Isatis tinctoria L.—From Botanical Description to Seed-Extracted Compounds and Their Applications: An Overview
Abstract
1. Introduction
2. Phenology and Botanical Description
2.1. International Names and Botanical Situation
2.2. Botanical Description of I. tinctoria L. Fruit
2.3. Phenology and Germination

2.4. Reproduction
3. Comparison of the Overall Chemical Composition of Isatis tinctoria and Isatis indigotica Seeds
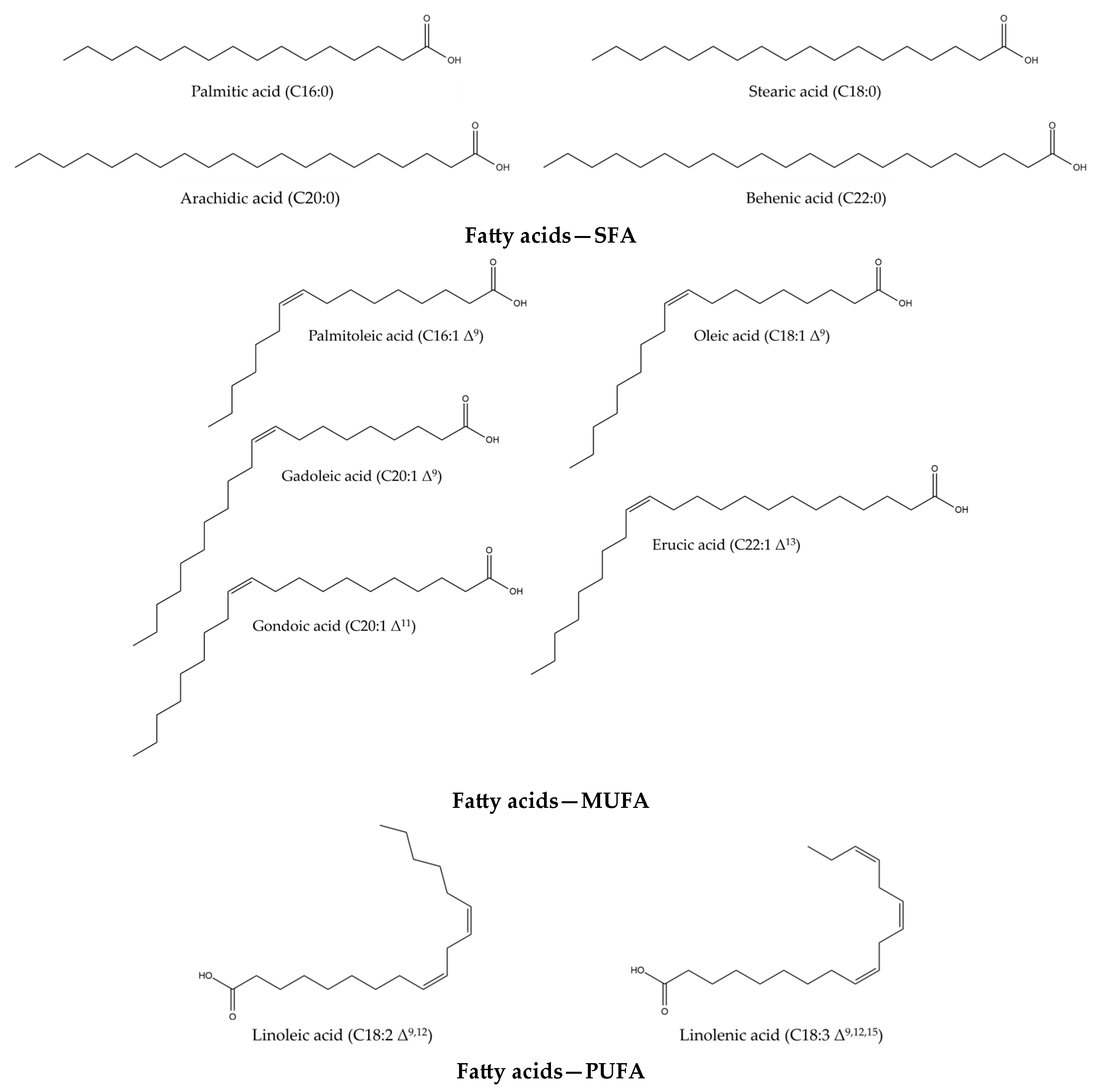
4. Fatty Acids
4.1. Functions and Properties of Fatty Acids
4.2. Oil Extraction and Analyses of Its Fatty Acid Contents
4.3. Fatty Acid Composition in Isatis tinctoria Seed Oil
5. Amino Acids
5.1. Functions and Properties of Amino Acids
5.2. Extraction and Analyses of Amino Acids
5.3. Amino Acid Composition
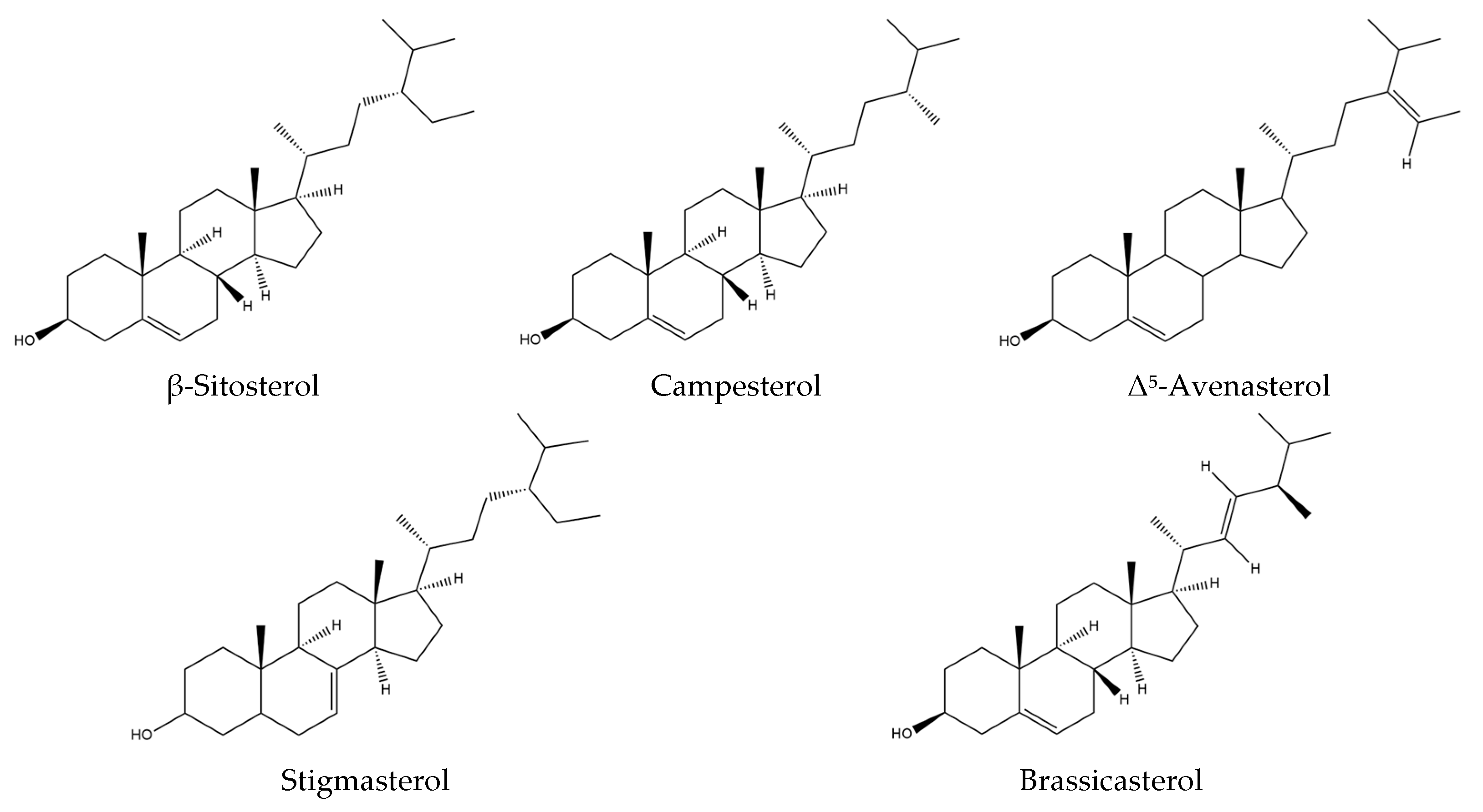
6. Phytosterols
6.1. Functions and Properties of Phytosterols
6.2. Extraction and Analyses of Phytosterols
6.3. Phytosterol Composition
7. Glucosinolates
7.1. Functions and Properties of Glucosinolates
7.2. Sample Preparation, Extraction and Analyses of Glucosinolates
7.3. Glucosinolate Composition
8. Antioxidant Potential
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, J.A.; Evans, R.A. Germination of Dyers Woad. Weed Sci. 1971, 19, 76–78. [Google Scholar] [CrossRef]
- Prasad, R. Indigo—The Crop that Created History and then Itself Became History. Indian. J. Hist. Sci. 2018, 53, 296–301. [Google Scholar] [CrossRef]
- eFlora. Available online: https://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200009571 (accessed on 11 March 2024).
- Sales, E.; Kanhonou, R.; Baixauli, C.; Giner, A.; Cooke, D.; Gilbert, K.; Arrillaga, I.; Segura, J.; Ros, R. Sowing date, transplanting, plant density and nitrogen fertilization affect indigo production from Isatis species in a Mediterranean region of Spain. Ind. Crops Prod. 2006, 23, 29–39. [Google Scholar] [CrossRef]
- Kokubun, T.; Edmonds, J.; John, P. Indoxyl Derivatives In Woad In Relation To Medieval Indigo Production. Phytochemistry 1998, 49, 79–87. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tozzi, S.; Nassi o Di Nasso, N. Differences in leaf yield and indigo precursors production in woad (Isatis tinctoria L.) and Chinese woad (Isatis indigotica Fort.) genotypes. Field Crops Res. 2007, 101, 285–295. [Google Scholar] [CrossRef]
- Giot, C.; Clausse, E.; Carbonnier, Y.; Vauquelin, R.; Joly, N.; Martin, P.; Schneider, P. Isatis tinctoria & Indigo; A Spatiotemporal Culture in France and Elsewhere-Historical Review. Am. J. Plant Sci. 2025, 16, 287–300. [Google Scholar] [CrossRef]
- Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M.; Withnall, R. Indigo, woad, and Tyrian Purple: Important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. [Google Scholar] [CrossRef]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Fatty acid and phytosterol accumulation during seed development in three oilseed species. Int. J. Food Sci. Technol. 2016, 51, 1820–1826. [Google Scholar] [CrossRef]
- Spataro, G.; Taviani, P.; Negri, V. Genetic Variation and Population Structure in a Eurasian Collection of Isatis tinctoria L. Genet. Resour. Crop Evol. 2007, 54, 573–584. [Google Scholar] [CrossRef]
- eFlora. Available online: https://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=200009571 (accessed on 11 March 2024).
- Fuller, A.T. An Autecological Study of Dyers Woad (Isatis tinctoria L.) on Utah Rangeland. Master’s Thesis, Utah State University, Logan, UT, USA, 1985; pp. 1–61. [Google Scholar] [CrossRef]
- Vauquelin, R.; Juillard-Condat, L.; Joly, N.; Jullian, N.; Choque, E.; Martin, P. Study of Woad (Isatis tinctoria L.)-Extracted Indoxyl Precursors Conversion into Dyes: Influence of the Oxidative Media on Indigo Recovery Yields and Indigotin/Indirubin Ratio Measured by HPLC-DAD Method. Molecules 2024, 29, 4804. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.F. The Woad Plant and its Dye. Nature 1931, 127, 658–659. [Google Scholar] [CrossRef]
- Gilbert, K.G.; Cooke, D.T. Dyes from plants: Past usage, present understanding and potential. Plant Growth Regul. 2001, 34, 57–69. [Google Scholar] [CrossRef]
- Baeyer, A.; Drewsen, V. Darstellung von Indigblau aus Orthonitrobenzaldehyd. Berichte Dtsch. Chem. Ges. 1882, 15, 2856–2864. [Google Scholar] [CrossRef]
- Steingruber, E. Indigo and Indigo Colorants. Ullmanns Encycl. Ind. Chem. 2004, 19, 55–63. [Google Scholar] [CrossRef]
- Bechtold, T.; Turcanu, A.; Geissler, S.; Ganglberger, E. Process balance and product quality in the production of natural indigo from Polygonum tinctorium Ait. applying low-technology methods. Bioresour. Technol. 2002, 81, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J.J. Dyer’s Woad. UC IPM Integr. Pest Manag. Home Gard. Landsc. Prof. 2017, 74175, 1–5. Available online: https://www.academia.edu/44904675/Dyers_Woad (accessed on 4 March 2024).
- Farah, K.O.; Tanaka, A.F.; West, N.E. Autecology and Population Biology of Dyers Woad (Isatis tinctoria). Weed Sci. 1988, 36, 186–193. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. PeerJ 2019, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Speranza, J.; Miceli, N.; Taviano, M.F.; Ragusa, S.; Kwiecień, I.; Szopa, A.; Ekiert, H. Isatis tinctoria L. (Woad): A Review of its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies. Plants 2020, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Bontaz-Carion, J.; Montaut, S.; Goetz, P. Isatis tinctoria L. Brassicaceae: Le pastel des teinturiers. Phytothérapie 2012, 10, 238–244. [Google Scholar] [CrossRef]
- Inventaire National du Patrimoine Naturel. Available online: https://inpn.mnhn.fr/espece/cd_nom/103817 (accessed on 2 July 2024).
- Info Flora. Available online: https://www.infoflora.ch/fr/flore/isatis-tinctoria.html (accessed on 2 July 2024).
- Biolib. Available online: http://www.biolib.de/sturm/flora/index.html (accessed on 6 January 2025).
- Plantes et Botanique. Available online: https://www.plantes-botanique.org/espece_isatis_tinctoria (accessed on 11 March 2024).
- Forest Service of the USDA. Available online: https://www.fs.usda.gov/database/feis/plants/forb/isatin/all.html#Botanical%20description (accessed on 10 July 2025).
- Kizil, S.; Turk, M.; Çakmak, Ö.; Özgüven, M.; Khawar, K.M. Microelement Contents and Fatty Acid Compositions of some Isatis Species Seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 175–178. Available online: https://www.notulaebotanicae.ro/index.php/nbha/article/view/3115 (accessed on 10 July 2025).
- Dolya, V.S.; Koreshchuk, K.E.; Fursa, N.S.; Golodner, D.N.; Kaminskii, N.A. Oils of three representatives of the family Cruciferae. Chem. Nat. Compd. 1972, 8, 377–378. [Google Scholar] [CrossRef]
- Li, T.; Qu, X.-Y.; Zhang, Q.-A.; Whang, Z.-Z. Ultrasound-assisted extraction and profile characteristics of seed oil from Isatis indigotica Fort. Ind. Crops Prod. 2012, 35, 98–104. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S.; Antichi, D.; Bagatta, M.; Matteo, R.; Lazzeri, L. Fatty acid and glucosinolate patterns of seed from Isatis indigotica Fortune as bioproducts for green chemistry. Ind. Crops Prod. 2015, 75, 51–58. [Google Scholar] [CrossRef]
- Miller, R.W.; VanEtten, C.H.; McGrew, C.; Wolff, I.A.; Jones, Q. Seed Meal Amino Acids, Amino Acid Composition of Seed Meals from Forty-One Species of Cruciferae. J. Agric. Food Chem. 1962, 10, 426–430. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Guan, L.; Zhang, L. Metabolome and transcriptome reveal the biosynthesis of flavonoids and amino acids in Isatis indigotica fruit during development. Physiol. Plant 2024, 176, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mohn, T.; Hamburger, M. Glucosinolate Pattern in Isatis tinctoria and I. indigotica Seeds. Planta Med. 2008, 74, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, M.; Guo, Q.; Wei, M.; Shi, H.; Wang, T.; Han, Z.; Liu, H.; Liu, C.; Huang, J. Classification of Isatis indigotica Fortune and Isatis tinctoria Linnaeus via comparative analysis of chloroplast genomes. BMC Genom. 2023, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iba, N. Les Esters D’acides Gras Polyinsaturés d’Isatis tinctoria (pastel): Mise au Point d’une Technique de Séparation—Generalisation à Différentes Huiles Insaturées. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 1990. [Google Scholar]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective Against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.M.; Vessby, B.; Uusitupa, M.; Berglund, L.; Pedersen, E.; Riccardi, G.; Rivellesse, A.A.; Tapsell, L.; Hermansen, K. Effects of dietary saturated, monounsaturated, and n−3 fatty acids on blood pressure in healthy subjects. Am. J. Clin. Nutr. 2006, 83, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Spataro, G.; Negri, V. Adaptability and variation in Isatis tinctoria L.: A new crop for Europe. Euphytica 2008, 163, 89–102. [Google Scholar] [CrossRef]
- Cosmile Europe. Available online: https://cosmileeurope.eu/fr/inci/ (accessed on 24 May 2024).
- Cosmetic Ingredient Review Safety. Available online: https://cir-reports.cir-safety.org/view-attachment/?id=3a3ac6dd-8c74-ec11-8943-0022482f06a6 (accessed on 28 August 2024).
- Publications Office of the European Union. Available online: https://op.europa.eu/en/publication-detail/-/publication/e332d361-c3e3-4bc0-9fd9-334ebdfbab12/language-en (accessed on 10 June 2024).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to oleic acid intended to replace saturated fatty acids (SFAs) in foods or diets and maintenance of normal blood LDL-cholesterol concentrations (ID 673, 728, 729, 1302, 4334) and maintenance of normal (fasting) blood concentrations of triglycerides (ID 673, 4334) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 1–17. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to linoleic acid and maintenance of normal blood cholesterol concentrations (ID 489) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1–12. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to linoleic acid and “molecule precursors regulating cell functions (prostaglandins, leucotrienes)” (ID 488, 4670), maintenance of normal blood LDL-cholesterol concentrations (ID 2899) and protection of the skin from UV-induced damage (ID 3659) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 1–15. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Opinion on the substantiation of health claims related to alpha linolenic acid and maintenance of normal blood cholesterol concentrations (ID 493) and maintenance of normal blood pressure (ID 625) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1–17. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on health claims already evaluated (ID 215, 568, 674, 712, 1398, 1633, 1974, 4191, 4192, 4193, 4236, 4335, 4698, 4704) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 1–22. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to the replacement of mixtures of saturated fatty acids (SFAs) as present in foods or diets with mixtures of monounsaturated fatty acids (MUFAs) and/or mixtures of polyunsaturated fatty acids (PUFAs), and maintenance of normal blood LDL cholesterol concentrations (ID 621, 1190, 1203, 2906, 2910, 3065) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 1–18. [Google Scholar] [CrossRef]
- Romdhane, M.; Gourdon, C. Investigation in solid–liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Mikolajczak, K.L.; Miwa, T.K.; Earle, F.R.; Wolff, I.A.; Jones, Q. Search for new industrial oils. V. Oils of cruciferae. J. Americ Oil Chem. Soc. 1961, 38, 678–681. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Ashraf, R.; Ghufran, S.; Akram, S.; Mushtaq, M.; Sultana, B. Cold pressed coriander (Coriandrum sativum L.) seed oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 345–356. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al-Juhaimi, F.Y.; Ahmed, I.A.M.; Osman, M.A.; Gassem, M.A. Effect of soxhlet and cold press extractions on the physico-chemical characteristics of roasted and non-roasted chia seed oils. J. Food Meas. Charact. 2019, 13, 648–655. [Google Scholar] [CrossRef]
- International Olive Council COI/T.20/Doc. No 33—Determination of Fatty Acid Methyl Esters by Gas Chromatography. 2017. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf (accessed on 14 April 2024).
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Milinsk, M.C.; Matsushita, M.; Visentainer, J.V.; de Oliveira, C.C.; de Souza, N.E. Comparative analysis of eight esterification methods in the quantitative determination of vegetable oil fatty acid methyl esters (FAME). J. Braz. Chem. Soc. 2008, 19, 1475–1483. [Google Scholar] [CrossRef]
- Gambert, A.-G.; Niţu, S.; Tămaș, A.; Fanani, M.L.; Dupré, J.; Delepine, C.; Chaveriat, L.; Martin, P.; Rusnac, L. Influence of the Extraction Process on the Characteristics of Romanian Mountain Walnut Oil. Am. J. Plant Sci. 2024, 15, 940–967. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar Srivastava, A.; Khare, P.; Kumar Nagar, H.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, C.H.; Miller, R.W.; Earle, F.R.; Wolffe, I.A.; Jones, Q. Plant Protein Constituents, Hydroxyproline Content of Seed Meals and Distribution of the Amino Acid in Kernel, Seed Coat, and Pericarp. J. Agric. Food Chem. 1961, 9, 433–435. [Google Scholar] [CrossRef]
- Hara, R.; Kino, K. Enzymatic reactions and microorganisms producing the various isomers of hydroxyproline. Appl. Microbiol. Biotechnol. 2020, 104, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, C.H.; Miller, R.W.; Wolff, I.A. Nutrients in Seed Meals, Amino Acid Composition of Twenty-Seven Selected Seed Meals. J. Agric. Food Chem. 1961, 9, 79–82. [Google Scholar] [CrossRef]
- Levine, M. A New Method for Isolation of Hydroxy-l-proline and l-Proline from Gelatin. J. Biol. Chem. 1959, 234, 1731–1732. [Google Scholar] [CrossRef] [PubMed]
- Spackman, D.H.; Stein, W.H. Moore Stanford. Automatic Recording Apparatus for Use in Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Becker, H.C.; Milner, R.T.; Nagel, R.H. A Method for the Determination of Nonprotein Nitrogen in Soybean Meal. Cereal Chem. 1940, 17, 447–457. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19401402307 (accessed on 14 April 2024).
- Ms, U.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Garcia-Llatas, G.; Alegría, A.; Barberá, R.; Cilla, A. Current methodologies for phytosterol analysis in foods. Microchem. J. 2021, 168, 1–16. [Google Scholar] [CrossRef]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Bouic, P.J. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Barkizatova, G.; Turgumbayeva, A.; Zhakipbekov, K.; Bekesheva, K.; Arystanov, Z.; Arystanova, T.; Kayupova, F.; Zhumalina, K.; Toxanbayeva, Z.; Ibragimova, A.; et al. Exploring the Pharmacological Potential of Lithospermum officinale L.: A Review of Phytochemicals and Ethnomedicinal Uses. Molecules 2024, 29, 1856. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Fink, C.S. Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingärtner, O.; Laufs, U.; et al. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Bin Sayeed, M.; Karim, S.; Sharmin, T.; Morshed, M. Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review Safety. Available online: https://cir-reports.cir-safety.org/view-attachment/?id=56e8bd9b-8c74-ec11-8943-0022482f06a6 (accessed on 29 August 2024).
- Nazir, S.; Chaudhary, W.; Mubashar, A.; Anjum, I.; Hameed, S.; Azhar, S. Campesterol: A Natural Phytochemical with Anti Inflammatory Properties as Potential Therapeutic Agent for Rheumatoid Arthritis: A Systematic Review: Campesterol: A Natural Phytochemical. Pak. J. Health Sci. 2023, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments. Biomedicines 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Vear, F.; Merah, O. Sterol content in sunflower seeds (Helianthus annuus L.) as affected by genotypes and environmental conditions. Food Chem. 2010, 121, 990–995. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.; Moreno-Fernández, D.A.; Castejon, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mumm, R.; Burow, M.; Bukovinszkine’Kiss, G.; Kazantzidou, E.; Wittstock, U.; Dicke, M.; Gershenzon, J. Formation of Simple Nitriles upon Glucosinolate Hydrolysis Affects Direct and Indirect Defense Against the Specialist Herbivore, Pieris rapae. J. Chem. Ecol. 2008, 34, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; du Plessis, J. Sinigrin and Its Therapeutic Benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Fruit and Vegetable Intake and Incidence of Bladder Cancer in a Male Prospective Cohort. J. Natl. Cancer Inst. 1999, 91, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Munday, C.M. Induction of Phase II Detoxification Enzymes in Rats by Plant-Derived Isothiocyanates: Comparison of Allyl Isothiocyanate with Sulforaphane and Related Compounds. J. Agric. Food Chem. 2004, 52, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W. Chemoprevention by Inducers of Carcinogen Detoxication Enzymes. Environ. Health Perspect. 1997, 105, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Aires, A.; Saavedra, M. Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wu, Y.; Dai, Z.; Ma, S. Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J. Ethnopharmacol. 2020, 251, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tarini, G.; Melo, A.S.; Fontana, L.F.; Da Silva, E.; Bolanho, B.C.; Moreno, B.P.; Sarragiotto, M.H.; Dias-Arieira, C.R. Aqueous extracts of Crambe abyssinica seed cake: Chemical composition and potential for nematode control. Ind. Crops Prod. 2020, 156, 1–9. [Google Scholar] [CrossRef]
- Ahmad, I.; Fatima, I.; Afza, N.; Malik, A.; Lodhi, M.A.; Choudhary, M.I. Urease and Serine Protease inhibitory alkaloids from Isatis tinctoria. J. Enzyme Inhib. Med. Chem. 2008, 23, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Moon, B.; Kim, S. Analysis of glucosinolates and their breakdown products from Mul-kimchis using UPLC-MS/MS. J. Food Compos. Anal. 2024, 125, 1–8. [Google Scholar] [CrossRef]
- McDanell, R.; McLean, A.E.M.; Hanley, A.B.; Heaney, R.K.; Fenwick, G.R. Chemical and biological properties of indole glucosinolates (glucobrassicins): A review. Food Chem. Toxicol. 1988, 26, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Valan Arasu, M.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, S.-J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Nartea, A.; Fanesi, B.; Pacetti, D.; Lenti, L.; Fiorini, D.; Lucci, P.; Frega, N.G.; Falcone, P.M. Cauliflower by-products as functional ingredient in bakery foods: Fortification of pizza with glucosinolates, carotenoids and phytosterols. Curr. Res. Food Sci. 2023, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; LeBlanc, G.A. Hypocholesterolemic properties of plant indoles. Biochem. Pharmacol. 1994, 47, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Mohn, T.; Cutting, B.; Ernst, B.; Hamburger, M. Extraction and analysis of intact glucosinolates--a validated pressurized liquid extraction/liquid chromatography-mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. J. Chromatogr. A 2007, 1166, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.-J.; Zhang, Y.-Y.; Xing, Y.; Yang, L.; Ni, H.; Li, H.-H. Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem. 2019, 300, 125–162. [Google Scholar] [CrossRef] [PubMed]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Müller, C.T.; Mewis, I. Development of a reliable extraction and quantification method for glucosinolates in Moringa oleifera. Food Chem. 2015, 166, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, H.; Yuan, Q. Separation of sinigrin from Indian mustard (Brassica juncea L.) seed using macroporous ion-exchange resin. Korean J. Chem. Eng. 2012, 29, 396–403. [Google Scholar] [CrossRef]
- Sut, S.; Boschiero, I.; Solana, M.; Malagoli, M.; Bertucco, A.; Dall’Acqua, S. Supercritical CO2 Extraction of Eruca sativa Using Cosolvents: Phytochemical Composition by LC-MS Analysis. Molecules 2018, 23, 3240. [Google Scholar] [CrossRef] [PubMed]
- Thies, W. Isolation of Sinigrin and Glucotropaeolin from Cruciferous Seeds. Lipid Fett. 1988, 90, 311–314. [Google Scholar] [CrossRef]
- Rochfort, S.; Caridi, D.; Stinton, M.; Trenerry, V.C.; Jones, R. The isolation and purification of glucoraphanin from broccoli seeds by solid phase extraction and preparative high performance liquid chromatography. J. Chromatogr. A 2006, 1120, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. High-speed countercurrent chromatography. Nature 1987, 326, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Sticher, O.; Veit, M.; Fröhlich, R.; Pauli, G.F. Evaluation of Glucoiberin Reference Material from Iberis amara by Spectroscopic Fingerprinting. J. Nat. Prod. 2002, 65, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.E.; Waser, J. Refinement of the crystal structure of sinigrin. Acta Crystallogr. B 1970, 26, 1030–1037. [Google Scholar] [CrossRef]
- Chidewe, C.; Castillo, U.F.; Sem, D.S. Structural Analysis and Antimicrobial Activity of Chromatographically Separated Fractions of Leaves of Sesamum angustifolium (Oliv.) Engl. J. Biol. Act. Prod. Nat. 2017, 7, 463–474. [Google Scholar] [CrossRef]
- Montaut, S.; Bleeker, R.S.; Jacques, C. Phytochemical constituents of Cardamine diphylla. Can. J. Chem. 2010, 88, 50–55. [Google Scholar] [CrossRef]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Popović, M.; Guillot, X.; Burčul, F.; Rollin, P. Microwave-Assisted versus Conventional Isolation of Glucosinolate Degradation Products from Lunaria annua L. and Their Cytotoxic Activity. Biomolecules 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Allart-Simon, I.; De Nicola, G.R.; Iori, R.; Renault, J.-H.; Rollin, P.; Nuzillard, J.-M. Advanced NMR-Based Structural Investigation of Glucosinolates and Desulfoglucosinolates. J. Nat. Prod. 2018, 81, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Grosser, K.; Van Dam, N.M. A Straightforward Method for Glucosinolate Extraction and Analysis with High-pressure Liquid Chromatography (HPLC). J. Vis. Exp. 2017, 121, 1–9. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.E.; Huang, X.-C.; Hansen, C.I.C.; Cipollini, D.; Ørgaard, M.; Matthes, A.; Geu-Flores, F.; Koch, M.A.; Agerbirk, N. Glucosinolate diversity within a phylogenetic framework of the tribe Cardamineae (Brassicaceae) unraveled with HPLC-MS/MS and NMR-based analytical distinction of 70 desulfoglucosinolates. Phytochemistry 2016, 132, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. Determination of Glucosinolates and Their Decomposition Products—Indoles and Isothiocyanates in Cruciferous Vegetables. Crit. Rev. Anal. Chem. 2010, 40, 202–216. [Google Scholar] [CrossRef]
- Gallaher, C.M.; Gallaher, D.D.; Peterson, S. Development and Validation of a Spectrophotometric Method for Quantification of Total Glucosinolates in Cruciferous Vegetables. J. Agric. Food Chem. 2012, 60, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Barickman, T.C.; Sams, C.E.; McElroy, J.S. Influence of Nitrogen and Sulfur on Biomass Production and Carotenoid and Glucosinolate Concentrations in Watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2007, 55, 10628–10634. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Leszczyńska, T.; Filipiak-Florkiewicz, A.; Sikora, E.; Pisulewski, P.M. Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Changes in Glucosinolate Concentrations, Myrosinase Activity, and Production of Metabolites of Glucosinolates in Cabbage (Brassica oleracea Var. capitata) Cooked for Different Durations. J. Agric. Food Chem. 2006, 54, 7628–7634. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Peschel, W.; Dieckmann, W.; Sonnenschein, M.; Plescher, A. High antioxidant potential of pressing residues from evening primrose in comparison to other oilseed cakes and plant antioxidants. Ind. Crops Prod. 2007, 25, 44–54. [Google Scholar] [CrossRef]


| Languages | Vernacular Names | References |
|---|---|---|
| None | Isatis | [23] |
| French | Waide Vouède Herbe du Lauraguais Indigo français Indigo des teinturiers | [23] |
| Pastel des teinturiers Guède (guedde or guesde) Herbe de Saint-Philippe | [23,24] | |
| Pastel | [25] | |
| English | Dyer’s woad | [23] |
| Woad | [23,24] | |
| German | Waid | [23] |
| Färberwaid | [23,25] | |
| Dutch | Wede | [23] |
| Flemish | Weede | [23] |
| Italian | Glasto commune (Glastum in latin) Guado | [23,25] |
| Spanish | Guasto Glasto Hierba pastel | [23] |
| Russian | Ijenack | [23] |
| Polish | Nilo | [23] |
| Chinese | Ban Lan Gen (roots) * Da Qing Ye (leaves) * Qing Dai (blue powder) | [23] |
| Characteristics | Comments | References |
|---|---|---|
| Plant life expectancy | Biennial or short-lived perennial species. (Figure 2) | [3,10,22,29] |
| First year of growing | Plant becomes a rosette. | [12] |
| Second year of growing | Stalk grows with flowers. Plant produces seeds before dying. | [12,19] |
| Dormancy periods | In summer under dry conditions and in winter under cold ones. | [20] |
| Dormancy duration | Indehiscent seeds. Seeds can survive eight years or longer. Germination rate decreases every year. | [1,19] |
| Dormancy inhibition | Water-soluble germination inhibitor in silicles removable by leaching. No long dormancy for seeds in growing lands. | [1,12] |
| Seed germination period | After a period of dormancy, the seeds germinate in the spring or fall. | [12,19] |
| Influence of sowing period | Stable seed production periods whatever the sowing period (spring or summer). | [4] |
| Chemical Classes | Isatis tinctoria | Isatis indigotica | ||
|---|---|---|---|---|
| Compounds | References | Compounds | References | |
| Fatty acids | Palmitic acid C16:0 Stearic acid C18:0 Arachidic acid C20:0 Behenic acid C22:0 Palmitoleic acid C16:1∆9 Oleic acid C18:1∆9 Gondoic acid C20:1∆11 /Gadoleic acid C20:1∆9 Erucic acid C22:1∆13 Linoleic acid C18:2∆9,12 Linolenic acid C18:3∆9,12,15 | [9,29,30] | Palmitic acid C16:0 Stearic acid C18:0 Arachidic acid C20:0 Behenic acid C22:0 Lignoceric acid C24:0 Palmitoleic acid C16:1∆9 Oleic acid C18:1∆9 Elaidic acid C18:1∆9 Vaccenic acid C18:1∆11 Linoleic acid C18:2∆9,12 Linolenic acid C18:3∆9,12,15 Gadoleic acid C20:1∆9 Erucic acid C22:1∆13 Nervonic acid C24:1∆15 11,14 Eicosadienoic C20:2∆11,14 Arachidonic acid C20:4∆5,8,11,14 | [31,32] |
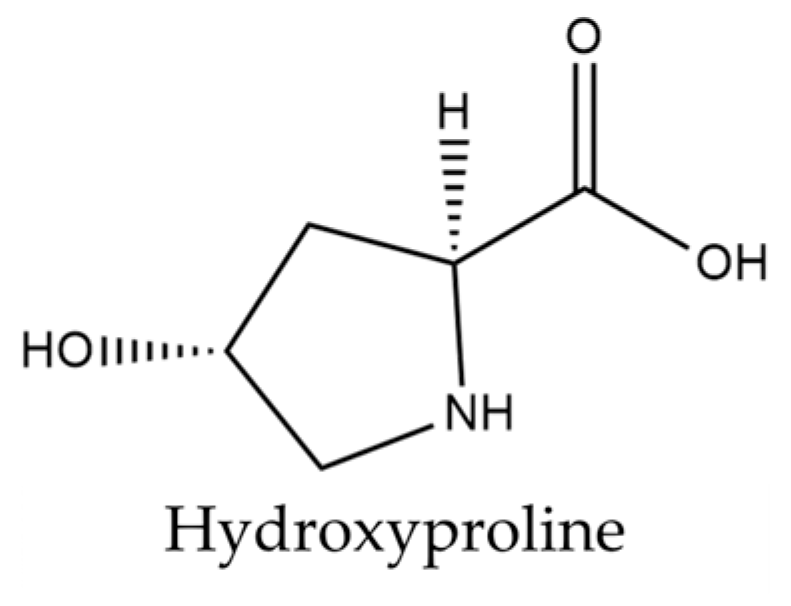
| Amino acid | Hydroxyproline Alanine Arginine Aspartic acid Cystine Glutamic acid Glycine Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Proline Serine Threonine Tyrosine Valine | [33] | Alanine Arginine Asparagine Citrulline Cysteine Glutamic acid Glutamine Glycine Histidine Isoleucine Leucine Lysine Methionine Ornithine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine | [34] |
| Phytosterols | β-Sitosterol Campesterol Δ5-Avenasterol Stigmasterol Brassicasterol Δ7-Avenasterol Δ7-Stigmastenol | [9] | No phytosterols described | / |
| Glucosinolates | Epiprogoitrin Progoitrin Epiglucoisatisin Glucoisatisin Gluconapin Glucobrassicin 4-Hydroxy-glucobrassicin Neoglucobrassicin | [35] | Epiprogoitrin Progoitrin Epiglucoisatisin Glucoisatisin Gluconapin Glucobrassicin 4-Hydroxy-glucobrassicin Neoglucobrassicin | [32] |
| Flavonoids | No phenolic compounds described | / | Taxifolin Cyanidin 3-glucoside Kaempferide Quercetin Astragalin Isovitexin 2″-O-beta-D-glucoside 1-O-Galloyl-beta-D-glucose (-)-Epigallocatechin Quercetin 3-beta-D-sophoroside Luteolin 7-glucoside Isoquercitrin Isorhamnetin Luteolin Naringenin Epicatechin Catechin Glycitein Procyanidin B2 Fisetin Eriodictyol | [34] |
| Chemical Classes | Compounds | Applications | Properties | References |
|---|---|---|---|---|
| Fatty acids —SFA | Palmitic acid C16:0 | Cosmetic uses | Skin emollient; surfactant—emulsifying agent | [41] |
| Cosmetic uses: leave-on products (eye makeup preparations, lipsticks, fragrance products); skin care products; rinse-off products (skin cleansing, shaving cream) | Fragrance agent; opacifying agent; surfactant—cleansing agent; surfactant—emulsifying agent | [42] | ||
| Stearic acid C18:0 | Cosmetic uses | Cleansing agent; fragrance agent; emulsion stabilizer; surfactant—emulsifying agent; surfactant—cleansing agent | [41] | |
| Cosmetic uses: leave-on products (eye makeup preparations, eyebrow pencil, eyeliners); skin care products; rinse-off products (bath soaps, detergents, shaving cream); cosmetic sprays (face, neck) | Fragrance agent; surfactant—cleansing agent; surfactant—emulsifying agent | [42] | ||
| Pharmaceutical uses: suppositories; coating enteric pills; ointments; coating bitter remedies; stearin soap (for opodeldoch) | / | |||
| Arachidic acid C20:0 | Cosmetic uses | Cleansing agent; opacifying agent; surfactant—emulsifying agent; surfactant—cleansing agent | [41] | |
| Cosmetic uses | Opacifying agent; surfactant—cleansing agent | [42] | ||
| Behenic acid C22:0 | Cosmetic uses | Cleansing agent; opacifying agent; surfactant—emulsifying agent; surfactant—cleansing agent | [41] | |
| Cosmetic uses: lipstick, eyebrow pencil | Opacifying agent; surfactant—cleansing agent | [42] | ||
| Fatty acids —MUFA | Palmitoleic acid C16:1 ∆9 | Cosmetic uses | Not referenced | [41,42] |
| Oleic acid C18:1 ∆9 | Cosmetic uses | Skin emollient; surfactant—emulsifying agent | [41] | |
| Cosmetic uses: spray deodorants | Fragrance agent; surfactant—cleansing agent | [42] | ||
| Pharmaceutical uses: emulsifying and solubilizing agent (in pharmaceutical acids); diagnostic aid (for pancreatic function); ointments | / | |||
| Pharmaceutical uses | Cholesterol stabilizer | [43,44] | ||
| Gadoleic acid C20:1∆9 | Cosmetic uses | Not referenced | [41,42] | |
| Gondoic acid C20:1∆11 | Cosmetic uses | Hair conditioner | [41] | |
| Erucic acid C22:1 ∆13 | Cosmetic uses | Skin care agent | [41] | |
| Cosmetic uses | Skin-conditioning agent—miscellaneous | [42] | ||
| Fatty acids —PUFA | Linoleic acid C18:2 ∆9,12 | Cosmetic uses | Antistatic agent; skin care agent; cleansing agent; hair conditioning agent; skin emollient; surfactant—cleansing agent | [41] |
| Cosmetic uses: leave-on skin care products; rinse-off skin cleansing products; skin care products (face, neck, body, hand) | Fragrance agent; hair conditioning agent; skin conditioning agent—miscellaneous; surfactant—cleansing agent | [42] | ||
| Pharmaceutical uses: vitamins | / | |||
| Pharmaceutical uses | Cholesterol stabilizer | [43,45,46] | ||
| Linolenic acid C18:3 ∆9,12,15 | Cosmetic uses | Antistatic agent; skin care agent; cleansing agent; fragrance agent; hair conditioning agent; skin emollient; surfactant—cleansing agent | [42] | |
| Cosmetic uses | Fragrance agent; hair conditioning agent; skin conditioning agent—miscellaneous; surfactant—cleansing agent | |||
| Food uses: dietary supplement/nutrient | / | |||
| Pharmaceutical uses | Cholesterol stabilizer | [43,47,48] |
| Chemical Classes | Compounds | Isatis tinctoria | Isatis indigotica | ||||
|---|---|---|---|---|---|---|---|
| Quantities | Sites of Collection | References | Quantities | Sites of Collection | References | ||
| Fatty acids —SFA | Palmitic acid C16:0 | 5.07 11.18 6 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 4.59 a/4.91 b 4.4 c/5.3 d/4.5 e/5.1 f | China 4 Italy 5 | [31] [32] |
| Stearic acid C18:0 | 2.35 2.17 2 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 2.1 c/1.5 d/1.4 e/1.7 f | Italy 5 | [32] | |
| Arachidic acid C20:0 | 1.68 1.22 2 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 1.33 a/1.28 b 1.5 c/1.1 d/1.0 e/1.3 f | China 4 Italy 5 | [31] [32] | |
| Behenic acid C22:0 | 0.48 0.66 Trace | France 1 Turkey 2 USA 3 | [9] [29] [51] | 0.7 c/0.5 d/0.4 e/0.7 f | Italy 5 | [32] | |
| Lignoceric acid C24:0 | / | / | / | 0.5 c/0.4 d/0.4 e/0.4 f | Italy 5 | [32] | |
| Total SFA | 9.58 15.23 10 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 5.92 a/6.19 b 9.2 c/8.8 d/7.7 e/9.2 f | China 4 Italy 5 | [31] [32] | |
| Fatty acids —MUFA | Palmitoleic acid C16:1 ∆9 | 0.180 | France 1 USA 3 | [9] [51] | 0.22 a/0.27 b | China 4 | [31] |
| Oleic acid C18:1 ∆9 | 16.51 14.64 16 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 27.6 c/12.9 d/18.4 e/14.0 f | Italy 5 | [32] | |
| Elaidic acid C18:1∆9 | n.d. | n.d. | n.d. | 20.30 a/19.60 b | China 4 | [31] | |
| Vaccenic acid C18:1∆11 | n.d. | n.d. | n.d. | 2.0 c/2.2 d/1.2 e/1.4 f | Italy 5 | [32] | |
| Gondoic acid C20:1∆11 */Gadoleic acid C20:1∆9 * | 10.01 10.40 13 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 11.89 a/11.32 b 10.9 c/8.2 d/10.5 e/8.7 f | China 4 Italy 5 | [31] [32] | |
| Erucic acid C22:1 ∆13 | 20.30 26.48 20 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 25.00 a/23.81 b 16.6 c/18.2 d/18.1 e/21.0 f | China 4 Italy 5 | [31] [32] | |
| Nervonic acid C24:1∆15 | n.d. | n.d. | n.d. | 1.4 c/2.2 d/3.3 e/2.1 f | Italy 5 | [32] | |
| Total MUFA | 47.01 51.52 49 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 57.41 a/55.00 b 58.4 c/43.7 d/51.4 e/47.3 f | China 4 Italy 5 | [31] [32] | |
| Fatty acids —PUFA | Linoleic acid C18:2 ∆9,12 | 12.40 2.74 12 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 9.75 a/10.44 b 10.9 c/15.6 d/11.6 e/11.5 f | China 4 Italy 5 | [31] [32] |
| 11,14 EicosadienoicC20:2∆11,14 | n.d. | n.d. | n.d. | 0.5 c/1.0 d/0.8 e/0.8 f | Italy 5 | [32] | |
| Linolenic acid C18:3 ∆9,12,15 | 31.00 14.05 28 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 26.92 a/28.37 b 19.8 c/28.6 d/26.8 e/29.2 f | China 4 Italy 5 | [31] [32] | |
| Arachidonic acidC20:4∆5,8,11,14 | n.d. | n.d. | n.d. | 0.4 c/0.7 d/0.6 e/0.8 f | Italy 5 | [32] | |
| Total PUFA | 43.41 16.79 40 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 36.67 a/38.81 b 31.1 c/44.9 d/39.0 e/41.5 f | China 4 Italy 5 | [31] [32] | |
| Total fatty acids | 100.00 86.84 ** 99 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 100 a/100 b 99.6 * c/99.1 * d/ 99.1 * e/99.2 * f | China 4 Italy 5 | [31] [32] | |
| Oil | Oil content (%) | 16.09 10.0 13 | France 1 Turkey 2 USA 3 | [9] [29] [51] | 37.35 c/37.60 d/ 36.40 e/35.85 f | Italy 5 | [32] |
| Protein | Protein content (%) | 12 | USA 3 | [51] | 36.33 c/36.49 d/ 36.84 e/36.46 f | Italy 5 | [32] |
| Physico-Chemical Characteristics | Isatis tinctoria Oil | Isatis indigotica Oil | |
|---|---|---|---|
| Ref. [30] 1 | Ref. [51] 2 | Ref. [31] 3 | |
| Density | 0.9187 | n.d. | n.d |
| Refractive index | 1.4760 | n.d. | 1.4725 a/1.4732 b |
| Relative viscosity | 9.85 | n.d. | n.d. |
| Acid index (mg KOH/g) | 1.19 | n.d. | 2.09 a/2.36 b |
| Saponification index (mg KOH/g) | 177.58 | n.d. | 171.00 a/174.68 b |
| Ester index (mg KOH/g) | 176.39 * | 171 * | 168.91 a*/172.32 b* |
| Peroxide index (meq O2/kg) | n.d. | n.d. | 5.48 a/5.37 b |
| Iodine index (%) | 130.48 | 136 | 105.41 a/102.60 b |
| MWFA (g/mol) | 947.836468 | 984.308772 | 963.572246 |
| Unsaponifiable (%) | 1.86 | n.d. | n.d. |
| Phosphatides (%) | 0.53 | n.d. | n.d. |
| Compound | Applications | Properties | References |
|---|---|---|---|
| Alanine | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Arginine | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Aspartic acid | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Cystine | Cosmetic uses | Antistatic agent; fragrance agent; hair conditioner; humectant | [41] |
| Glutamic acid | Cosmetic uses | Antistatic agent; hair conditioner, | [41] |
| Glycine | Cosmetic uses | Antistatic agent; skin care agent; buffer agent; hair conditioner | [41] |
| Histidine | Cosmetic uses | Antistatic agent; skin care agent; humectant | [41] |
| Hydroxyproline | Cosmetic uses | Antistatic agent; skin care agent; hair conditioner; surfactant—cleanser | [41] |
| Pharmaceutical uses | Pharmaceutical synthesis reagent; potential anti-cancer agent | [63] | |
| Isoleucine | Cosmetic uses | Antistatic agent; skin care agent; hair conditioner | [41] |
| Leucine | Cosmetic uses | Antistatic agent; skin care agent; hair conditioner | [41] |
| Lysine | Cosmetic uses | Antistatic agent; skin care agent; hair conditioner | [41] |
| Methionine | Cosmetic uses | Antistatic agent; skin care agent; hair conditioner | [41] |
| Phenylalanine | Cosmetic uses | Skin care agent; fragrance agent; hair conditioner | [41] |
| Proline | Cosmetic uses | Skin care agent; hair conditioner | [41] |
| Serine | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Threonine | Cosmetic uses | Antistatic agent; curl or stretch agent; hair conditioner | [41] |
| Tyrosine | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Valine | Cosmetic uses | Antistatic agent; skin care agent; fragrance agent; hair conditioner | [41] |
| Chemical Classes | Comments/Compounds | Quantities | |
|---|---|---|---|
| Isatis tinctoria [33] 1 | Isatis indigotica [34] 2 | ||
| Oila | b | 12.6% c | n.d. |
| Proteins | In whole seed a,b | 12.5% c | n.d. |
| In extracted meal a,b | 14.3% c | n.d. | |
| Amino acids | Nitrogen distribution as % of total nitrogen | 69.8% c | n.d. |
| Alanine Arginine Aspartic acid Cystine Glutamic acid Glycine Histidine Hydroxyproline Isoleucine Leucine Lysine Methionine Phenylalanine Proline Serine Threonine Tyrosine Valine | 232 377 421 148 872 315 139 148 211 370 327 102 233 346 225 212 137 283 | n.d. p. d/n.p. e p. d/n.p. e n.p. d/p. e p. d/n.p. e n.d. n.p. d/p. e n.d. p. d/n.p. e p. d/n.p. e n.p. d/n. e p. d/n.p. e n.p. d/p. e p. d/n.p. e p. d/n.p. e p. d/n.p. e p. d/n.p. e n.d. | |
| Compounds | Applications | Properties | References |
|---|---|---|---|
| β-Sitosterol | Cosmetic uses | Skin care agent; fragrance agent; light stabilizing agent; emulsion stabilizer | [41] |
| Cosmetic and pharmaceutical uses | Antioxidant; antimicrobial; angiogenic; immunomodulatory; antidiabetic; anti-inflammatory; anti-cancer; antinociceptive | [78] | |
| Cosmetic uses | Fragrance agent; skin-conditioning agent—miscellaneous | [79] | |
| Food uses: food products (heart health benefits) | Coronary heart disease risk reducer | ||
| Campesterol | No cosmetic uses referenced | No cosmetic properties referenced | [41] |
| Pharmaceutical uses | Alleviator of arthritis symptoms 1 | [80] | |
| Cosmetic uses | Skin-conditioning agent—emollient (in mixture of phytosterols obtained from rapeseed) | [79] | |
| Food uses: food products (heart health benefits) | Coronary heart disease risk reducer | ||
| Δ5-Avenasterol | No cosmetic uses referenced | No cosmetic properties referenced | [41,79] |
| Cosmetic uses | Antioxidant | [68] | |
| Cosmetic and pharmaceutical uses | Cholesterol-lowering effector; cardiovascular diseases reducer; anti-inflammatory | [73] | |
| Stigmasterol | No cosmetic uses referenced | No cosmetic properties referenced | [41] |
| Cosmetic and pharmaceutical uses | Anti-inflammatory; antidiabetic; antioxidant; cholesterol-lowering effector; anti-tumoral | [81] | |
| Cosmetic uses | Skin-conditioning agent—emollient (in mixture of phytosterols obtained from soybean) | [79] | |
| Food uses: food products (heart health benefits) | Coronary heart disease risk reducer | ||
| Brassicasterol | No cosmetic uses referenced | No cosmetic properties referenced | [41] |
| Pharmaceutical uses | Nutritional and biological values; anti-infector 2; cardiovascular diseases reducer | [82] | |
| Cosmetic uses | Skin-conditioning agent—emollient (in mixture of phytosterols obtained from rapeseed) | [79] |
| Compounds | Quantities |
|---|---|
| β-Sitosterol | 68.25 ± 0.12 |
| Campesterol | 20.32 ± 0.07 |
| Δ5-Avenasterol | 14.82 ± 0.12 |
| Stigmasterol | 3.41 ± 0.02 |
| Brassicasterol | 3.37 ± 0.04 |
| Total phytosterols (desmethysterols) | 114.11 ± 0.32 |
| Chemical Classes | Compounds | Applications | Properties | References |
|---|---|---|---|---|
| Epiprogoitrin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Aliphatic glucosinolates | Pharmaceutical uses: antiviral drug resources (virus strain A/California/7/2009 (H1N1)). | Anti-viral efficacy (in vitro and in ovo) | [92] | |
| Pharmaceutical uses: nematode control (Meloidogyne hapla) | Inhibition of infectious juveniles | [93] | ||
| Progoitrin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Pharmaceutical uses: antiviral drug resources (virus strain A/California/7/2009 (H1N1). | Anti-viral efficacy (in vitro and in ovo) | [92] | ||
| Gluconapin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Epiglucoisatisin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Indolic glucosinolates | Pharmaceutical uses | Potential agent in the treatment of urease- and protease-associated complications | [94] | |
| Glucoisatisin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Glucobrassicin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Pharmaceutical uses | Anti-cancer, antioxidant, antibacterial and anti-inflammatory | [95] | ||
| Pharmaceutical uses: anti-tumoral drug | Tumor inhibition agent | [96,97,98] | ||
| Pharmaceutical/food uses: enrichment of pizza (cauliflower byproduct flours for fortification level) | Chemoprotective agent in pre-clinical models (breakdown products) | [97,99] | ||
| Pharmaceutical/food uses: lowering of blood LDL/VLDL 1 cholesterol levels (through the consumption of glucobrassicin-rich vegetables) | Cholesterol-lowering effector | [100] | ||
| 4-Hydroxy- glucobrassicin | No cosmetic uses referenced | No cosmetic properties referenced | [41] | |
| Neoglucobrassicin | No cosmetic uses referenced | No cosmetic properties referenced | [41] |
| Chemical Classes | Compounds | Quantities | ||
|---|---|---|---|---|
| Isatis tinctoria [35] 1 | Isatis indigotica [35] 1 | Isatis indigotica [32] 2 | ||
| Aliphatic glucosinolates | Epiprogoitrin | 130 | 50 | 115.42 a/112.90 b/18.56 c/68.77 d |
| Progoitrin | 90 | 140 | 28.64 a/27.65 b/64.20 c/34.70 d | |
| Gluconapin | 55 | 10 | 3.16 a/0.79 b/32.89 c/31.03 d | |
| Total aliphatic glucosinolates | 275 | 200 | 147.22 a/141.34 b/115.65 c/134.5 d | |
| Indolic glucosinolates | Epiglucoisatisin Glucoisatisin | 90 both isomers together | 120 both isomers together | 0.95 a/0.65 b/0.53 c/0.44 d |
| Glucobrassicin | 45 | n.d. | 0.10 a/0.07 b/4.37 c/0.70 d | |
| 4-Hydroxy-glucobrassicin | 15 | 10 | 3.44 a/2.30 b/2.57 c/2.36 d | |
| Neoglucobrassicin | 10 | n.d. | 0.26 a/0.21 b/0.12 c/0.14 d | |
| Total indolic glucosinolates | 160 | 130 | 4.75 a/3.23 b/7.59 c/3.64 d | |
| Total glucosinolates | 435 | 330 | 151.96 a/144.54 b/123.24 c/138.13 d | |
| EM (%) | TPC (mg/GAE g) | DPPH (%) | NTZ (%) | Rancimat (%) | ||
|---|---|---|---|---|---|---|
| Isatis tinctoria extracts | Hexane | 9.63 | 10.91 ± 3.00 | 2.51 ± 0.19 | 2.50 ± 0.76 | −2.72 ± 2.48 1 |
| Water (60 °C) | 32.94 | nd | nd | nd | nd | |
| Propylene glycol | 10.59 | 74.61 ± 3.03 | 7.14 ± 0.75 | 15.08 ± 2.30 | 36.03 ± 3.06 2 | |
| Ethanol (75%) | 13.72 | 106.10 ± 2.10 | 6.88 ± 1.12 | 14.53 ± 2.82 | 30.07 ± 4.34 | |
| Isopropanol | 1.12 | 60.63 ± 0.65 | 6.08 ± 0.37 | 10.10 ± 0.47 | 25.93 ± 0.830 | |
| Standard references | BHT | / | / | 10.56 ± 1.32 | 19.34 ± 4.70 | 611.98 ± 37.11 |
| Grape seed | / | 790.00 ± 53.08 | 79.14 ± 0.99 | 47.46 ± 2.31 | 0.80 ± 1.18 | |
| Green tea | / | 446.79 ± 27.40 | 61.79 ± 1.14 | 38.34 ± 1.97 | 247.30 ± 12.78 | |
| Rosemary | / | 142.10 ± 1.98 | 19.42 ± 0.19 | 4.30 ± 8.60 | 32.28 ± 1.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupré, J.; Joly, N.; Vauquelin, R.; Lequart, V.; Choque, É.; Jullian, N.; Martin, P. Isatis tinctoria L.—From Botanical Description to Seed-Extracted Compounds and Their Applications: An Overview. Plants 2025, 14, 2304. https://doi.org/10.3390/plants14152304
Dupré J, Joly N, Vauquelin R, Lequart V, Choque É, Jullian N, Martin P. Isatis tinctoria L.—From Botanical Description to Seed-Extracted Compounds and Their Applications: An Overview. Plants. 2025; 14(15):2304. https://doi.org/10.3390/plants14152304
Chicago/Turabian StyleDupré, Justine, Nicolas Joly, Romain Vauquelin, Vincent Lequart, Élodie Choque, Nathalie Jullian, and Patrick Martin. 2025. "Isatis tinctoria L.—From Botanical Description to Seed-Extracted Compounds and Their Applications: An Overview" Plants 14, no. 15: 2304. https://doi.org/10.3390/plants14152304
APA StyleDupré, J., Joly, N., Vauquelin, R., Lequart, V., Choque, É., Jullian, N., & Martin, P. (2025). Isatis tinctoria L.—From Botanical Description to Seed-Extracted Compounds and Their Applications: An Overview. Plants, 14(15), 2304. https://doi.org/10.3390/plants14152304








