Abstract
Botrytis cinerea is one of the phytopathogenic fungi of the greatest economic importance worldwide. Essential oils (EOs) have been proposed as a sustainable alternative to reduce the growth of phytopathogenic fungi. Nevertheless, few studies exist about its mechanisms of action. This study evaluated the antifungal activity of EOs from Citrus reticulata, Citrus limon, Citrus sinensis, and Citrus paradisi peels and their encapsulation inside solid lipid nanoparticles (SLNs). Accordingly, Citrus EOs were mainly constituted by monoterpene hydrocarbons, where limonene was the most abundant in all EOs. C. reticulata and C. limon EOs reduced the mycelial growth at above 54% after 96 h. The other EOs did not significantly impact the phytopathogen. C. reticulata EO increased the hyphae damage by 40%, but the spore germination was reduced by only 8.34%. It also significantly increased the pH, the electrical conductivity, and the release of intracellular absorbing material and soluble proteins in B. cinerea cultures. Contrary, the esterase, mitochondrial, and succinate dehydrogenase activities decreased at above 50%. C. reticulata EO into SLN reduced the mycelial growth of B. cinerea by 90–97%. These results show that the EO of C. reticulata alters the physiological and metabolic activities of B. cinerea to reduce its growth.
1. Introduction
Botrytis cinerea is considered the second most important and destructive phytopathogen by the scientific community due to its economic and global impact on the loss of fruits and vegetables [1,2]. B. cinerea is a destructive necrotrophic plant pathogen that produces gray mold diseases, resulting in significant yield and quality losses (>50%) in vegetables and fruits [3]. This phytopathogen can infect a wide range of plant species, causing diseases in over 1400 plant species [2]. The germination of conidia and germ tubes on the surface of plants under appropriate environmental conditions facilitates infection in host tissue or cells [4]. The conidia of B. cinerea germinate on plant surfaces under appropriate temperatures (15–25 °C) and humidity (80%). The appressorium located at the tip of the germ tube invades host tissues or cells to complete the infection process. In the early stage, B. cinerea produces an asymptomatic biotrophic phase, which is followed by a necrotrophic phase as plant organs mature [5]. This fungus releases cell-wall-degrading enzymes, low-molecular-weight compounds, toxins, and phytotoxic metabolites that facilitate the infection process [6]. The control of gray mold is conventionally based on the application of synthetic fungicides, including hydroxyanilides, anilinopyrimidines, dicarboximides, and carboxamides, among others, which collectively account for approximately 10% of the global fungicide market [7,8]. Nevertheless, the adverse impact of synthetic fungicides on the ecosystem and human health has led to the search for sustainable alternatives [7,9]. In this context, essential oils (EOs) have emerged as an eco-friendly alternative to overcome the limitations and mitigate the harmful impact of synthetic fungicides in controlling fungal phytopathogens [10]. EOs are a natural mixture of hydrophobic secondary metabolites (around 20–60) with volatile and aromatic properties, including hydrocarbons, terpenes, and terpenoids produced naturally by plants or fruits [11]. EOs can be extracted from different plant organs, including flowers, seeds, leaves, fruits, roots, shoots, grasses, wood, and bark [12]. In this sense, EOs constitute an eco-friendly tool due to their biodegradable and biocompatible properties, which enable them to mitigate the adverse impacts of synthetic fungicides. EOs of citrus species are composed of secondary metabolites with an aromatic nature, comprising 85–99% volatile and 1–15% non-volatile components [13]. Volatile constituents contain monoterpene hydrocarbons (70–95%) and D-limonene. The antifungal mechanisms of EOs are associated with the alteration of ergosterol synthesis, modification of morphology, dysfunction of ATPase activity, and the production of reactive oxygen species [11]. Other studies have reported that the antifungal activity of EOs could be associated with the interaction and subsequent formation of hydrogen bonds between hydroxyl groups and the active site of fungal enzymes [14]. Recently, it has been reported that the EO of citrus species exhibits antimicrobial properties due to its capacity to damage cell membranes, inhibit the respiratory chain, and alter cell constituents (i.e., DNA, proteins, lipids) [13,15]. For example, Simas et al. [16] demonstrated that EOs of Citrus limon and Citrus limonia at 312 μg mL−1 completely inhibited the mycelial growth of B. cinerea due to the disorganization in the fungal cell membrane [16]. Besides, the vapor phase released from 625 µL L−1 of EO of lemongrass inhibited the growth of B. cinerea [17]. Nevertheless, studies on the impact of the EOs of citrus on controlling B. cinerea are scarce, and their action mechanisms have not been fully elucidated. Furthermore, the application of EO in agriculture is scarce due to its lipophilic nature and sensitivity to environmental conditions, which prevents its direct application. In this sense, solid lipid nanoparticles (SLNs) (size: 50 to 1000 nm) have been proposed for the encapsulation of EO due to it immobilizing and protecting the active agent within solid lipids [18]. The SLNs are characterized by their biodegradable nature, stability, water solubility, and protection of the active agent against chemical, photochemical, and oxidative degradation [19]. In this context, this study focused on (1) evaluating the EOs from the peel of Citrus reticulata Blanco (Mandarin), Citrus limon Risso (Lemon), Citrus sinensis L. (Sweet orange), and Citrus paradisi Macfad. (Grapefruit) for controlling the growth of B. cinerea, (2) elucidating their potential action mechanisms of EO of citrus species, and (3) prospecting SLN to apply EO in agricultural systems.
2. Results
2.1. Characterization of the Essential Oils
EOs from peels of C. reticulata, C. limon, C. paradisi, and C. sinensis were analyzed by gas–liquid chromatography coupled with mass spectrometry (GC/MS) (Table 1). Eleven, twelve, nine, and eight compounds, corresponding to 99.64%, 99.29%, 99.95%, and 99.92% of the detected compounds were identified in the C. reticulata, C. limon, C. paradisi, and C. sinensis EOs, respectively.

Table 1.
Chemical composition of the Citrus essential oils.
Six monoterpene hydrocarbons (99.36%) and five sesquiterpenes (0.28%)—four non-oxygenated (0.21%) and one oxygenated (0.07%)—were present in the C. reticulata EO. Similarly, seven monoterpene hydrocarbons (97.78%) and five sesquiterpenes (1.51%)—four non-oxygenated (1.42%) and one oxygenated (0.09%)—were in the C. limon EO. Four monoterpene hydrocarbons (99.55%) and five sesquiterpenes (0.40%)—four non-oxygenated (0.38%) and one oxygenated (0.02%)—were in the C. paradisi EO. Six monoterpenes (99.88%)—four non-oxygenated (95.97%) and two oxygenated (3.91%)— and two sesquiterpene hydrocarbons (0.04%) were present in the C. sinensis EO.
The monoterpene hydrocarbon limonene was the most abundant compound in all Citrus EOs, followed by γ-terpinene (26.29%) in C. reticulata EO, and by β-pinene (21.89%) and γ-terpinene (12.85%) in C. limon EO. The major content of limonene was found in C. paradisi and C. sinensis EOs, with 98.51% and 95.23%, respectively (Table 1).
2.2. Inhibition of Mycelial Growth and Spore Germination
The EO of C. reticulata at 1000 μL L−1 reduced the mycelial growth between 62 and 64% after 72 and 96 h of exposure (Figure 1a). Concentrations of 400, 600, and 800 μL L−1 of the same EO similarly reduced the mycelial growth of B. cinerea on hours 72 and 96 and reduced the antifungal activity on hour 168. The EO of C. limon at concentrations from 800 to 1000 μL L−1 inhibited the mycelial growth of B. cinerea between 44 and 54% on hour 72, but the antifungal activity decreased on hour 168 (Figure 1b). The lower concentrations of EO of C. limon (400 and 600 μL L−1) had a similar antifungal activity behavior. It was observed that the EO of C. paradisi at 400 μL L−1 increased the mycelial growth between 41 and 56% on hours 72 and 96, respectively (Figure 1c). Similarly, the other concentrations produced an increase in the mycelial growth of B. cinerea. Finally, the application of EO of C. sinensis did not affect mycelial growth during the 168 h evaluated (Figure 1d). In summary, the results showed that the EO of C. reticulata presented the best activity in reducing mycelial growth, whereby it was selected for the following assay. Nevertheless, the spore germination was slightly reduced by around 8.34% with exposure for 24 h at 1000 μL L−1 of EO of C. reticulata (Figure 2).
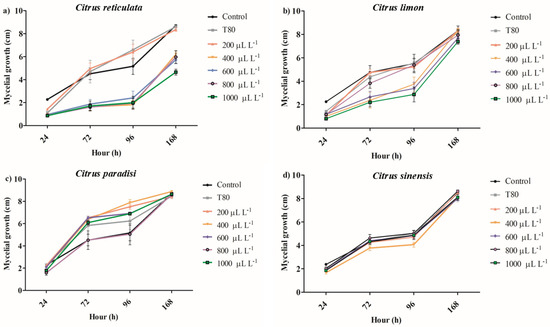
Figure 1.
The impact of different concentrations of essential oils of (a) Citrus reticulata, (b) Citrus limon, (c) Citrus paradisi, and (d) Citrus sinesis on mycelial growth of Botrytis cinerea. (mean values ± standard error n = 4).
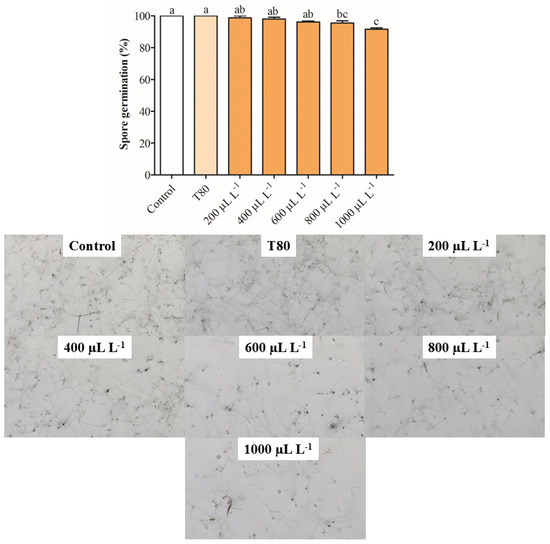
Figure 2.
Inhibition of spore germination of B. cinerea exposed to different concentrations of C. reticulata EO. Different letters above bars indicate significant differences according to the Tukey test (p < 0.05) (mean values ± standard error, n = 3).
2.3. Dry Weight, Electrical Conductivity, and pH
Figure 3a shows that the dry weight of B. cinerea decreased by approximately 89% with exposure to 800 and 1000 μL L−1, while concentrations of 400 and 600 μL L−1 reduced this parameter by 37–45%. The fungus exposure at 200 μL L−1 of EO of C. reticulata had no antifungal effect on B. cinerea. The electrical conductivity and pH of B. cinerea cultures were measured to investigate potential alterations in the cell membrane with exposure to the EO. The electrical conductivity in cultures of B. cinerea slightly increased by 5–12% with 800 and 1000 μL L−1 of EO of C. reticulata, respectively (Figure 3b). Meanwhile, concentrations from 200 to 600 μL L−1 did not change significantly the electrical conductivity of the cultures of B. cinerea. Regarding pH, the cultures of B. cinerea exposed to 800 and 1000 μL L−1 of EO increased this parameter by 11%, while concentrations between 200 and 600 μL L−1 had no impact on B. cinerea cultures (Figure 3c).
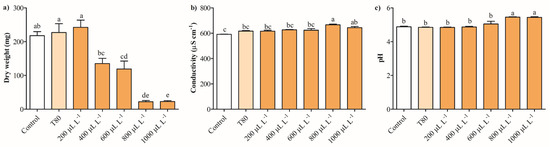
Figure 3.
Effects of C. reticulata EO on (a) dry weight and (b,c) release of cell constituents of B. cinerea measurement through extracellular pH and conductivity. Different letters above bars indicate significant differences according to the Tukey test (p < 0.05) (mean values ± standard error, n = 3).
2.4. The Release of Intracellular Constituents and Potential Physiological Mechanisms
Figure 4a shows that the release of intracellular absorbing material OD260nm in cultures of B. cinerea exposed to 200- 600 μL L−1 of EO of C. reticulata partially increases between 13 and 38%, while concentrations of 800 and 1000 μL L−1 enhanced 1-fold after 2 h. No significant differences were found in the release of intracellular absorbing material OD260nm of cultures of B. cinerea after 4 h of exposure to EO of C. reticulata. Similarly, the release of intracellular soluble proteins increased significantly in cells of B. cinerea exposed to 200 and 600 μL L−1 of EO of C. reticulata with 2 h of exposure, and no differences were found after 4 h (Figure 4b). Additionally, Mitotracker orange, Propidium iodide, and Calcein AM fluorescence staining were performed to explore mitochondrial activity, cell viability (hyphae damage), and esterase activity of cultures of B. cinerea exposed to EO of C. reticulata. Figure 5 shows that the cultures of B. cinerea exposed to EO of C. reticulata at 1000 μL L−1 decreased mitochondrial activity, esterase activity, and succinate dehydrogenase by 57%, 52%, and 65%, respectively. Simultaneously, the hypha damage of B. cinerea increased by 40% with the application of EO.
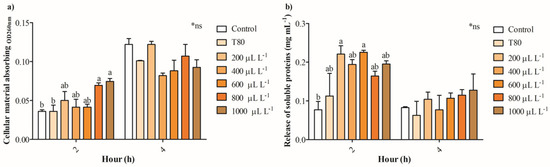
Figure 4.
The impact of essential oil from C. reticulata peels on (a) intracellular absorbing material OD260nm and (b) extracellular soluble proteins. Different letters above bars indicate significant differences according to the Tukey test (p < 0.05) (mean values ± standard error, n = 3). *ns indicate no statistically significant differences.
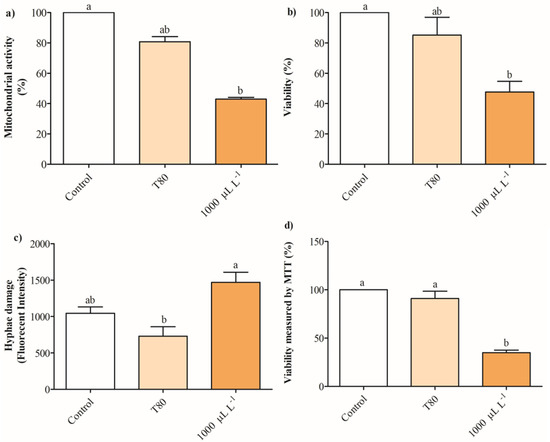
Figure 5.
The potential action mechanisms of the C. reticulata EO to suppress the growth of B. cinerea were measured through (a) mitochondrial activity, (b) viability, (c) hyphae damage, and (d) intracellular esterases measured by the MTT technique. Different letters above bars indicate significant differences according to the Tukey test (p < 0.05) (mean values ± standard error, n = 3).
2.5. Characterization and Evaluation of SLN Loaded with the EO of C. reticulata
SLN formulation and C. reticulata EO-loaded SLN had a mean hydrodynamic size of 262 and 406 nm, respectively. Both SLNs demonstrated high homogeneity in particle size, as indicated by their polydispersity index (PDI) values, which ranged from 0.18 to 0.20 (Figure 6a,c). Moreover, the encapsulation efficiency ranged from 90 to 93%. Meanwhile, the δ-potential of SLNs obtained had values from −27.1 to −29.0 mV, showing similar stability and supporting that the encapsulation of EO of C. reticulata does not modify the stability of the particle (Figure 6b,d). Further, the stability of the SLN formulation was also measured for 3 weeks, with values ranging from −17.7 mV to −25.5 mV, indicating that hydrophobic compounds could be encapsulated stably over time.
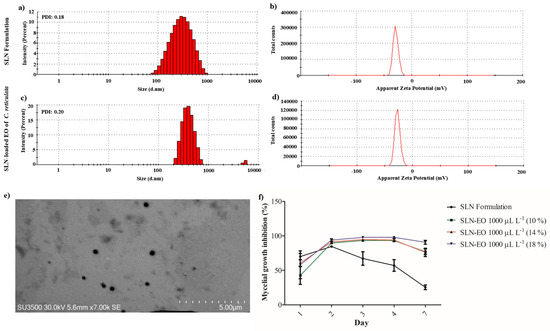
Figure 6.
Physicochemical characterization and antifungal activity of solid lipid nanoparticles (SLNs) loaded with C. reticulata EO. (a,c) Hydrodynamic size distribution and (b,d) ζ-potential of SLNs, (e) representative photograph of SLN captured by scanning transmission electron microscopy (STEM), and (f) the mycelial growth inhibition of B. cinerea modulated the controlled release of the EO of C. reticulata from SLN (mean values ± standard error, n = 3).
The photographic capture by scanning transmission electron microscopy (STEM) evidenced the spherical shape and homogeneous surface of the SLN formulation (Figure 6e). Figure 6e shows that on day 2, there was a small significant effect of C. reticulata-loaded SLN compared to the formulation of SLN (94% versus 84%). It was evidenced that SLN-loaded with EO of C. reticulata reduced the mycelial growth of B. cinerea between 90 and 97%. In contrast, the impact of the SLN formulation decreased significantly over time, from 67% to 25% (Figure 6f).
3. Discussion
Fungi species are responsible for 70–80% of plant diseases caused by phytopathogenic microorganisms, which reduce the growth and yield of agricultural crops [20,21]. Phytopathogenic fungi release low-molecular-weight secondary metabolites, which produce disease symptoms in vegetable tissues, including chlorosis, necrosis, growth inhibition, and leaf spotting [22]. In particular, B. cinerea is considered the second most important phytopathogenic fungus worldwide due to its widespread distribution, resulting in significant losses during the pre- and post-harvest stages of vegetables and fruits of economic importance [1,6]. Until now, synthetic fungicides have demonstrated the greatest efficiency in controlling phytopathogenic fungi, but their adverse effects on the ecosystem and human health limit their application [21]. Studies on formulating products based on natural molecules have recently increased. In this context, the application of EO has emerged as a sustainable alternative to reduce the infection of B. cinerea and the application of synthetic fungicides due to it is generally recognized as safe (GRAS) [23]. Studies have shown the efficient antifungal activity of EO extracted from plants such as thyme, oregano, clove, lavender, rosemary, and eucalyptus [24,25]. However, studies comparing the efficiency of EO of citrus species in inhibiting the growth of B. cinerea are scarce even though they have been reported as an important by-product of citrus processing due to their several biological activities, including broad-spectrum antimicrobial, anti-viral, anti-insecticidal, anti-inflammatory, anti-cancer, and immunomodulatory, among others [26]. The effects of EOs from citrus species have been reported in fungal species, including Penicillium italicum, Penicillium expansum, B. cinerea, Geotrichum citri-aurantii, and Pyricularia oryzae, among others [27,28]. Based on the above, this study was focused on evaluating EOs from the peel of citrus species, prospecting the potential physiological mechanisms and nanoencapsulation techniques to apply in agricultural systems.
The genus Citrus, belonging to the Rutaceae family, consists of various species commonly known as mandarins, oranges, lemons, and pomelos. These species are categorized differently under different classification systems. Swingle’s classification identified 16 distinct species, while Tanaka’s horticultural classification recognized a more extensive 162 species [29,30]. Despite its complicated phylogeny situation, citrus peel is a by-product with valuable applications due to its rich chemical composition. The potential of citrus peel lies mainly in EOs, which are characterized by their strong and pleasant aroma and are used in various foods, beverages, and pharmaceuticals [31]. The chemical composition of Citrus EOs has been extensively studied for the last decades [32]. These EOs are complex mixtures of volatile organic compounds rich in monoterpene and sesquiterpene hydrocarbons [33]. Particularly, the EOs obtained from citrus peel always show limonene, a monoterpene hydrocarbon, as the most abundant compound, generally representing about 60–95% of the oil [32]. The EOs analyzed in the present study were no exception. Here, limonene was also the most abundant compound in all analyzed Citrus EOs, representing 66.6%, 59.7%, 98.5%, and 95.2% of the C. reticulata (Mandarin), C. limon (Lemon), C. paradisi (Grapefruit), and C. sinensis (Sweet orange) EOs, respectively. The latter two oils showed the major content of limonene. Limonene was followed by γ-terpinene (26.3%) in C. reticulata EO, and by β-pinene (21.9%) and γ-terpinene (12.9%) in C. limon EO. It is in accordance with literature where limonene is the most common compound, followed by β-myrcene, 3-carene, α-pinene and β-pinene, γ-terpinene, linalool, β-terpineol, and β-citronellol [32].
Comparing among species, Dosoky and Setzer [26] reported that the peel of mandarin fruits contains mainly monoterpenes hydrocarbons, accounting for 86.62% of the oil, and lower than levels detected in other Citrus fruits like sweet orange (83.9–95.9%), bitter orange (89.7–94.7%), and grapefruit (84.8–95.4%). In the present study, C. reticulata EO contained 99.4% of monoterpene hydrocarbons, like other Citrus fruits. Moreover, monoterpenes hydrocarbons in the peel of lemon, grapefruit, and sweet orange represented 96.0–99.5% of the oils, according to the literature [26].
Particularly to C. reticulata (Mandarin) EO studied here, limonene was the most abundant compound (66.6%), followed by γ-terpinene (26.3%), α-pinene (2.5%), β-pinene, (2.2%), o-cymene (1.1%), and β-myrcene (0.6%). This composition is quite similar to the reported by Dosoky and Setzer [26], where the most prevalent terpene in mandarin was limonene (95%), followed by γ-terpinene (16.4–22.7%), α-pinene (2.0–2.7%), β-pinene, (1.4–2.1%), β-myrcene (1.5–1.8%), and linalool (0.67%). However, the content of any component of EO in mandarin and other fruits may differ depending on the variety, ripening stage, extraction method, and plant structure [34].
In relation to biological activities, the EOs of citrus species studied here had a differential activity to inhibit the mycelial growth of B. cinerea. For example, the EO of C. sinensis did not affect mycelial growth, while the EO of C. paradisi stimulated the mycelial growth at concentrations greater than 200 µL L−1. The EO of C. limon and C. reticulata reduced the mycelial growth after 72 and 96 h of exposure. It notes that only EO of C. reticulata at 1000 µL L−1 maintained the reduction of mycelial growth of B. cinerea during the period of evaluation. These results are in concordance with the reported by De-Montijo-Prieto et al. [35], where the EO of C. sinensis, C. limon, and C. paradisi had a low capacity to inhibit mycelial growth (3.4–18.4%). However, this study differs in its antifungal activity, reporting a 3.6% reduction in the mycelial growth of B. cinerea. Additionally, the results are different from those reported by Badawy et al. [36], who evidenced that EO of C. limon and C. sinensis (313–431 mg L−1) had the best antifungal activity against B. cinerea compared to C. paradisi (809 mg L−1) according to effective concentration using 50% growth inhibition (EC50) values. Other studies have shown that concentrations of 11.3% v/v × 10−2 of EO of C. sinensis and C. limon inhibited mycelial growth between 88% and 99% after 3 days of exposure [37]. Furthermore, higher concentrations of EO of C. limon at 20% and 35% reduced mycelial growth by 100% after 7 days of incubation [38]. The volatile exposure to 1.25 µL of EO of C. limon for plates reduced the mycelial growth of B. cinerea by around 70% (minimum inhibitory concentration: 312 µg mL−1) [16]. In summary, the results of mycelial growth inhibition in B. cinerea show a differential behavior compared to other studies, which can be attributed to exposure contact, EO concentration, and the evaluation period. It is noteworthy that the EO of C. reticulata was selected for the following assays because it showed the greatest activity in inhibiting the mycelial growth of B. cinerea. Unfortunately, the selected EO had a low effect on reducing spore germination of B. cinerea, which is in concordance with the finding reported by Badawi et al. [36].
The studies have indicated that inhibiting mycelial growth and spore germination strongly depends on the EO and its chemical composition. Recently, Lin et al. [39] reported that limonene can act as an antifungal agent reducing the growth of many phytopathogen fungi through the damage of cell walls and cell membranes, promoting the production of ROS, altering energy metabolism and respiration, producing DNA damage and the leakage of intracellular contents. However, in the present study, the EOs of C. paradisi and C. sinensis, which presented the highest amount of limonene, were not capable of inhibiting the mycelial growth of B. cinerea. Contrary, EOs from C. reticulate and C. limon inhibited mycelial growth. These EOs were constituted by lesser amounts of limonene but also presented γ-terpinene as well as other minor compounds. Additionally, γ-terpinene was absent in C. paradisi and C. sinensis EOs. γ-terpinene was found in the EOs from pharmacologically active plants like Citrus deliciosa Tenore, Origanumonites L., and Protiumicicariba (DC.) Marchand. Due to the liposolubility of γ-terpinene, it can be easily absorbed through biological members [40]. Then, the above would suggest that a combination of γ-terpinene and limonene could be responsible for the inhibition of the mycelial growth of B. cinerea. It is known that the effects of single compounds are not necessarily the same as the whole mixture, the interactions between molecules may lead to antagonistic, additive/non-interactive, or synergistic effects [41]. In this case, limonene mixed with γ-terpinene and other minor compounds in the EO showed a synergistic effect. Moreover, minor compounds also could be involved in antifungal activity. α-Pinene and β-pinene, both constituents of the C. reticulate and C. limon EOs, have shown antifungal activity against other phytopathogens. Lee et al. [42] showed that α-pinene reduced the growth of Colletotrichum gloeosporioides by 100% with exposure to doses of 10, 5, 2.5, and 1.25 µL by increasing the overproduction of ROS, the disruption of the cell membrane, and decreasing the ergosterol content. Furthermore, β-pinene has been reported for its efficient antifungal activity against Rhizoctonia solani (IC50 values of 2.439 and 1.857 µg mL−1) [43]. However, further studies evaluating the antifungal activity of pure terpenes against B. cinerea, considering mixtures of those at different concentrations could be really valuable to elucidate the antifungal mechanisms.
Notwithstanding the above, the presence of monoterpenes in EO has an important role in inhibiting mycelial growth because it increases the lipidic peroxides, including alkoxyl, hydroxyl, and alkoperoxyl radicals [36]. In addition, phenolic compounds (i.e., thymol, eugenol, and carvacrol) contribute to the alteration of function and permeability of cell membrane proteins [44]. Phenols with free -OH group can modify amino acid residues of proteins and interact with protein targets of fungal cells [45]. Terpenes and phenolic compounds enable EO to accumulate in the hydrocarbon molecules of the lipid bilayer of fungal cells, facilitating its entry into fungal cells [10]. The significant reduction in the dry weight of B. cinerea with the exposure to 800 and 1000 µL L−1 of EO of C. reticulata for 7 days supported efficient antifungal activity. Once the EO crosses the cell membrane, it can alter various physiological and metabolic functions, thereby reducing the growth of the phytopathogenic fungi. In this sense, this study determined the electrical conductivity and pH of cultures of B. cinerea exposed to different concentrations of EO of C. reticulata as indirect parameters to analyze cell membrane permeability. The fungal cell membrane maintains cellular and molecular functions to ensure adequate homeostasis and protection for survival [45,46]. Therefore, alterations in the fluidity and integrity of the fungi cell membrane respond to the initial adverse impact of C. reticulata EO to trigger the alteration in intracellular compartments.
The extracellular pH and conductivity evidenced that treatments of 800 and 1000 µL L−1 of EO of C. reticulata produce an alteration in the membrane cell of B. cinerea, which is attributed to an intracellular leakage of Mg2+, K+, and Ca2+ [47]. Pavoni et al. [48] reported that an increase in extracellular pH in fungi cultures is due to an enhancement of ionic permeability and alteration of osmotic pressure. Otherwise, the increase in electrical conductivity can be attributed to alterations in the cell membrane and the release of intracellular content [49,50]. The increase of these parameters is in concordance with the results obtained with the exposure of B. cinerea cultures to EO of Litsea cubeba, O. vulgare, and T. vulgaris [51,52]. Additionally, the cell membrane integrity of B. cinerea was evaluated by measuring the release of cellular material, as indicated by an OD260nm absorption and soluble proteins [50]. The release of intracellular material or the leakage of cytoplasmic content is attributed to an imbalance in intracellular osmotic pressure resulting from alterations in cell membrane permeability [24]. The exposure of B. cinerea cultures to high concentration of EO of C. reticulata increased the release of intracellular constituents, confirming the disruption of cell membrane integrity after 2 h. Both parameters are indicators of severe damage in fungi cell membranes, supporting the efficient antifungal activity of EO of C. reticulata. These results are attributed to an imbalance in osmotic pressure between the extracellular and intracellular compartments due to the loss of permeability, which was evidenced by increased pH and electrical conductivity in cultures of B. cinerea [53]. The results showed that the EO of C. reticulata 1000 µL L−1 has the best antifungal activity against B. cinerea through the alteration of the cell membrane permeability and the release of cytoplasmic content. Therefore, this concentration was used to investigate the potential mechanisms of action triggered by the EO of C. reticulata in inhibiting B. cinerea.
Propidium iodide was applied to determine the hyphae damage of B. cinerea exposed to EO, where the dye binds to double-stranded DNA in dead cells due to the alteration in the cell membrane [54]. Cell membrane damage in B. cinerea increased by 40% with the exposure to EO of C. reticulata, confirming the alteration of cell membrane integrity. Similar results were obtained with the exposure of B. cinerea cells to vapor release of EO of Cymbopogon citratus, T. vulgaris, and O. heracleoticum at 50 µL L−1 [55]. In addition, the EO of Solidago canadensis L. at 16.5 mL L−1 produced the same impact on the cell membrane integrity of B. cinerea [56]. Additionally, Calcein AM was applied to B. cinerea cultures exposed to EO of C. reticulata to determine cell viability through esterase activity. Calcein AM is a cell-penetrating fluorescent dye used to determine cell viability in eukaryotic cells. Non-fluorescent calcein AM is converted to green fluorescent calcein in living cells, which is followed by the hydrolysis of the acetoxymethyl ester by intracellular esterases [57]. The results showed a decrease in intracellular esterase activity of ~ 50% in cultures of B. cinerea exposed to the EO. This result is in concordance with the exposure of B. cinerea to EO of T. vulgaris and O. vulgare in the range concentration from 300 to 500 µL L−1 [52].
Previous studies have demonstrated that EO leads to abnormal metabolisms due to the dysfunction of mitochondrial activity [45]. The results obtained in this study evidenced that 1000 µL L−1 of EO of C. reticulata decreased 57% mitochondrial activity, suggesting a disruption in the respiratory chain and tricarboxylic acid (TCA) cycle pathways. The EO can alter the ATPase and dehydrogenase activities, decreasing the energy production and biochemical reactions in mitochondria [58]. It has been reported that phenolic compounds in the EO cause hyperpolarization and dysfunction of mitochondria, leading to the depletion of adenosine triphosphate (ATP) [59]. This review also indicated that EO can modulate mitochondrial activity by altering mitochondrial REDOX balance, inhibiting mitochondrial enzymes, disrupting mitochondrial membranes, and suppressing oxidative phosphorylation. Previously, Li et al. [60] reported that the EO of tea tree increased the mitochondrial membrane permeability and decreased enzymatic activities of the TCA cycle, including isocitrate dehydrogenase, d-ketoglutarate dehydrogenase, citrate synthetase, malic dehydrogenase, ATPase, and succinate dehydrogenase. In this sense, succinate dehydrogenase activity in B. cinerea exposed to the EO was determined using the 3-(4,5-Dimethylthiazol-2-yl) 2,5-diphenyltetrazolium (MTT) technique. MTT reagent can cross through the cell and mitochondrial inner membranes of viable cells and be reduced to formazan in living cells [61]. The EO of C. reticulata decreased the succinate dehydrogenase activity of B. cinerea by 65%, confirming an alteration in mitochondrial activity due to the alteration of essential enzymes to maintain the cell metabolic activity. Succinate dehydrogenase, also known as complex II, participates in the TCA cycle and electron transport chain, which is also a target enzyme of some chemical fungicides [62]. Specifically, the TCA cycle corresponds to metabolic reactions in which mitochondria generate ATP in organisms that are oxidative [63]. Similarly, the EO of tea tree between 1 and 2 mL L−1 decreased the succinate dehydrogenase activity in B. cinerea [60,64]). Additionally, Hu et al. [58] demonstrated that the EO of Curcuma longa inhibited succinate dehydrogenase and other dehydrogenase activities. In summary, the results obtained in this study evidenced that the EO of C. reticulata had a significant antifungal activity to reduce the growth of B. cinerea by altering physiological functions, including the permeability of cell membrane, the release of intracellular material, the dysfunction in esterase activity, and altering the mitochondrial activity and enzymes related to the TCA cycle.
Once the antifungal action of EO of C. reticulata was proven, its application was prospected through its encapsulation in lipid nanoparticles. The controlled release of EO of C. limon from nanosystems exhibited greater fungicidal potential than that of EO alone [65]. Furthermore, vapor-based citrus EOs offer greater advantages than direct application by reducing toxicity and facilitating application [66]. In this study, we used SLN for the encapsulation of EO of C. reticulata due to its ability to protect and control the release of compounds, according to its interaction with the lipid matrix [18]. Additionally, SLN is characterized by its biodegradability, low production cost, and approval by the Food and Drug Administration (FDA) for its use in biological systems. In this study, SLNs were formulated using Tween 80 and glyceryl tristearate with a high-revolution homogenization and ultrasonication methodology. The incorporation of 1000 µL L−1 of EO of C. reticulata into SLN increased the hydrodynamic size from 263 to 406 nm. On the contrary, Fuentes et al. [67] showed that the encapsulation of EO of Mentha piperita at 500, 700, and 900 µL L−1 reduced the hydrodynamic size. It has been reported that the size of SLN depends on the interaction of the compounds with the lipid matrix [68]. The homogeneity of SLN size was measured by PDI, where values less than 0.1 showed monodispersion, PDI values from 0.1 to 0.4 represent moderate polydispersity, and values higher than 0.4 are highly polydisperse [69]. The SLN samples exhibited a PDI between 0.18 and 0.20, indicating good particle size homogeneity and minimal aggregation in the colloidal solution [70]. Meanwhile, high values of ζ-potential (−27.1 to −29.0 mV) obtained in this study reflected the stability of EO of C. reticulata-loaded SLN due to the high energy barrier and favored good stability.
The antifungal activity against B. cinerea was evaluated once the characterization and stability of SLN were verified. In recent years, studies have demonstrated the efficient antifungal activity of EO released from lipid nanoparticles [71]. In this study, the results supported the controlled release of EO of C. reticulata from SLN exhibited stable antifungal activity during the evaluation period. This result concurs with previous reports from other studies. For example, the EO of cuminum and cyminum incorporated into nanoemulsions had significant antifungal activity against Aspergillus flavus [72]. Furthermore, clove, thyme, cinnamon, and rosemary EO-loaded nano-structured lipid carriers decreased the mycelial growth of Fusarium oxysporum [73]. Similarly, Vakili-Ghartavol et al. [74] showed that the EO of Mentha × piperita L. encapsulated into SLN had a significant antifungal activity against Rhizoctonia solani and Rhizopus stolonifer.
In summary, this study provided important evidence about the significant activity of the EO of C. reticulata to reduce the mycelial growth of B. cinerea compared to the EO of C. sinensis, C. paradisi, and C. limon. The EO of C. reticulata altered the physiological and metabolic activities of B. cinerea, modifying the biological process to reduce mycelial growth. Additionally, the results evidenced that the encapsulation of EO of C. reticulata into SLN can be an eco-friendly alternative to prospect its application in agricultural systems to mitigate the use of synthetic fungicides. However, further studies considering pure compounds and mixtures at different concentrations could be valuable to elucidate the antifungal mechanisms of the EOs against B. cinerea.
4. Materials and Methods
4.1. Characterization of the Essential Oils by GC/MS
The EOs from peels of C. reticulata, C. limon, C. paradisi, and C. sinensis were acquired commercially from Qenkón Aromatherapy® company (Santiago, Chile) (https://qenkon.cl/, accessed on 14 June 2025). The analysis of constituents from these EOs was conducted by gas chromatography/mass spectrometry (GC/MS) using a Thermo Scientific TRACE 1300 Series gas–liquid chromatography (Waltham, MA, USA) coupled to a Thermo Scientific ISQ 7000 single quadrupole mass detector, with an integrated data system (Xcalibur 4.2.47, Thermo Fisher Scientific Inc., Waltham, MA, USA). A 30 m long BPX5 capillary column (0.25 μm film thickness × 0.25 mm inner diameter, SGE Forte, Trajan Scientific and Medical, Ringwood, Victoria, Australia) was used for separation, and He at 1.00 mL min−1 was the carrier gas. The operating conditions included the following: Injector, transfer line, ion source, and detector temperature: 250 °C; oven temperature program: Hold at 40 °C for 1 min. After, it increased to 250 °C at 5 °C min−1, and then maintained for 5 min. The mass spectra were obtained at an ionization voltage of 70 eV. The recording conditions employed a scan time of 1.5 s and a mass range of 30 to 400 amu. The identification of the compounds was carried out by comparison of the mass spectra for each detected compound with those in the NIST ver. 2.0 library database (NIST, Gaithersburg, MD, USA). In others, by comparison of the retention times and the mass spectra for each detected compound with those of standards [75], and by comparison of the calculated retention index with those reported in the literature [76] considering the same type of stationary phase. The retention indices were calculated by means of C9–26 n-alkane standards (100 µg mL−1 in n-hexane) (Sigma-Aldrich, St. Louis, MO, USA) using the equation described by Kovats and Keulemans [77].
4.2. B. cinerea Strain
B. cinerea (CCCT 21.01) was acquired from the Chilean Culture Collection of Type Strains (CCCT-UFRO) belonging to Scientific and Technological Bioresource Nucleus BIOREN at Universidad de La Frontera (Temuco, Chile) and maintained on potato dextrose agar medium (PDA) (Difco® Laboratories, Detroit, MI, USA) at 24 °C ± 2 °C under dark conditions.
4.3. Inhibition of B. cinerea Mycelium Growth
The inhibition of B. cinerea mycelial growth was performed by the Kirby–Bauer methodology. The inoculum of B. cinerea was previously grown on PDA (Difco® Laboratories, Detroit, MI, USA) for 7 days at 25 °C in dark conditions. Then, a fungal disc (5 mm) of mycelium inoculum was extracted from the edge of the colony culture and placed in the center of a Petri dish (Greiner Bio-One, Kremsmünster, Austria) containing PDA amended with treatments. The treatments were solutions of the C. limon, C. paradisi, C. sinensis, and C. reticulata EOs (Qenkón Aromatherapy company, Santiago, Chile) at 200, 400, 600, 800, and 1000 µL L−1 in 0.05% Tween-80 (Difco® Laboratories, Detroit, MI, USA). Petri dishes with PDA containing 0.05% Tween-80 inoculated with a 5 mm disc of fungal mycelium were considered the first control, and inoculated Petri dishes not exposed to EO or 0.05% Tween-80 were the second control. After, the Petri dishes were sealed with Parafilm (Heathrow Scientific®, Vernon Hills, IL, USA) and placed in an incubator (Labwit, Shanghai, China) at 25 °C ± 2 °C for 7 days in dark conditions [67]. Five replicates were considered for each treatment and control.
4.4. Inhibition of Spore Germination of B. cinerea
The inoculum of B. cinerea was previously grown on PDA for 14 days for conidia production. Sterile distilled water (3 mL) was added to the Petri dish, and the fungi culture was gently scraped to obtain the spores. A solution of spores was adjusted to 104–105 conidia mL−1 on a hemocytometer slide under an optical microscope Leica DM5000 integrated with an ICC50 camera (Leica Microsystems, Wetzlar, Germany). Then, 40 µL of spore solution at 104–105 conidia mL−1 was placed on the surface of microscopic slides containing 1 mL of PDA with the selected EO solution at 200, 400, 600, 800, and 1000 µL L−1 in 0.05% Tween-80. The first and second controls described in point 4.3 were also used here. Three slides for each treatment were used and placed in Petri dishes. Next, Petri dishes were sealed with Parafilm® and incubated at 25 °C ± 2 °C under dark conditions. The spore germination was evaluated after 24 h using a Leica DM5000 optical microscope integrated with an ICC50 camera (Leica Microsystems, Wetzlar, Germany). Five focus areas were analyzed for each replicate. The fungal conidia were considered germinated when the germ tube lengths were equal or when multiple germ tubes were derived from the same conidia [78]. Three replicates were implemented for each treatment and control.
4.5. Evaluation of Dry Weight, pH, and Electrical Conductivity to Investigate Potential Alterations in the Cell Membrane
Dry weight, pH, and electrical conductivity were measured to prospect the physiological impact of selected EO on the culture of B. cinerea. For this, 100 μL of conidial solution (104–105 conidia mL−1) was added to 50 mL of potato dextrose broth (PDB) (Difco® Laboratories, Detroit, MI, USA) containing solutions of C. reticulata EO at 200, 400, 600, 800, or 1000 μL L−1 diluted in 0.05% Tween-80. The controls previously described in point 4.3 were also implemented here. Then, treatments and controls were incubated at 25 °C ± 2 °C and 70 rpm on an orbital shaker for 1 week (Labwit, Shanghai, China) for 1 week. Simultaneously, extracellular pH and electrical conductivity measures of the B. cinerea cultures were carried out using a portable PCT-407 multiparameter instrument (EZODO, Taipei, Taiwan) [79]. After, the B. cinerea cultures were centrifuged at 4000 rpm for 15 min and then, supernatants were discarded. The samples were cleaned with deionized water on a vacuum filtration system and then dried at 50 °C for 48 h to determine the dry weight in an analytical balance (Radwag, Radom, Poland) [79]. Three replicates were conducted for each treatment and controls.
4.6. Release of Constituents Absorbing at OD260nm and Soluble Proteins
A solution of 100 μL of spore (104–105 conidia mL−1) was inoculated into 900 μL of PDB containing the C. reticulata EO at 200, 400, 600, 800, or 1000 μL L−1 in 0.05% Tween-80. The controls previously described in point 4.3 were also implemented here. B. cinerea cultures were homogenized at 25 °C ± 2 °C and centrifugated at 70 rpm for 2 h and 4 h in an orbital shaker. Then, the fungi cultures were centrifuged at 10,000 rpm for 5 min. Supernatants were used to measure the absorbed constituents at OD260nm and soluble proteins through Bradford methodology [63,80]. Accordingly, 132 μL of Bradford reagent (Sigma-Aldrich, Steinheim, Germany) was added to 68 μL of fungi spore solution. The bovine serum albumin (Sigma-Aldrich, Steinheim, Germany) standard concentrations from 0 to 2 mg mL−1 were used to perform a calibration curve. The total protein concentration was measured at 595 nm in a spectrophotometer Epoch (Biotek Instrument, Winooski, VT, USA) integrated with the Gen 5 software (version 2.00.18). Three replicates were implemented for each treatment and control.
4.7. Fluorescent Staining to Explore Mitochondrial Activity, Cell Viability, and Esterase Activity of Cultures of B. cinerea
Cultures of B. cinerea with 50 mL of PDB containing a solution of C. reticulata EO at 1000 μL L−1 dissolved in Tween-80 were inoculated with 100 µL of B. cinerea spores at 104–105 conidia mL−1. The controls described in point 4.3 were used here. Cultures of B. cinerea were incubated at 25 °C ± 2 °C in an orbital shaker (200 rpm) for 48 h. The cultures were centrifuged, and then the fungi biomass was washed with phosphate-buffered saline (PBS) 1X (Gibco®, Thermo Fisher Scientific, New York, NY, USA). After, 500 µL of samples for each B. cinerea culture was extracted for fluorescent staining. Samples were incubated in the following conditions: (1) 0.1 µg mL−1 of MitoTracker™ Orange CMTMRos (Invitrogen, Thermo Fisher, Waltham, MA, USA) for 30 min at 30 °C ± 2 °C, (2) 10 µg mL−1 of Propidium iodide (Invitrogen, ThermoFisher, Waltham, MA, USA) for 30 min, and (3) 1 µg mL−1 of Calcein-AM (InvitrogenTM, Thermo Fisher Scientific, USA) [52,81,82]. The intensity of the fluorescence was determined in a multimodal microplate reader model Feyond-a300 (Allsheng Instruments Co., Ltd., Hangzhou, China) using the following excitation and emission wavelengths: 554/575 nm for MitoTracker™ Orange, 535/617 nm for propidium iodide, and 477/520 nm for Calcein-AM. Three replicates were used for each treatment and control.
4.8. Succinate Dehydrogenase Activity
Cultures were performed inoculating 100 μL of B. cinerea spores (104–105 conidia mL−1) in 50 mL of PDB containing the EO of C. reticulata at 1000 μL L−1 in Tween-80. The controls described in point 4.3 were also used here. The B. cinerea cultures were incubated at 25 °C for 72 h and after, centrifuged at 5000 rpm for 10 min. The supernatants were discarded, and spores were collected from the pellet. Next, 300 μL of MTT bromide reagent (ThermoFisher Scientific Inc., Waltham, MA, USA) at 0.5 mg mL−1 in PBS was added to the spores. Fungal spore samples were incubated at 25 °C ± 2 °C and centrifuged at 200 rpm for 4 h under dark conditions. Samples were centrifuged at 5000 rpm for 10 min and 300 μL of isopropanol–hydrochloric acid solution (95:5) was added to the pellet [83]. Each solution was analyzed at 560 nm using a spectrophotometer Epoch (Biotek Instrument, Winooski, VT, USA) integrated with the Gen 5 software (version 2.00.18).
4.9. Formulation and Characterization of SLN Loaded with the EO of C. reticulata
SLNs were formulated using high-shear homogenization followed by the ultrasonication method. The aqueous phase was Tween-80 at 1.5% w v−1 (Difco® Laboratories, Detroit, MI, USA) dissolved in 40 mL of distilled water. The lipid phase was 250 mg of glyceryl tristearate (Sigma-Aldrich®, St. Louis, MO, USA) dissolved in 5 mL of n-hexane (MerckMilipore®, Bedford, MA, USA). Both aqueous and lipid solutions were maintained at 70 °C, and the EO of C. reticulata was applied to the lipid phase to obtain a final concentration at 1000 μL L−1. The aqueous phase was gently added to the lipid phase, and the resulting solution was homogenized at 10,000 rpm for 5 min by an Ultraturrax OV5 (VELP®, Scientifica, Usmate Velate, Italy) and after, sonicated six times for 1 min at 35% amplitude (5 s on/off). The stability of the SLN formulation was confirmed through δ-potential measurements for 3 weeks, with values ranging from −17.7 mV to −25.5 mV. Finally, the formulation of SLN loaded with the EO of C. reticulata was stored at 4 °C in a pharmaceutical refrigerator (Refrigerator, 290 L, HYC-290, Haier®, Qingdao, China) [84]. The hydrodynamic size, polydispersity index (PDI), and charge surface (ζ-potential) were determined by dynamic dispersion of light (DLS) using a Zetasizer Nano ZS90 (Malvern Instruments, Inc., Malvern, UK) [47]. For morphological characterization, a scanning transmission electron microscopy (STEM) (HITACHI SU3500, Hitachi®, Tokyo, Japan) was conducted. To calculate the encapsulation efficiency (EE), 30 mL of SLN solution was centrifuged at 9000 rpm for 15 min at 4 °C to separate the lipid fraction from the aqueous solution. Then, 100 mg supernatant was diluted in 2 mL of distilled water (MerckMilipore, Bedford, MA, 275 USA) and analyzed at 285 nm using an Epoch microplate spectrophotometer (BioTek 276 Instruments, Winooski, VT, USA). A calibration curve was prepared using 50, 100, 200, 250, 277, 500, and 750 μL L−1 of C. reticulata EO dissolved in n-hexane to obtain the regression equation. EE was determined using Equation (1), where Wa is the concentration of EO applied to formulate SLN and Ws is the concentration quantified in the supernatant [84].
4.10. Antifungal Activity of SLN Loaded with the EO of C. reticulata
Fungi mycelia disks of 5 mm in diameter were extracted from the edges of a B. cinerea colony culture growth during 7 days on PDA (Difco® Laboratories, Detroit, MI, USA). The B. cinerea mycelia disk was placed on the surface of the PDA medium supplemented with the C. reticulata EO-loaded SLN at 1000 μL L−1. Untreated plates (without EO-loaded SLN) were considered the first control, and plates with SLN formulation (without EO) were the second control. Then, the plates were sealed with Parafilm (Heathrow Scientific™, Vernon Hills, IL, USA) and incubated at 25 °C ± 2 °C for 4 days.
4.11. Statistical Analysis
All bioassays were performed with three replicates for each treatment and control. The data were subjected to an analysis of variance (ANOVA) and mean separations were performed using a Tukey test (p ≤ 0.05), using Statistix v10 software.
Author Contributions
Conceptualization, J.E., J.M.F., I.J.-F., G.T., A.Q., M.C.D., O.R. and P.F.; Methodology, S.C., J.E., J.M.F., I.J.-F. and P.F.; Software, G.T. and P.F.; Validation, O.R.; Formal analysis, J.E., G.T., D.N. and P.F.; Investigation, S.C., J.E., I.J.-F., D.N. and P.F.; Resources, J.E., A.Q., M.C.D., O.R. and P.F.; Data curation, G.T., M.C.D., O.R. and P.F.; Writing—original draft, J.E. and P.F.; Writing—review & editing, J.E., G.T., O.R. and P.F.; Visualization, J.E., G.T., O.R. and P.F.; Supervision, G.T., O.R. and P.F.; Project administration, J.E. and P.F.; Funding acquisition, J.E., G.T., O.R. and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID/FONDAP grant number 15130015 and ANID/FONDAP grant number 1523A0001, and the APC was partially funded by Dirección de Investigación—Vicerrectoría de Investigación y Postgrado, VRIP-UFRO.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| EO | Essential oil |
| SLN | Solid lipid nanoparticle |
| GC/MS | Gas–liquid chromatography coupled to mass spectrometry |
| OD | Optical density |
| PDI | Polydispersity index |
| STEM | Scanning transmission electron microscopy |
| GRAS | Generally recognized as safe |
| TCA | Tricarboxylic acid |
| ATP | Adenosine triphosphate |
| MTT | 3- (4,5-Dimethylthiazol-2-yl) 2,5-diphenyltetrazolium |
| FDA | Food and Drug Administration |
| PDA | Potato dextrose agar |
| PDB | Potato dextrose broth |
| PBS | Phosphate-buffered saline |
References
- Dean, R.; van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Dwivedi, M.; Singh, P.; Pandey, A.K. Botrytis fruit rot management: What have we achieved so far? Food Microbiol. 2024, 122, 104564. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing Softly: A Roadmap of Botrytis cinerea Pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Li, T.; Zhou, J.; Li, J. Combined effects of temperature and humidity on the interaction between tomato and Botrytis cinerea revealed by integration of histological characteristics and transcriptome sequencing. Hortic. Res. 2023, 10, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Veloso, J.; Van Kan, J.A.L. Many Shades of Grey in Botrytis–Host Plant Interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea—Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal Activity of Eugenol Derivatives against Botrytis Cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal Activity of Thirty Essential Oils to Control Pathogenic Fungi of Postharvest Decay. Antibiotics 2024, 13, 28. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef] [PubMed]
- Tabti, L.; El Amine Dib, M.; Djabou, N.; Gaouar Benyelles, N.; Paolini, J.; Costa, J.; Muselli, A. Control of fungal pathogens of Citrus sinensis L. by essential oil and hydrosol of Thymus capitatus L. J. Appl. Bot. Food Qual. 2014, 87, 279–285. [Google Scholar]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Simas, D.L.; de Amorim, S.H.; Goulart, F.R.; Alviano, C.S.; Alviano, D.S.; da Silva, A.J.R. Citrus species essential oils and their components can inhibit or stimulate fungal growth in fruit. Ind. Crops Prod. 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Tančinová, D.; Mašková, Z.; Mendelová, A.; Foltinová, D.; Barboráková, Z.; Medo, J. Antifungal Activities of Essential Oils in Vapor Phase against Botrytis cinerea and Their Potential to Control Postharvest Strawberry Gray Mold. Foods 2022, 11, 2945. [Google Scholar] [CrossRef]
- Almawash, S. Solid Lipid Nanoparticles, an Effective Carrier for Classical Antifungal Drugs. Saudi Pharm. J. 2023, 31, 1167–1180. [Google Scholar] [CrossRef]
- Sharma, S.; Mulrey, L.; Byrne, M.; Jaiswal, A.K.; Jaiswal, S. Encapsulation of Essential Oils in Nanocarriers for Active Food Packaging. Foods 2022, 11, 2337. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Rodrigo, S.; García-Latorre, C.; Santamaria, O. Metabolites Produced by Fungi against Fungal Phytopathogens: Review, Implementation and Perspectives. Plants 2022, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Basak, S.; Guha, P. A review on antifungal activity and mode of action of essential oils and their delivery as nano-sized oil droplets in food system. J. Food Sci. Technol. 2018, 55, 4701–4710. [Google Scholar] [CrossRef]
- Taheri, P.; Soweizy, M.; Tarighi, S. Application of Essential Oils to Control Some Important Fungi and Bacteria Pathogenic on Cereals. J. Nat. Pestic. Res. 2023, 6, 100052. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal activity of citrus essential oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef]
- Moura, V.S.; Olandin, L.D.; Mariano, B.S.; Rodrigues, J.; Devite, F.T.; Arantes, A.C.C.; Queiroga, C.L.; Sartoratto, A.; de Azevedo, F.A.; Bastianel, M. Antifungal Activity of Citrus Essential Oil in Controlling Sour Rot in Tahiti Acid Lime Fruits. Plants 2024, 13, 3075. [Google Scholar] [CrossRef]
- Malik, S.K.; Kumar, S.; Singh, I.P.; Dhariwal, O.P.; Chaudhury, R. Socio-economic importance, domestication trends and in situ conservation of wild Citrus species of Northeast India. Genet. Resour. Crop Evol. 2013, 60, 1655–1671. [Google Scholar] [CrossRef]
- Palangasinghe, P.C.; Liyanage, W.K.; Wickramasinghe, M.P.; Palangasinghe, H.R.; Shih, H.-C.; Shiao, M.-S.; Chiang, Y.-C. Reviews on Asian citrus species: Exploring traditional uses, biochemistry, conservation, and disease resistance. Ecol. Genet. Genom. 2024, 32, 100269. [Google Scholar] [CrossRef]
- Vasquez-Gomez, K.L.; Mori-Mestanza, D.; Caetano, A.C.; Idrogo-Vasquez, G.; Culqui-Arce, C.; Auquiñivin-Silva, E.A.; Castro-Alayo, E.M.; Cruz-Lacerna, R.; Perez-Ramos, H.A.; Balcázar-Zumaeta, C.R.; et al. Exploring Chemical Properties of Essential Oils from Citrus Peels Using Green Solvent. Heliyon 2024, 10, e40088. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, R.E.; Sued, N.; Arena, M.E. Citrus paradisi and Citrus reticulata essential oils interfere with Pseudomonas aeruginosa quorum sensing in vivo on Caenorhabditis elegans. Phytomed. Plus 2022, 2, 100160. [Google Scholar] [CrossRef]
- Sanli, I.; Ozkan, G.; Şahin-Yeşilçubuk, N. Green extractions of bioactive compounds from citrus peels and their applications in the food industry. Food Res. Int. 2025, 212, 116352. [Google Scholar] [CrossRef]
- De-Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Brito, C.; Hansen, H.; Espinoza, L.; Faúndez, M.; Olea, A.F.; Pino, S.; Díaz, K. Assessing the Control of Postharvest Gray Mold Disease on Tomato Fruit Using Mixtures of Essential Oils and Their Respective Hydrolates. Plants 2021, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on ‘Golden Delicious’, ‘Pink Lady’ and ‘Granny Smith’ apples. J. Plant Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Zochedh, A.; Priya, M.; Shunmuganarayanan, A.; Thandavarayan, K.; Sultan, A.B. Investigation on structural, spectroscopic, DFT, biological activity and molecular docking simulation of essential oil Gamma-Terpinene. J. Mol. Struct. 2022, 1268, 133651. [Google Scholar] [CrossRef]
- Połeć, K.; Wyżga, B.; Olechowska, K.; Hąc-Wydro, K. On the synergy/antagonism of selected terpenes in the effect on lipid membranes studied in model systems. J. Mol. Liq. 2022, 349, 118473. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, M.-W.; Cho, S.B.; Hwang, K.; Park, I.-K. Antifungal mode of action of bay, allspice, and ajowan essential oils and their constituents against Colletotrichum gloeosporioides via overproduction of reactive oxygen species and downregulation of ergosterol biosynthetic genes. Ind. Crops Prod. 2023, 197, 116684. [Google Scholar] [CrossRef]
- Li, J.; Tian, X.; Gao, Y.; Shang, S.; Feng, J.; Zhang, X. A value-added use of volatile turpentine: Antifungal activity and QSAR study of β-pinene derivatives against three agricultural fungi. RSC Adv. 2015, 5, 66947–66955. [Google Scholar] [CrossRef]
- Allizond, V.; Cavallo, L.; Roana, J.; Mandras, N.; Cuffini, A.M.; Tullio, V.; Banche, G. In Vitro Antifungal Activity of Selected Essential Oils against Drug-Resistant Clinical Aspergillus spp. Strains. Molecules 2023, 28, 7259. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Navarro, B.B.; Canton, L.; Maraschin, M.; Di Piero, R.M. Antifungal activity of palmarosa (Cymbopogon martinii), tea tree (Melaleuca alternifolia) and star anise (Illicium verum) essential oils against Penicillium expansum and their mechanisms of action. LWT-Food Sci. Technol. 2019, 105, 385–392. [Google Scholar] [CrossRef]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 2019, 53, 101101. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Kou, Z.; Zhang, J.; Lan, Q.; Liu, L.; Su, X.; Islam, R.; Tian, Y. Antifungal activity and mechanism of palmarosa essential oil against pathogen Botrytis cinerea in the postharvest onions. J. Appl. Microbiol. 2023, 134, lxad290. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Deng, J.; Liu, X.; Zhou, J.; Li, X. Antibacterial activity of Litsea cubeba essential oil and its mechanism against Botrytis cinerea. RSC Adv. 2019, 9, 28987–28995. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Jofré, I.; Espinoza, J.; Levío-Raimán, M.; Tortella, G.; Oliveira, H.; Diez, M.; Quiroz, A.; Rublar, O. The efficient activity of plant essential oils for inhibiting Botrytis cinerea and Penicillium expansum: Mechanistic insights into antifungal activity. Microbiol. Res. 2023, 277, 127486. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Nidhin, P.T.; Ali, S.M.; Bera, A.; Katoch, M. The antifungal mechanism of Monarda citriodora essential oil, hexanal and their combined vapours on Aspergillus foetidus. Biocatal. Agric. Biotechnol. 2023, 54, 102894. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Rodriguez-Canales, M.; Rodriguez-Monroy, M.A.; Hernandez-Hernandez, A.B.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Cruz-Sanchez, T.; Garcia-Tovar, C.G.; Canales-Martinez, M.M. Effect of the Essential Oils of Bursera morelensis and Lippia graveolens and Five Pure Compounds on the Mycelium, Spore Production, and Germination of Species of Fusarium. J. Fungi 2022, 8, 617. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods 2021, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shao, X.; Wei, Y.; Li, Y.; Xu, F.; Wang, H. Solidago canadensis L. Essential Oil Vapor Effectively Inhibits Botrytis cinerea Growth and Preserves Postharvest Quality of Strawberry as a Food Model System. Front. Microbiol. 2016, 7, 1179. [Google Scholar] [CrossRef]
- Uggeri, J.; Gatti, R.; Belletti, S.; Scandroglio, R.; Corradini, R.; Rotoli, B.M.; Orlandini, G. Calcein-AM is a detector of intracellular oxidative activity. Histochem. Cell Biol. 2000, 122, 499–505. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2007, 220, 1–8. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Zheng, Y.; Chun, H.S. Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics 2022, 11, 1727. [Google Scholar] [CrossRef]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorg. Med. Chem. 2021, 50, 116476. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shao, X.; Li, Y.; Wei, Y.; Xu, F.; Wang, H. Metabolomic Analysis and Mode of Action of Metabolites of Tea Tree Oil Involved in the Suppression of Botrytis cinerea. Front. Microbiol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Li, Z.; Shao, X.; Wei, Y.; Dai, K.; Xu, J.; Xu, F.; Wang, H. Transcriptome analysis of Botrytis cinerea in response to tea tree oil and its two characteristic components. Appl. Microbiol. Biotechnol. 2020, 104, 2163–2178. [Google Scholar] [CrossRef]
- Sedeek, M.S.; Al-Mahallawi, A.M.; Hussien, R.A.A.; Ali, A.M.A.; Naguib, I.A.; Mansour, M.K. Hexosomal Dispersion: A Nano-Based Approach to Boost the Antifungal Potential of Citrus Essential Oils against Plant Fungal Pathogens. Molecules 2021, 26, 6284. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A. Antifungal Activity of the Volatile Phase of Essential Oils: A Brief Review. Nat. Prod. Commun. 2007, 2, 1297–1302. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Jofré, I.; Tortella, G.; Benavides-Mendoza, A.; Diez, M.C.; Rubilar, O.; Fincheira, P. The mechanistic insights of essential oil of Mentha piperita to control Botrytis cinerea and the prospection of lipid nanoparticles to its application. Microbiol. Res. 2024, 286, 127792. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.D.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.-i.; Sakai-Kato, K. Current Status and Challenges of Analytical Methods for Evaluation of Size and Surface Modification of Nanoparticle-Based Drug Formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Bouqellah, N.A.; Abdulmajeed, A.M.; Rashed, A.F.K.; Mattar, E.; Al-Sarraj, F.; Abdulfattah, A.M.; Hassan, M.M.; Baazeem, A.; Al-Harthi, H.F.; Musa, A.; et al. Optimizing encapsulation of garlic and cinnamon essential oils in silver nanoparticles for enhanced antifungal activity against Botrytis cinerea pathogenic disease. Physiol. Mol. Plant Pathol. 2025, 136, 102522. [Google Scholar] [CrossRef]
- Silva, E.F.d.; Santos, F.A.L.d.; Pires, H.M.; Bastos, L.M.; Ribeiro, L.N.d.M. Lipid Nanoparticles Carrying Essential Oils for Multiple Applications as Antimicrobials. Pharmaceutics 2025, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sharmila, J.S.; Golla, G.; Arunachalam, L.; Singh, T.; Karthikeyan, G.; Shabthi, A.; Malaichamy, K. Encapsulation of essential oil to prepare environment friendly nanobio-fungicide against Fusarium oxysporum f.sp. lycopersici: An experimental and molecular dynamics approach. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132681. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M. Synthesis, Characterization, and in Vitro Antifungal Activity of Solid Lipid Nanoparticles Containing Mentha × piperita L. Essential Oil. J. Agric. Sci. Technol. 2024, 26, 667–680. [Google Scholar]
- Espinoza, J.; Soto, I.; Arriagada, J.; Lizama, M.; Aninao, N.; Aniñir, W.; Ungerfeld, E.M.; Chacón-Fuentes, M.; Quiroz, A. Volatile Cues from Fresh Cattle Dung Can Drive Horn Fly Egg-Laying and Fecal Attraction to Horn Flies, Haematobia irritans (Diptera: Muscidae). Insects 2025, 16, 129. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kovats, E.; Keulemans, A.I.M. The Kováts Retention Index System. Anal. Chem. 1964, 36, 31A–41A. [Google Scholar]
- Areco, A.V.; Achimón, F.; Almirón, C.; Nally, C.M.; Zunino, P.M.; Yaryura, P. Antifungal activity of essential oils rich in ketones against Botrytis cinerea: New strategy for biocontrol. Biocatal. Agric. Biotechnol. 2024, 59, 103233. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Pasten, A.; López-Ramírez, L.A.; Mora-Montes, H.M.; Rubio-Astudillo, J.; Silva-Moreno, E.; Castillo, L. Botrytis cinerea PMT4 Is Involved in O-Glycosylation, Cell Wall Organization, Membrane Integrity, and Virulence. J. Fungi 2025, 11, 71. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, X.; Li, L.; Li, D.; Wang, M.; Shi, H. Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish. Chemosphere 2021, 272, 129577. [Google Scholar] [CrossRef]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Jakob, C.A.; Aebi, M.; Tuor, U. The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] Assay Is a Fast and Reliable Method for Colorimetric Determination of Fungal Cell Densities. Appl. Environ. Microbiol. 1999, 65, 3727–3729. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Quiroz, A.; Medina, C.; Tortella, G.; Hermosilla, E.; Diez, M.C.; Rubilar, O. Plant growth induction by volatile organic compound released from solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124739. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).