Effects of Cadmium on the Accumulation and Phytotoxicity of Uranium in Radish (Raphanus sativus L.) Seedlings
Abstract
1. Introduction
2. Results
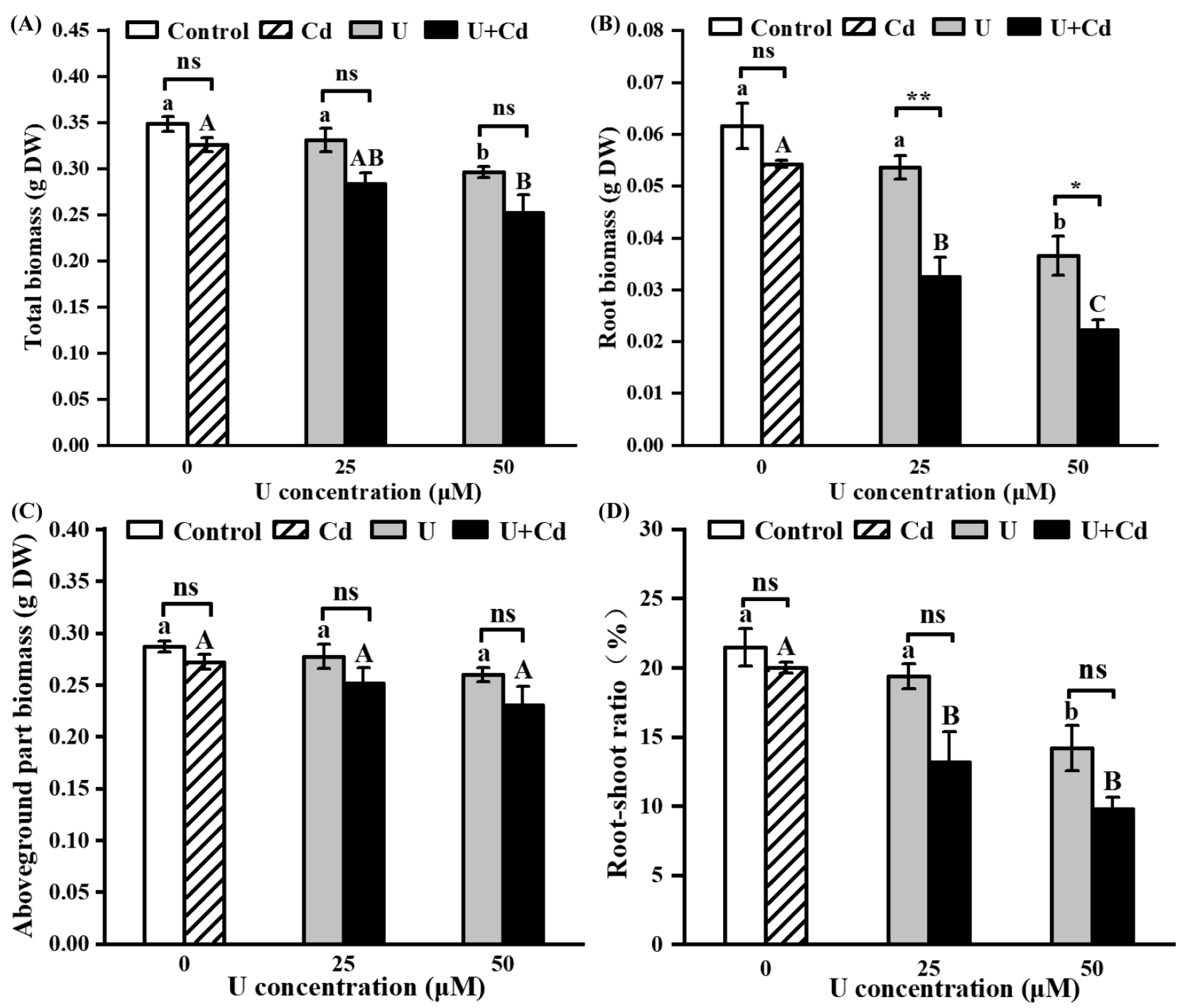
2.1. Phytotoxicity of U and Cd in Radish Seedlings
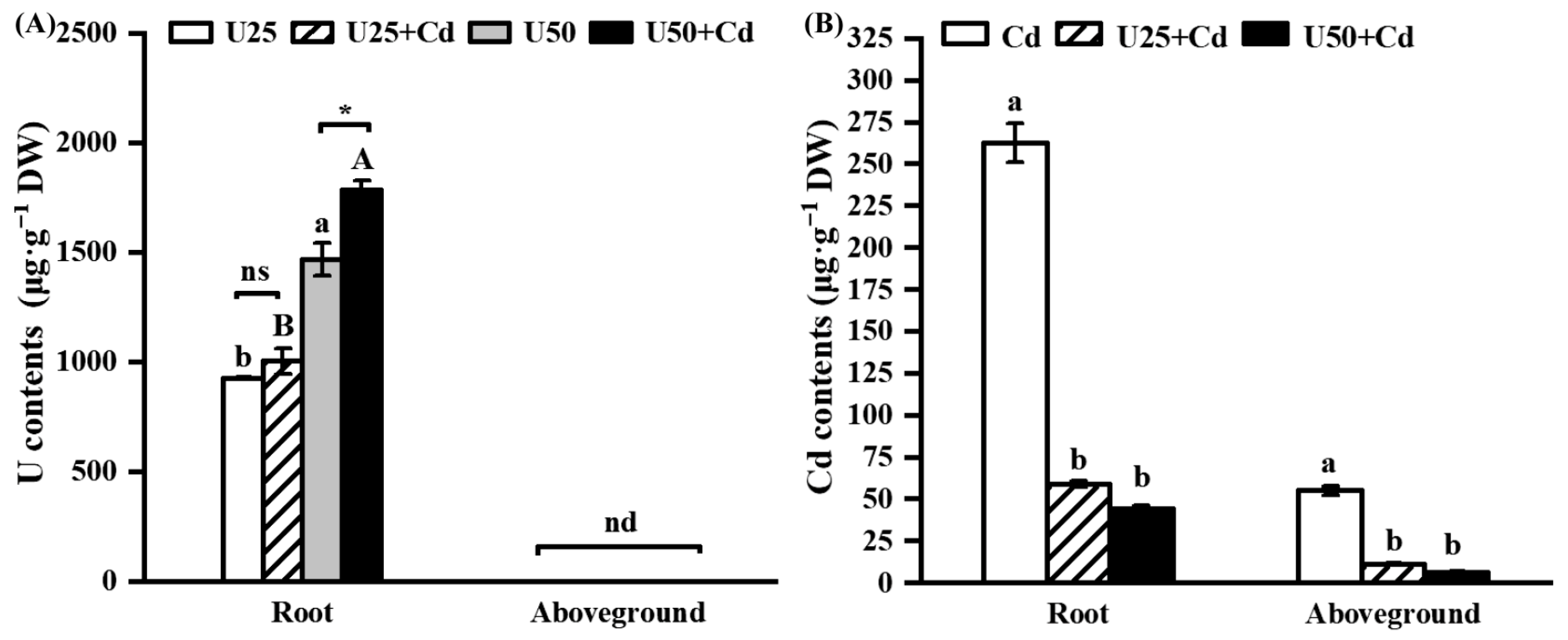
2.2. U and Cd Accumulation in Radish Seedlings
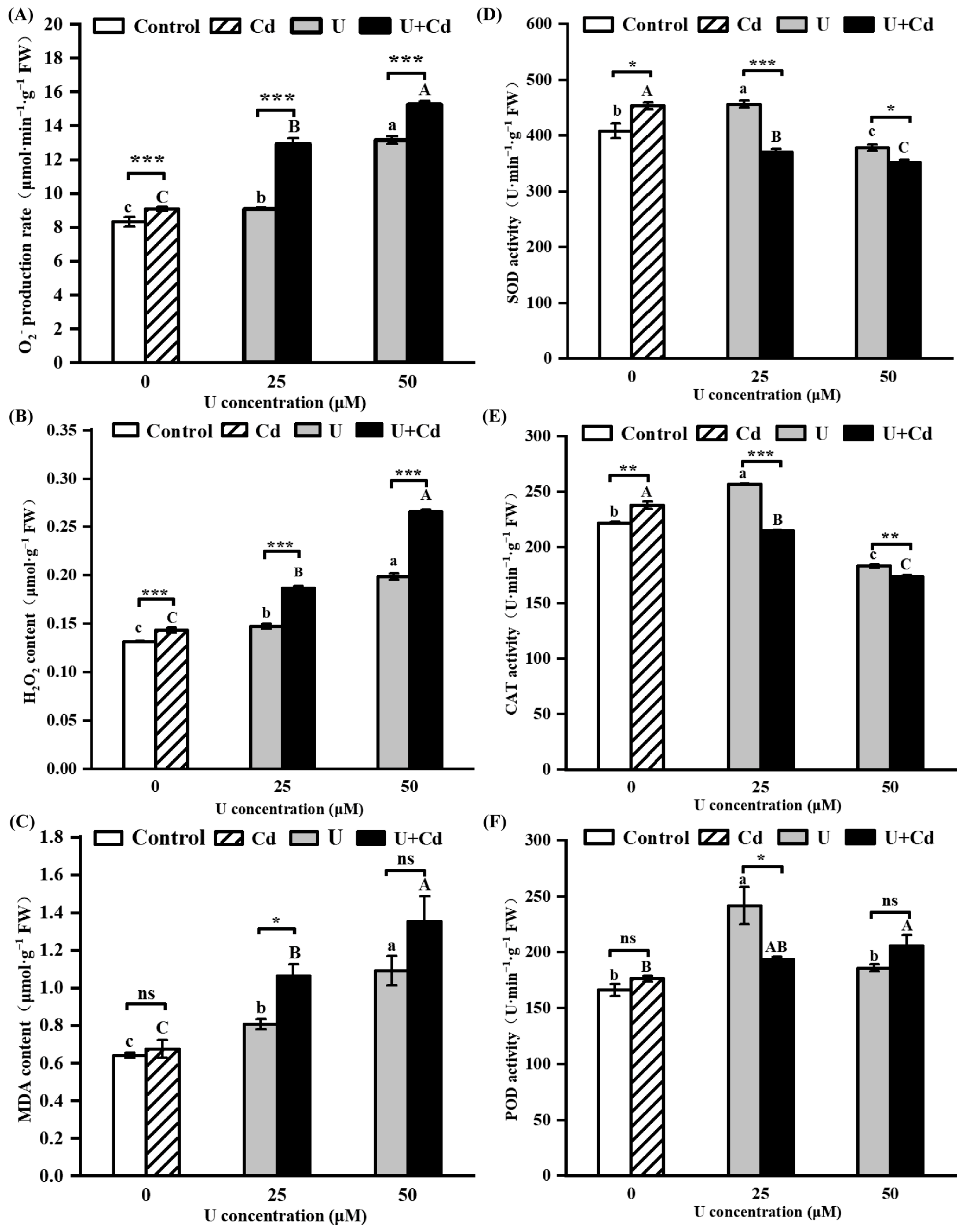
2.3. U and Cd Interfere with the ROS Metabolism in Radish Roots
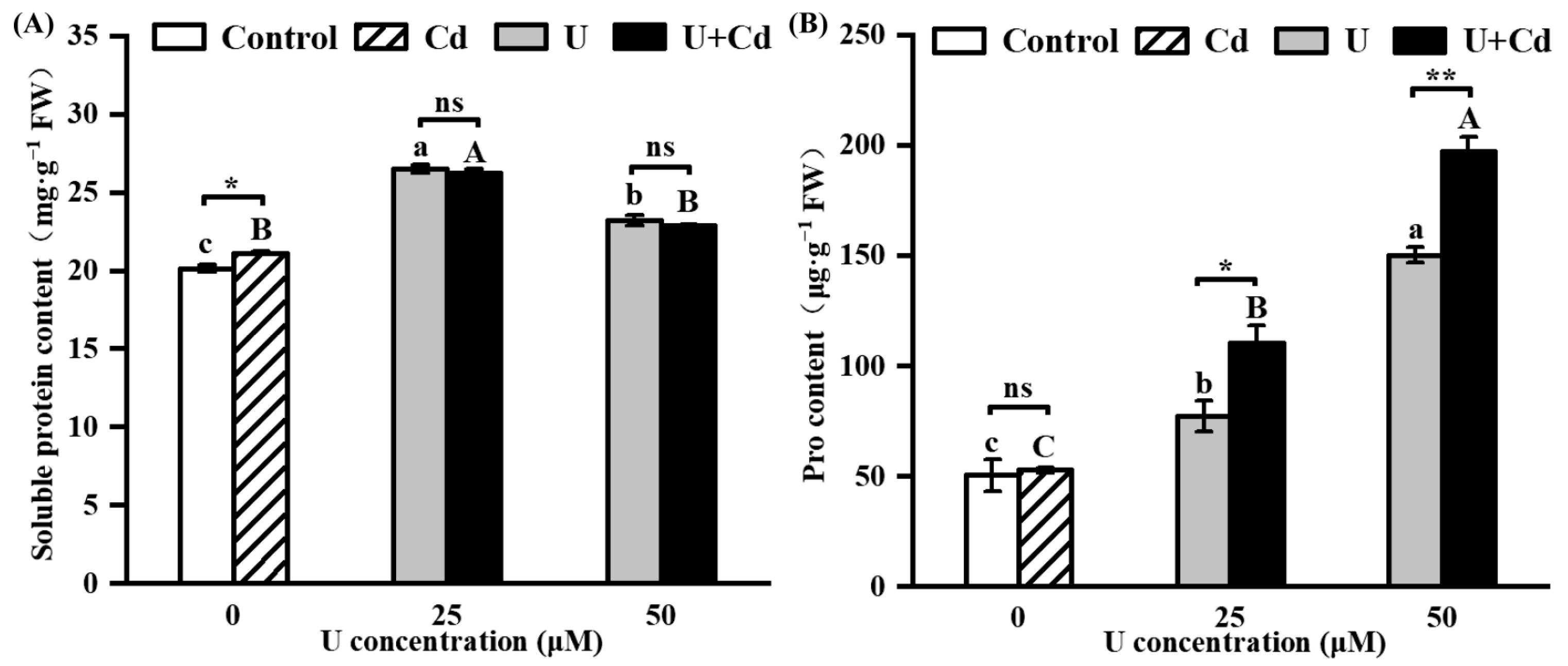
2.4. U and Cd Interfere with Soluble Protein and Pro Accumulation in Radish Roots
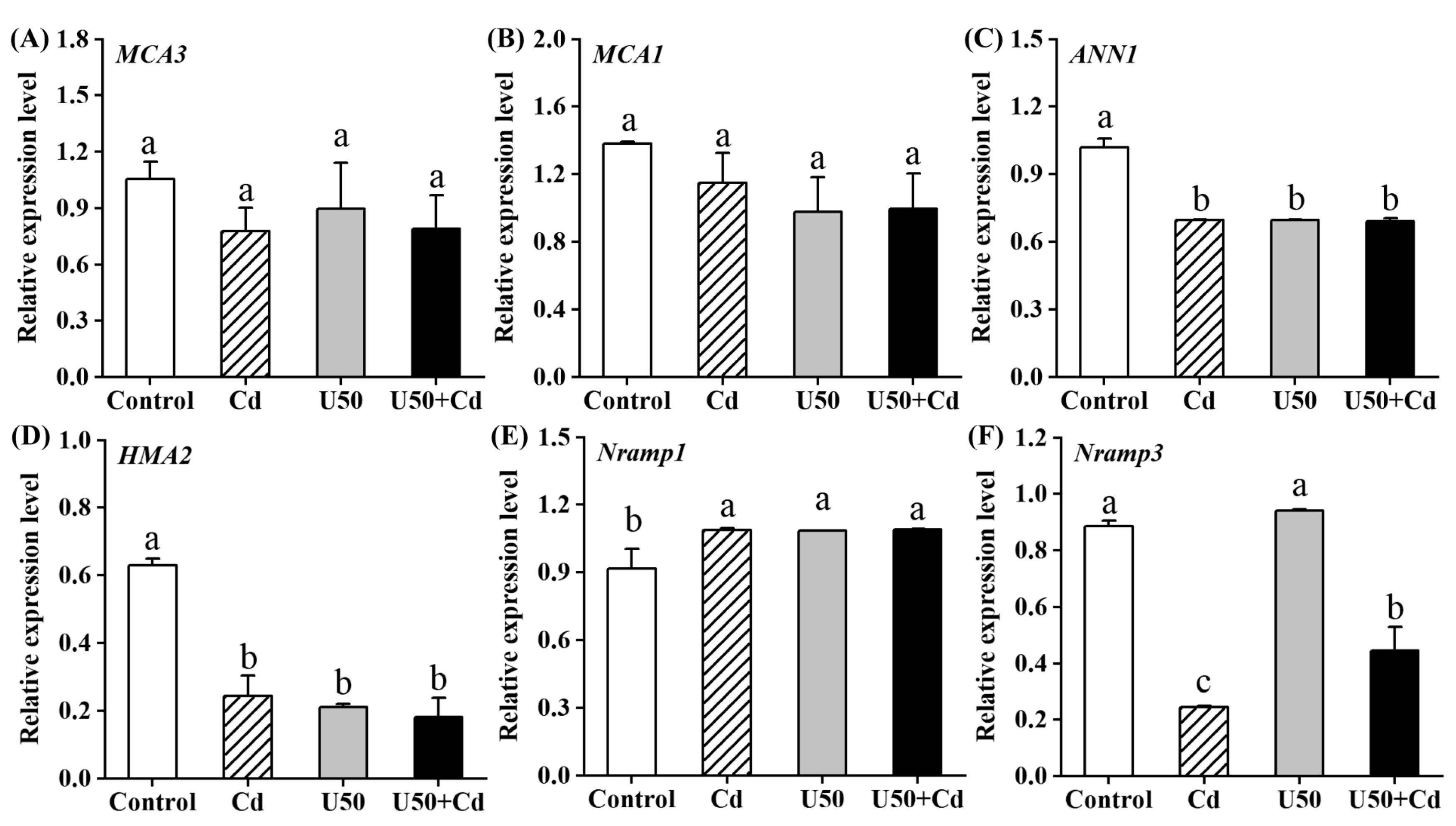
2.5. Relative Expression Level of Genes Involved in U and Cd Transportation
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Plant Culture
4.2. Experimental Design
4.3. Experimental Methods
4.3.1. Detection of Root Activity
4.3.2. Biomass Determination
4.3.3. Determination of Physiological Parameters of the Radish Roots
4.3.4. U and Cd Content Detection
4.3.5. Analysis of Gene Expression
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Wang, J.; Li, H.; Shen, C.-C.; Chen, Y.; Wang, C.; Ye, H.; Long, J.; Song, G.; Wu, Y. Surface Sediment Contamination by Uranium Mining/Milling Activities in South China. CLEAN Soil Air Water 2015, 43, 414–420. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Navarro-León, E.; Oviedo-Silva, J.; Ruiz, J.M.; Blasco, B. Possible role of HMA4a TILLING mutants of Brassica rapa in cadmium phytoremediation programs. Ecotox. Environ. Saf. 2019, 180, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Beiyuan, J.; Guo, X.; Wu, H.; Fang, L. Environmental and health risk assessment of potentially toxic trace elements in soils near uranium (U) mines: A global meta-analysis. Sci. Total Environ. 2022, 816, 151556. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef]
- Serre, N.B.C.; Alban, C.; Bourguignon, J.; Ravanel, S. Uncovering the physiological and cellular effects of uranium on the root system of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 157, 121–130. [Google Scholar] [CrossRef]
- Liu, Z.; Tran, K.-Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotox. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Gupta, D.K.; Vuković, A.; Semenishchev, V.S.; Inouhe, M.; Walther, C. Uranium accumulation and its phytotoxicity symptoms in Pisum sativum L. Environ. Sci. Pollut. Res. 2020, 27, 3513–3522. [Google Scholar] [CrossRef]
- Piršelová, B.; Ondrušková, E. Effect of Cadmium Chloride and Cadmium Nitrate on Growth and Mineral Nutrient Content in the Root of Fava Bean (Vicia faba L.). Plants 2021, 10, 1007. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Smeets, K.; Remans, T.; Van Hees, M.; Wannijn, J.; Vangronsveld, J.; Cuypers, A. Effects of uranium and phosphate concentrations on oxidative stress related responses induced in Arabidopsis thaliana. Plant Physiol. Biochem. 2008, 46, 987–996. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Ma, Q.; Lai, J.-l.; Luo, X.-g.; Ji, X.-h. Cytotoxic and genotoxic evaluation and the toxicological mechanism of uranium in Vicia faba root. Environ. Exp. Bot. 2020, 179, 104227. [Google Scholar] [CrossRef]
- Aydin, D.; Yalçin, E.; Çavuşoğlu, K. Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci. Rep. 2022, 12, 15845. [Google Scholar] [CrossRef]
- Deng, M.; Wang, S.; Huang, H.; Ye, D.; Zhang, X.; Wang, Y.; Zheng, Z.; Liu, T.; Li, T.; Yu, H. Hydrogen peroxide mediates cadmium accumulation in the root of a high cadmium-accumulating rice (Oryza sativa L.) line. J. Hazard. Mater. 2023, 448, 130969. [Google Scholar] [CrossRef]
- Pinto, F.R.; Mourato, M.P.; Sales, J.R.; Moreira, I.N.; Martins, L.L. Oxidative stress response in spinach plants induced by cadmium. J. Plant Nutr. 2017, 40, 268–276. [Google Scholar] [CrossRef]
- Berthet, S.; Villiers, F.; Alban, C.; Serre, N.B.C.; Martin-Laffon, J.; Figuet, S.; Boisson, A.-M.; Bligny, R.; Kuntz, M.; Finazzi, G.; et al. Arabidopsis thaliana plants challenged with uranium reveal new insights into iron and phosphate homeostasis. New Phytol. 2018, 217, 657–670. [Google Scholar] [CrossRef]
- Lai, J.-l.; Liu, Z.-w.; Li, C.; Luo, X.-g. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. J. Hazard. Mater. 2021, 409, 124997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Huang, P.; Yan, X.; Chukwuma, A.; Yang, S.; Yang, Z.; Li, H.; Yang, W. Inhibitory effect of exogenous mineral elements (Si, P, Zn, Ca, Mn, Se, Fe, S) on rice Cd accumulation and soil Cd bioavailability in Cd-contaminated farmlands: A meta-analysis. Chemosphere 2023, 343, 140282. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, N.; Horemans, N.; Biermans, G.; Saenen, E.; Wannijn, J.; Nauts, R.; Van Hees, M.; Vandenhove, H. Uranium affects photosynthetic parameters in Arabidopsis thaliana. Environ. Exp. Bot. 2014, 97, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, J.-L.; Ji, X.-H.; Luo, X.-G. Unraveling response mechanism of photosynthetic metabolism and respiratory metabolism to uranium-exposure in Vicia faba. J. Hazard. Mater. 2020, 398, 122997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, J.; Li, L.; Yin, Y.; Xue, B.; Li, J.; Sun, F. Exploration of the Effects of Cadmium Stress on Photosynthesis in Oenanthe javanica (Blume) DC. Toxics 2024, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Todorenko, D.; Volgusheva, A.; Timofeev, N.; Kovalenko, I.; Matorin, D.; Antal, T. Multiple in vivo Effects of Cadmium on Photosynthetic Electron Transport in Pea Plants. Photochem. Photobiol. 2021, 97, 1516–1526. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Horemans, N.; Wannijn, J.; Bujanic, A.; Vangronsveld, J.; Cuypers, A. Study of oxidative stress related responses induced in Arabidopsis thaliana following mixed exposure to uranium and cadmium. Plant Physiol. Biochem. 2010, 48, 879–886. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, L.; Zhou, Z.; Xu, G.; Chen, W.; Cao, Y.; Li, C.; Fu, Q.; Fan, W.; Hu, S. Moso bamboo alleviates Uranium/Cadmium stress through altering the rhizosphere micro-environment and regulating roots carbon and nitrogen metabolism. Environ. Res. 2025, 276, 121452. [Google Scholar] [CrossRef]
- Li, S.; Xiong, F.; Ouyang, C.; Zeng, T.; Li, L.; Xie, S. Accumulation features and the physiologic-biochemical mechanism for Cd and U toxic tolerability in Rohdea japonica. J. Saf. Environ. 2017, 17, 2432–2437. [Google Scholar] [CrossRef]
- Liu, C.; Wen, L.; Cui, Y.; Ahammed, G.J.; Cheng, Y. Metal transport proteins and transcription factor networks in plant responses to cadmium stress. Plant Cell Rep. 2024, 43, 218. [Google Scholar] [CrossRef]
- Sarthou, M.C.M.; Devime, F.; Baggio, C.; Figuet, S.; Alban, C.; Bourguignon, J.; Ravanel, S. Calcium-permeable cation channels are involved in uranium uptake in Arabidopsis thaliana. J. Hazard. Mater. 2022, 424, 127436. [Google Scholar] [CrossRef]
- Chen, X.; Xie, M.-t.; Li, Q.-l.; Dang, Y.-x.; Peng, S.; Tan, Y.-y.; Wang, M.-y.; Fan, Y.-m.; Lai, J.-l.; Wu, G. New insights into plant physiological responses to uranium: An integrative analysis of autophagy, DNA repair, and antioxidant systems in radish. Plant Physiol. Biochem. 2025, 221, 109641. [Google Scholar] [CrossRef]
- Tońska, E.; Klepacka, J.; Michalak, J.; Toński, M. Lead and cadmium in conventional and organic carrots. Proc. Nutr. Soc. 2020, 79, E324. [Google Scholar] [CrossRef]
- Van Netten, C.; Morley, D.R. Uptake of Uranium, Molybdenum, Copper, and Selenium by the Radish from Uranium-Rich Soils. Arch. Environ. Health 1983, 38, 172–175. [Google Scholar] [CrossRef]
- Han, Y.; Lee, J.; Kim, C.; Park, J.; Lee, M.; Yang, M. Uranium Rhizofiltration by Lactuca sativa, Brassica campestris L., Raphanus sativus L., Oenanthe javanica under Different Hydroponic Conditions. Minerals 2021, 11, 41. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Q.; Shi, J.; Sun, J.; Liao, M.a.; Mei, L. Intercropping different varieties of radish can increase cadmium accumulation in radish. Environ. Toxicol. Chem. 2014, 33, 1950–1955. [Google Scholar] [CrossRef]
- Zarei, A.; Rokni, H.R. Data on vermicompost effect on the uptake of cadmium from soil by the roots of radish (Raphanus sativus). MethodsX 2024, 13, 102917. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, Y.; Pan, S. Differences in enrichment and soil safety thresholds of five vegetables grown in Cd-polluted soil of Chengdu Plain, China. Environ. Geochem. Health 2025, 47, 229. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Khan, F.A.; Rehman, F.; Masoodi, A.; Ansari, A.A.; Varshney, D.; Naushin, F.; Naikoo, M.I. Roles of Brassicaceae in Phytoremediation of Metals and Metalloids. In Phytoremediation: Management of Environmental Contaminants, Volume 1; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 201–215. [Google Scholar]
- Li, C.; Wang, M.; Luo, X.; Liang, L.; Han, X.; Lin, X. Accumulation and effects of uranium on aquatic macrophyte Nymphaea tetragona Georgi: Potential application to phytoremediation and environmental monitoring. J. Environ. Radioactiv. 2019, 198, 43–49. [Google Scholar] [CrossRef]
- Hou, J.; Wang, C.; Zhou, Y.; Li, S.; Hayat, T.; Alsaedi, A.; Wang, X. Effects of uranium stress on physiological and biochemical characteristics in seedlings of six common edible vegetables. J. Radioanal. Nucl. Chem. 2018, 316, 1001–1010. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, B.; Wu, Y.; Sun, H.; Yang, Y. Cadmium phytoremediation potential of turnip compared with three common high Cd-accumulating plants. Environ. Sci. Pollut. Res. 2017, 24, 21660–21670. [Google Scholar] [CrossRef]
- Wu, G.; Chen, X.; Zheng, T.; Xiao, P.-x.; Zhong, N.-y.; Yang, X.-l.; Li, Y.; Li, W. Effects of U on the growth, reactive oxygen metabolism and osmotic regulation in radish (Raphanus sativus L.). Environ. Sci. Pollut. Res. 2022, 29, 55081–55091. [Google Scholar] [CrossRef]
- Duhan, S.S.; Khyalia, P.; Solanki, P.; Laura, J.S. Uranium Sources, Uptake, Translocation in the soil-plant System and Its Toxicity in Plants and Humans: A Critical Review. Orient. J. Chem. 2023, 39, 303–319. [Google Scholar] [CrossRef]
- Altaf, M.A.; Naz, S.; Kumar, R.; Sardar, H.; Nawaz, M.A.; Kumar, A.; Lal, P.; Ahmad, R.; Hayat, F.; Wani, M.A.; et al. Unraveling the mechanisms of cadmium toxicity in horticultural plants: Implications for plant health. S. Afr. J. Bot. 2023, 163, 433–442. [Google Scholar] [CrossRef]
- Yao, T.; Wang, D.; Li, Z. Effects of single and combined pollution of uranium and cadmium on growth and physiological characteristics of Oenanthe javanica. J. Agro-Environ. Sci. 2016, 35, 871–877. [Google Scholar] [CrossRef]
- Misson, J.; Henner, P.; Morello, M.; Floriani, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Février, L. Use of phosphate to avoid uranium toxicity in Arabidopsis thaliana leads to alterations of morphological and physiological responses regulated by phosphate availability. Environ. Exp. Bot. 2009, 67, 353–362. [Google Scholar] [CrossRef]
- Wu, X.; Gao, B.; Lyu, X.; Zeng, X.; Wu, J.; Sun, Y. Insight into the mechanism of phosphate and cadmium co-transport in natural soils. J. Hazard. Mater. 2022, 435, 129095. [Google Scholar] [CrossRef]
- You, Y.; Ju, C.; Wang, L.; Wang, X.; Ma, F.; Wang, G.; Wang, Y. The mechanism of arbuscular mycorrhizal enhancing cadmium uptake in Phragmites australis depends on the phosphorus concentration. J. Hazard. Mater. 2022, 440, 129800. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; He, W.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Effect of phosphorus supplementation on growth, nutrient uptake, physiological responses, and cadmium absorption by tall fescue (Festuca arundinacea Schreb.) exposed to cadmium. Ecotox. Environ. Saf. 2021, 213, 112021. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, X.; Zheng, L.; Ren, H.; Li, W.; Lei, Y.; Plenković-Moraj, A.; Chen, K. Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress. Forests 2022, 13, 1123. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhainsa, K.C.; D’Souza, S.F. Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresour. Technol. 2010, 101, 2573–2579. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ĺ.u.; Garnczarska, M.g. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef]
- Xiao, P.-x.; Chen, X.; Zhong, N.-y.; Zheng, T.; Wang, Y.-m.; Wu, G.; Zhang, H.; He, B. Response of Vicia faba to short-term uranium exposure: Chelating and antioxidant system changes in roots. J. Plant Res. 2023, 136, 413–421. [Google Scholar] [CrossRef]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytorem. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Edayilam, N.; Ferguson, B.; Montgomery, D.; Al Mamun, A.; Martinez, N.; Powell, B.A.; Tharayil, N. Dissolution and Vertical Transport of Uranium from Stable Mineral Forms by Plants as Influenced by the Co-occurrence of Uranium with Phosphorus. Environ. Sci. Technol. 2020, 54, 6602–6609. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhong, Y.; Chen, J.; Qi, X. Deciphering the functional roles of transporter proteins in subcellular metal transportation of plants. Planta 2023, 258, 17. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Lu, L. Advances in Genes-Encoding Transporters for Cadmium Uptake, Translocation, and Accumulation in Plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Ohtaki, H.; Radnai, T. Structure and dynamics of hydrated ions. Chem. Rev. 1993, 93, 1157–1204. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mertens, A.; Horemans, N.; Saenen, E.; Nauts, R.; Cuypers, A. Calcium affects uranium responses in Arabidopsis thaliana: From distribution to toxicity. Plant Physiol. Biochem. 2022, 185, 101–111. [Google Scholar] [CrossRef]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Feng, B.; Zhao, Z.; Zhang, Y.; Yin, K.; Liu, Y.; Zhang, X.; Liu, J.; Li, J.; Zhao, R.; et al. Populus euphratica R2R3-MYB transcription factor RAX2 binds ANN1 promoter to increase cadmium enrichment in Arabidopsis. Plant Sci. 2024, 344, 112082. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, Z.; Liu, J.; Zhang, Y.; Zhang, Y.; Yu, D.; Hou, S.; Zhang, Y.; Yao, J.; Zhang, H.; et al. Ectomycorrhizal Fungal Strains Facilitate Cd2+ Enrichment in a Woody Hyperaccumulator under Co-Existing Stress of Cadmium and Salt. Int. J. Mol. Sci. 2021, 22, 11651. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zheng, L.; Wang, Y.; Chen, X.; Zhang, W. A Functional Study Identifying Critical Residues Involving Metal Transport Activity and Selectivity in Natural Resistance-Associated Macrophage Protein 3 in Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 1430. [Google Scholar] [CrossRef]
- Thomine, S.; Wang, R.; Ward, J.M.; Crawford, N.M.; Schroeder, J.I. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Oomen, R.J.F.J.; Wu, J.; Lelièvre, F.; Blanchet, S.; Richaud, P.; Barbier-Brygoo, H.; Aarts, M.G.M.; Thomine, S. Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 2009, 181, 637–650. [Google Scholar] [CrossRef]
- Zorrig, W.; Abdelly, C.; Berthomieu, P. The phylogenetic tree gathering the plant Zn/Cd/Pb/Co P1B-ATPases appears to be structured according to the botanical families. Comptes Rendus. Biol. 2011, 334, 863–871. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Qiu, G.; Li, H.; Wang, Y.; Chen, X.; Fu, Q.; Guo, B. Inhibition Roles of Calcium in Cadmium Uptake and Translocation in Rice: A Review. Int. J. Mol. Sci. 2023, 24, 11587. [Google Scholar] [CrossRef]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, impact, adaptive mechanisms, and phytoremediation of heavy metals by plants: Role of transporters in heavy metal sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef] [PubMed]
- Przybyla-Toscano, J.; Chetouhi, C.; Pennera, L.; Boursiac, Y.; Galeone, A.; Devime, F.; Balliau, T.; Santoni, V.; Bourguignon, J.; Alban, C.; et al. New insights into uranium stress responses of Arabidopsis roots through membrane- and cell wall-associated proteome analysis. Chemosphere 2025, 370, 143873. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Chen, X.; Sun, M.; Yang, C.-H.; Xu, M.-Y.; Lai, F.-X.; Fu, S.-Q.; Fan, Y.-M.; Guo, X.-P.; Li, Q.; et al. Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study. Plants 2025, 14, 1802. [Google Scholar] [CrossRef]
- Lai, J.-l.; Liu, Z.-w.; Luo, X.-g. A metabolomic, transcriptomic profiling, and mineral nutrient metabolism study of the phytotoxicity mechanism of uranium. J. Hazard. Mater. 2020, 386, 121437. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Z. Pollution characteristics and risk assessment of uranium and heavy metals of agricultural soil around the uranium tailing reservoir in Southern China. J. Radioanal. Nucl. Chem. 2018, 318, 923–933. [Google Scholar] [CrossRef]
- Yan, X.; Luo, X. Radionuclides distribution, properties, and microbial diversity of soils in uranium mill tailings from southeastern China. J. Environ. Radioactiv. 2015, 139, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, G.; Tian, J.; Zhang, Y.; Jiang, C.; Zhang, W. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agr. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Lai, J.-l.; Luo, X.-g. High-efficiency antioxidant system, chelating system and stress-responsive genes enhance tolerance to cesium ionotoxicity in Indian mustard (Brassica juncea L.). Ecotox. Environ. Saf. 2019, 181, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Guo, X.; Yan, S.; Liao, J.; Wu, W.; Chen, X.; Lai, F.; Wen, Y.; Yuan, S.; Zhao, J. Bacillus cereus GW-01 promotes Pak Choi (Brassica chinensis L.) growth by reducing β-Cypermethrin and regulating plant metabolism, phyllosphere and rhizosphere microbial communities. Food Chem. 2025, 492, 145521. [Google Scholar] [CrossRef]
- Lai, J.-l.; Zhang-xuan, D.; Xiao-hui, J.I.; Xue-gang, L. Absorption and interaction mechanisms of uranium & cadmium in purple sweet potato(Ipomoea batatas L.). J. Hazard. Mater. 2020, 400, 123264. [Google Scholar] [CrossRef]
- Khan, M.H.; Warwick, P.; Evans, N. Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 2006, 63, 1165–1169. [Google Scholar] [CrossRef]
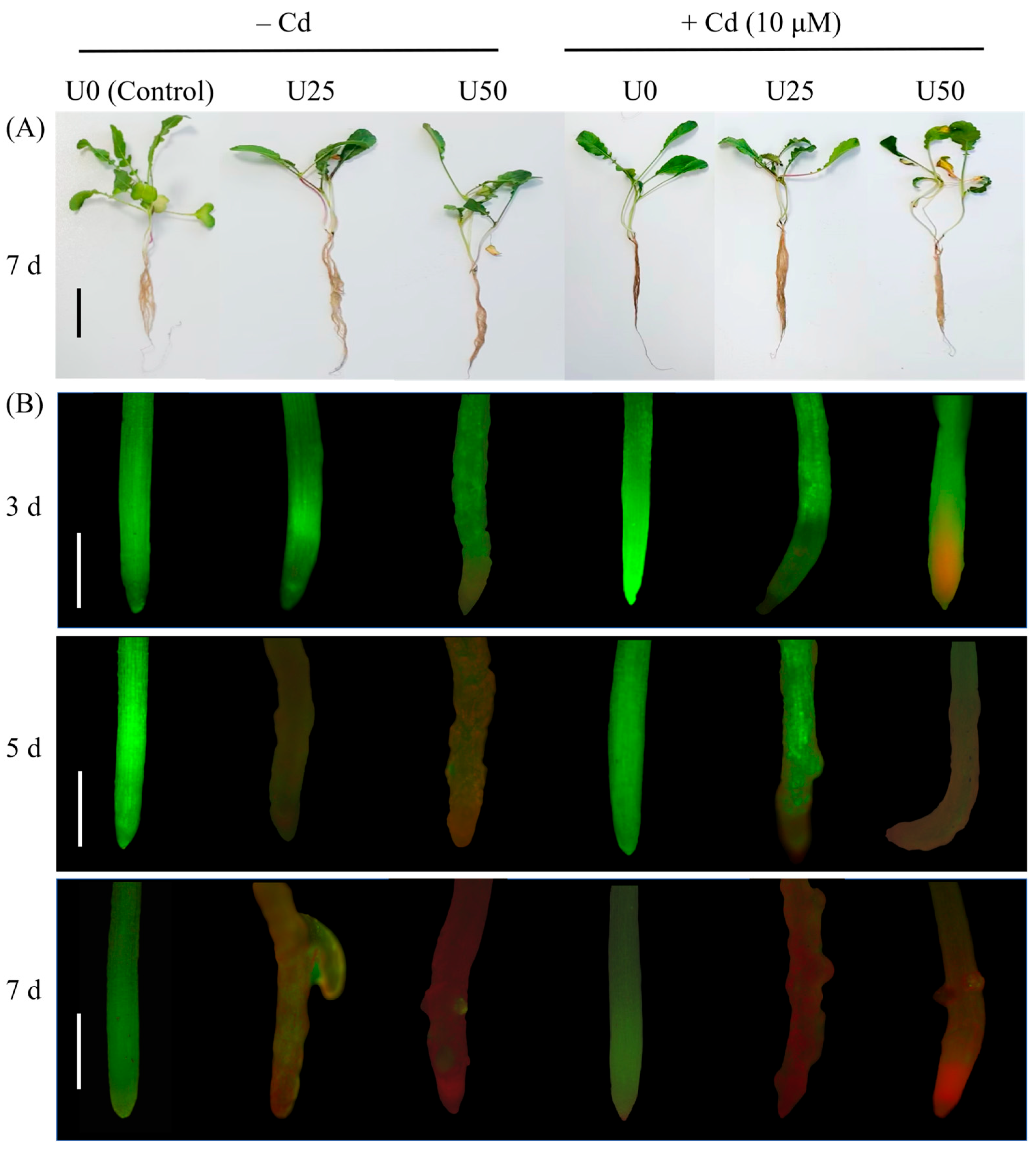
| Treatments | U0 | U25 | U50 | Cd | U25 + Cd | U50 + Cd |
|---|---|---|---|---|---|---|
| U concentration (μM) | 0 | 25 | 50 | 0 | 25 | 50 |
| Cd concentration (μM) | 0 | 0 | 0 | 10 | 10 | 10 |
| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| ACT7 | GATGGGTCAGAAAGATGC | CTGTTGGCTTTAGGGTTA |
| MCA1 | TCCGTATCGTATCCCTAACA | GGAGCCGTGACCAGAGT |
| MCA3 | GTCCTTGATTTACCCTCTG | GTTCCACCATTTGTTCCT |
| ANN1 | AATGCTACTTTCAATCGCTAC | GCTGTTCCTCCTCTGATACTC |
| Nramp1 | CAATCTGGAGCACAATACA | CAGCAACAACCCATAGC |
| Nramp3 | AATCGGTCCTTTGTATCAGA | ATGCCACGAGCAATCAG |
| HMA2 | GTAATAACCGTGGGAGC | GATTGGAGCCATTCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.-P.; Chen, X.; Tu, C.-X.; Fan, Y.-M.; Wang, M.-X.; Zhao, Z.-Q.; Yang, S.-Y.; Cui, L.-L.; Wu, G.; Lai, J.-L.; et al. Effects of Cadmium on the Accumulation and Phytotoxicity of Uranium in Radish (Raphanus sativus L.) Seedlings. Plants 2025, 14, 2711. https://doi.org/10.3390/plants14172711
Guo X-P, Chen X, Tu C-X, Fan Y-M, Wang M-X, Zhao Z-Q, Yang S-Y, Cui L-L, Wu G, Lai J-L, et al. Effects of Cadmium on the Accumulation and Phytotoxicity of Uranium in Radish (Raphanus sativus L.) Seedlings. Plants. 2025; 14(17):2711. https://doi.org/10.3390/plants14172711
Chicago/Turabian StyleGuo, Xin-Peng, Xi Chen, Chun-Xia Tu, Yu-Meng Fan, Ming-Xuan Wang, Zheng-Qin Zhao, Shi-Yi Yang, Lan-Lan Cui, Guo Wu, Jin-Long Lai, and et al. 2025. "Effects of Cadmium on the Accumulation and Phytotoxicity of Uranium in Radish (Raphanus sativus L.) Seedlings" Plants 14, no. 17: 2711. https://doi.org/10.3390/plants14172711
APA StyleGuo, X.-P., Chen, X., Tu, C.-X., Fan, Y.-M., Wang, M.-X., Zhao, Z.-Q., Yang, S.-Y., Cui, L.-L., Wu, G., Lai, J.-L., & Li, Q. (2025). Effects of Cadmium on the Accumulation and Phytotoxicity of Uranium in Radish (Raphanus sativus L.) Seedlings. Plants, 14(17), 2711. https://doi.org/10.3390/plants14172711







