Temporal-Resolution Dynamics of Polyphenolic During the Pepper Graft Healing
Abstract
1. Introduction
2. Results
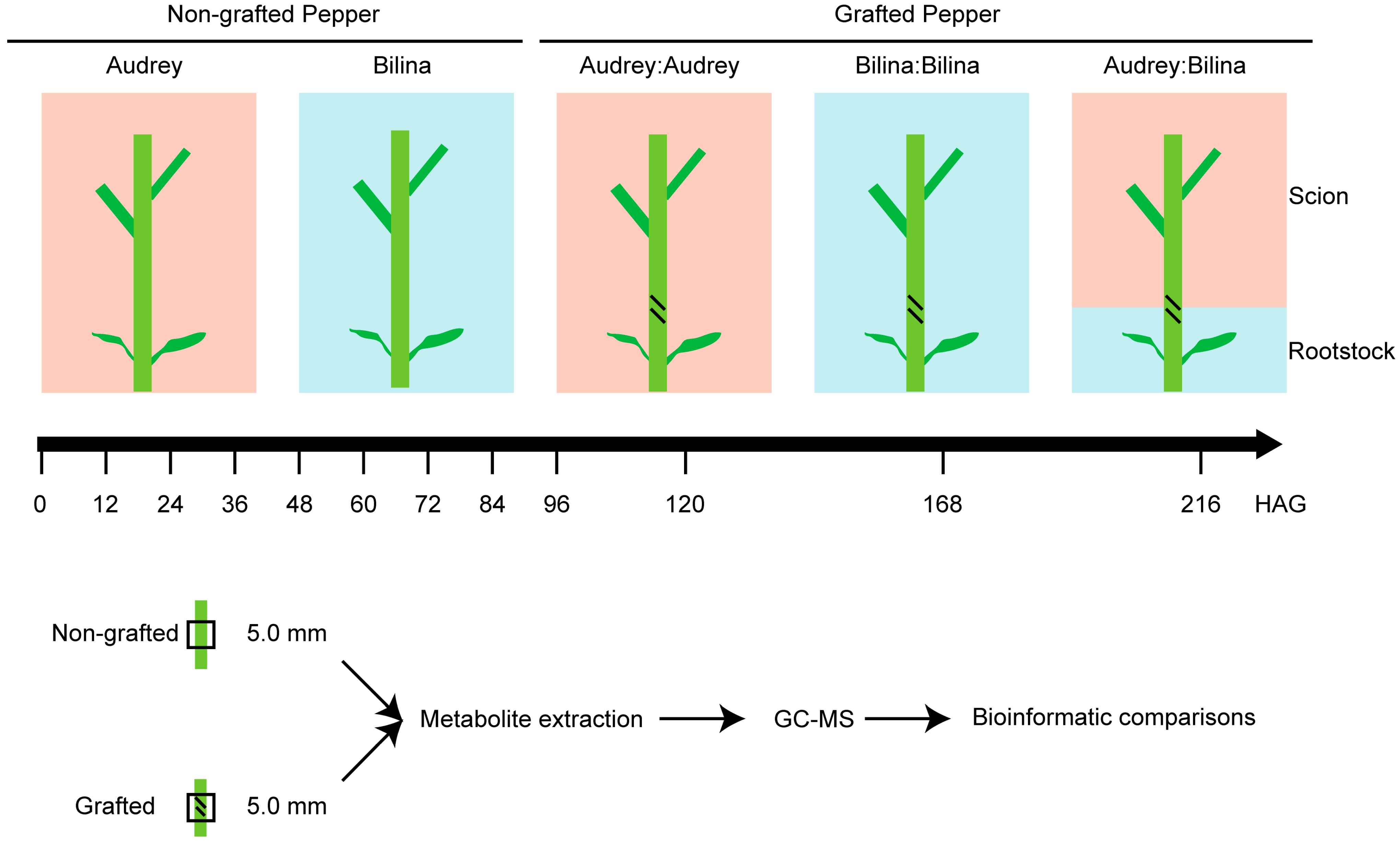
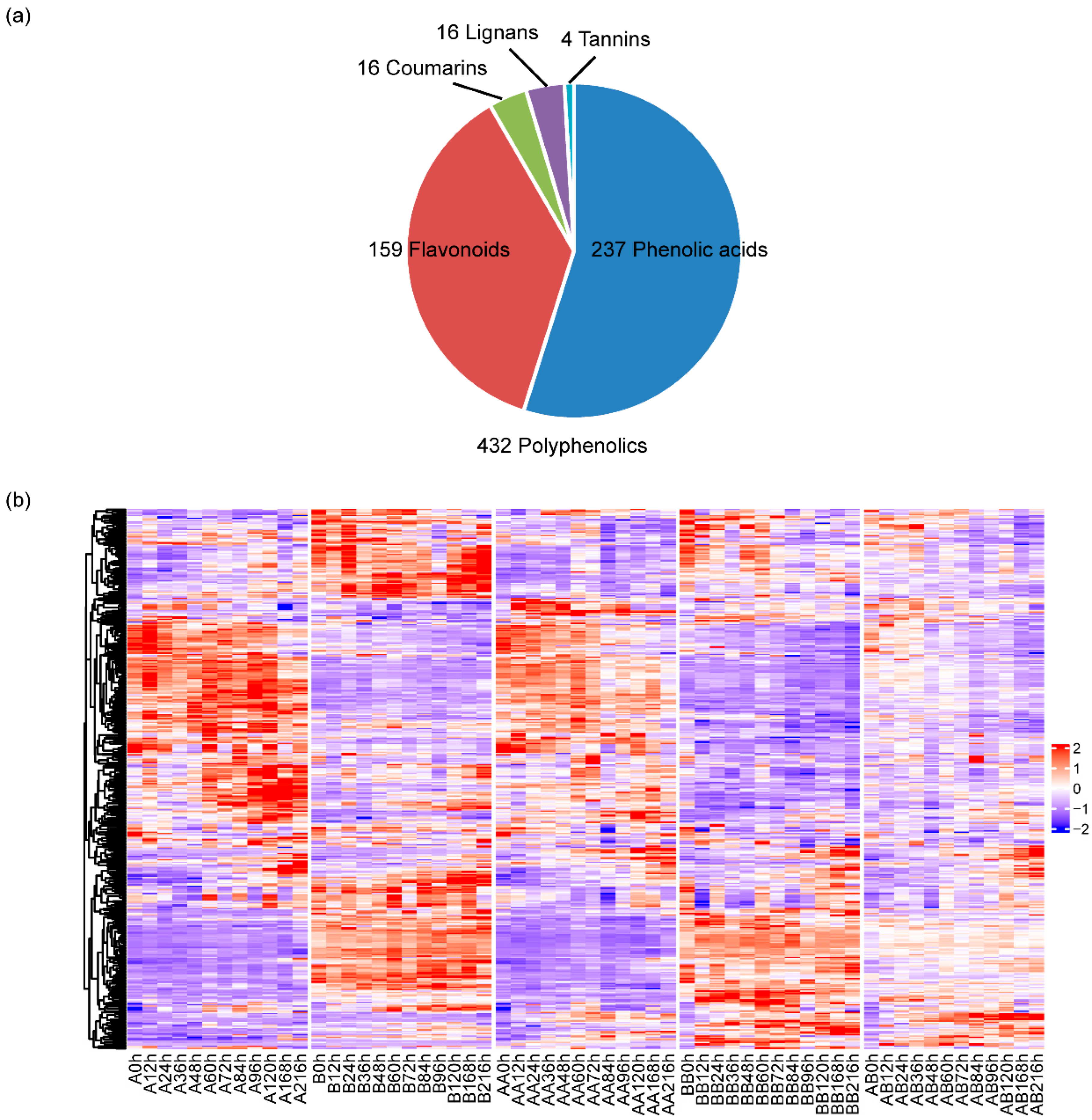
2.1. The Generation of Pepper Graft Healing Time-Course Polyphenolics Metabolome Data
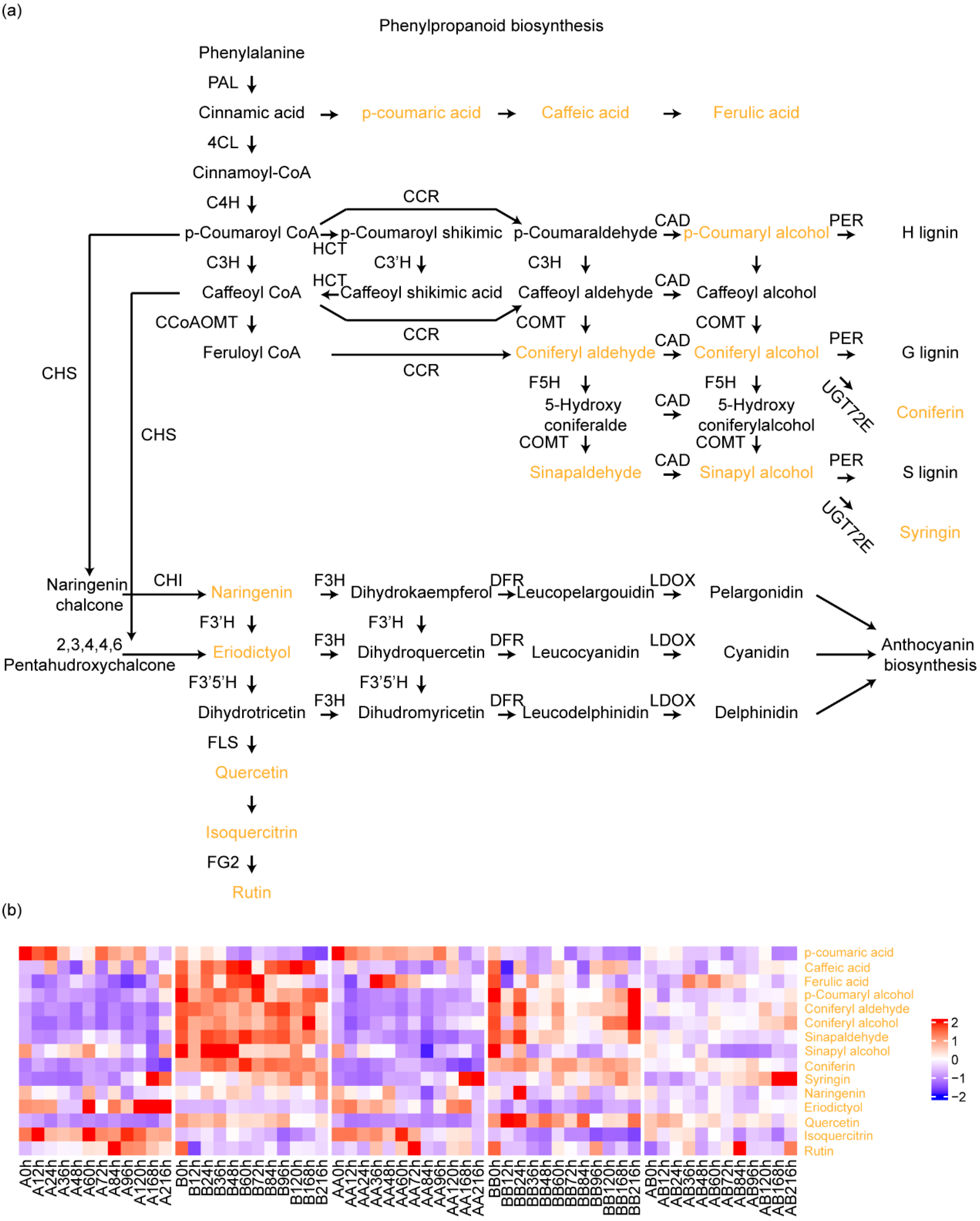
2.2. Accumulation Profiles of the Metabolites Involved in Phenylpropanoid Biosynthesis
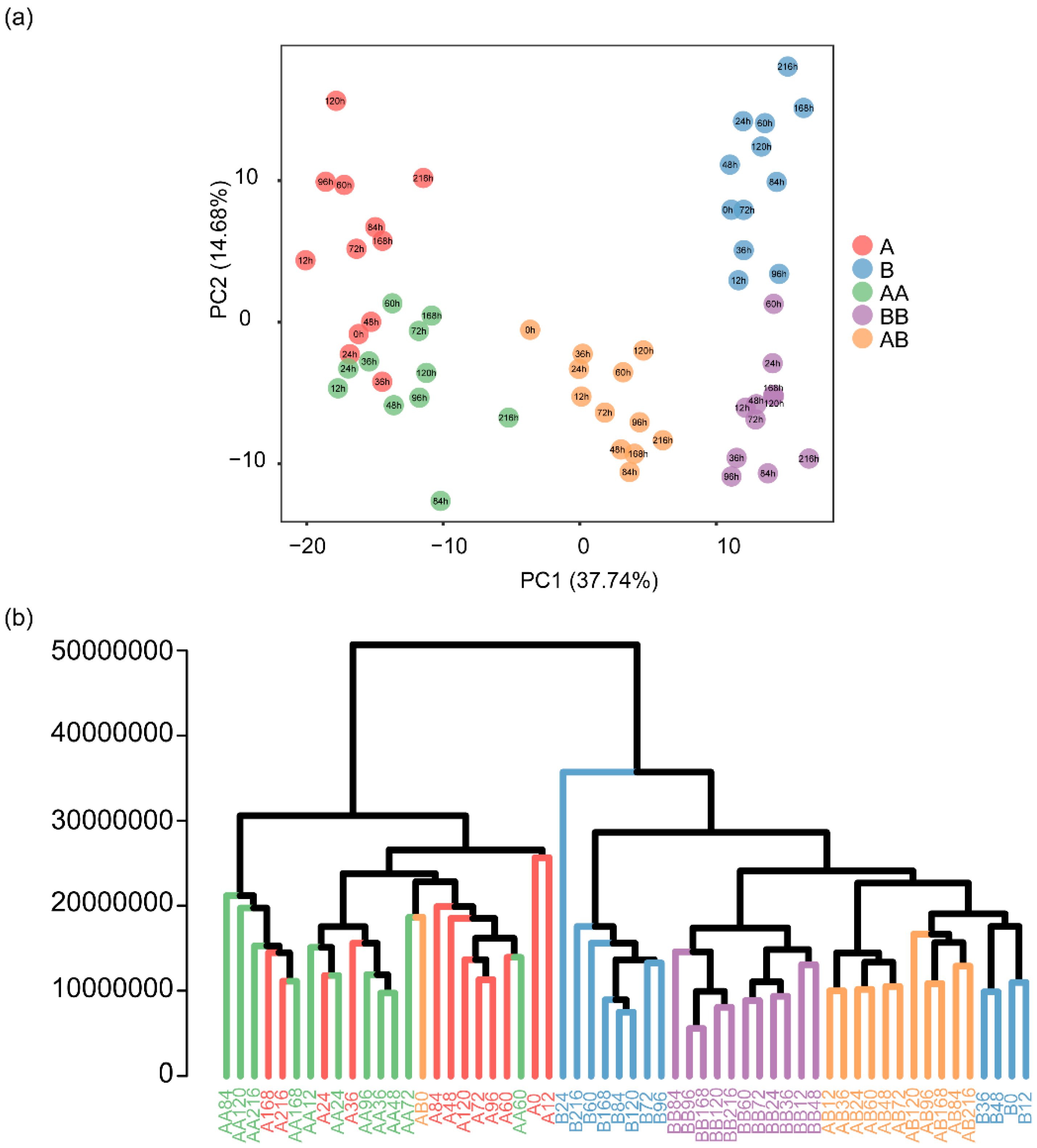
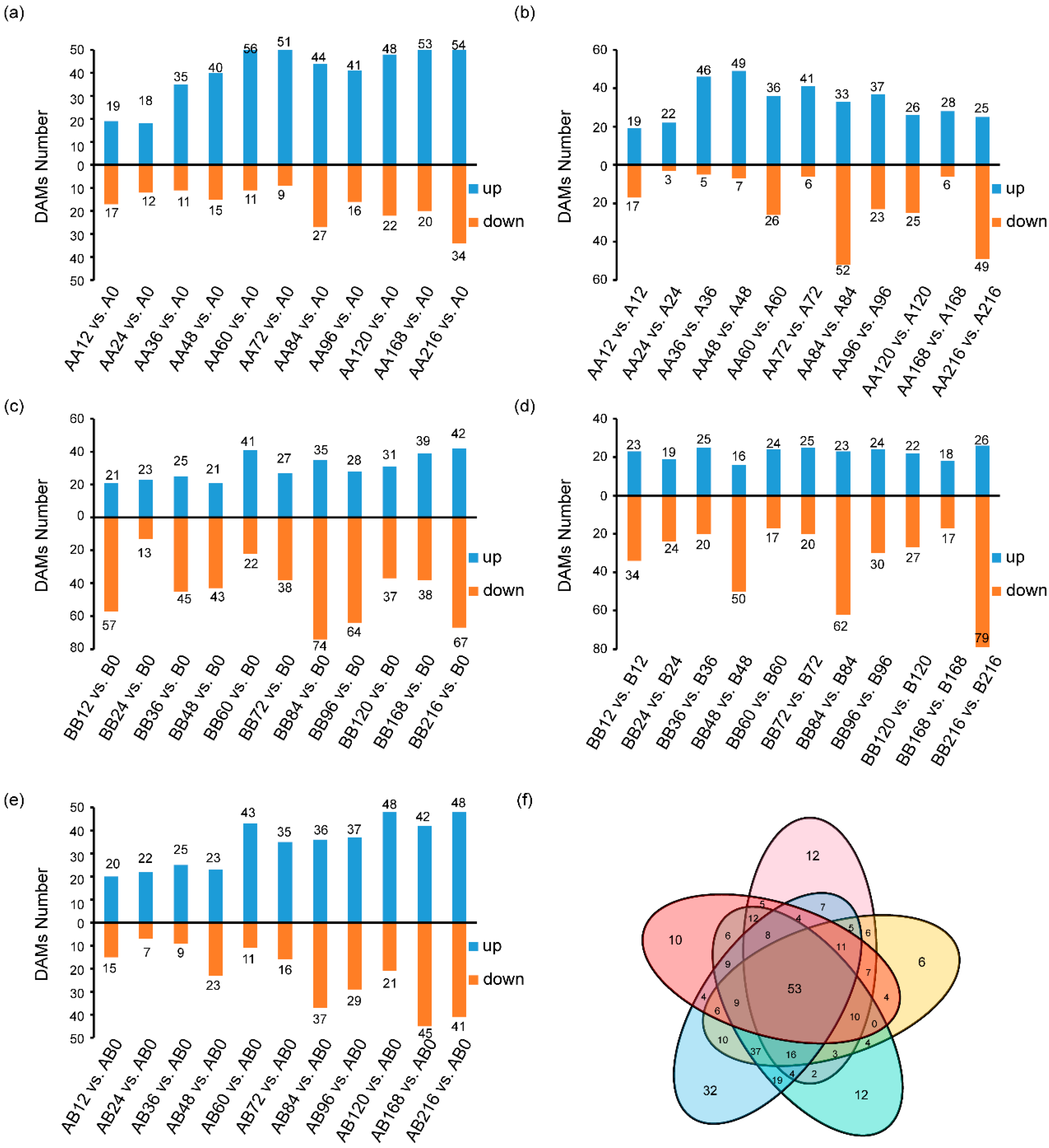
2.3. Differential Metabolite Screening During Pepper Graft Healing
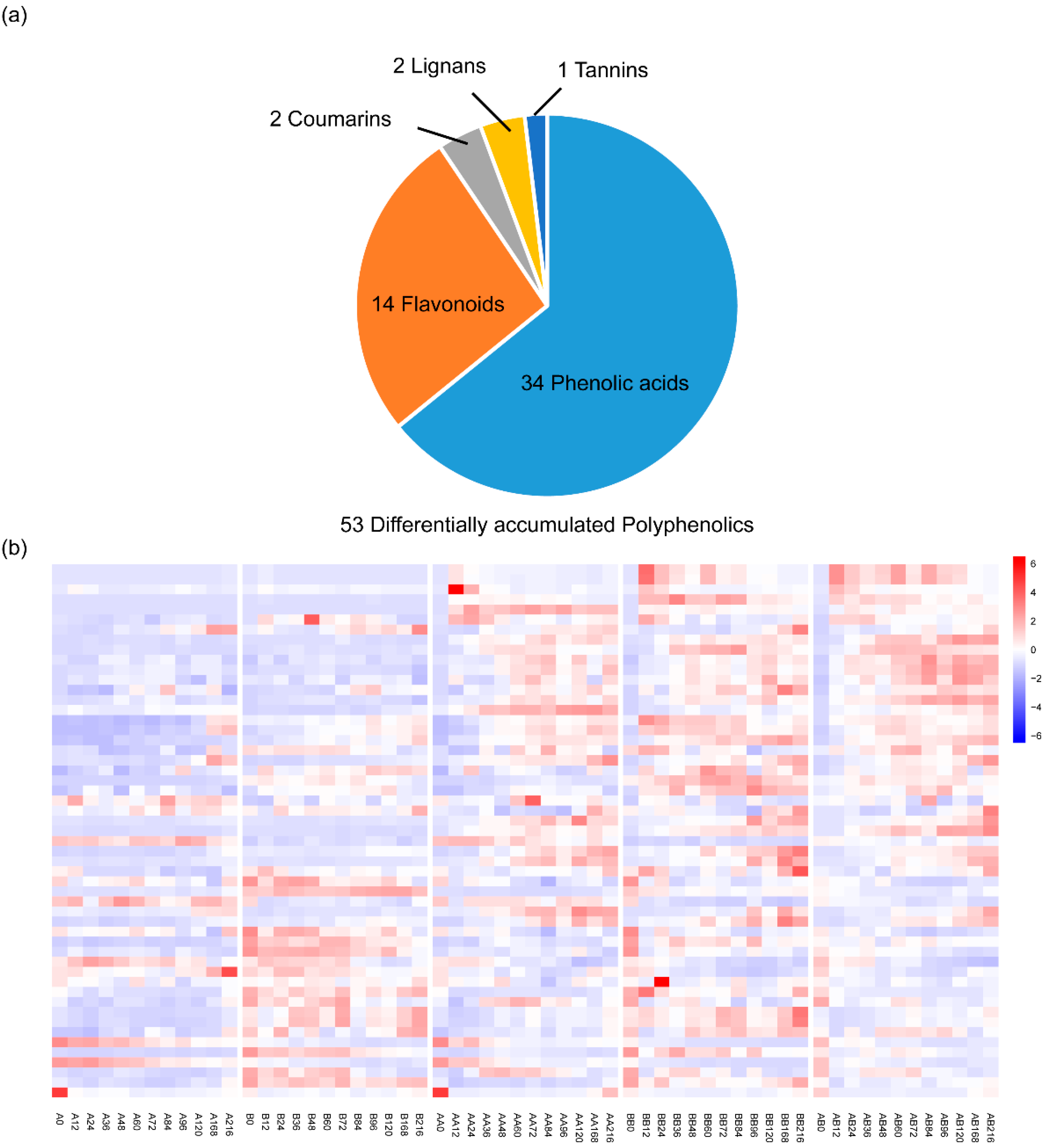
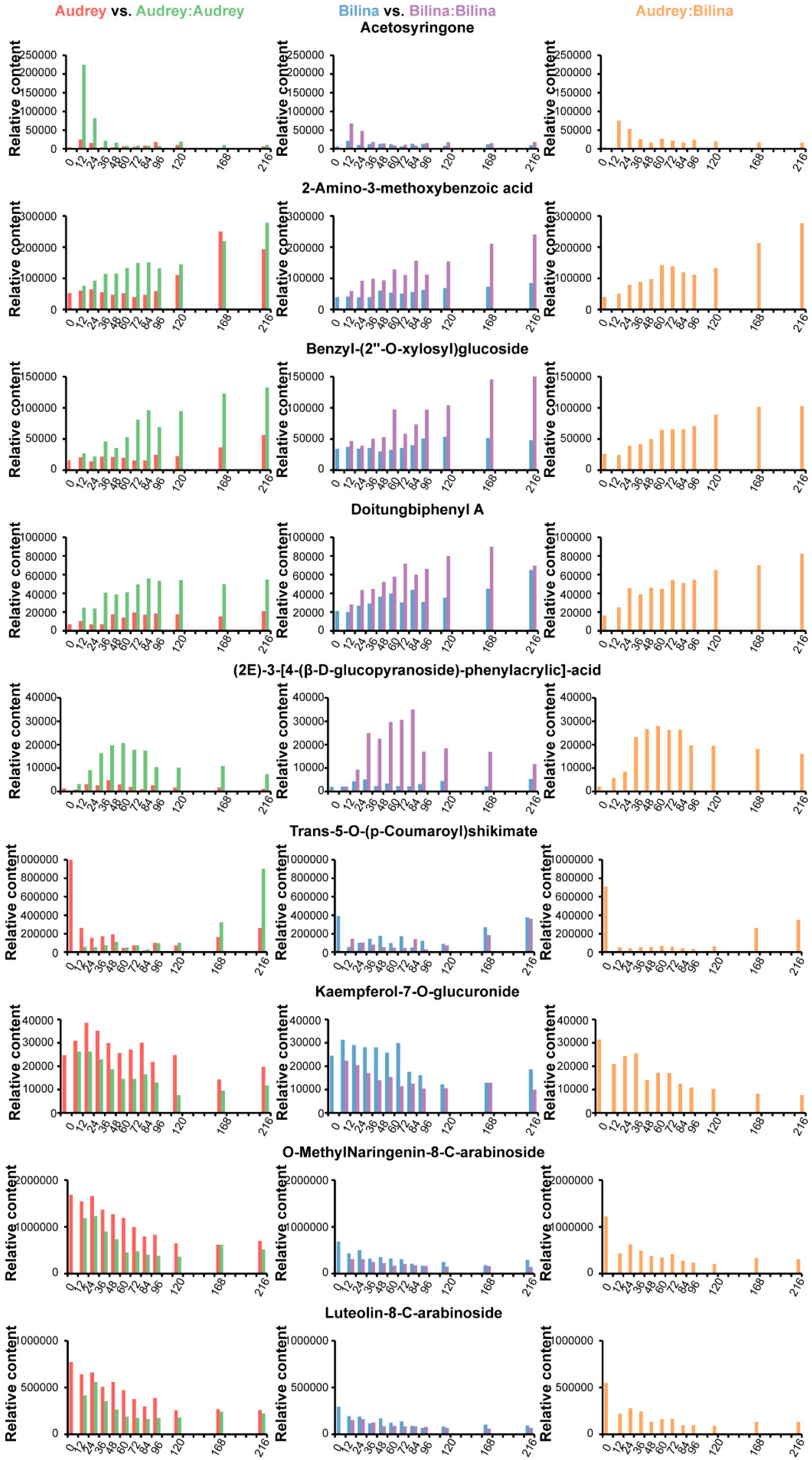
2.4. The Responded Polyphenolics During Pepper Graft Healing
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation and Extraction Process
4.3. UPLC Analytical Conditions
4.4. ESI-Q TRAP-MS/MS Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Sun, Z.; Li, T.; Fan, T.; Zhou, Z.; Liu, J.; Chen, X.; Wang, A. Identifying a Biocontrol Bacterium with Disease-Prevention Potential and Employing It as a Powerful Biocontrol Agent Against Fusarium oxysporum. Int. J. Mol. Sci. 2025, 26, 700. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, Y.; Liu, J.; Zhao, T.; Jia, L.; Chen, Z. Advances in Understanding the Graft Healing Mechanism: A Review of Factors and Regulatory Pathways. Hortic. Res. 2024, 11, uhae175. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2021, 34, 535–556. [Google Scholar] [CrossRef]
- Thomas, H.R.; Gevorgyan, A.; Hermanson, A.; Yanders, S.; Erndwein, L.; Norman- Ariztía, M.; Sparks, E.E.; Frank, M. Graft incompatibility between pepper and tomato elicits an immune response and triggers localized cell death. Hortic. Res. 2024, 11, uhae255. [Google Scholar] [CrossRef]
- Mozafarian, M.; Hawrylak-Nowak, B.; Kappel, N. Effect of Different Rootstocks on the Salt Stress Tolerance and Fruit Quality of Grafted Eggplants (Solanum melongena L.). Plants 2023, 12, 3631. [Google Scholar] [CrossRef]
- Miao, L.; Li, Q.; Sun, T.S.; Chai, S.; Wang, C.L.; Bai, L.Q.; Sun, M.T.; Li, Y.S.; Qin, X.; Zhang, Z.H.; et al. Sugars promote graft union development in the heterograft of cucumber onto pumpkin. Hortic. Res. 2021, 8, 146. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Zhang, R.; Liu, M.; Wang, C.; Liu, Z.; Xiang, C.; Lu, X.; Zhang, X.; Li, X.; et al. Rootstock-scion exchanging mRNAs participate in the pathways of amino acids and fatty acid metabolism in cucumber under early chilling stress. Hortic. Res. 2022, 9, uhac031. [Google Scholar] [CrossRef]
- Meng, J.; Wu, S.; Wang, X.; Yu, X.; Jiang, R. Effects of Different Rootstocks on Plant Growth and Fruit Quality of Watermelon. Agric. Biotechnol. 2019, 8, 64–68. [Google Scholar]
- Lu, J.; Cheng, F.; Huang, Y.; Bie, Z. Grafting Watermelon onto Pumpkin Increases Chilling Tolerance by Up Regulating Arginine Decarboxylase to Increase Putrescine Biosynthesis. Front. Plant Sci. 2022, 12, 812396. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Liu, C.; Guo, L.; Wang, J.; Wu, X.; Li, L.; Bie, Z.; Huang, Y. Compatibility evaluation and anatomical observation of melon grafted onto eight Cucurbitaceae species. Front. Plant Sci. 2021, 12, 762889. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Pina, A.; Fevereiro, P.; Kragler, F. A phenotypic search on graft compatibility in grapevine. Agronomy 2020, 10, 706. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Mao, C.; Xiang, L.; Zhang, X.; Jiang, F.; Cheng, Y.; Li, T. Integrative Analyses of Metabolome and Transcriptome Reveal Scion–Stock Asymmetry Reduction and Shift of Sugar Metabolism During Graft Junction Formation in Malus Domestica (‘Hanfu’) Homograft. Int. J. Mol. Sci. 2025, 26, 5290. [Google Scholar] [CrossRef]
- He, W.; Xie, R.; Wang, Y.; Chen, Q.; Wang, H.; Yang, S.; Luo, Y.; Zhang, Y.; Tang, H.; Gmitter, F.G., Jr.; et al. Comparative transcriptomic analysis on compatible/incompatible grafts in Citrus. Hortic. Res. 2022, 9, uhab072. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-Union Development: A Delicate Process That Involves Cell–Cell Communication between Scion and Stock for Local Auxin Accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2447–E2456. [Google Scholar] [CrossRef] [PubMed]
- Notaguchi, M.; Kurotani, K.; Sato, Y.; Tabata, R.; Kawakatsu, Y.; Okayasu, K.; Sawai, Y.; Okada, R.; Asahina, M.; Ichihashi, Y.; et al. Cell-Cell Adhesion in Plant Grafting Is Facilitated by b-1,4-Glucanases. Science 2020, 369, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.I.; Notaguchi, M. Cell-to-cell connection in plant grafting—Molecular insights into symplasmic reconstruction. Plant Cell Physiol. 2021, 62, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.; Tripathi, A.; Singh, P.; Jones, M.R.W.; Nanda, A.K.; Musseau, C.; Craze, M.; Bowden, S.; Walker, J.F.; Bentley, A.R.; et al. Monocotyledonous plants graft at the embryonic root-shoot interface. Nature 2022, 602, 280–286. [Google Scholar] [CrossRef]
- Luna-Garcia, L.R.; Robledo-Torres, V.; Gonzalez-Cortes, A.; Mendoza-Villarreal, R.; Paredes-Jacome, J.R. Effect of Pepper Rootstocks as a Sustainable Alternative to Improve Yield and Fruit Quality. Horticulturae 2023, 9, 795. [Google Scholar] [CrossRef]
- Dussarrat, T.; Prigent, S.; Latorre, C.; Bernillon, S.; Flandin, A.; Díaz, F.P.; Cassan, C.; Van Delft, P.; Jacob, D.; Varala, K. Predictive metabolomics of multiple Atacama plant species unveils a core set of generic metabolites for extreme climate resilience. New Phytol. 2022, 234, 1614–1628. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Pal, S.; Yu, J.; Zhou, Y.; Tran, L.-S.P.; Xia, X. The hormonal, metabolic, and environmental regulation of plant shoot branching. New Crops 2024, 1, 100028. [Google Scholar] [CrossRef]
- Yang, W.; Wen, D.; Yang, Y.; Li, H.; Yang, C.; Yu, J.; Xiang, H. Metabolomics and transcriptomics combined with physiology reveal key metabolic pathway responses in tobacco roots exposed to NaHS. BMC Plant Biol. 2024, 24, 680. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W.; et al. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant 2020, 13, 1203–1218. [Google Scholar] [CrossRef]
- Von Steimker, J.; Tripodi, P.; Wendenburg, R.; Tringovska, I.; Nankar, A.N.; Stoeva, V.; Pasev, G.; Klemmer, A.; Todorova, V.; Bulut, M.; et al. The genetic architecture of the pepper metabolome and the biosynthesis of its signature capsianoside metabolites. Curr. Biol. 2024, 34, 4209–4223.e4203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mao, L.; Yang, B.; Cui, Q.; Dai, Y.; Li, X.; Chen, Y.; Dai, X.; Zou, X.; Ou, L.; et al. A multi-omics approach identifies bHLH71-like as a positive regulator of yellowing leaf pepper mutants exposed to high-intensity light. Hortic. Res. 2023, 10, uhad098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. A draft genome, resequencing, and metabolomes reveal the genetic background and molecular basis of the nutritional and medicinal properties of loquat (Eriobotrya japonica (Thunb.) Lindl). Hortic. Res. 2021, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P.K.W. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014, 143, 33–39. [Google Scholar] [CrossRef]
- Hudina, M.; Orazem, P.; Jakopic, J.; Stampar, F. The phenolic content and its involvement in the graft incompatibility process of various pear rootstocks (Pyrus communis L.). J. Plant Physiol. 2014, 171, 76–84. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Cui, Q.; Xie, L.; Dong, C.; Gao, L.; Shang, Q. Stage-specific events in tomato graft formation and the regulatory effects of auxin and cytokinin. Plant Sci. 2021, 304, 110803. [Google Scholar] [CrossRef]
- Zeng, B.; Jiang, T.; Xiong, W.; Che, H.; Sun, S. Protective properties of polyphenols in food allergy: A review. Allergy 2022, 78, 1654–1656. [Google Scholar] [CrossRef]
- Song, C.; Acuña, T.; Adler-Agmon, M.; Rachmilevitch, S.; Barak, S.; Fait, A. Leveraging a graft collection to develop metabolome-based trait prediction for the selection of tomato rootstocks with enhanced salt tolerance. Hortic. Res. 2022, 9, uhac061. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Zhou, X.; Pan, M.; Xu, J.; Liu, M.; Wang, M.; Liu, G.; Xu, T.; Wang, Y.; et al. Grafting with Rootstocks Promotes Phenolic Compound Accumulation in Grape Berry Skin During Development Based on Integrative Multi-Omics Analysis. Hortic. Res. 2022, 9, uhac055. [Google Scholar] [CrossRef]
- Loupit, G.; Valls Fonayet, J.; Prigent, S.; Prodhomme, D.; Spilmont, A.-S.; Hilbert, G.; Franc, C.; De Revel, G.; Richard, T.; Ollat, N.; et al. Identifying Early Metabolite Markers of Successful Graft Union Formation in Grapevine. Hortic. Res. 2022, 9, uhab070. [Google Scholar] [CrossRef]
- Loupit, G.; Fonayet, J.V.; Lorensen, M.D.B.B.; Franc, C.; De Revel, G.; Janfelt, C.; Cookson, S.J. Tissue-specific stilbene accumulation is an early response to wounding/grafting as revealed by using spatial and temporal metabolomics. Plant Cell Environ. 2023, 46, 3871–3886. [Google Scholar] [CrossRef]
- Johkan, M.; Mitukuri, K.; Yamasaki, S.; Mori, G.; Oda, M. Causes of defoliation and low survival rate of grafted sweet pepper plants. Sci. Hortic. 2009, 119, 103–107. [Google Scholar] [CrossRef]
- Fernández-Garcia, N.; Carvajal, M.; Olmos, E. Graft union formation in tomato plants: Peroxidase and catalase involvement. Ann. Bot. 2004, 93, 53–60. [Google Scholar] [CrossRef]
- Xie, L.; Dong, C.; Shang, Q. Gene co-expression network analysis reveals pathways associated with graft healing by asymmetric profiling in tomato. BMC Plant Biol. 2019, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Var, I.; Kayar, B.; Eker, E.; Kafkas, E.; Zarifikhosroshahi, M.; Köksal, F. Evaluation of Polyphenolic Profile and Antibacterial Activity of Pomegranate Juice in Combination with Rifampin (R) against MDR-TB Clinical Isolates. Curr. Pharm. Biotechnol. 2019, 20, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhao, S.; Li, Y.; Deng, Y.; Shi, Y.; Chen, X.; Li, Y.; Li, H.; Chen, C.; Wang, X.; et al. Phenolic acid–induced phase separation and translation inhibition mediate plant interspecific competition. Nat. Plants 2023, 9, 1481–1499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Duan, Y.; Shang, Q. Temporal-Resolution Dynamics of Polyphenolic During the Pepper Graft Healing. Plants 2025, 14, 2656. https://doi.org/10.3390/plants14172656
Zhang F, Duan Y, Shang Q. Temporal-Resolution Dynamics of Polyphenolic During the Pepper Graft Healing. Plants. 2025; 14(17):2656. https://doi.org/10.3390/plants14172656
Chicago/Turabian StyleZhang, Feng, Yundan Duan, and Qingmao Shang. 2025. "Temporal-Resolution Dynamics of Polyphenolic During the Pepper Graft Healing" Plants 14, no. 17: 2656. https://doi.org/10.3390/plants14172656
APA StyleZhang, F., Duan, Y., & Shang, Q. (2025). Temporal-Resolution Dynamics of Polyphenolic During the Pepper Graft Healing. Plants, 14(17), 2656. https://doi.org/10.3390/plants14172656





