Chelidonium majus L.: A Current Perspective on Isoquinoline Alkaloids, Emerging Phytochemicals, Alkaloid Biosynthesis, and Biological Activities
Abstract
1. Introduction
2. Emerging Phytochemicals and Biological Functions
2.1. Isoquinoline Alkaloids
2.2. Lignanamides
2.3. Polyphenols and Other Phytochemicals
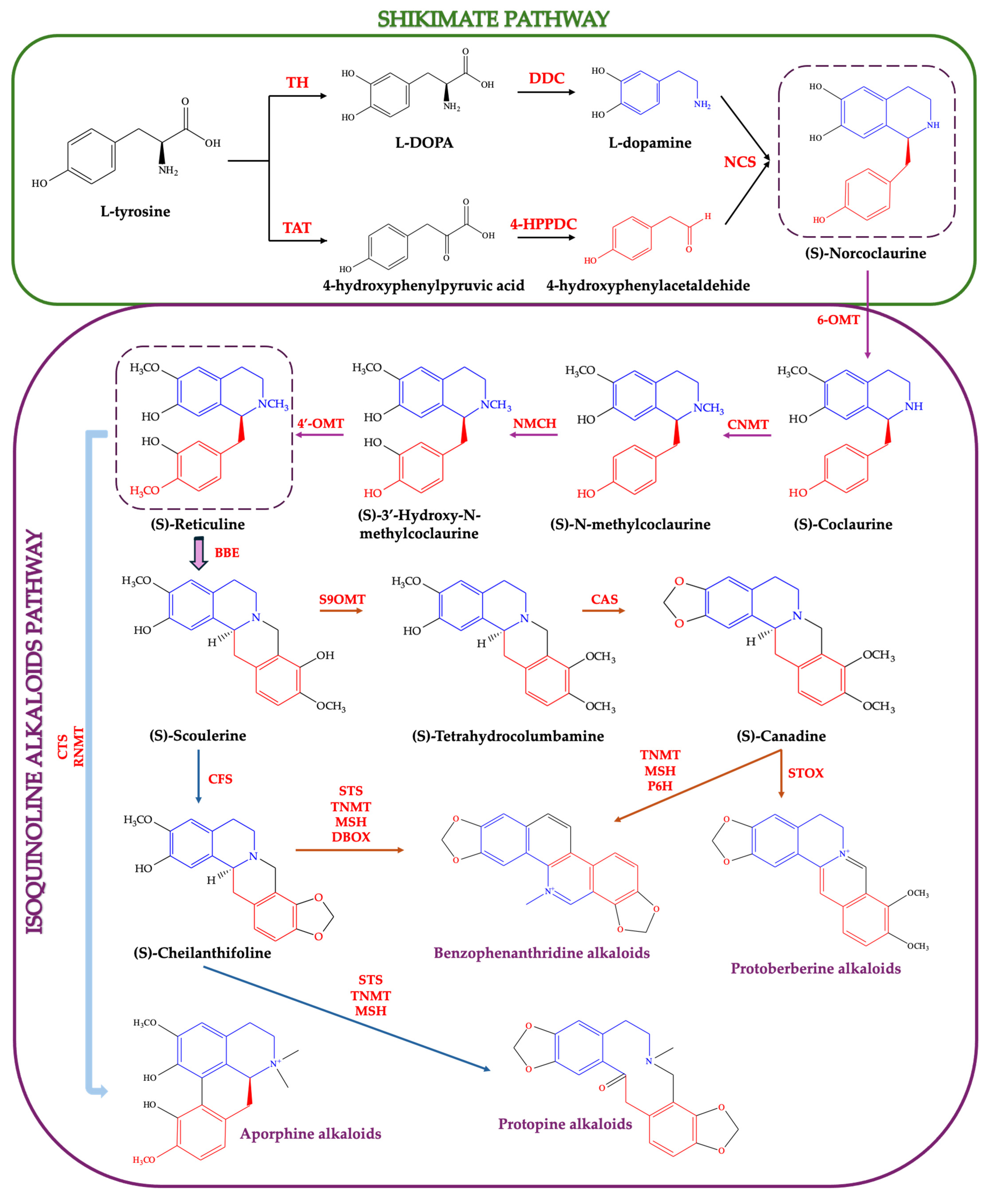
3. Biosynthesis of Isoquinoline Alkaloids
4. Recent Data on the Bioactivity of Chelidonium majus
4.1. Anticancer Activity
4.2. Anti-Inflammatory and Analgesic Activities
4.3. Antiviral Activity
4.4. Antimicrobial and Antifungal Activities
4.5. Antioxidant Activity
4.6. Immunomodulatory Activity
4.7. Insecticidal Activity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APG IV | Angiosperm Phylogeny Group fourth version |
| CC50 | Cytotoxic concentration 50% |
| CDK1 | Cyclin-dependent kinase 1 |
| COX2 | Cyclooxygenase-2 |
| EGFR | Epidermal growth factor receptor |
| GPVI | Platelet glycoprotein VI |
| HEI-OC1 | House Ear Institute-Organ of Corti 1 |
| HPLC-DAD | High-performance liquid chromatography with photodiode-array detection |
| HPV | Human papillomavirus |
| IL-8 | Interleukine-8 |
| JNK | c-Jun N-terminal kinases |
| LSD1 | Lysine-specific demethylase 1A |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| p21, p53 | Cyclin-dependent kinase inhibitor |
| PGE2 | Prostaglandin E2 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor necrosis factor α |
| TRPA1 | Transient receptor potential ankyrin 1 |
| VEGF | Vascular endothelial growth factor |
| ZIKV | Zika virus |
References
- Sadybekov, A.V.; Katritch, V. Computational Approaches Streamlining Drug Discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Naithani, U.; Guleria, V. Integrative Computational Approaches for Discovery and Evaluation of Lead Compound for Drug Design. Front. Drug Discov. 2024, 4, 1362456. [Google Scholar] [CrossRef]
- Southey, M.W.Y.; Brunavs, M. Introduction to Small Molecule Drug Discovery and Preclinical Development. Front. Drug Discov. 2023, 3, 1314077. [Google Scholar] [CrossRef]
- Salm, S.; Rutz, J.; van den Akker, M.; Blaheta, R.A.; Bachmeier, B.E. Current State of Research on the Clinical Benefits of Herbal Medicines for Non-Life-Threatening Ailments. Front. Pharmacol. 2023, 14, 1234701. [Google Scholar] [CrossRef]
- Zare, G.; Diker, N.Y.; Arituluk, Z.C.; Tatli Cankaya, I.I. Chelidonium majus L. (Papaveraceae) Morphology, Anatomy and Traditional Medicinal Uses in Turkey. İstanbul J. Pharm. 2021, 51, 123–132. [Google Scholar] [CrossRef]
- Samatadze, T.E.; Yurkevich, O.Y.; Hazieva, F.M.; Konyaeva, E.A.; Morozov, A.I.; Zoshchuk, S.A.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological, Microanatomical and Molecular Cytogenetic Characterization of the Medicinal Plant Chelidonium majus L. Plants 2020, 9, 1396. [Google Scholar] [CrossRef]
- Borges, A.; Calvo, M.L.M.; Vaz, J.A.; Calhelha, R.C. Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings. Materials 2024, 17, 4199. [Google Scholar] [CrossRef] [PubMed]
- Tutin, T.G. (Ed.) Flora Europaea: Plantaginaceae to Compositae (and Rubiaceae), Vol. 4; Cambridge University Press: Cambridge, UK, 1964. [Google Scholar]
- Colombo, M.L.; Tomè, F. Chelidonium majus L. (Greater Celandine): In Vitro Culture and the Production of Sanguinarine, Coptisine, and Other Isoquinoline Alkaloids. In Medicinal and Aromatic Plants VIII; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1995; Volume 33. [Google Scholar] [CrossRef]
- Achilova, S.S.; Rabbimov, A. Some Biological Characteristics of the Plant of the Large Blood Pump—Chelidonium majus L. in the Conditions of the Zarafshon Oasis. Int. J. Virol. Mol. Biol. 2024, 13, 37–38. [Google Scholar] [CrossRef]
- Wu, J.; Peng, L.; Dong, S.; Xia, X.; Zhao, L. Transcriptome Analysis of Chelidonium majus Elaiosomes and Seeds Provide Insights into Fatty Acid Biosynthesis. PeerJ 2019, 7, e6871. [Google Scholar] [CrossRef] [PubMed]
- Rahmonov, O.; Srodek, D.; Pytel, S.; Kupka, T.; Makieieva, N. Mineral Composition of Chelidonium majus L. and Soils in Urban Areas. Appl. Sci. 2025, 15, 4718. [Google Scholar] [CrossRef]
- Zielińska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s Ups and Downs—21 Centuries of Medicinal Uses of Chelidonium majus From the Viewpoint of Today’s Pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus—An Integrative Review: Traditional Knowledge versus Modern Findings. Forsch. Komplementmed. 2010, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Power, S.; Barritt, A. A Yellow Flower With Jaundice Power: Liver Injury Attributed to Greater Celandine. ACG Case Rep. J. 2024, 11, e01005. [Google Scholar] [CrossRef] [PubMed]
- Moro, P.A.; Cassetti, F.; Giugliano, G.; Falce, M.T.; Mazzanti, G.; Menniti-Ippolito, F.; Raschetti, R.; Santuccio, C. Hepatitis from Greater Celandine (Chelidonium majus L.): Review of Literature and Report of a New Case. J. Ethnopharmacol. 2009, 124, 328–332. [Google Scholar] [CrossRef]
- Pantano, F.; Mannocchi, G.; Marinelli, E.; Gentili, S.; Graziano, S.; Busardò, F.P.; di Luca, N.M. Hepatotoxicity Induced by Greater Celandine (Chelidonium majus L.): A Review of the Literature. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 46–52. [Google Scholar]
- Maggini, V.; Lombardi, N.; Crescioli, G.; Gallo, E.; Sivelli, F.; Gensini, G.F.; Vannacci, A.; Firenzuoli, F. Chelidonium majus: Relevant safety aspects of a hepatotoxic plant, trawling the web. Phytother. Res. 2019, 33, 2465–2469. [Google Scholar] [CrossRef]
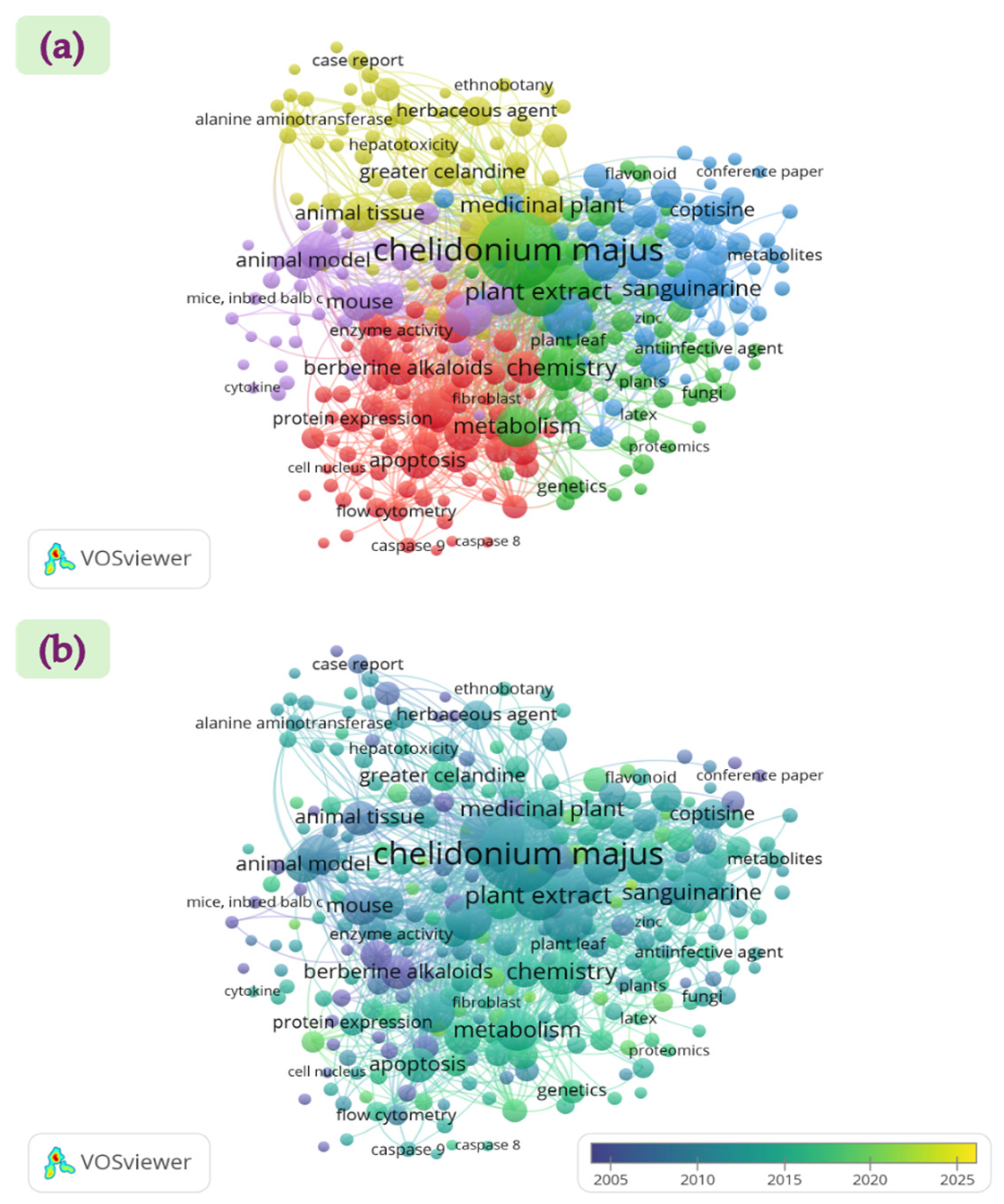
- VOSviewer Software. Version 1.6.20. Available online: www.vosviewer.com (accessed on 11 July 2025).
- Maji, A.K.; Banerji, P. Chelidonium majus L. (Greater Celandine)—A Review on Its Phytochemical and Therapeutic Perspectives. Int. J. Herb. Med. 2015, 3, 10–27. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, Y.P.; Wang, M.; Wang, Z.B.; Kuang, H.X. Alkaloids in Chelidonium majus L: A Review of Its Phytochemistry, Pharmacology, and Toxicology. Front. Pharmacol. 2024, 15, 1440979. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tong, L.-T.; Tu, P.-F.; Chen, L.-L.; Xu, X.; Song, Y.; Yang, X.-X.; Guo, Z.-B.; Zou, X.; Sun, C.-X.; et al. Lignanamides: A Comprehensive Review of Chemical Constituents, Biological Activities, Extraction Methods and Synthetic Pathway. Food Chem. 2024, 460, 140459. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Shao, Z.-X.; An, L.-J.; Xue, J.-J.; Li, D.-H.; Li, Z.-L.; Hua, H.-M. New Lignanamides and Alkaloids from Chelidonium majus and Their Anti-Inflammation Activity. Fitoterapia 2019, 139, 104359. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Sampaio, M.; Lima, J.; Andrade, P.B. Box–Behnken Factorial Design to Obtain a Phenolic-Rich Extract from the Aerial Parts of Chelidonium majus L. Talanta 2014, 130, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovska, V.; Sile, I.; Kronberga, A.; Nakurte, I.; Mezaka, I.; Dambrova, M.; Pugovics, O.; Grinberga, S. The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants. Plants 2021, 10, 1971. [Google Scholar] [CrossRef]
- Rahmonov, O.; Środek, D.; Pytel, S.; Makieieva, N.; Kupka, T. Relationships between Heavy Metal Concentrations in Greater Celandine (Chelidonium majus L.) Tissues and Soil in Urban Parks. Int. J. Environ. Res. Public Health 2023, 20, 3887. [Google Scholar] [CrossRef] [PubMed]
- Rahmonov, O.; Środek, D.; Pytel, S.; Kupka, T.; Makieieva, N. Accumulation of heavy metals in soil and Chelidonium majus L. in the urban environment. J. Elem. 2023, 28, 1329–1351. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Shao, J.; Wang, C.; Dong, B.; Cui, H.; Dai, D. Chelidonine Reduces IL-1β-Induced Inflammation and Matrix Catabolism in Chondrocytes and Attenuates Cartilage Degeneration and Synovial Inflammation in Rats. Braz. J. Med. Biol. Res. 2023, 56, e12604. [Google Scholar] [CrossRef]
- Jang, H.J.; Yang, J.H.; Hong, E.; Jo, E.; Lee, S.; Lee, S.; Choi, J.S.; Yoo, H.S.; Kang, H. Chelidonine Induces Apoptosis via GADD45a-p53 Regulation in Human Pancreatic Cancer Cells. Integr. Cancer Ther. 2021, 20, 15347354211006191. [Google Scholar] [CrossRef]
- Xie, Y.J.; Gao, W.N.; Wu, Q.B.; Yao, X.J.; Jiang, Z.B.; Wang, Y.W.; Wang, W.J.; Li, W.; Hussain, S.; Liu, L.; et al. Chelidonine Selectively Inhibits the Growth of Gefitinib-Resistant Non-Small Cell Lung Cancer Cells through the EGFR-AMPK Pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Chen, P.; Ji, X.-Y.; Feng, J.-T.; Wang, X.-Q.; Zhang, B. The Synergistic Mechanism of Chelidonium majus Alkaloids on Melanoma Treatment via a Multi-Strategy Insight. Molecules 2024, 29, 5412. [Google Scholar] [CrossRef]
- Hao, J.; Qin, X.; Guan, L.; Chen, S.; Hao, X.; Zhang, P.; Bai, H.; Zhao, W.; Huang, Z.; Chu, S.; et al. Chelerythrine Inhibits NR2B NMDA Receptor Independent of PKC Activity. Biochem. Biophys. Res. Commun. 2024, 739, 150914. [Google Scholar] [CrossRef]
- Loe, M.W.C.; Lee, R.C.H.; Chin, W.X.; Min, N.; Teo, Z.Y.; Ho, S.X.; Yi, B.; Chu, J.J.H. Chelerythrine Chloride Inhibits Zika Virus Infection by Targeting the Viral NS4B Protein. Antivir. Res. 2023, 219, 105732. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, M.; Li, X.; Sun, Z.; Li, Y.; Wang, X.; Wang, T. Anti-Microbial and Anti-Biofilm Activities of Combined Chelerythrine-Sanguinarine and Mode of Action Against Candida albicans and Cryptococcus neoformans In Vitro. Colloids Surf. B Biointerfaces 2020, 191, 111003. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.; Wang, W.; Li, Y.; Ma, W.; Sun, S. In Vitro and In Vivo Activity of Chelerythrine Against Candida albicans and Underlying Mechanisms. Future Microbiol. 2019, 14, 1545–1557. [Google Scholar] [CrossRef]
- He, H.; Zhuo, R.; Dai, J.; Wang, X.; Huang, X.; Wang, H.; Xu, D. Chelerythrine Induces Apoptosis via ROS-Mediated Endoplasmic Reticulum Stress and STAT3 Pathways in Human Renal Cell Carcinoma. J. Cell. Mol. Med. 2020, 24, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Valipour, M.; Zarghi, A.; Ebrahimzadeh, M.A.; Irannejad, H. Therapeutic Potential of Chelerythrine as a Multi-Purpose Adjuvant for the Treatment of COVID-19. Cell Cycle 2021, 20, 2321–2336. [Google Scholar] [CrossRef]
- Mao, X.; Zhao, G.; Wang, Q.; He, J.; Liu, Y.; Liu, T.; Li, W.; Peng, Y.; Zheng, J. Chelerythrine Chloride is an Affinity-Labeling Inactivator of CYP3A4 by Modification of Cysteine239. J. Med. Chem. 2024, 67, 2802–2811. [Google Scholar] [CrossRef]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Habartova, K.; Cahlikova, L.; Rezacova, M. Chelidonine and Homochelidonine Induce Cell Death through Cell Cycle Checkpoints and MAP Kinase Pathways. Nat. Prod. Commun. 2017, 12, 1934578X1701200910. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, N.; Kou, S.; Gao, L.; Peng, B.; Dai, Y.; Zheng, J. Sanguinarine Synergistically Potentiates Aminoglycoside-Mediated Bacterial Killing. Microb. Biotechnol. 2022, 15, 2055–2070. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, X.; Chen, X.; Fang, S.; Ding, Q.; Gao, Z. Sanguinarine is an Agonist of TRPA1 Channel. Biochem. Biophys. Res. Commun. 2021, 534, 226–232. [Google Scholar] [CrossRef]
- Zhang, S.; Leng, T.; Zhang, Q.; Zhao, Q.; Nie, X.; Yang, L. Sanguinarine Inhibits Epithelial Ovarian Cancer Development via Regulating Long Non-Coding RNA CASC2-EIF4A3 Axis and/or Inhibiting NF-κB Signaling or PI3K/AKT/mTOR Pathway. Biomed. Pharmacother. 2018, 102, 302–308. [Google Scholar] [CrossRef]
- Shu, D.; Zhu, Y.; Lu, M.; He, A.-D.; Chen, J.-B.; Ye, D.-S.; Liu, Y.; Zeng, X.-B.; Ma, R.; Ming, Z.-Y. Sanguinarine Attenuates Collagen-Induced Platelet Activation and Thrombus Formation. Biomedicines 2021, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chen, Y.; Liu, S.; Liu, L.; Yu, Z.; Yin, L.; Fu, L.; Deng, M.; Liang, S.; Lü, M. Sanguinarine Inhibits Melanoma Invasion and Migration by Targeting the FAK/PI3K/AKT/mTOR Signalling Pathway. Pharm. Biol. 2023, 61, 696–709. [Google Scholar] [CrossRef]
- Mengzhe, Y.; Beibei, Z.; Zhenqiang, L.; Nannan, C.; Anqiao, L.; Jianyu, Y.; Xingzhe, G.; Xianyu, B.; Yuanjiao, H.; Aijun, J.; et al. Sanguinarine Suppresses Cell Proliferation, Migration, and Invasion in Nasopharyngeal Carcinoma Inhibiting mTOR Signaling. J. Tradit. Chin. Med. 2022, 42, 687–692. [Google Scholar] [CrossRef]
- Lu, Q.; Luo, S.; Shi, Z.; Yu, M.; Guo, W.; Li, C. Nitidine Chloride, a Benzophenanthridine Alkaloid from Zanthoxylum nitidum (Roxb.) DC., Exerts Multiple Beneficial Properties, Especially in Tumors and Inflammation-Related Diseases. Front. Pharmacol. 2022, 13, 1046402. [Google Scholar] [CrossRef]
- Khan, H.; Hadda, T.B.; Touzani, R. Diverse Therapeutic Potential of Nitidine, A Comprehensive Review. Curr. Drug Metab. 2018, 19, 986–991. [Google Scholar] [CrossRef]
- Shen, C.; Kuang, Y.; Xu, S.; Li, R.; Wang, J.; Zou, Y.; Wang, C.; Xu, S.; Liang, L.; Lin, C.; et al. Nitidine Chloride Inhibits Fibroblast Like Synoviocytes-Mediated Rheumatoid Synovial Inflammation and Joint Destruction by Targeting KCNH1. Int. Immunopharmacol. 2021, 101 Pt A, 108273. [Google Scholar] [CrossRef]
- Lin, C.; Ge, L.; Tang, L.; He, Y.; Moqbel, S.A.A.; Xu, K.; Ma, D.; Zhou, X.; Ran, J.; Wu, L. Nitidine Chloride Alleviates Inflammation and Cellular Senescence in Murine Osteoarthritis Through Scavenging ROS. Front. Pharmacol. 2022, 13, 919940. [Google Scholar] [CrossRef]
- Lian, C.; Huang, Y.; Hu, P.; Cao, Y.; Zhang, Z.; Feng, F.; Zhang, J. Nitidine Chloride Triggers Autophagy and Apoptosis of Ovarian Cancer Cells through Akt/mTOR Signaling Pathway. Curr. Pharm. Des. 2023, 29, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological Properties and Clinical Applications of Berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine—A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of Inflammation by Berberine: Molecular Mechanism and Network Pharmacology Analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Huang, S.-Y.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Saimaiti, A.; Zhao, C.-N.; Shang, A.; Zhang, Y.-J.; Gan, R.-Y.; et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules 2022, 27, 4523. [Google Scholar] [CrossRef]
- Gasmi, A.; Asghar, F.; Zafar, S.; Oliinyk, P.; Khavrona, O.; Lysiuk, R.; Peana, M.; Piscopo, S.; Antonyak, H.; Pen, J.J.; et al. Berberine: Pharmacological Features in Health, Disease and Aging. Curr. Med. Chem. 2024, 31, 1214–1234. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Wang, T.; Yu, L.; Wang, K.; Qiu, F. Inactivation of CYP2D6 by Berberrubine and the Chemical Mechanism. Biochemistry 2024, 63, 3078–3089. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cui, J.; Ma, H.; Abuduaini, N.; Huang, Y.; Tang, L.; Wang, W.; Zhang, Y.; Wang, Y.; Lu, W.; et al. Berberrubine Is a Novel and Selective IMPDH2 Inhibitor That Impairs the Growth of Colorectal Cancer. Biochem. Pharmacol. 2023, 218, 115868. [Google Scholar] [CrossRef]
- Miao, Z.; Chang, D.; Du, X.; Sun, C. Berberrubine Protects Against Cisplatin-Induced Ototoxicity by Promoting Folate Biosynthesis. Front. Pharmacol. 2025, 15, 1496917. [Google Scholar] [CrossRef]
- Chu, Y.; Nie, Q.; Zhou, X.; Yang, J.; Fang, J.; Zhang, J. Berberrubine as a Novel TrxR Inhibitor Enhances Cisplatin Sensitivity in the Treatment of Non-Small Cell Lung Cancer. Bioorg. Chem. 2025, 158, 108329. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Q.Q.; Gu, D.M.; Yuan, X.; Wang, Y.H.; Guo, L.C. Canadine Inhibits Epithelial Mesenchymal Transformation of HPV-Negative Cervical Cancer. Tissue Barriers 2024, 12, 2256641. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; He, Y.; Li, S.; Feng, S.; Liu, Z.; Zhang, N.; Liu, M.; Wang, Q. Canadine Platinum(IV) Complexes Targeting Epithelial-Mesenchymal Transition as Antiproliferative and Antimetastatic Agents. J. Med. Chem. 2024, 67, 12868–12886. [Google Scholar] [CrossRef]
- Anjum, F.; Sulaimani, M.N.; Shafie, A.; Mohammad, T.; Ashraf, G.M.; Bilgrami, A.L.; Alhumaydhi, F.A.; Alsagaby, S.A.; Yadav, D.K.; Hassan, M.I. Bioactive Phytoconstituents as Potent Inhibitors of Casein Kinase-2: Dual Implications in Cancer and COVID-19 Therapeutics. RSC Adv. 2022, 12, 7872–7882. [Google Scholar] [CrossRef]
- Velayutham, N.K.; Thamaraikani, T.; Wahab, S.; Khalid, M.; Ramachawolran, G.; Abullais, S.S.; Wong, L.S.; Sekar, M.; Gan, S.H.; Ebenezer, A.J.; et al. Stylopine: A Potential Natural Metabolite to Block Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) in Osteosarcoma Therapy. Front. Pharmacol. 2023, 14, 1150270. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, S.; Li, K.; Wu, G.; Zheng, X.; Zheng, J.; Lu, X.; Zhang, L.; Li, J.; Su, Z.; et al. Coptisine Protects Against Hyperuricemic Nephropathy through Alleviating Inflammation, Oxidative Stress and Mitochondrial Apoptosis via PI3K/Akt Signaling Pathway. Biomed. Pharmacother. 2022, 156, 113941. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, Z.; Zhang, H.; Lu, Z.; Zhang, Y.; Li, M.; Kang, J.; Yang, Z.; Ma, L.; Ma, L.; et al. Coptisine Mitigates Diabetic Nephropathy via Repressing the NRLP3 Inflammasome. Open Life Sci. 2023, 18, 20220568. [Google Scholar] [CrossRef]
- Meng, J.; Song, X.; Xing, X.; Chen, J.; Lou, D. Coptisine Prevents Angiotensin II-Induced Endothelial Cell Injury and Senescence via the lncRNA SNHG12/miR-603/NAMPT Pathway. Exp. Ther. Med. 2023, 27, 68. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Zhang, X.; Vong, C.T.; Wang, Y.; Cheang, W.S. Coptisine Attenuates Diabetes—Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress. Molecules 2021, 26, 4210. [Google Scholar] [CrossRef] [PubMed]
- He, M.-F.; Liang, J.-H.; Shen, Y.-N.; Zhang, C.-W.; Yang, K.-Y.; Liu, L.-C.; Xie, Q.; Hu, C.; Song, X.; Wang, Y. Coptisine Inhibits Influenza Virus Replication by Upregulating p21. Molecules 2023, 28, 5398. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Luo, Y.; Xu, F.; Gao, F.; Sun, Y.; Yang, B.; Kuang, H. Protopine Ameliorates OVA-Induced Asthma through Modulating TLR4/MyD88/NF-κB Pathway and NLRP3 Inflammasome-Mediated Pyroptosis. Phytomedicine 2024, 126, 155410. [Google Scholar] [CrossRef]
- Nie, C.; Wang, B.; Wang, B.; Lv, N.; Yu, R.; Zhang, E. Protopine Triggers Apoptosis via the Intrinsic Pathway and Regulation of ROS/PI3K/Akt Signalling Pathway in Liver Carcinoma. Cancer Cell Int. 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.; OuYang, C.; Wu, X.; Xie, Y.; Xie, J. Protopine Alleviates Lipopolysaccharide-Triggered Intestinal Epithelial Cell Injury through Retarding the NLRP3 and NF-κB Signaling Pathways to Reduce Inflammation and Oxidative Stress. Allergol. Immunopathol. 2022, 50, 84–92. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, M.; Kan, Y.; Li, B.; Xu, R.; Wu, Y.; Wang, S.; Zheng, X.; Feng, W. Protopine Protects Mice against LPS-Induced Acute Kidney Injury by Inhibiting Apoptosis and Inflammation via the TLR4 Signaling Pathway. Molecules 2020, 25, 15. [Google Scholar] [CrossRef]
- Huang, W.; Kong, L.; Cao, Y.; Yan, L. Identification and Quantification, Metabolism and Pharmacokinetics, Pharmacological Activities, and Botanical Preparations of Protopine: A Review. Molecules 2022, 27, 215. [Google Scholar] [CrossRef]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef]
- Zhong, L.; Qin, Y.; Liu, M.; Sun, J.; Tang, H.; Zeng, Y.; Zhang, J.; Wang, W.; Liang, G.; Zhao, X. Magnoflorine Improves Cognitive Deficits and Pathology of Alzheimer’s Disease via Inhibiting of JNK Signaling Pathway. Phytomedicine 2023, 112, 154714. [Google Scholar] [CrossRef]
- Liang, H.; Chang, X.; Xia, R.; Wu, W.; Guo, H.; Yang, M. Magnoflorine Attenuates Cerebral Ischemia-Induced Neuronal Injury via Autophagy/Sirt1/AMPK Signaling Pathway. Evid. Based Complement. Alternat. Med. 2022, 2022, 2131561. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Zhou, Y.; Gu, R.; Lu, G.; Zhang, C. Magnoflorine Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response via the NF-κB/MAPK Signaling Pathways. J. Inflamm. Res. 2023, 16, 2271–2296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, Z.; Mao, T.; Song, Y.; Qu, Y.; Chen, X.; Chen, K.; Liu, K.; Zhang, C. Magnoflorine Ameliorates Chronic Kidney Disease in High-Fat and High-Fructose-Fed Mice by Promoting Parkin/PINK1-Dependent Mitophagy to Inhibit NLRP3/Caspase-1-Mediated Pyroptosis. J. Agric. Food Chem. 2024, 72, 12775–12787. [Google Scholar] [CrossRef]
- Hong, S.; Park, J.; Park, M.; Park, J.M.; Lee, H. Exploring the Antioxidant and Antimicrobial Potential of Chelidonium majus Extracts against Oral Microorganisms. Biocatal. Agric. Biotechnol. 2024, 59, 103231. [Google Scholar] [CrossRef]
- Bilušić, T.; Šola, I.; Čikeš Čulić, V. Identification of Flavonoids, Antioxidant and Antiproliferative Activity of Aqueous Infusions of Chelidonium majus L., Lamium album L., Leonurus cardiaca L. and Lysimachia nummularia L. Food Technol. Biotechnol. 2024, 62, 49–58. [Google Scholar] [CrossRef]
- Farid, M.; Alia, H.F.M.; Abdelgayed, S.S. Chemical Characterization of Chelidonium majus Flowers with Hepatotoxicity Protective Study in Male Albino Rats. Int. J. Vet. Sci. 2019, 8, 218–223. [Google Scholar]
- Kim, D.-S.; Kim, S.-J.; Kim, M.-C.; Jeon, Y.-D.; Um, J.-Y.; Hong, S.-H. The Therapeutic Effect of Chelidonic Acid on Ulcerative Colitis. Biol. Pharm. Bull. 2012, 35, 666–671. [Google Scholar] [CrossRef]
- Makieieva, N.; Kupka, T.; Spaleniak, G.; Korchowiec, J.; Rachiy, B.; Lis, T.; Błażejowski, J.; Grech, E.; Zawada, A.; Korchowiec, B. Experimental and Theoretical Characterization of Chelidonic Acid Structure. Struct. Chem. 2022, 33, 2133–2145. [Google Scholar] [CrossRef]
- Gomaa, H.F.; Fadl, N.N.; Elmashad, W.M.A.; Abouelfadl, D.M.; Mannaa, F.A.; Abdel-Wahhab, K.G. Protective Efficiency of Chelidonium majus Extract against Hepatoimmune and DNA Changes Induced by Aflatoxin B1. J. Appl. Pharm. Sci. 2022, 12, 140–149. [Google Scholar]
- Tian, Y.; Kong, L.; Li, Q.; Wang, Y.; Wang, Y.; An, Z.; Ma, Y.; Tian, L.; Duan, B.; Sun, W.; et al. Structural Diversity, Evolutionary Origin, and Metabolic Engineering of Plant Specialized Benzylisoquinoline Alkaloids. Nat. Prod. Rep. 2024, 41, 1787–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, J.; Chen, Y. Advances in the Biosynthesis of Naturally Occurring Benzylisoquinoline Alkaloids. Front. Plant Sci. 2025, 16, 1548471. [Google Scholar] [CrossRef]
- Wang, Y.; Subrizi, F.; Carter, E.M.; Sheppard, T.D.; Ward, J.M.; Hailes, H.C. Enzymatic Synthesis of Benzylisoquinoline Alkaloids Using a Parallel Cascade Strategy and Tyrosinase Variants. Nat. Commun. 2022, 13, 5436. [Google Scholar] [CrossRef]
- Minami, H.; Kim, J.; Ikezawa, N.; Takemura, T.; Katayama, T.; Kumagai, H.; Sato, F. Microbial Production of Plant Benzylisoquinoline Alkaloids. Proc. Natl. Acad. Sci. USA 2008, 105, 7393–7398. [Google Scholar] [CrossRef]
- Bradley, S.A.; Zhang, J.; Jensen, M.K. Deploying Microbial Synthesis for Halogenating and Diversifying Medicinal Alkaloid Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 594126. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. [Google Scholar] [CrossRef]
- Jiao, X.; Fu, X.; Li, Q.; Gao, S.; Zhao, C.; Wang, J.; Wu, J.; Xu, Y.; Li, Z.; Yan, Y. De Novo Production of Protoberberine and Benzophenanthridine Alkaloids through Metabolic Engineering of Yeast. Nat. Commun. 2024, 15, 8759. [Google Scholar] [CrossRef]
- Barton, B.H.; Castle, T. The British Flora Medica, or History of the Medicinal Plants of Great Britain; E. Cox: London, UK, 1845. [Google Scholar]
- Mayer, J.G.; Uehleke, B.; Saum, K. Handbuch der Klosterheilkunde; Verlag Zabert Sandmann: München, Germany, 2003. [Google Scholar]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; Greater Celandine; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548684/ (accessed on 11 July 2025).
- Petruczynik, A.; Tuzimski, T.; Plech, T.; Misiurek, J.; Szalast, K.; Szymczak, G. Comparison of Anticancer Activity and HPLC-DAD Determination of Selected Isoquinoline Alkaloids from Thalictrum foetidum, Berberis sp. and Chelidonium majus Extracts. Molecules 2019, 24, 3417. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A.; Plech, T.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Misiurek, J.; Buszewski, B.; Waksmundzka-Hajnos, M. Determination of Selected Isoquinoline Alkaloids from Chelidonium majus, Mahonia aquifolium and Sanguinaria canadensis Extracts by Liquid Chromatography and Their In Vitro and In Vivo Cytotoxic Activity against Human Cancer Cells. Int. J. Mol. Sci. 2023, 24, 6360. [Google Scholar] [CrossRef]
- Nile, S.H.; Wang, H.; Nile, A.; Lin, X.; Dong, H.; Venkidasamy, B.; Sieniawska, E.; Enkhtaivan, G.; Kai, G. Comparative Analysis of Metabolic Variations, Antioxidant Potential and Cytotoxic Effects in Different Parts of Chelidonium majus L. Food Chem. Toxicol. 2021, 156, 112483. [Google Scholar] [CrossRef]
- Nawrot, R.; Warowicka, A.; Rudzki, P.J.; Musidlak, O.; Dolata, K.M.; Musijowski, J.; Stolarczyk, E.U.; Goździcka-Józefiak, A. Combined Protein and Alkaloid Research of Chelidonium majus Latex Reveals CmMLP1 Accompanied by Alkaloids with Cytotoxic Potential to Human Cervical Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 11838. [Google Scholar] [CrossRef]
- Terzic, M.; Fayez, S.; Fahmy, N.M.; Eldahshan, O.A.; Uba, A.I.; Ponniya, S.K.M.; Selvi, S.; Nilofar; Koyuncu, I.; Yüksekdağ, Ö.; et al. Chemical Characterization of Three Different Extracts Obtained from Chelidonium majus L. (Greater Celandine) with Insights into Their In Vitro, In Silico and Network Pharmacological Properties. Fitoterapia 2024, 174, 105835. [Google Scholar] [CrossRef]
- Sadeghpour Natanzi, M.; Parsania, M.; Ashrafi, F.; Pouriayevali, M.H. Evaluation of Chelidonium majus L. Alkaloid Effect on VEGF Gene Expression and Cell Apoptosis in the Burkitt Lymphoma Cell Line. Iran J. Ped. Hematol. Oncol. 2025, 15, 347–357. [Google Scholar]
- Salehi, S.; Schallmayer, E.; Bandomir, N.; Kärcher, A.; Güth, J.F.; Heitel, P. Screening of Chelidonium majus isoquinoline alkaloids reveals berberine and chelidonine as selective ligands for the nuclear receptors RORβ and HNF4α, respectively. Arch. Pharm. 2024, 357, e2300756. [Google Scholar] [CrossRef]
- Mikołajczak, P.Ł.; Kędzia, B.; Ożarowski, M.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Białas, W.; Gryszczyńska, A.; Buchwald, W.; Szulc, M.; et al. Evaluation of Anti-Inflammatory and Analgesic Activities of Extracts from Herb of Chelidonium majus L. Cent.-Eur. J. Immunol. 2015, 40, 400–410. [Google Scholar] [CrossRef]
- Zielińska, S.; Czerwińska, M.E.; Dziągwa-Becker, M.; Dryś, A.; Kucharski, M.; Jezierska-Domaradzka, A.; Płachno, B.J.; Matkowski, A. Modulatory Effect of Chelidonium majus Extract and Its Alkaloids on LPS-Stimulated Cytokine Secretion in Human Neutrophils. Molecules 2020, 25, 842. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fan, T.; Zhuge, R.; Li, H.; Li, G.; Zhou, L.; Xu, L.; Hao, X.; Gu, W.; Wang, J. Chelerythrine Chloride Alleviated Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Glycolytic Pathway Through Targeting Glyceraldehyde-3-Phosphate Dehydrogenase. Molecules 2025, 30, 2572. [Google Scholar] [CrossRef]
- Musidlak, O.; Warowicka, A.; Broniarczyk, J.; Adamczyk, D.; Goździcka-Józefiak, A.; Nawrot, R. The Activity of Chelidonium majus L. Latex and Its Components on HPV Reveal Insights into the Antiviral Molecular Mechanism. Int. J. Mol. Sci. 2022, 23, 9241. [Google Scholar] [CrossRef]
- Gardin, N.E.; Braga, A.J. Greater Celandine (Chelidonium majus L.) for COVID-19: A Twenty-Case Series. Phytother. Res. 2021, 35, 3792–3798. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, X.; Guo, X.; Yang, Y.; Fan, X.; Han, Y.; Liu, Y. Evaluation of the In Vitro and In Vivo Antimicrobial Activity of Alkaloids Prepared from Chelidonium majus L. Using MRSA-Infected C. elegans as a Model Host. Fitoterapia 2024, 175, 105944. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, Y.; Zhang, M.; Wang, Z. Nematicidal Potential of Berberine and Sanguinarine from Chelidonium majus against Meloidogyne incognita. Asian Pac. J. Trop. Biomed. 2025, 15, 102384. [Google Scholar] [CrossRef]
- Sadecki, P.; Mangal, M.; Chintapenta, L.K.; Novakowski, S.K.; Massefski, W.W.; van der Donk, W.A. The Greater Celandine: Identification and Characterization of an Antimicrobial Peptide from Chelidonium majus. J. Nat. Prod. 2024, 87, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Vlase, G.; Vlase, T.; Ciocalteu, M.-V.; Bejenaru, C.; Buema, G.; Bejenaru, L.E.; Boia, E.R.; Dumitru, A.; Boia, S. Romanian Wild-Growing Chelidonium majus—An Emerging Approach to a Potential Antimicrobial Engineering Carrier System Based on AuNPs: In Vitro Investigation and Evaluation. Plants 2024, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Khodabande, Z.; Jafarian, V.; Sariri, R. Antioxidant Activity of Chelidonium majus Extract at Phenological Stages. Appl. Biol. Chem. 2017, 60, 497–503. [Google Scholar] [CrossRef]
- Dostemessova, A.; Canpolat, Ş.; Kurmanbayeva, M.; Sadykova, D.; Yürümez Canpolat, E.; Ildız, E.; Izbastina, K.; İşlek, C. Biological Activity and Alkaloid Composition of Chelidonium majus L. Growing at Three Sites in the Eastern Part of Kungey Alatau Ridge (Kazakhstan). Appl. Biol. Res. 2025, 27, 80–93. [Google Scholar] [CrossRef]
- Zou, C.; Lv, C.; Wang, Y.; Cao, C.; Zhang, G. Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pestic. Biochem. Physiol. 2017, 142, 123–132. [Google Scholar] [CrossRef]

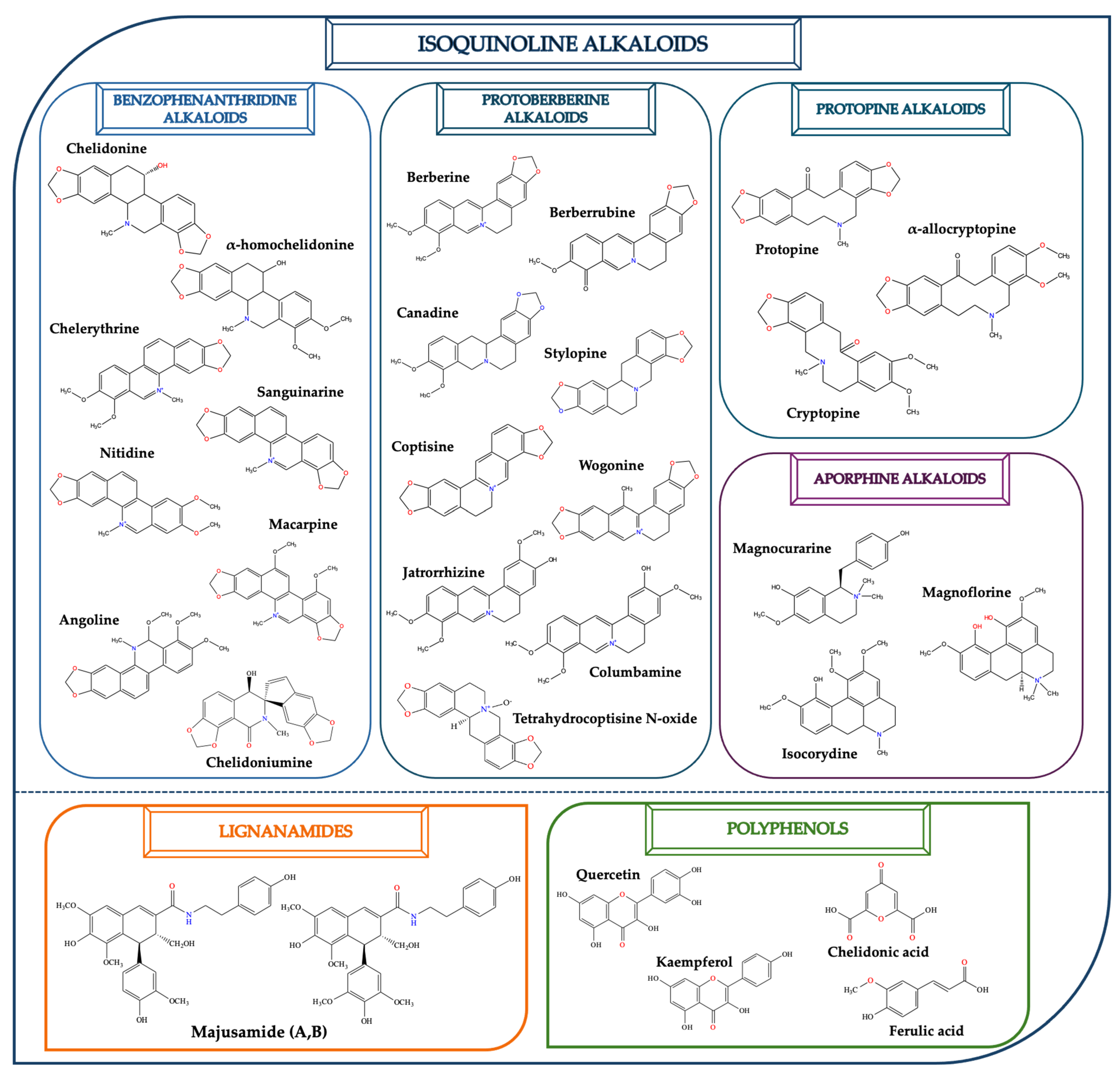
| Phytochemical Group | Phytoconstituent | Biological Functions | References |
|---|---|---|---|
| Benzophenanthridines | 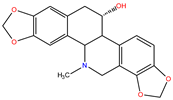 Chelidonine |
| [29,30,31,32] |
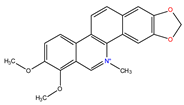 Chelerythrine |
| [33,34,35,36,37,38,39] | |
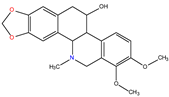 α-homochelidonine |
| [40] | |
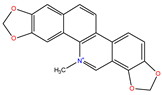 Sanguinarine |
| [41,42,43,44,45,46] | |
 Nitidine |
| [47,48,49,50,51] | |
| Protoberberines | 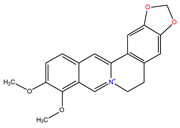 Berberine |
| [52,53,54,55,56] |
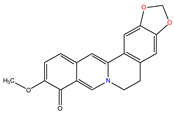 Berberrubine |
| [57,58,59,60,61] | |
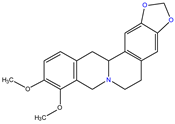 Canadine |
| [62,63] | |
 Stylopine |
| [64,65] | |
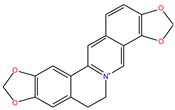 Coptisine |
| [66,67,68,69,70] | |
| Protopines | 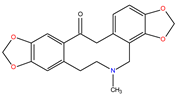 Protopine |
| [71,72,73,74,75] |
| Aporphine | 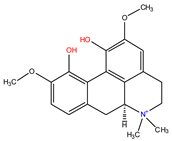 Magnoflorine |
| [76,77,78,79,80] |
| Polyphenols | 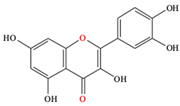 Quercetin |
| [13,81,82] |
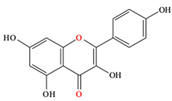 Kaempferol |
| [13,82,83] | |
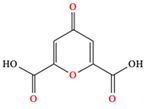 Chelidonic acid |
| [84,85] | |
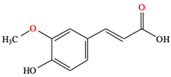 Ferulic acid |
| [86] |
| Biological Activities | Plant Part | Solvent/Extract Type | Extraction Method | References |
|---|---|---|---|---|
| Anticancer activity | Aerial part |
|
| [97] |
| Aerial part |
|
| [98] | |
| Whole plant |
|
| [99] | |
| Different parts (e.g., leaf, stem, flower, pod and root) |
|
| ||
| Whole plant tissue |
|
| [100] | |
| Milky sap (latex) |
|
| ||
| Aerial parts |
|
| [101] | |
| Whole plant |
|
| [102] | |
| Anti-inflammatory and analgesic activities | Whole plant |
|
| [104] |
| Roots and root collars |
|
| [105] | |
| Antiviral activity | Latex |
|
| [107] |
| Antimicrobial and antifungal activities | Whole plant |
|
| [81] |
| Whole plant |
|
| [109] | |
| Aerial and belowground parts |
|
| [111] | |
| Antioxidant activity | Roots, aerial parts |
|
| [115] |
| Immunomodulatory activity | Roots and root collars |
|
| [105] |
| Insecticidal activity | Aerial parts |
|
| [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanu, R.; Liga, S.; Tripon, M.R.; Huiban, F.; Iliescu, D.; Dehelean, C.A.; Camelia, T. Chelidonium majus L.: A Current Perspective on Isoquinoline Alkaloids, Emerging Phytochemicals, Alkaloid Biosynthesis, and Biological Activities. Plants 2025, 14, 2627. https://doi.org/10.3390/plants14172627
Romanu R, Liga S, Tripon MR, Huiban F, Iliescu D, Dehelean CA, Camelia T. Chelidonium majus L.: A Current Perspective on Isoquinoline Alkaloids, Emerging Phytochemicals, Alkaloid Biosynthesis, and Biological Activities. Plants. 2025; 14(17):2627. https://doi.org/10.3390/plants14172627
Chicago/Turabian StyleRomanu, Ramona, Sergio Liga, Maria Roberta Tripon, Florin Huiban, Dan Iliescu, Cristina Adriana Dehelean, and Tulcan Camelia. 2025. "Chelidonium majus L.: A Current Perspective on Isoquinoline Alkaloids, Emerging Phytochemicals, Alkaloid Biosynthesis, and Biological Activities" Plants 14, no. 17: 2627. https://doi.org/10.3390/plants14172627
APA StyleRomanu, R., Liga, S., Tripon, M. R., Huiban, F., Iliescu, D., Dehelean, C. A., & Camelia, T. (2025). Chelidonium majus L.: A Current Perspective on Isoquinoline Alkaloids, Emerging Phytochemicals, Alkaloid Biosynthesis, and Biological Activities. Plants, 14(17), 2627. https://doi.org/10.3390/plants14172627









