; Zeng, F.; Chen, G.; Chen, Z.-H.; Deng, F. The vascular preferentially expressed OsCOPT7 mediates the long-distance transport of copper in rice. Plant Soil 2025. [Google Scholar] [CrossRef]
- Senovilla, M.; Castro-Rodríguez, R.; Abreu, I.; Escudero, V.; Kryvoruchko, I.; Udvardi, M.K.; Imperial, J.; González-Guerrero, M. Medicago truncatula copper transporter 1 (MtCOPT1) delivers copper for symbiotic nitrogen fixation. New Phytol. 2018, 218, 696–709. [Google Scholar] [CrossRef]
- Huang, X.Y.; Deng, F.; Yamaji, N.; Pinson, S.R.; Fujii-Kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.L.; Salt, D.E.; Ma, J.F. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef]
- Polesel, M.; Ingles-Prieto, A.; Christodoulaki, E.; Ferrada, E.; Doucerain, C.; Altermatt, P.; Knecht, M.; Kuhn, M.; Steck, A.L.; Wilhelm, M.; et al. Functional characterization of SLC39 family members ZIP5 and ZIP10 in overexpressing HEK293 cells reveals selective copper transport activity. Biometals 2023, 36, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, N.; Xiong, H.; Qiu, W.; Nakanishi, H.; Kobayashi, T.; Nishizawa, N.K.; Zuo, Y. The Yellow Stripe-Like (YSL) Gene Functions in Internal Copper Transport in Peanut. Genes 2018, 9, 635. [Google Scholar] [CrossRef]
- Hussain, Q.; Ye, T.; Shang, C.; Li, S.; Khan, A.; Nkoh, J.N.; Mustafa, A.E.-Z.M.A.; Elshikh, M.S. NRAMP gene family in Kandelia obovata: Genome-wide identification, expression analysis, and response to five different copper stress conditions. Front. Plant Sci. 2023, 14, 1318383. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, Y.; Chen, X.; Xiao, N.; Jiang, W.; Tang, H.; Zhou, H.; Qiu, X.; Xu, J.; Zeng, F.; et al. The differential partition of copper in cell wall and symplastic space contributes to the natural variation of copper toxicity tolerance in rice. Plant Soil 2025, 510, 583–601. [Google Scholar] [CrossRef]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, impact, adaptive mechanisms, and phytoremediation of heavy metals by plants: Role of transporters in heavy metal sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gong, Y.; Liu, Q.; Yang, S.; Ma, J.; Zhao, L.; Hou, H. Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 2020, 244, 125516. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- dos Santos Savaio, S.; Barreiro, A.; Núñez-Delgado, A.; Suluda, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique. Processes 2025, 13, 902. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Li, Z.; et al. A global meta-analysis of heavy metal(loid)s pollution in soils near copper mines: Evaluation of pollution level and probabilistic health risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef]
- Apori, O.S.; Hanyabui, E.; Asiamah, Y.J. Remediation Technology for Copper Contaminated Soil: A Review. Asian Soil Res. J. 2018, 1, ASRJ.45322. [Google Scholar] [CrossRef]
- Franco, A.; Buoso, S.; Zanin, L.; Pinton, R.; Tomasi, N. Copper Toxicity in Maize: The Severity of the Stress is Reduced Depending on the Applied Fe-Chelating Agent. J. Plant Growth Regul. 2023, 42, 1567–1581. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Liu, Y.; Wu, J.F.; Yan, X.; Rong, Q.L.; Lu, Z.H. Effects of the content of Cu on grain filling properties and grain yield in paddy soils. J. Nucl. Agric. Sci. 2023, 37, 188–195. [Google Scholar]
- Kopittke, P.M.; Blamey, F.P.; Asher, C.J.; Menzies, N.W. Trace metal phytotoxicity in solution culture: A review. J. Exp. Bot. 2010, 61, 945–954. [Google Scholar] [CrossRef]
- Zhou, H.; Thiele, D.J. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 2001, 276, 20529–20535. [Google Scholar] [CrossRef]
- Sancenón, V.; Puig, S.; Mira, H.; Thiele, D.J.; Peñarrubia, L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol. Biol. 2003, 51, 577–587. [Google Scholar] [CrossRef]
- Lee, J.; Peña, M.M.; Nose, Y.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef]
- Dumay, Q.C.; Debut, A.J.; Mansour, N.M.; Saier, M.H., Jr. The copper transporter (Ctr) family of Cu+ uptake systems. J. Mol. Microbiol. Biotechnol. 2006, 11, 10–19. [Google Scholar] [PubMed]
- Kampfenkel, K.; Kushnir, S.; Babiychuk, E.; Inzé, D.; Van Montagu, M. Molecular Characterization of a Putative Arabidopsis thaliana Copper Transporter and Its Yeast Homologue(*). J. Biol. Chem. 1995, 270, 28479–28486. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.; Xiao, J.; Wang, S. Molecular and functional analyses of COPT/Ctr-type copper transporter-like gene family in rice. BMC Plant Biol. 2011, 11, 69. [Google Scholar] [CrossRef]
- Sanz, A.; Pike, S.; Khan, M.A.; Carrió-Seguí, À.; Mendoza-Cózatl, D.G.; Peñarrubia, L.; Gassmann, W. Copper uptake mechanism of Arabidopsis thaliana high-affinity COPT transporters. Protoplasma 2018, 256, 161–170. [Google Scholar] [CrossRef]
- Klaumann, S.; Nickolaus, S.D.; Fürst, S.H.; Starck, S.; Schneider, S.; Ekkehard Neuhaus, H.; Trentmann, O. The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 2011, 192, 393–404. [Google Scholar] [CrossRef]
- Jung, H.I.; Gayomba, S.R.; Rutzke, M.A.; Craft, E.; Kochian, L.V.; Vatamaniuk, O.K. COPT6 is a plasma membrane transporter that functions in copper homeostasis in Arabidopsis and is a novel target of SQUAMOSA promoter-binding protein-like 7. J. Biol. Chem. 2012, 287, 33252–33267. [Google Scholar] [CrossRef]
- Ding, J.; Ji, C.; Yu, L.; Wang, C.; Ding, G.; Wang, S.; Shi, L.; Xu, F.; Cai, H. OsMYB84, a transcriptional regulator of OsCOPT2 and OsHMA5, modulates copper uptake and transport and yield production in rice. Crop J. 2024, 12, 456–469. [Google Scholar] [CrossRef]
- Yao, S.; Kang, J.; Guo, G.; Yang, Z.; Huang, Y.; Lan, Y.; Zhou, T.; Wang, L.; Wei, C.; Xu, Z.; et al. The key micronutrient copper orchestrates broad-spectrum virus resistance in rice. Sci. Adv. 2022, 8, eabm0660. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-i.; Gayomba, S.R.; Yan, J.; Vatamaniuk, O.K. Brachypodium distachyon as a model system for studies of copper transport in cereal crops. Front. Plant Sci. 2014, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; He, W.; Qian, Q.; Shang, L. Genetic resource utilization in wild rice species: Genomes and gene bank. New Crops 2025, 2, 100065. [Google Scholar] [CrossRef]
- Jiang, W.; He, J.; Babla, M.; Wu, T.; Tong, T.; Riaz, A.; Zeng, F.; Qin, Y.; Chen, G.; Deng, F.; et al. Molecular evolution and interaction of 14-3-3 proteins with H+-ATPases in plant abiotic stresses. J. Exp. Bot. 2024, 75, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, H.; Abou-Elwafa, S.F.; Qiao, Y.; Chen, L.; Alshehri, M.A.; Wu, Y.; Jiang, W.; Tan, W. Molecular evolution and interaction of ROS with ion transport for plant abiotic stresses. New Plant Prot. 2024, 1, e22. [Google Scholar] [CrossRef]
- Fan, X.; Tang, H.; Chen, X.; Zeng, F.; Chen, G.; Chen, Z.H.; Qin, Y.; Deng, F. Allene oxide synthase 1 contributes to limiting grain arsenic accumulation and seedling detoxification in rice. Stress Biol. 2023, 3, 52. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wang, W.; Li, D.; Hu, X.; Wang, H.; Wei, M.; Liu, Q.; Wang, Z.; Li, C. Genome-wide identification and expression profiling of the copper transporter gene family in Populus trichocarpa. Plant Physiol. Biochem. PPB 2015, 97, 451–460. [Google Scholar] [CrossRef]
- Aller, S.G.; Eng, E.T.; De Feo, C.J.; Unger, V.M. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J. Biol. Chem. 2004, 279, 53435–53441. [Google Scholar] [CrossRef]
- Puig, S. Function and Regulation of the Plant COPT Family of High-Affinity Copper Transport Proteins. Adv. Bot. 2014, 2014, 476917. [Google Scholar] [CrossRef]
- Zhu, M.; Hsu, C.W.; Peralta Ogorek, L.L.; Taylor, I.W.; La Cavera, S.; Oliveira, D.M.; Verma, L.; Mehra, P.; Mijar, M.; Sadanandom, A.; et al. Single-cell transcriptomics reveal how root tissues adapt to soil stress. Nature 2025, 642, 721–729. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Jiang, S.; Kang, J.; Li, D.; Wang, K.; Xie, S.; Tong, C.; Liu, C.; Hu, G.; et al. A single-cell multi-omics atlas of rice. Nature 2025, 644, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.C.; Xu, Z.G.; Liu, Y.J.; Wang, L.; Ming, X.; Wu, Z.Y.; Lian, H.; Han, Y.W.; Xu, J.; Zhang, Z.D.; et al. A unified cell atlas of vascular plants reveals cell-type foundational genes and accelerates gene discovery. Cell 2025. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Transporters involved in mineral nutrient uptake in rice. J. Exp. Bot. 2016, 67, 3645–3653. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Perea-García, A.; Mayo de Andrés, S.; Garcia-Molina, A.; Dorcey, E.; Rodríguez-Navarro, S.; Pérez-Amador, M.A.; Puig, S.; Peñarrubia, L. Comparison of global responses to mild deficiency and excess copper levels in Arabidopsis seedlings. Met. Integr. Biometal Sci. 2013, 5, 1234–1246. [Google Scholar] [CrossRef]
- Pilon, M.; Cohu, C.M.; Ravet, K.; Abdel-Ghany, S.E.; Gaymard, F. Essential transition metal homeostasis in plants. Curr. Opin. Plant Biol. 2009, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Deng, F.; Yamaji, N.; Xia, J.; Ma, J.F. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Choi, H.; Segami, S.; Cho, H.T.; Martinoia, E.; Maeshima, M.; Lee, Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. Cell Mol. Biol. 2009, 58, 737–753. [Google Scholar]
- Williams, L.E.; Mills, R.F. P(1B)-ATPases—An ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005, 10, 491–502. [Google Scholar] [CrossRef]
- Li, W.; Lacey, R.F.; Ye, Y.; Lu, J.; Yeh, K.C.; Xiao, Y.; Li, L.; Wen, C.K.; Binder, B.M.; Zhao, Y. Triplin, a small molecule, reveals copper ion transport in ethylene signaling from ATX1 to RAN1. PLoS Genet. 2017, 13, e1006703. [Google Scholar] [CrossRef]
- Mayerhofer, H.; Sautron, E.; Rolland, N.; Catty, P.; Seigneurin-Berny, D.; Pebay-Peyroula, E.; Ravaud, S. Structural Insights into the Nucleotide-Binding Domains of the P1B-type ATPases HMA6 and HMA8 from Arabidopsis thaliana. PLoS ONE 2016, 11, e0165666. [Google Scholar]
- Sautron, E.; Mayerhofer, H.; Giustini, C.; Pro, D.; Crouzy, S.; Ravaud, S.; Pebay-Peyroula, E.; Rolland, N.; Catty, P.; Seigneurin-Berny, D. HMA6 and HMA8 are two chloroplast Cu+-ATPases with different enzymatic properties. Biosci. Rep. 2015, 35, e00201. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Q.; Wang, L.; Du, Q.; Ackah, M.; Guo, P.; Zheng, D.; Wu, M.; Zhao, W. Functional Characterization of MaZIP4, a Gene Regulating Copper Stress Tolerance in Mulberry (Morus atropurpurea R.). Life 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-D.; Xie, Y.; Zhang, H.; Zhang, S.; Zhao, F.-J. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects Cd distribution to rice grain. Plant Soil 2022, 476, 79–95. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, L. Effects of Deficiency of Copper and Zinc on OsNRAMPs Expression and Main Metal-Ions Uptake in Oryza sativa L. Plant Physiol. Commun. 2011, 47, 879–884. [Google Scholar]
- Chen, X.; Zhao, Y.; Zhong, Y.; Chen, J.; Qi, X. Deciphering the functional roles of transporter proteins in subcellular metal transportation of plants. Planta 2023, 258, 17. [Google Scholar] [CrossRef]
- Waters, B.M.; Grusak, M.A. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol. 2008, 177, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Chiecko, J.; Punshon, T.; Lanzirotti, A.; Lahner, B.; Salt, D.E.; Walker, E.L. Successful reproduction requires the function of Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol. 2010, 154, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, W.; Yang, Y.; Shen, Z.; Ma, J.F.; Zheng, L. OsYSL16 is Required for Preferential Cu Distribution to Floral Organs in Rice. Plant Cell Physiol. 2018, 59, 2039–2051. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, C.; Wang, K.; Fang, Z. Efficient oxidative degradation of fluconazole by a heterogeneous Fenton process with Cu-V bimetallic catalysts. Chem. Eng. J. 2020, 380, 122516. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.; Liu, Y.; Wang, X.; Xu, Q.; Deng, X. Genome-wide identification of sweet orange (Citrus sinensis) histone modification gene families and their expression analysis during the fruit development and fruit-blue mold infection process. Front. Plant Sci. 2015, 6, 607. [Google Scholar] [CrossRef]
- Javed, M.T.; Stoltz, E.; Lindberg, S.; Greger, M. Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ. Sci. Pollut. Res. Int. 2013, 20, 1876–1880. [Google Scholar]
- Chen, J.; Shafi, M.; Wang, Y.; Wu, J.; Ye, Z.; Liu, C.; Zhong, B.; Guo, H.; He, L.; Liu, D. Organic acid compounds in root exudation of Moso Bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ. Sci. Pollut. Res. Int. 2016, 23, 20977–20984. [Google Scholar] [CrossRef]
- Noreen, S.; Akhter, M.S.; Yaamin, T.; Arfan, M. The ameliorative effects of exogenously applied proline on physiological and biochemical parameters of wheat (Triticum aestivum L.) crop under copper stress condition. J. Plant Interact. 2018, 13, 221–230. [Google Scholar]
- Yadav, P.; Kaur, R.; Kanwar, M.K.; Sharma, A.; Verma, V.; Sirhindi, G.; Bhardwaj, R. Castasterone confers copper stress tolerance by regulating antioxidant enzyme responses, antioxidants, and amino acid balance in B. juncea seedlings. Ecotoxicol. Environ. Saf. 2018, 147, 725–734. [Google Scholar] [CrossRef]
- Zhu, K.; Peng, Y.; Chen, Z.; Zhang, Q.; Xu, Q.; Wang, W.; He, K.; Chen, X. Effects of β-aminobutyric acid on tobacco growth under copper stress. Tob. Sci. Technol. 2015, 49, 8–13. [Google Scholar]
- Shangguan, X.; Qi, Y.; Wang, A.; Ren, Y.; Wang, Y.; Xiao, T.; Shen, Z.; Wang, Q.; Xia, Y. OsGLP participates in the regulation of lignin synthesis and deposition in rice against copper and cadmium toxicity. Front. Plant Sci. 2022, 13, 1078113. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, C.; Tang, F.; Zhou, Y.; Zhu, C.; Ding, Y. The cell wall functions in plant heavy metal response. Ecotoxicol. Environ. Saf. 2025, 299, 118326. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Shangguan, X.; Yan, J.; Yu, X.; Jing, W.; Peng, K.; Chen, Y.; Shen, Z.; Xia, Y. The pectin methylesterase OsPME14 modifies the cell wall to confer copper tolerance in Oryza sativa L. Plant J. Cell Mol. Biol. 2025, 122, e70173. [Google Scholar]
- Wu, Z.; Cui, C.; Xing, A.; Xu, X.; Sun, Y.; Tian, Z.; Li, X.; Zhu, J.; Wang, G.; Wang, Y. Identification and response analysis of xyloglucan endotransglycosylase/hydrolases (XTH) family to fluoride and aluminum treatment in Camellia sinensis. BMC Genom. 2021, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin Methylesterases: Cell Wall Remodeling Proteins Are Required for Plant Response to Heat Stress. Front. Plant Sci. 2018, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Fan, Y.; Wei, S.; Niu, Y.; Li, Y.; Huang, H.; Chen, Y.; Tang, Z.; Liu, L.; Qu, C.; et al. Comprehensive analysis of polygalacturonase genes offers new insights into their origin and functional evolution in land plants. Genomics 2021, 113, 1096–1108. [Google Scholar] [CrossRef]
- Rather, B.A.; Masood, A.; Sehar, Z.; Majid, A.; Anjum, N.A.; Khan, N.A. Mechanisms and Role of Nitric Oxide in Phytotoxicity-Mitigation of Copper. Front. Plant Sci. 2020, 11, 675. [Google Scholar] [CrossRef]
- Gouiaa, S.; Khoudi, H. Expression of V-PPase proton pump, singly or in combination with a NHX1 transporter, in transgenic tobacco improves copper tolerance and accumulation. Environ. Sci. Pollut. Res. 2019, 26, 37037–37045. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.Y.; Lee, Y.; An, G. Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 2007, 145, 831–842. [Google Scholar] [CrossRef]
- Liu, X.; Feng, S.; Zhang, B.; Wang, M.; Cao, H.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C.; Wang, K.; Zhao, J.; Shao, J.; Chen, H.; Zhou, M.; Zhu, X. Metal Tolerance Protein Encoding Gene Family in Fagopyrum tartaricum: Genome-Wide Identification, Characterization and Expression under Multiple Metal Stresses. Plants 2022, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Abbasi, G.H.; Hayat, T.; Ali, B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2014, 22, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-h.; Yang, N.; Wan, Q.-w.; Teng, R.-m.; Duan, A.-q.; Wang, Y.-h.; Zhuang, J. Exogenous melatonin positively regulates lignin biosynthesis in Camellia sinensis. Int. J. Biol. Macromol. 2021, 179, 485–499. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-Wide Analysis and Expression Profile of Superoxide Dismutase (SOD) Gene Family in Rapeseed (Brassica napus L.) under Different Hormones and Abiotic Stress Conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Du, X.; Zhang, J.; Zou, Y.; Liu, Y.; Li, Y.; Lin, H.; Li, H.; Liu, D.; et al. Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa. Int. J. Mol. Sci. 2022, 23, 3340. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Jin, Y.; Wang, C.; Yang, J.; Qi, H. Drought-induced ABA, H2O2 and JA positively regulate CmCAD genes and lignin synthesis in melon stems. BMC Plant Biol. 2021, 21, 83. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Z.; Zhou, X. WRKY Transcription Factors in Response to Metal Stress in Plants: A Review. Int. J. Mol. Sci. 2024, 25, 10952. [Google Scholar] [CrossRef]
- Djemal, R.; Khoudi, H. The ethylene-responsive transcription factor of durum wheat, TdSHN1, confers cadmium, copper, and zinc tolerance to yeast and transgenic tobacco plants. Protoplasma 2022, 259, 19–31. [Google Scholar] [CrossRef]
- Raldugina, G.N.; Maree, M.; Mattana, M.; Shumkova, G.; Mapelli, S.; Kholodova, V.P.; Karpichev, I.V.; Kuznetsov, V.V. Expression of rice OsMyb4 transcription factor improves tolerance to copper or zinc in canola plants. Biol. Plant. 2018, 62, 511–520. [Google Scholar] [CrossRef]
- Garcia-Molina, A.; Xing, S.; Huijser, P. Functional characterisation of Arabidopsis SPL7 conserved protein domains suggests novel regulatory mechanisms in the Cu deficiency response. BMC Plant Biol. 2014, 14, 231. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, C.; Du, J.; Xu, L.; Guo, Z.; Li, D.; Cai, H.; Guo, H.; Li, L. The carboxy terminal transmembrane domain of SPL7 mediates interaction with RAN1 at the endoplasmic reticulum to regulate ethylene signalling in Arabidopsis. New Phytol. 2022, 236, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Schulten, A.; Pietzenuk, B.; Quintana, J.; Scholle, M.; Feil, R.; Krause, M.; Romera-Branchat, M.; Wahl, V.; Severing, E.; Coupland, G.; et al. Energy status-promoted growth and development of Arabidopsis require copper deficiency response transcriptional regulator SPL7. Plant Cell 2022, 34, 3873–3898. [Google Scholar] [CrossRef]
- Ji, C.; Li, H.; Ding, J.; Yu, L.; Jiang, C.; Wang, C.; Wang, S.; Ding, G.; Shi, L.; Xu, F.; et al. Rice transcription factor OsWRKY37 positively regulates flowering time and grain fertility under copper deficiency. Plant Physiol. 2024, 195, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Casero, D.; Singh, V.; Wilson, G.T.; Grande, A.; Yang, H.; Dodani, S.C.; Pellegrini, M.; Huijser, P.; Connolly, E.L.; et al. Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 2012, 24, 738–761. [Google Scholar]
- Yamasaki, H.; Hayashi, M.; Fukazawa, M.; Kobayashi, Y.; Shikanai, T. SQUAMOSA Promoter Binding Protein-Like7 Is a Central Regulator for Copper Homeostasis in Arabidopsis. Plant Cell 2009, 21, 347–361. [Google Scholar] [CrossRef]
- Cai, Y.; Ping, H.; Zhao, J.; Li, C.; Li, Y.; Liang, G. IRON MAN interacts with Cu-DEFICIENCY INDUCED TRANSCRIPTION FACTOR 1 to maintain copper homeostasis. New Phytol. 2024, 242, 1206–1217. [Google Scholar] [CrossRef]
- Garcia-Molina, A.; Xing, S.; Huijser, P. A conserved KIN17 curved DNA-binding domain protein assembles with SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE7 to adapt Arabidopsis growth and development to limiting copper availability. Plant Physiol. 2014, 164, 828–840. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Li, J.; Cai, H.; Deng, X.W.; Li, L. MicroRNA408 is critical for the HY5-SPL7 gene network that mediates the coordinated response to light and copper. Plant Cell 2014, 26, 4933–4953. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Liang, G. FIT and bHLH Ib transcription factors modulate iron and copper crosstalk in Arabidopsis. Plant Cell Environ. 2021, 44, 1679–1691. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Carrió-Seguí, A.; Abdel-Ghany, S.E.; Pilon, M.; Peñarrubia, L. Expression of the Intracellular COPT3-Mediated Cu Transport Is Temporally Regulated by the TCP16 Transcription Factor. Front. Plant Sci. 2018, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Munyaneza, V.; Kant, S.; Wang, S.; Wang, X.; Cai, H.; Wang, C.; Shi, L.; Wang, S.; Xu, F.; et al. Transcription factor AtNAC002 positively regulates Cu toxicity tolerance in Arabidopsis thaliana. J. Hazard. Mater. 2024, 480, 136186. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Balyan, S.; Kumar, M.; Mutum, R.D.; Raghuvanshi, U.; Agarwal, P.; Mathur, S.; Raghuvanshi, S. Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22. Sci. Rep. 2017, 7, 15446. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wu, S.; Bai, A.N.; Zhang, Y.M.; Zhang, Y.; Yao, X.F.; Yang, T.; Chen, M.M.; Liu, J.L.; Li, L.; et al. OsSPL9 promotes Cu uptake and translocation in rice grown in high-Fe red soil. New Phytol. 2025, 246, 2207–2221. [Google Scholar] [CrossRef]
- Ding, J.; Ji, C.; Wang, C.; Wang, S.; Ding, G.; Shi, L.; Xu, F.; Cai, H. OsMYB67 Knockout Promotes Rice Heading and Yield by Facilitating Copper Distribution in Panicles. Plant Cell Environ. 2025, 48, 5664–5679. [Google Scholar] [CrossRef]
- Shangguan, X.; Tian, Z.; Wang, Y.; Xiao, T.; Yu, X.; Jing, W.; Peng, K.; Shen, Z.; Hu, Z.; Xia, Y. Transcription factor OsWRKY72 is involved in Cu/Cd toxicity by regulating lignin synthesis in rice. Crop J. 2024, 12, 1471–1482. [Google Scholar] [CrossRef]
- Gong, X.R.; Zhang, S.N.; Ye, L.N.; Luo, J.J.; Zhang, C. Cross talk between Cu excess and Fe deficiency in the roots of rice. Gene 2023, 874, 147491. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Liu, X.; Zhu, Y.; Bai, Y.; Shan, D.; Zheng, X.; Wang, L.; Zhang, H.; Wang, C.; Yan, T.; et al. MdWRKY11 improves copper tolerance by directly promoting the expression of the copper transporter gene MdHMA5. Hortic. Res. 2020, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Kropat, J.; Malasarn, D.; Grossoehme, N.E.; Chen, X.; Giedroc, D.P.; Merchant, S.S. The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell 2010, 22, 4098–4113. [Google Scholar] [CrossRef]
- Nagae, M.; Nakata, M.; Takahashi, Y. Identification of negative cis-acting elements in response to copper in the chloroplastic iron superoxide dismutase gene of the moss Barbula unguiculata. Plant Physiol. 2008, 146, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Zheng, Y.X.; Chen, S.J.; Liu, J.; Wang, P.F.; Wang, Y.H.; Guo, T.C.; Kang, G.Z. TaWRKY74 participates copper tolerance through regulation of TaGST1 expression and GSH content in wheat. Ecotoxicol. Environ. Saf. 2021, 221, 112469. [Google Scholar] [CrossRef]
- Alam, I.; Manghwar, H.; Zhang, H.; Yu, Q.; Ge, L. Identification of GOLDEN2-like transcription factor genes in soybeans and their role in regulating plant development and metal ion stresses. Front. Plant Sci. 2022, 13, 1052659. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Tang, Q.; Zhang, J.; Chen, X.; Tong, T.; Zheng, Q.; Hao, L.; Deng, F.; Chen, G.; Chen, Z.-H.; et al. Molecular Evolution of Cu Transporters and Transcription Factors in Plant Response to Copper Stress. Plants 2025, 14, 2710. https://doi.org/10.3390/plants14172710
Tang H, Tang Q, Zhang J, Chen X, Tong T, Zheng Q, Hao L, Deng F, Chen G, Chen Z-H, et al. Molecular Evolution of Cu Transporters and Transcription Factors in Plant Response to Copper Stress. Plants. 2025; 14(17):2710. https://doi.org/10.3390/plants14172710
Chicago/Turabian StyleTang, Haiyang, Qianqian Tang, Jin Zhang, Xuan Chen, Tao Tong, Qingfeng Zheng, Li Hao, Fenglin Deng, Guang Chen, Zhong-Hua Chen, and et al. 2025. "Molecular Evolution of Cu Transporters and Transcription Factors in Plant Response to Copper Stress" Plants 14, no. 17: 2710. https://doi.org/10.3390/plants14172710
APA StyleTang, H., Tang, Q., Zhang, J., Chen, X., Tong, T., Zheng, Q., Hao, L., Deng, F., Chen, G., Chen, Z.-H., Zeng, F., Qin, Y., & Jiang, W. (2025). Molecular Evolution of Cu Transporters and Transcription Factors in Plant Response to Copper Stress. Plants, 14(17), 2710. https://doi.org/10.3390/plants14172710









