Effects of the Agrobacterium rhizogenes rolC Gene Insertion on Secondary Metabolites Profile and In Vitro Biological Activity of Acmella oleracea (L.) R.K. Jansen
Abstract
1. Introduction
2. Results
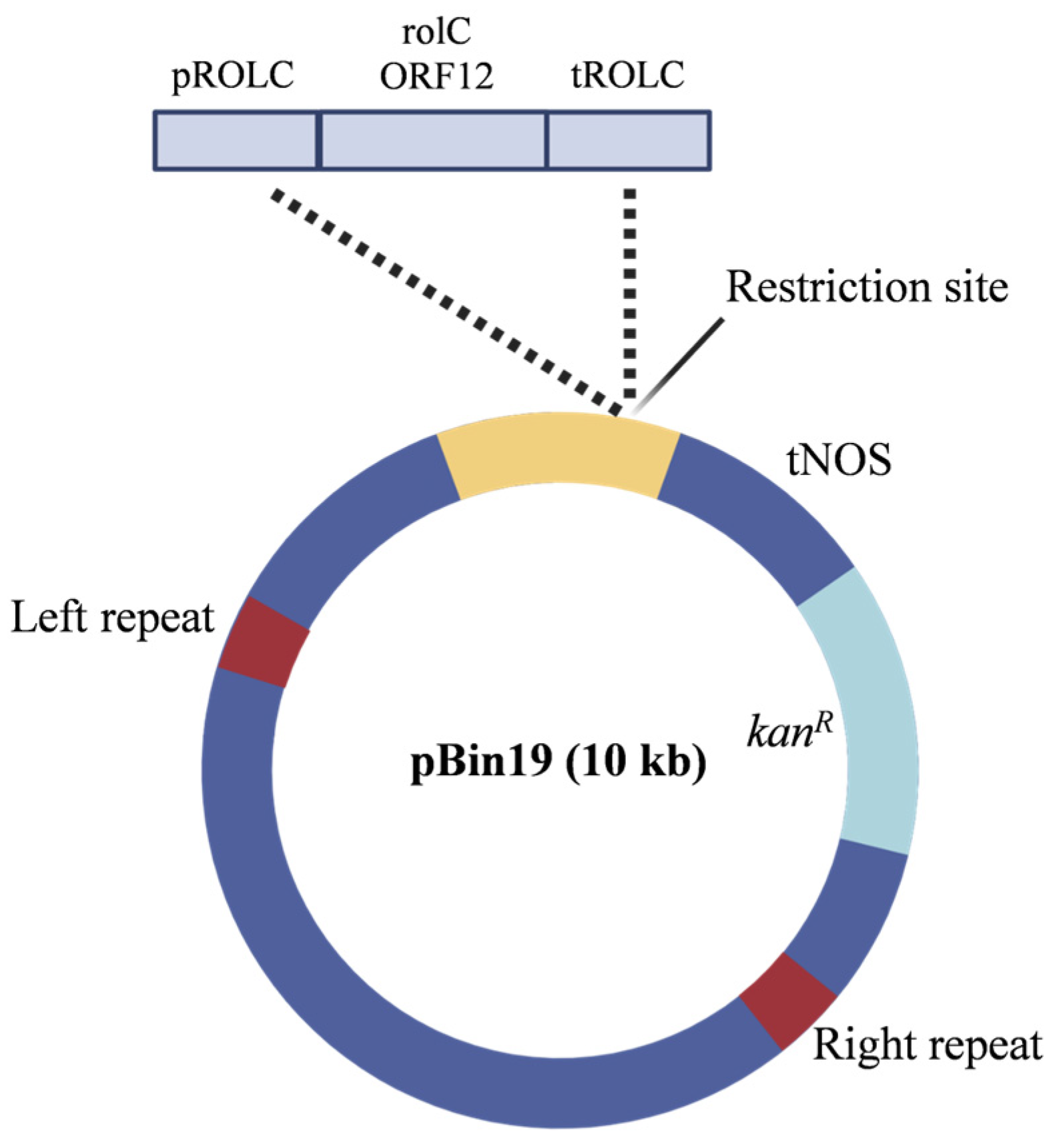
2.1. Transformation and Molecular Analysis of rolC−Transgenic Plants
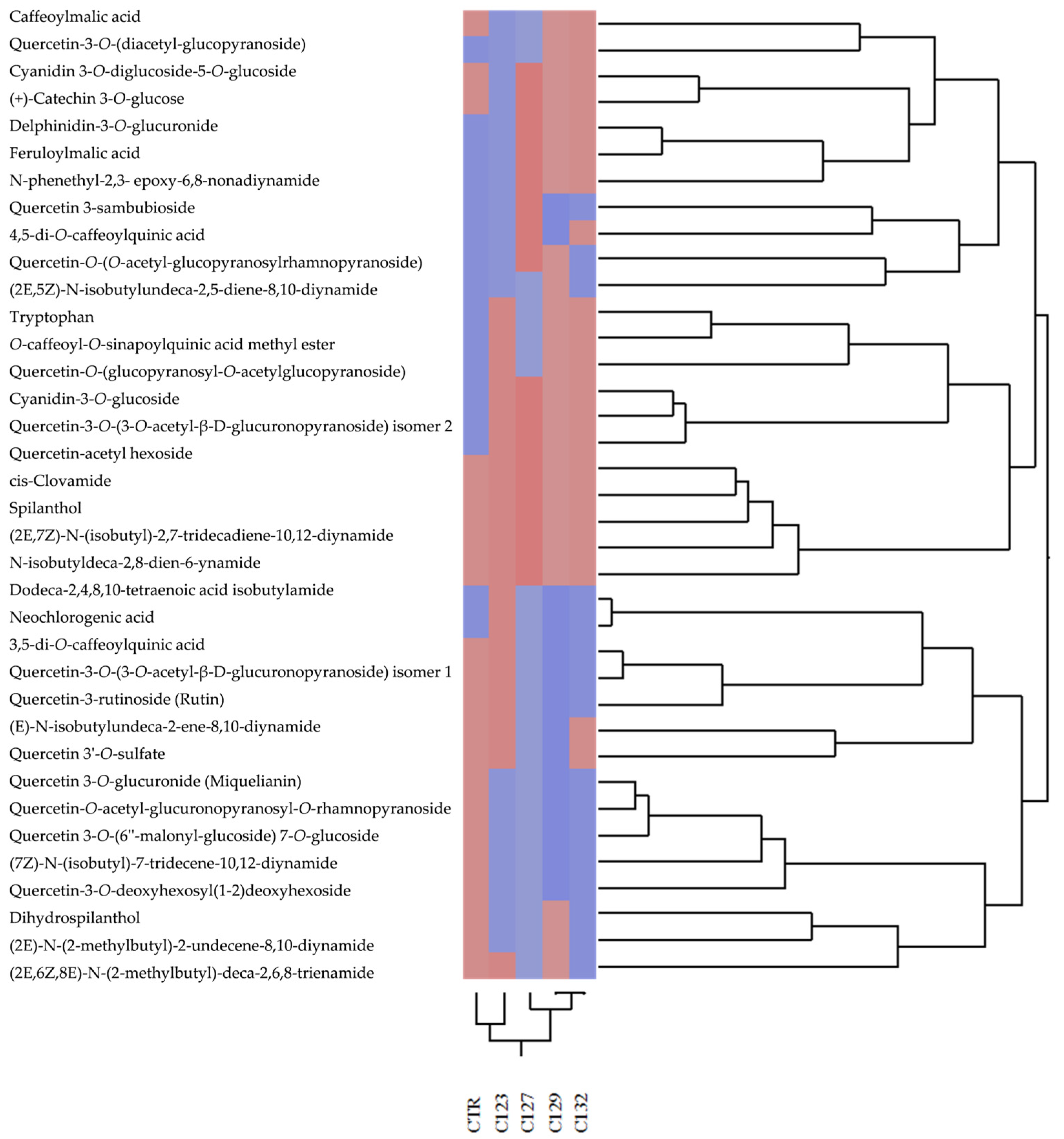
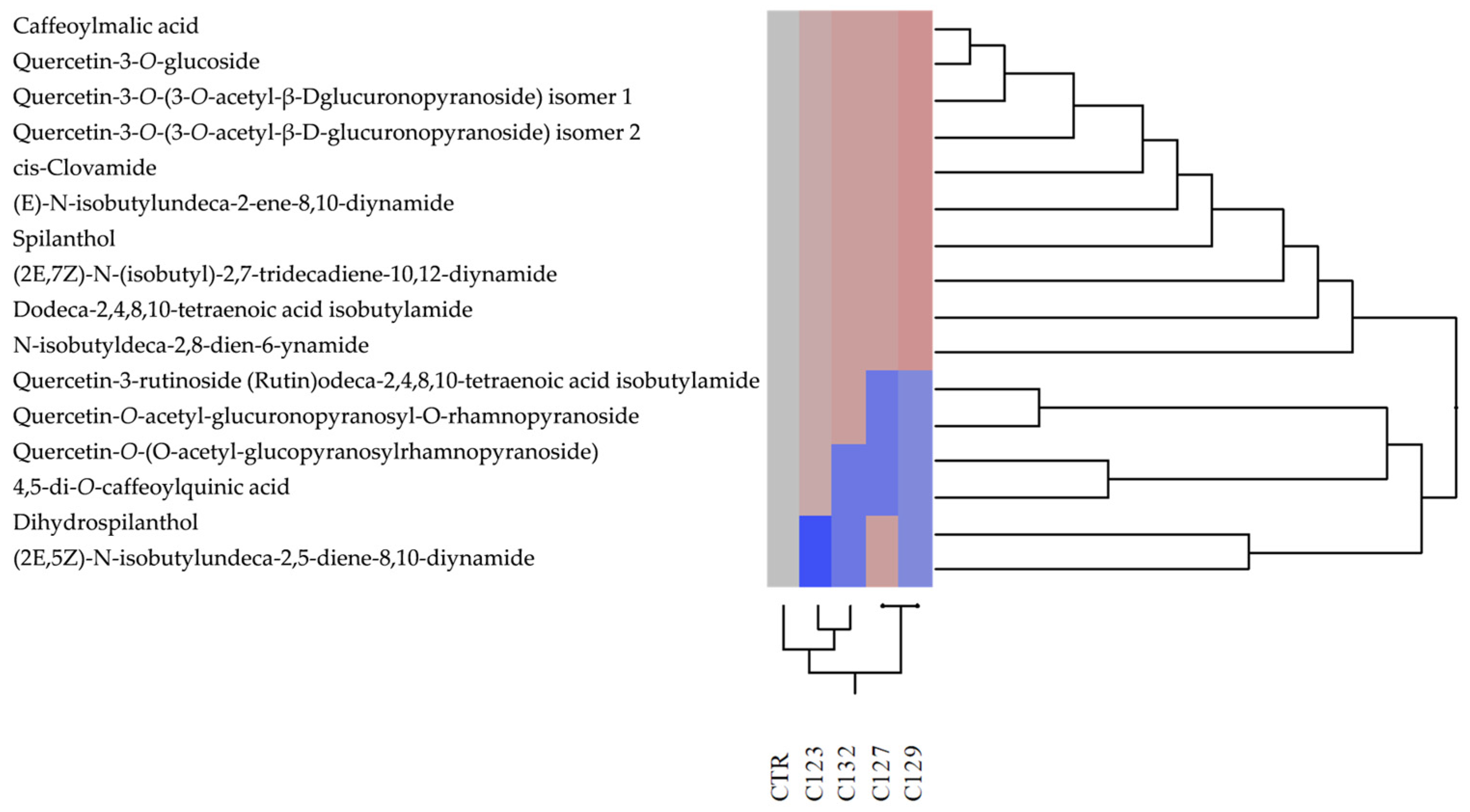
2.2. Extraction and Phytochemical Characterization of Food−Grade Extracts
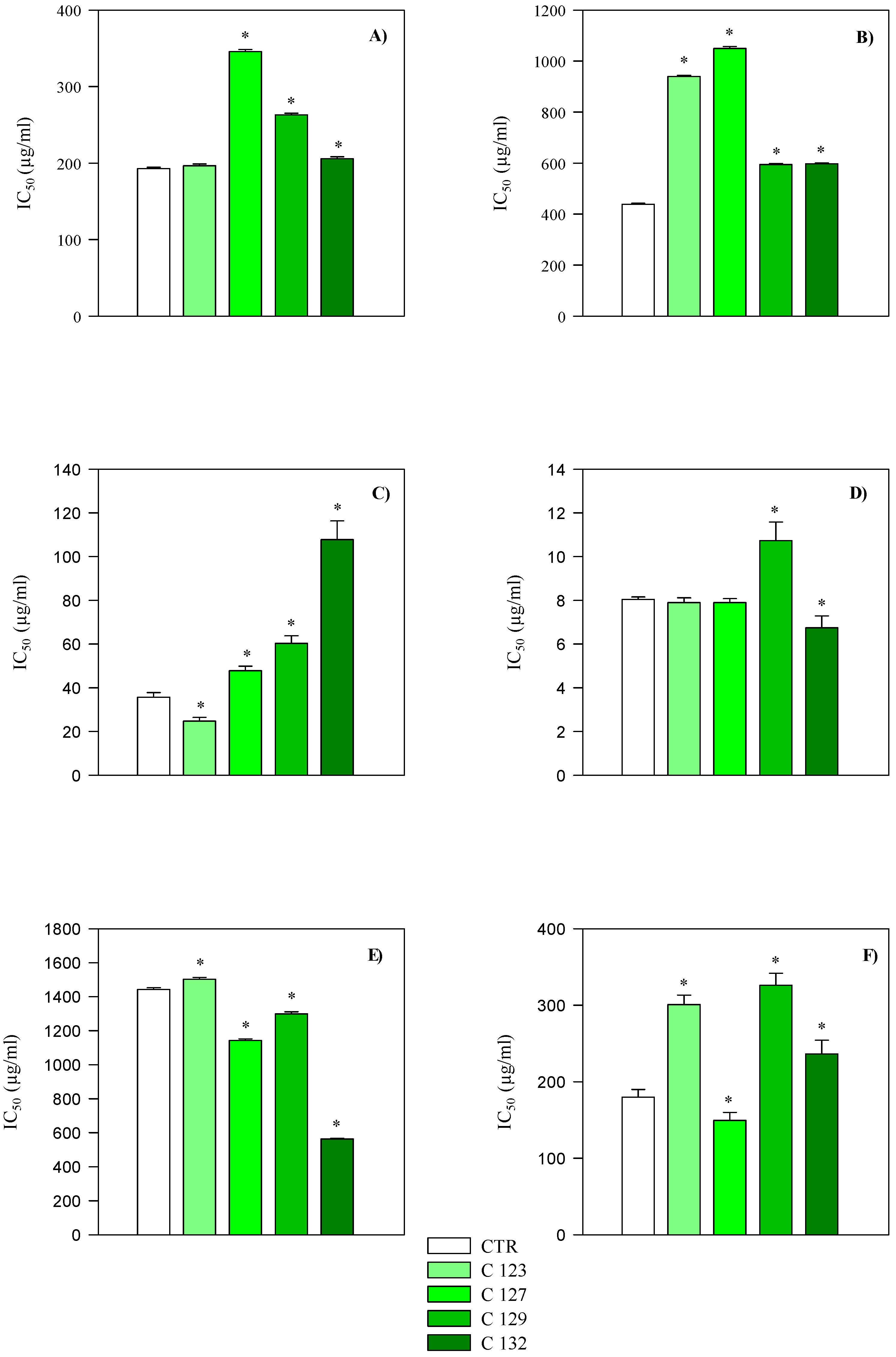
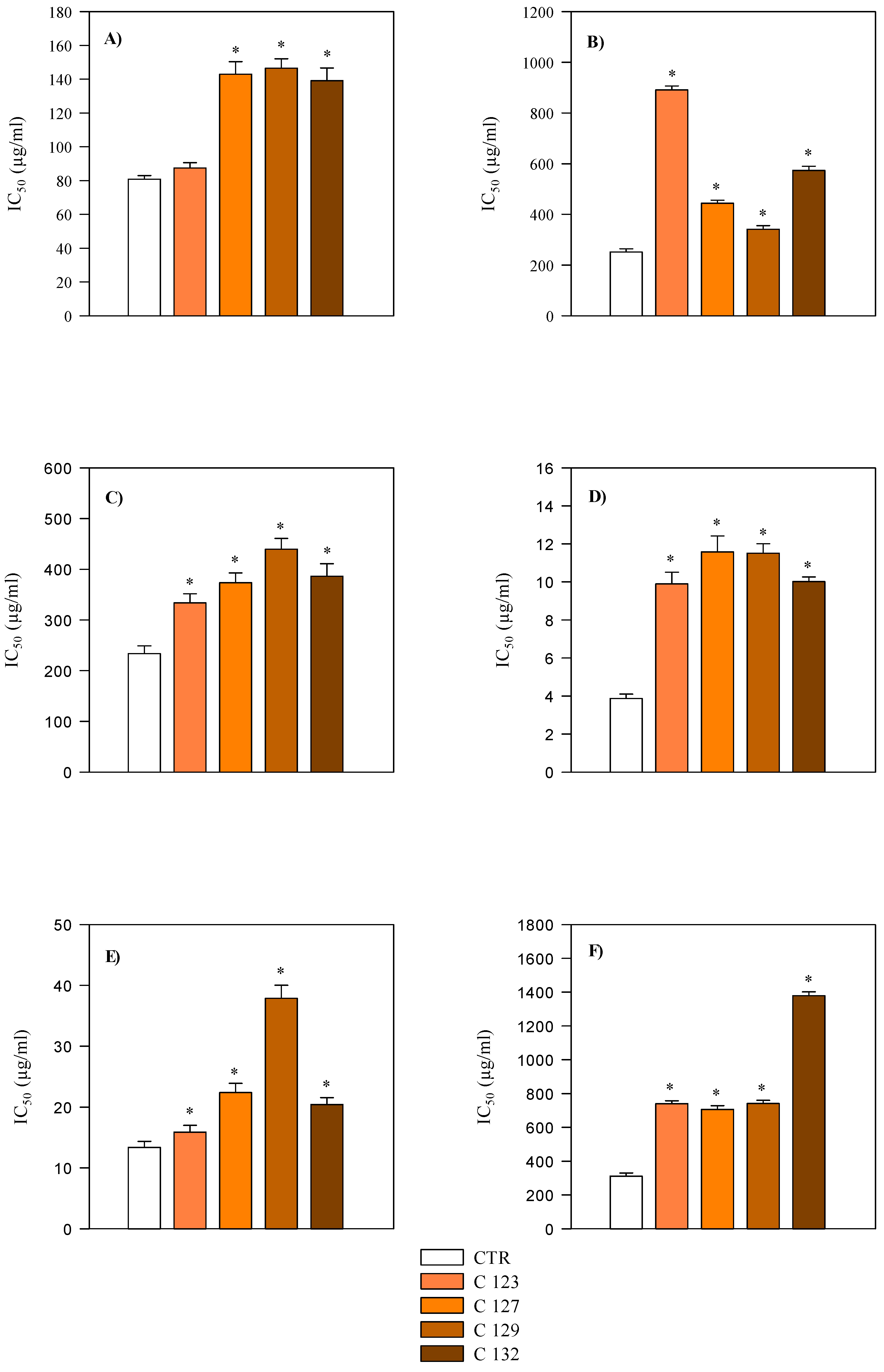
2.3. Evaluation of Antioxidant and Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Plant Transformation
4.2. Molecular Analysis of Transformants
4.3. Preparation of Food−Grade Extracts
4.4. Phytochemical Analyses
4.4.1. Total Phenolics
4.4.2. Total Flavonoids
4.4.3. Vanillin Index
4.4.4. Proanthocyanidins
4.4.5. LC−DAD−ESI−MS Analysis
4.5. Determination of Antioxidant and Anti−Inflammatory Activity
4.5.1. FRAP
4.5.2. DPPH
4.5.3. TEAC
4.5.4. ORAC
4.5.5. BCB
4.5.6. ICA
4.5.7. ADA
4.5.8. PIA
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | Albumin Denaturation Assay |
| AP | Aerial Parts |
| BCB | β−Carotene Bleaching Assay |
| BHT | Butylated HydroxyToluene |
| CcE | Cyanidin Chloride Equivalents |
| CE | Catechin Equivalents |
| CTR | Control (non-transformed plants) |
| DE | Dry Extract |
| DPPH | 2,2−Diphenyl−1−picrylhydrazyl |
| EDTA | Ethylenediaminetetraacetic Acid |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| HPLC-DAD | High−Performance Liquid Chromatography with Diode Array Detection |
| ICA | Iron−Chelating Activity |
| IC50 | Half−maximal Inhibitory Concentration |
| LC-DAD-ESI-MS | Liquid Chromatography−Diode Array Detection−Electrospray Ionization−Mass Spectrometry |
| ORAC | Oxygen Radical Absorbance Capacity |
| PBS | Phosphate Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PI | Polymerization Index |
| PIA | Protease Inhibition Assay |
| RE | Rutin Equivalents |
| RNA | Ribonucleic Acid |
| R | Roots |
| rolC | Root oncogenic locus C gene |
| RT | Retention Time |
| TEAC | Trolox Equivalent Antioxidant Capacity |
References
- Jansen, R.K. The Systematics of Acmella (Asteraceae−Heliantheae). Syst. Bot. Monogr. 1985, 8, 1–115. [Google Scholar] [CrossRef]
- Singh, M.; Chaturvedi, R. Screening and Quantification of an Antiseptic Alkylamide, Spilanthol from in Vitro Cell and Tissue Cultures of Spilanthes acmella Murr. Ind. Crops Prod. 2012, 36, 321–328. [Google Scholar] [CrossRef]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of Phytochemical, Physico−Chemical and Bioactivity of Different Parts of Acmella oleracea Murr. (Asteraceae), a Natural Remedy for Toothache. Ind. Crops Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Martins, C.P.S.; Melo, M.T.P.; Honório, I.C.G.; D’Ávila, V.A.; Carvalho Júnior, W.G.O. Caracterização Morfológica e Agronômica de Acessos de Jambu (Spilanthes oleracea L.) nas Condições do Norte de Minas Gerais. Rev. Bras. Plantas Med. 2012, 14, 410–413. [Google Scholar] [CrossRef]
- Tiwari, K.L.; Jadhav, S.K.; Joshi, V. An Updated Review on Medicinal Herb Genus Spilanthes. J. Chin. Integr. Med. 2011, 9, 1170–1178. [Google Scholar] [CrossRef]
- Paulraj, J.; Govindarajan, R.; Palpu, P. The Genus Spilanthes Ethnopharmacology, Phytochemistry, and Pharmacological Properties: A Review. Adv. Pharmacol. Sci. 2013, 2013, 510298. [Google Scholar]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. High Therapeutic Potential of Spilanthes acmella: A Review. EXCLI J. 2013, 12, 291. [Google Scholar]
- Lalchhandama, K.; Lalthanpuii, P.B.; Zokimi, Z. The Toothache Plant (Acmella oleracea) Exhibits Anthelmintic Activity on Both Parasitic Tapeworms and Roundworms. Pharmacogn. Mag. 2020, 16, 193. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant: Identification, Applications, and Use as an Emerging Food Source—Review. Food Rev. Int. 2020, 37, 399–414. [Google Scholar] [CrossRef]
- Turcov, D.; Trifari, A.; Puitel, A.C.; Cimpoesu, R.; Zbranca−Toporas, A.; Maxim, C.; Suteu, D.; Barna, A.S. Preliminary Studies about Valorization of Acmella oleracea Bioactive Content in Modern Dermato−Cosmetic Applications to Combat Skin Oxidative Stress. Intl. J. Mol. Sci. 2024, 25, 8886. [Google Scholar] [CrossRef]
- Dallazen, J.L.; Maria−Ferreira, D.; da Luz, B.B.; Nascimento, A.M.; Cipriani, T.R.; de Souza, L.M.; Felipe, L.P.G.; Silva, B.J.G.; Nassini, R.; de Paula Werner, M.F. Pharmacological Potential of Alkylamides from Acmella oleracea Flowers and Synthetic Isobutylalkyl Amide to Treat Inflammatory Pain. Inflammopharmacology 2020, 28, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gerber, E. Über die Chemischen Bestandteile der Parakresse (Spilanthes olearacea, Jacquin). Arch. Pharm. 1903, 241, 270–289. [Google Scholar] [CrossRef]
- Lalthanpuii, P.B.; Lalruatfela, B.; Vanlaldinpuia, K.; Lalremsanga, H.T.; Lalchhandama, K. Antioxidant and Cytotoxic Properties of Acmella oleracea. Int. J. Phytomed. Ind. 2018, 10, 353–358. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Santos, P.; Seabra, I.J.; Júnior, R.N.C.; Braga, M.E.M.; de Sousa, H.C. Spilanthol from Spilanthes acmella Flowers, Leaves, and Stems Obtained by Selective Supercritical Carbon Dioxide Extraction. J. Supercrit. Fluids 2012, 61, 62–70. [Google Scholar] [CrossRef]
- Simas, N.K.; Dellamora, E.D.C.L.; Schripsema, J.; Lage, C.L.S.; de Oliveira Filho, A.M.; Wessjohann, L.; Porzel, A.; Kuster, R.M. Acetylenic 2−Phenylethylamides and New Isobutylamides from Acmella oleracea (L.) R. K. Jansen, a Brazilian Spice with Larvicidal Activity on Aedes aegypti. Phytochemistry 2013, 6, 67–72. [Google Scholar] [CrossRef]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, Pharmacology, and Toxicology of Spilanthes acmella: A Review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar]
- Joshi, V.; Sharma, G.D.; Jadhav, S.K. Alkamides: Multifunctional Bioactive Agents in Spilanthes spp. J. Sci. Res. 2020, 64, 198–206. [Google Scholar] [CrossRef]
- Barbosa, A.F.; de Carvalho, M.G.; Smith, R.E.; Sabaa-Srur, A.U.O. Spilanthol: Occurrence, Extraction, Chemistry, and Biological Activities. Rev. Bras. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Sut, S.; Ferrarese, I.; Shrestha, S.S.; Kumar, G.; Slaviero, A.; Sello, S.; Altissimo, A.; Pagni, L.; Gattesco, F.; Dall’Acqua, S. Comparison of Biostimulant Treatments in Acmella oleracea Cultivation for Alkylamides Production. Plants 2020, 9, 818. [Google Scholar] [CrossRef]
- Rios−Chavez, P.; Ramirez−Chavez, E.; Armenta−Salinas, C.; Molina−Torres, J. Acmella radicans var. radicans: In Vitro Culture Establishment and Alkamide Content. In Vitro Cell. Dev. Biol. Plant 2003, 39, 37–41. [Google Scholar]
- Saritha, K.V.; Naidu, C.V. Direct Shoot Regeneration from Leaf Explants of Spilanthes acmella. Biol. Plant. 2008, 52, 334–338. [Google Scholar] [CrossRef]
- Deka, P.; Kalita, M.C. In Vitro Clonal Propagation and Organogenesis in Spilanthes acmella (L.) Murray: A Herbal Pesticidal Plant of North−East India. J. Plant Biochem. Biotechnol. 2005, 14, 69–71. [Google Scholar] [CrossRef]
- Pandey, V.; Agrawal, V. Efficient Micropropagation Protocol of Spilanthes acmella L. Possessing Strong Antimalarial Activity. Vitr. Cell. Dev. Biol. Plant 2009, 45, 491–499. [Google Scholar] [CrossRef]
- Almeida, S.P.; Souza, J.M.; Amorim, A.M.; de Gusmão, S.A.L.; Souza, R.O.; Santos, A.S. In Vitro Culture of Jambu with Different Growth Regulators. Hortic. Bras. 2020, 38, 134–138. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Metabolic Engineering Approaches for Production of Biochemicals in Food and Medicinal Plants. Curr. Opin. Biotechnol. 2014, 26, 174–182. [Google Scholar] [CrossRef]
- Alok, A.; Jain, P.; Kumar, J.; Yajnik, K.; Bhalothia, P. Genome Engineering in Medicinally Important Plants Using CRISPR/Cas9 Tool. In Genome Engineering via CRISPR-Cas9 System, 1st ed.; Singh, V., Dhar, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–161. [Google Scholar]
- Li, C.; Wang, M. Application of Hairy Root Culture for Bioactive Compounds Production in Medicinal Plants. Curr. Pharm. Biotechnol. 2021, 22, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Shi, J.; Xia, Z.; Lu, B.; Shi, S.; Fu, G. Precise Control of Variable−Height Laser Metal Deposition Using a Height Memory Strategy. J. Manuf. Process. 2020, 57, 222–232. [Google Scholar] [CrossRef]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The Never Ending Story of rol Genes: A Century After. Plant Cell Tiss. Org. Cult. 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Dilshad, E.; Cusido, R.M.; Ramirez Estrada, K.; Bonfill, M.; Mirza, B. Genetic Transformation of Artemisia carvifolia Buch with rol Genes Enhances Artemisinin Accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure–Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, T.V.; Sokornova, S.V. Agrobacterium rhizogenes−Mediated Transformation of Plants for Improvement of Yields of Secondary Metabolites. In Bioprocessing of Plant In Vitro Systems; Pavlov, A., Bley, T., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018; pp. 161–202. [Google Scholar]
- Dilshad, E.; Noor, H.; Nosheen, N.; Gilani, S.R.; Ali, U.; Khan, M.A. Influence of rol Genes for Enhanced Biosynthesis of Potent Natural Products. In Chemistry of Biologically Potent Natural Products and Synthetic Compounds; Shahid-ul-Islam, Butola, B.S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 379–404. [Google Scholar]
- Palazón, J.; Cusidó, R.M.; Roig, C.; Piñol, M.T. Expression of the rolC Gene and Nicotine Production in Transgenic Roots and Their Regenerated Plants. Plant Cell Rep. 1998, 17, 384–390. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)−Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A. Plant Polyphenols: Are They the New Magic Bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef]
- Maggini, V.; Bettini, P.; Firenzuoli, F.; Bogani, P. An Efficient Method for the Genetic Transformation of Acmella oleracea L. (Spilanthes acmella Linn.) with Agrobacterium tumefaciens. Plants 2021, 10, 198. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Wallroth, M.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A Simple and General Method for Transferring Genes into Plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Ooms, G.; Hooykaas, P.J.J.; Van Veen, R.J.M.; Van Beelen, P.; Regensburg−Tuink, A.J.G.; Schilperoort, R.A. Octopine Ti Plasmid Deletion Mutants of Agrobacterium tumefaciens with Emphasis on the Right Side of the T Region. Plants 1982, 7, 15–19. [Google Scholar] [CrossRef]
- Bevan, M. Binary Agrobacterium Vectors for Plant Transformation. Nucleic Acids Res. 1984, 12, 8711–8721. [Google Scholar] [CrossRef]
- Cardarelli, M.; Mariotti, D.; Pomponi, M.; Spanò, L.; Capone, I.; Costantino, P. Agrobacterium rhizogenes Genes Capable of Inducing Hairy Root Phenotype. Mol. Gen. Genet. 1987, 209, 475–480. [Google Scholar] [CrossRef]
- Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients 2023, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant Composition in Cherry and High−Pigment Tomato Cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Ingegneri, M.; Germanò, M.P.; Miori, L.; Battistini, G.; Betuzzi, F.; Malaspina, P.; Trombetta, D.; Cornara, L. Pharmacognostic Evaluation of Monarda didyma L. Growing in Trentino (Northern Italy) for Cosmeceutical Applications. Plants 2024, 13, 112. [Google Scholar] [CrossRef]
- Nascimento, L.E.S.; Arriola, N.D.A.; da Silva, L.A.L.; Faqueti, L.G.; Sandjo, L.P.; de Araújo, C.E.S.; Biavatti, M.W.; Barcelos-Oliveira, J.L.; Dias de Mello Castanho Amboni, R. Phytochemical Profile of Different Anatomical Parts of Jambu (Acmella oleracea (L.) R. K. Jansen): A Comparison Between Hydroponic and Conventional Cultivation Using PCA and Cluster Analysis. Food Chem. 2020, 332, 127393. [Google Scholar] [CrossRef]
- Cornara, L.; Malaspina, P.; Betuzzi, F.; Di Gristina, E.; D’Arrigo, M.; Ingegneri, M.; Trombetta, D.; Smeriglio, A. The Influence of Pedo−Climatic Conditions on the Micromorphological, Phytochemical Features, and Biological Properties of Leaves of Saponaria sicula Raf. Int. J. Mol. Sci. 2023, 24, 11693. [Google Scholar] [CrossRef]



| Clone | fg rolC mRNA/μg Total RNA |
|---|---|
| rolC−123 | 1.6 |
| rolC−127 | 9.14 |
| rolC−129 | 0.105 |
| rolC−132 | 0.282 |
| Extracts | Total Phenols (g GAE a/100 g DE b) | Total Flavonoids (g RE c/100 g DE) | Vanillic Index (g CE d/100 g DE) | Proanthocyanidins (g CcE e/100 g DE) | PI f |
|---|---|---|---|---|---|
| Aerial parts | |||||
| CTR | 4.22 ± 0.34 | 2.01 ± 0.14 | 1.61 ± 0.03 | 0.01 ± 0.00 | 161.00 |
| C123 | 4.33 ± 0.13 | 2.97 ± 0.22 ** | 2.66 ± 0.03 *** | 0.02 ± 0.00 ** | 133.00 |
| C127 | 2.62 ± 0.15 *** | 2.46 ± 0.02 ** | 1.84 ± 0.02 * | 0.02 ± 0.00 ** | 92.00 |
| C129 | 2.79 ± 0.08 *** | 2.24 ± 0.01 | 1.34 ± 0.03 ** | 0.03 ± 0.00 *** | 44.67 |
| C132 | 4.48 ± 0.26 | 2.22 ± 0.03 | 1.66 ± 0.02 | 0.01 ± 0.00 | 166.00 |
| Roots | |||||
| CTR | 8.44 ± 0.12 | 2.25 ± 0.13 | 0.318 ± 0.01 | 0.02 ± 0.00 | 15.90 |
| C123 | 4.27 ± 0.18 *** | 0.85 ± 0.01 *** | 0.068 ± 0.00 *** | 0.01 ± 0.00 ** | 6.80 |
| C127 | 3.25 ± 0.03 *** | 1.66 ± 0.04 *** | 0.773 ± 0.02 *** | 0.01 ± 0.00 ** | 77.30 |
| C129 | 3.05 ± 0.17 *** | 0.73 ± 0.06 *** | 0.455 ± 0.02 *** | 0.01 ± 0.00 ** | 45.50 |
| C132 | 2.86 ± 0.11 *** | 0.74 ± 0.02 *** | 0.273 ± 0.01 ** | 0.01 ± 0.00 ** | 27.30 |
| Metabolite | RT a min | [M-H]− m/z | [M-H]+ m/z | CTR | C123 | C127 | C129 | C132 |
|---|---|---|---|---|---|---|---|---|
| Caffeoylmalic acid | 1.0 | − | 297 | + | − | − | + | + |
| Neochlorogenic acid b | 1.6 | 353 | − | − | + | − | − | − |
| Cyanidin 3−O−diglucoside−5−o−glucoside | 21.3 | − | 774 | + | − | + | + | + |
| Tryptophan | 22.6 | − | 205 | − | + | − | + | + |
| Quercetin−3−O−(diacetyl−glucopyranoside) | 24.1 | 547 | − | − | − | − | + | + |
| Cyanidin−3−O−glucoside b | 25.0 | − | 450 | − | + | + | + | + |
| 3,5−di−O−caffeoylquinic acid | 26.6 | 515 | − | − | + | − | − | − |
| Quercetin−3−O−glucuronide (Miquelianin) | 29.0 | − | 479 | + | − | − | − | − |
| Delphinidin−3−O−glucuronide | 30.0 | − | 508 | − | − | + | + | + |
| Quercetin−3−O−(3−O−acetyl−β−D−glucopyranoside) isomer 1 | 30.3 | − | 521 | + | + | − | − | − |
| Quercetin−rutinoside (Rutin) b | 31.2 | − | 611 | + | + | − | − | − |
| Quercetin−O−acetil−glucopyranosyl−O−rhamnopyranoside | 32.1 | − | 667 | + | − | − | − | − |
| Quercetin 3−O−(6″−malonil−glucoside) 7−O−glucoside | 32.4 | − | 713 | + | − | − | − | − |
| Quercetin 3−sambubioside | 32.8 | 651 | 653 | − | − | + | − | − |
| Feruloyl malic acid | 33.2 | 309 | 311 | − | − | + | + | + |
| Quercetin−3−O−(3−O−acetil−β−D−glucopyranoside) isomer 2 | 33.8 | 519 | − | − | + | + | + | + |
| 4,5−di−O−caffeokyninic acid | 34.7 | − | 517 | − | − | + | − | + |
| Quercetin−acetyl esoside | 37.0 | 505 | − | − | + | + | + | + |
| cis−Clovamide | 38.5 | 358 | − | + | + | + | + | + |
| Quercetin−O−(O−acetyl−glucopyranosyl rhamnopyranoside) | 42.5 | − | 653 | − | − | + | + | − |
| (+)−Catechin 3−O−glucoside | 43.6 | − | 453 | + | − | + | + | + |
| Quercetin 3′−O−sulfate | 46.1 | 381 | − | + | + | − | − | + |
| Methyl ester of O−caffeoyl−O−sinapoilkinic acid | 49.6 | 573 | − | − | + | − | + | + |
| (E)−N−isobutylundeca−2−(E)−en−8,10−dynamide | 50.2 | − | 232 | + | + | − | − | − |
| Dihydrospilanthol | 51.7 | − | 224 | + | − | − | + | − |
| Spilanthol b | 52.9 | − | 222 | + | + | + | + | + |
| (2E,4E)−N−isobutylundeca−2,5−dien−8,10−diynamide | 53.5 | − | 230 | − | − | − | + | − |
| (2E,7Z)−N−Isobutyl−2,7−tridecadiene−10,12−diynamide | 54.4 | − | 258 | + | + | + | + | + |
| (7Z)−N−(isobutyl)−7−tridecene−10,12−dynamide | 54.9 | − | 260 | + | − | − | − | − |
| N−isobutyl deca−2,8−dien−6−inamide | 55.3 | − | 220 | + | + | + | + | + |
| Quercetin−3−O−deoxyhexosyl(1−2)deoxyhexoside | 57.9 | 593 | − | + | − | − | − | − |
| Dodeca−2,4,8,10−tetraenoic isobutylamide acid | 59.3 | − | 248 | + | + | + | + | + |
| ((2E)−N−(2−Methylbutyl)−2−undecene−8,10−diynamide | 63.2 | − | 246 | + | − | − | + | − |
| (2E,6E,8E)−N−(2−methylbutyl)deca−2,6,8−trienamide | 66.4 | − | 236 | + | + | − | + | − |
| N−phenylethyl−2,3−epoxy−6,8−nonadiynamide | 68.8 | − | 508 | − | − | + | + | + |
| N−phenylethyl−2,3−epoxy−6,8−nonadiynamide | 76.2 | − | 268 | + | + | − | − | + |
| Quercetin−O−(glucopyranosyl−O−acetylglucopyranoside) | 79.3 | − | 669 | − | + | − | + | + |
| Metabolite | RT a min | [M−H]− m/z | [M−H]+ m/z | CTR | C123 | C127 | C129 | C132 |
|---|---|---|---|---|---|---|---|---|
| Caffeoylmalic acid | 1.0 | − | 297 | + | + | + | + | + |
| Quercetin−3−O−glucoside b | 23.3 | 462 | − | + | + | + | + | + |
| Quercetin−3−O−(3−O−acetyl−β−D−glucopyranoside) isomer 1 | 30.3 | − | 521 | + | + | + | + | + |
| Quercetin−rutinoside (Rutin) b | 31.2 | − | 611 | + | + | − | − | + |
| Quercetin−O−acetil−glucopyranosyl−O−rhamnopyranoside | 32.1 | − | 667 | + | + | − | − | + |
| Quercetin−O−(O−acetyl− glucopyranosyl rhamnopyranoside) | 32.8 | 651 | 653 | + | + | − | − | − |
| Quercetin−3−O−(3−O−acetil−β−D− glucopyranoside) isomer 2 | 33.8 | 519 | − | + | + | + | + | + |
| 4,5−di−O−caffeokyninic acid | 34.7 | − | 517 | + | + | − | − | − |
| cis−Clovamide | 38.5 | 358 | − | + | + | + | + | + |
| (E)−N−isobutylundeca−2−(E)−en−8,10−dynamide | 50.2 | − | 232 | + | + | + | + | + |
| Dihydrospilanthol | 51.7 | − | 224 | + | − | + | − | − |
| Spilanthol b | 52.9 | − | 222 | + | + | + | + | + |
| 2E,4E)−N−isobutylundeca−2,5−dien−8,10−diynamide | 53.5 | − | 230 | + | − | + | − | − |
| (2E,7Z)−N−Isobutyl−2,7−tridecadiene−10,12−diynamide | 54.4 | − | 258 | + | + | + | + | + |
| N−isobutyl deca−2,8−dien−6−inamide | 55.3 | − | 220 | + | + | + | + | + |
| Dodeca−2,4,8,10−tetraenoic isobutylamide acid | 59.3 | − | 248 | + | + | + | + | + |
| Hydroalcoholic Extracts | Aerial Parts (g/100 g) | Roots (g/100 g) |
|---|---|---|
| CTR | 5.70 ± 0.08 | 0.077 ± 0.00 |
| C123 | 2.90 ± 0.04 *** | 0.064 ± 0.00 ** |
| C127 | 1.29 ± 0.02 *** | 0.019 ± 0.00 *** |
| C129 | 0.17 ± 0.01 *** | 0.051 ± 0.00 *** |
| C132 | 4.88 ± 0.05 ** | 0.028 ± 0.00 *** |
| Extracts | DPPH | FRAP | ICA | TEAC | ORAC | BCB |
|---|---|---|---|---|---|---|
| Aerial parts | IC50 mg/mL | IC50 µg/mL | ||||
| CTR | 0.44 (0.32–0.59) | 0.19 (0.16–0.23) | 0.18 (0.15–0.22) | 1.44 (0.91–2.29) | 8.04 (2.50–14.40) | 35.65 (29.92–42.47) |
| C123 | 0.94 (0.57–1.56) | 0.20 (0.08–0.47) | 0.30 (0.24–0.38) | 1.50 (0.91–2.49) | 7.90 (1.02–11.25) | 24.73 (17.18–35.61) |
| C127 | 1.05 (0.61–1.79) | 0.35 (0.29–0.41) | 0.15 (0.13–0.18) | 1.14 (0.77–1.69) | 7.90 (0.48–13.01) | 47.82 (34.25–66.77) |
| C129 | 0.59 (0.41–0.86) | 0.26 (0.22–0.32) | 0.33 (0.26–0.41) | 1.30 (0.84–2.00) | 10.73 (9.15–12.58) | 60.33 (40.25–90.44) |
| C132 | 0.60 (0.40–0.88) | 0.20 (0.16–0.24) | 0.24 (0.19–0.29) | 0.56 (0.41–0.77) | 6.74 (6.60–8.12) | 107.77 (65.06–178.52) |
| Roots | IC50 (mg/mL) | IC50 µg/mL | ||||
| CTR | 0.25 (0.19–0.33) | 0.08 (0.07–0.09) *** | 0.31 (0.25–0.39) *** | 0.23 (0.16–0.34) *** | 3.87 (2.30–6.50) | 13.39 (10.38–17.26) *** |
| C123 | 0.89 (0.55–1.45) | 0.09 (0.06–0.12) | 0.74 (0.57–0.97) *** | 0.33 (0.26–0.43) *** | 9.90 (7.66–12.79) | 15.89 (12.51–20.19) *** |
| C127 | 0.44 (0.30–0.66) | 0.14 (0.12–0.17) *** | 0.71 (0.54–0.92) *** | 0.37 (0.28–0.48) *** | 11.58 (8.49–15.80) | 22.38 (16.30–31.08) *** |
| C129 | 0.34 (0.26–0.46) | 0.15 (0.12–0.17) *** | 0.74 (0.58–0.95) *** | 0.44 (0.33–0.59) *** | 11.51 (8.69–15.25) | 37.89 (25.50–56.31) |
| C132 | 0.57 (0.38–0.86) | 0.14 (0.12–0.16) | 1.38 (1.06–1.79) *** | 0.39 (0.30–0.51) *** | 10.02 (7.34–13.68) | 20.42 (14.13–29.50) *** |
| IC50 (µg/mL) | ||||||
| Standard a | 6.09 (5.71–8.18) | 3.74 (1.55–5.01) | 6.54 (5.74–7.46) | 3.95 (2.39–6.09) | 0.79 (0.13–1.68) | 0.37 (0.15–0.76) |
| ADA | PIA | |
|---|---|---|
| Aerial parts | IC50 mg/mL | |
| CTR | 3.56 (2.90–4.36) | 0.38 (0.31–0.46) |
| C123 | 2.92 (2.28–3.75) | 0.48 (0.36–0.56) |
| C127 | 3.94 (2.99–5.20) | 0.51 (0.39–0.66) |
| C129 | 4.91 (3.70–6.53) | 0.46 (0.38–0.55) |
| C132 | 7.24 (5.78–9.08) | 0.44 (0.35–0.54) |
| Roots | IC50 mg/mL | |
| CTR | 2.05 (1.62–2.59) *** | 0.18 (0.07–0.46) |
| C123 | 2.18 (1.69–2.82) | 0.30 (0.26–0.36) |
| C127 | 0.72 (0.54–0.95) *** | 0.53 (0.40–0.69) |
| C129 | 0.60 (0.43–0.83) *** | 0.56 (0.43–0.74) |
| C132 | 1.14 (0.89–1.45) *** | 0.37 (0.15–0.89) |
| Diclofenac sodium (IC50 µg/mL) | 39.44 (25.28–54.21) | 35.35 (21.95–56.93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettini, P.P.; Imbesi, M.; Bogani, P.; Maggini, V.; Firenzuoli, F.; Firenzuoli, F.; Trombetta, D.; Smeriglio, A. Effects of the Agrobacterium rhizogenes rolC Gene Insertion on Secondary Metabolites Profile and In Vitro Biological Activity of Acmella oleracea (L.) R.K. Jansen. Plants 2025, 14, 1373. https://doi.org/10.3390/plants14091373
Bettini PP, Imbesi M, Bogani P, Maggini V, Firenzuoli F, Firenzuoli F, Trombetta D, Smeriglio A. Effects of the Agrobacterium rhizogenes rolC Gene Insertion on Secondary Metabolites Profile and In Vitro Biological Activity of Acmella oleracea (L.) R.K. Jansen. Plants. 2025; 14(9):1373. https://doi.org/10.3390/plants14091373
Chicago/Turabian StyleBettini, Priscilla Paola, Martina Imbesi, Patrizia Bogani, Valentina Maggini, Filippo Firenzuoli, Fabio Firenzuoli, Domenico Trombetta, and Antonella Smeriglio. 2025. "Effects of the Agrobacterium rhizogenes rolC Gene Insertion on Secondary Metabolites Profile and In Vitro Biological Activity of Acmella oleracea (L.) R.K. Jansen" Plants 14, no. 9: 1373. https://doi.org/10.3390/plants14091373
APA StyleBettini, P. P., Imbesi, M., Bogani, P., Maggini, V., Firenzuoli, F., Firenzuoli, F., Trombetta, D., & Smeriglio, A. (2025). Effects of the Agrobacterium rhizogenes rolC Gene Insertion on Secondary Metabolites Profile and In Vitro Biological Activity of Acmella oleracea (L.) R.K. Jansen. Plants, 14(9), 1373. https://doi.org/10.3390/plants14091373







