The Resistance of Germinating Pea (Pisum sativum L.) Seeds to Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Effects of Bio-AgNPs on Seedlings’ Growth
2.2. Effect of Bio-AgNPs on Seedlings’ Viability and Antioxidant System
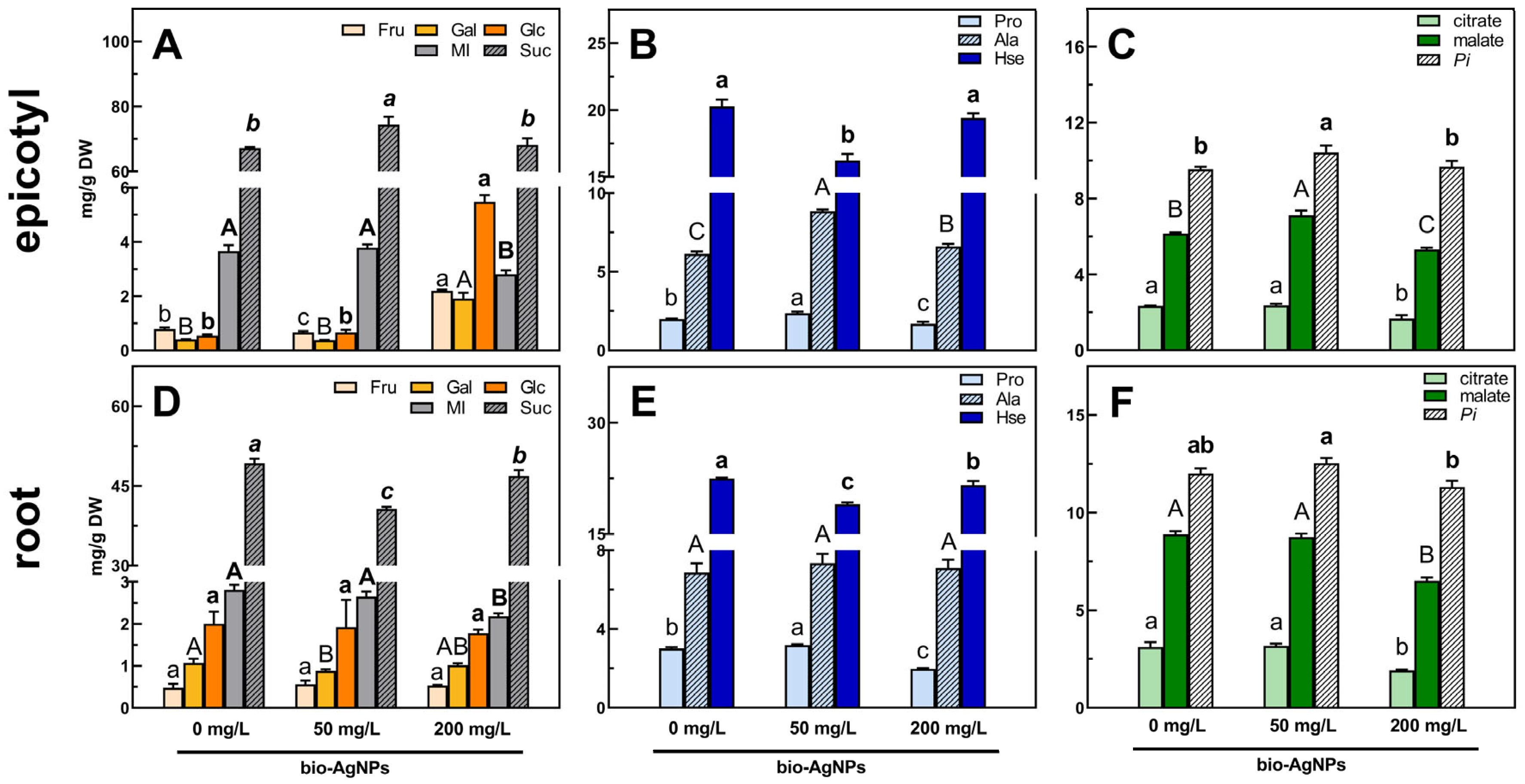
2.3. Polar Metabolites Profiles
2.3.1. Metabolic Profile of Control Seedlings
2.3.2. Changes in Metabolic Profiles After Exposure to Bio-AgNPs
Soluble Carbohydrates
Amino Acids
Organic Acids and Remaining Compounds
3. Discussion
3.1. Species-Specific Effect of Bio-AgNPs on Seedling Growth
3.2. Seedlings’ Viability, ROS Generation, and Antioxidant System
3.3. Bio-AgNPs Affected Metabolic Profiles of Pea Seedlings
4. Materials and Methods
4.1. Biologically Synthesized Silver Nanoparticles
4.2. Plant Material
4.3. Presence of Reactive Oxygen Species and Cell Viability
4.3.1. Fluorescence Staining
4.3.2. Histochemical Staining
4.4. Antioxidant Enzyme Activity
4.5. Total Antioxidant Capacity
4.6. Metabolite Profiling
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bio-AgNPs | Biologically synthesized silver nanoparticles |
| NPs | Nanoparticles |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| GPOX | Guaiacol peroxidase |
| SOD | Superoxide dismutase |
| TEAC | Trolox equivalent antioxidant capacity |
| TAC | Total antioxidant capacity |
| PCA | Principal component analysis |
| TIPMs | Total identified polar metabolites |
| TSCs | Total soluble carbohydrates |
| TAAs | Total amino acids |
| TOAs | Total organic acids |
| TRCs | Total remaining compounds |
References
- Siddiqi, K.S.; Husen, A. Plant response to silver nanoparticles: A critical review. Crit. Rev. Biotechnol. 2022, 42, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mahra, S.; Sharma, S.; Mathew, S.; Sharma, S. Interaction of silver nanoparticles with plants: A focus on the phytotoxicity, underlying mechanism, and alleviation strategies. Plant Nano Biol. 2024, 9, 100082. [Google Scholar] [CrossRef]
- Saleem, S.; Solanki, B.; Khan, M.S. Beyond agrochemicals: Potential of nanoparticles as nanofertilizer and nanopesticide in legumes. Theor. Exp. Plant Physiol. 2025, 37, 7. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green synthesis of nanoparticles and their energy storage, environmental, and biomedical applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Gong, X.; Jadhav, N.D.; Lonikar, V.V.; Kulkarni, A.N.; Zhang, H.; Sankapal, B.R.; Ren, J.; Xu, B.B.; Pathan, H.M.; Ma, Y.; et al. An overview of green synthesized silver nanoparticles towards bioactive antibacterial, antimicrobial and antifungal applications. Adv. Colloid Interface Sci. 2024, 323, 103053. [Google Scholar] [CrossRef]
- Mathur, P.; Chakraborty, R.; Aftab, T.; Roy, S. Engineered nanoparticles in plant growth: Phytotoxicity concerns and the strategies for their attenuation. Plant Physiol. Biochem. 2023, 199, 107721. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant mediated green synthesis of CuO nanoparticles: Comparison of toxicity of engineered and plant mediated CuO nanoparticles towards Daphnia magna. Nanomater. 2016, 6, 205. [Google Scholar] [CrossRef]
- Jassim, A.Y.; Wang, J.; Chung, K.W.; Loosli, F.; Chanda, A.; Scott, G.I.; Baalousha, M. Comparative assessment of the fate and toxicity of chemically and biologically synthesized silver nanoparticles to juvenile clams. Colloids Surf. B Biointerfaces 2022, 209, 18–21. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef] [PubMed]
- Sattar, D.S.; Jalal Ud Din, M.; Sattar, J. The effect of silver nanoparticles on growth, bioactive compounds, antioxidants, scanning electron microscope and functional properties on germination of cowpea beans (Vigna unguiculata (L.) Walp). J. Food Chem. Nanotechnol. 2024, 10, 16–25. [Google Scholar] [CrossRef]
- Koley, R.; Mondal, A.; Mondal, N.K. Green synthesized silver nanoparticles mediated regulation on hydrolytic enzymes and ROS homeostasis promote growth of three pulses (Cicer arietinum L., Pisum sativum L., and Vigna radiata L.). Energy Ecol. Environ. 2023, 8, 537–555. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B.; Stałanowska, K.; Horbowicz, M. The imbibition of pea (Pisum sativum L.) seeds in silver nitrate reduces seed germination, seedlings development and their metabolic profile. Plants 2022, 11, 1877. [Google Scholar] [CrossRef]
- Noori, A.; Hasanuzzaman, M.; Roychowdhury, R.; Sarraf, M.; Afzal, S.; Das, S.; Rastogi, A. Silver nanoparticles in plant health: Physiological response to phytotoxicity and oxidative stress. Plant Physiol. Biochem. 2024, 209, 108538. [Google Scholar] [CrossRef]
- Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Use of Lactobacillus paracasei isolated from whey for silver nanocomposite synthesis: Antiradical and antimicrobial properties against selected pathogens. J. Dairy Sci. 2021, 104, 2480–2498. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Głowacka, K.; Stałanowska, K.; Railean-Plugaru, V.; Hrobowicz, M.; Pomastowski, P.; Buszewski, B. The effect of bio-synthesized silver nanoparticles on germination, early seedling development, and metabolome of wheat (Triticum aestivum L.). Molecules 2022, 27, 2303. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The size-dependent effects of silver nanoparticles on germination, early seedling development and polar metabolite profile of wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef]
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant. 2020, 170, 415–432. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitr. 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Wang, Y.; Ye, W.; Kong, X.; Yin, Z. Small particles, big effects: How nanoparticles can enhance plant growth in favorable and harsh conditions. J. Integr. Plant Biol. 2024, 66, 1274–1294. [Google Scholar] [CrossRef] [PubMed]
- Stałanowska, K.; Railean, V.; Pomastowski, P.; Pszczółkowska, A.; Okorski, A.; Lahuta, L.B. Seeds priming with bio-silver nanoparticles protects pea (Pisum sativum L.) seedlings against selected fungal pathogens. Int. J. Mol. Sci. 2024, 25, 11402. [Google Scholar] [CrossRef]
- Stałanowska, K.; Szablińska-Piernik, J.; Pszczółkowska, A.; Railean, V.; Wasicki, M.; Pomastowski, P.; Lahuta, L.B.; Okorski, A. Antifungal properties of bio-AgNPs against D. pinodes and F. avenaceum infection of pea (Pisum sativum L.) seedlings. Int. J. Mol. Sci. 2024, 25, 4525. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S.; El-Mehasseb, I.M. Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanz. 2020, 72, 113–127. [Google Scholar] [CrossRef]
- Debnath, B.; Sarkar, S.; Das, R. Effects of saponin capped triangular silver nanocrystals on the germination of Pisum sativum, Cicer arietinum, Vigna radiata seeds & their subsequent growth study. IET Nanobiotechnology 2020, 14, 25–32. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-kholy, A.S. Ultrastructural and molecular implications of ecofriendly made silver nanoparticles treatments in pea (Pisum sativum L.). J. Genet. Eng. Biotechnol. 2022, 20, 5. [Google Scholar] [CrossRef]
- Manivannan, A.; Kim, J.H.; Kim, D.S.; Lee, E.S.; Lee, H.E. Deciphering the nutraceutical potential of Raphanus sativus—A comprehensive overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Seilkhan, A.; Ydyrys, A.; Rad, J.S.; Alshehri, M.M.; et al. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid. Med. Cell. Longev. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Plant Sci. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Modlitbová, P.; Hlaváček, A.; Švestková, T.; Pořízka, P.; Šimoníková, L.; Novotný, K.; Kaiser, J. The effects of photon-upconversion nanoparticles on the growth of radish and duckweed: Bioaccumulation, imaging, and spectroscopic studies. Chemosphere 2019, 225, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, J.; Wu, Q.; Huang, Y. Effects of silver nanoparticles on germination and seedling growth of radish (Raphanus sativus L.). In Proceedings of the 2nd International Conference on Civil, Materials and Environmental Sciences, London, UK, 13–14 March 2015; Atlantis Press: Dordrecht, The Netherlands, 2015; pp. 604–608. [Google Scholar]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle exposure and hermetic dose-responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Bazoobandi, A.; Fotovat, A.; Halajnia, A.; Philippe, A. Effect of coating agents of silver nanoparticles on their accumulation in and growth of radish (Raphanus sativus L.). J. Agric. Sci. Technol. 2022, 24, 1473–1485. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of silver nanoparticles with different surface properties on monocots and dicots model plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Huang, X.; Keller, A.A. Dissolution and Aggregation of Metal Oxide Nanoparticles in Root Exudates and Soil Leachate: Implications for Nanoagrochemical Application. Environ. Sci. Technol. 2021, 55, 13443–13451. [Google Scholar] [CrossRef]
- Yang, J.; Duan, H.; Wang, X.; Zhang, H.; Zhang, Z. Effects of Rice Root Exudates on Aggregation, Dissolution and Bioaccumulation of Differently-Charged Ag Nanoparticles. RSC Adv. 2022, 12, 9435–9444. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus-Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Garnczarska, M.; Zalewski, T.; Bednarski, W.; Ratajczak, L.; Jurga, S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J. Plant Physiol. 2006, 163, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Roach, T.; Beckett, R.P.; Whitaker, C.; Minibayeva, F.V. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of reactive oxygen species and mitochondria in seed germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Aro, E.M.; Tiwari, A. True oxygen reduction capacity during photosynthetic electron transfer in thylakoids and intact leaves. Plant Physiol. 2022, 189, 112–128. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Wu, J.; Wang, G.; Vijver, M.G.; Bosker, T.; Peijnenburg, W.J.G.M. Foliar versus root exposure of AgNPs to lettuce: Phytotoxicity, antioxidant responses and internal translocation. Environ. Pollut. 2020, 261, 114117. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Samrot, A.V.; Ram Singh, S.P.; Deenadhayalan, R.; Rajesh, V.V.; Padmanaban, S.; Radhakrishnan, K. Nanoparticles, a double-edged sword with oxidant as well as antioxidant properties—A review. Oxygen 2022, 2, 591–604. [Google Scholar] [CrossRef]
- Jouili, H.; Bouazizi, H.; Ferjani, E. El Plant peroxidases: Biomarkers of metallic stress. Acta Physiol. Plant. 2011, 33, 2075–2082. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Beszterda, M.; Goliński, P. Nonenzymatic antioxidants in plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 201–234. ISBN 9780127999630. [Google Scholar]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnology 2023, 21, 75. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Benson, A.; Thirunavukkarasu, S.; Joe, M.M.; Dhanaraj, S. Pseudomonas plecoglossicida based silver nanoparticles: Characterization, effect on growth and antioxidative enzymes on Pisum sativum L. Agric. Conspec. Sci. 2024, 89, 17–25. [Google Scholar]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.U.R.; Wattoo, F.H.; Hussain, M.; Ejaz, M.; Saira, H. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defense system in wheat (Triticum aestivum L.) under heat stress. IET Nanobiotechnol. 2019, 13, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Szablińska-Piernik, J.; Lahuta, L.B. Changes in polar metabolites during seed germination and early seedling development of pea, cucumber, and wheat. Agriculture 2023, 13, 2278. [Google Scholar] [CrossRef]
- Górecki, R.J.; Fordoński, G.; Halmajan, H.; Horbowicz, M.; Jones, R.G.; Lahuta, L.B.; Horbowicz, M. Seed physiology and biochemistry. In Carbohydrates in Grain Legume Seeds: Improving Nutritional Quality and Agronomic Characteristics; Hedley, C.L., Ed.; CABI Books: Wallingford, UK, 2001; pp. 117–143. ISBN 978-0-85199-467-3. [Google Scholar]
- Blöchl, A.; Peterbauer, T.; Richter, A. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J. Plant Physiol. 2007, 164, 1093–1096. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Goszczyńska, J. Inhibition of raffinose family oligosaccharides and galactosyl pinitols breakdown delays germination of winter vetch (Vicia villosa Roth.) seeds. Acta Soc. Bot. Pol. 2009, 78, 203–208. [Google Scholar] [CrossRef]
- Jahan, I.; Matpan Bekler, F.; Tunç, A.; Güven, K. The effects of silver nanoparticles (AgNPs) on thermophilic bacteria: Antibacterial, morphological, physiological and biochemical investigations. Microorganisms 2024, 12, 402. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R. Sugars play a critical role in abiotic stress tolerance in plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress in Plants; Wani, S.H., Ed.; Elsevier Inc.: London, UK, 2018; pp. 17–38. ISBN 9780128130667. [Google Scholar]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Saxena, S.C.; Salvi, P.; Kaur, H.; Verma, P.; Petla, B.P.; Rao, V.; Kamble, N.; Majee, M. Differentially expressed myo-inositol monophosphatase gene (CaIMP) in chickpea (Cicer arietinum L.) encodes a lithium-sensitive phosphatase enzyme with broad substrate specificity and improves seed germination and seedling growth under abiotic stresses. J. Exp. Bot. 2013, 64, 5623–5639. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Pociecha, E.; Gorczyca, A.; Dziurka, M.; Matras, E.; Oćwieja, M. Silver nanoparticles and silver ions differentially affect the phytohormone balance and yield in wheat. Agriculture 2021, 11, 729. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Larson, L.A.; Beevers, H. Amino acid metabolism in young pea seedlings. Plant Physiol. 1965, 40, 424–432. [Google Scholar] [CrossRef]
- Joy, K.W.; Prabha, C. The role of transamination in the synthesis of homoserine in peas. Plant Physiol. 1986, 82, 99–102. [Google Scholar] [CrossRef]
- Melcher, I.M. Homoserine synthesis in dark-grown and light-grown seedlings of Pisum sativum. New Phytol. 1985, 100, 157–162. [Google Scholar] [CrossRef]
- Ta, T.C.; Joy, K.W.; Ireland, R.J. Amino acid metabolism in pea leaves. Plant Physiol. 1984, 74, 822–826. [Google Scholar] [CrossRef][Green Version]
- Jander, G.; Joshi, V. Recent progress in deciphering the biosynthesis of aspartate-derived amino acids in plants. Mol. Plant 2010, 3, 54–65. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signaling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Qiu, T.A.; Gallagher, M.J.; Hudson-Smith, N.V.; Wu, J.; Krause, M.O.P.; Fortner, J.D.; Haynes, C.L. Research highlights: Unveiling the mechanisms underlying nanoparticle-induced ROS generation and oxidative stress. Environ. Sci. Nano 2016, 3, 940–945. [Google Scholar] [CrossRef]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef]
- Gahlowt, P.; Tripathi, D.K.; Singh, S.P.; Gupta, R.; Singh, V.P. GABA in plants: Developmental and stress resilience perspective. Physiol. Plant. 2024, 176, e14116. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, e1862565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; García-Sánchez, F. Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids aspartic acid, glutamic acid and alanine. Front. Plant Sci. 2021, 11, 581234. [Google Scholar] [CrossRef]
- Ma, W.; Fang, X.; Qiu, M.; Hareem, M.; Erden, Z.; Toprak, Ç.C.; Alarfaj, A.A. Mitigating drought stress in fenugreek through synergistic effects of alanine and potassium-enriched biochar. BMC Plant Biol. 2025, 25, 139. [Google Scholar] [CrossRef]
- Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Li, X.; Zhang, M.; Zhang, Z.; Lu, T.; Pan, X.; Qian, H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci. Total Environ. 2018, 644, 1070–1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Baek, K.-H.; Jan, A.T. Soil and mineral nutrients in plant health: A prospective study of iron and phosphorus in the growth and development of plants. Curr. Issues Mol. Biol. 2024, 46, 5194–5222. [Google Scholar] [CrossRef]
- Benabdellah, K.; Ruiz-Lozano, J.M.; Aroca, R. Hydrogen peroxide effects on root hydraulic properties and plasma membrane aquaporin regulation in Phaseolus vulgaris. Plant Mol. Biol. 2009, 70, 647–661. [Google Scholar] [CrossRef]
- Truernit, E.; Bauby, H.; Dubreucq, B.; Grandjean, O.; Runions, J.; Barthélémy, J.; Palauqui, J.C. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 2008, 20, 1494–1503. [Google Scholar] [CrossRef]
- Weiller, F.; Moore, J.P.; Young, P.; Driouich, A.; Vivier, M.A. The Brassicaceae species Heliophila coronopifolia produces root border-like cells that protect the root tip and secrete defensin peptides. Ann. Bot. 2017, 119, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Chen, B.X.; Chen, Z.J.; Gao, Y.T.; Chen, Z.; Liu, J. Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int. J. Mol. Sci. 2017, 18, 110. [Google Scholar] [CrossRef]
- Ruf, M.; Brunner, I. Vitality of tree fine roots: Reevaluation of the tetrazolium test. Tree Physiol. 2003, 23, 257–263. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Mika, A.; Lüthje, S. Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiol. Online 2014, 132, 1489–1498. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Michalska, A.; Ceglinska, A.; Amarowicz, R.; Piskula, M.K.; Szawara-Nowak, D.; Zielinski, H. Antioxidant contents and antioxidative properties of traditional rye breads. J. Agric. Food Chem. 2007, 55, 734–740. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Weckwerth, W. COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 2012, 8, 81–93. [Google Scholar] [CrossRef]
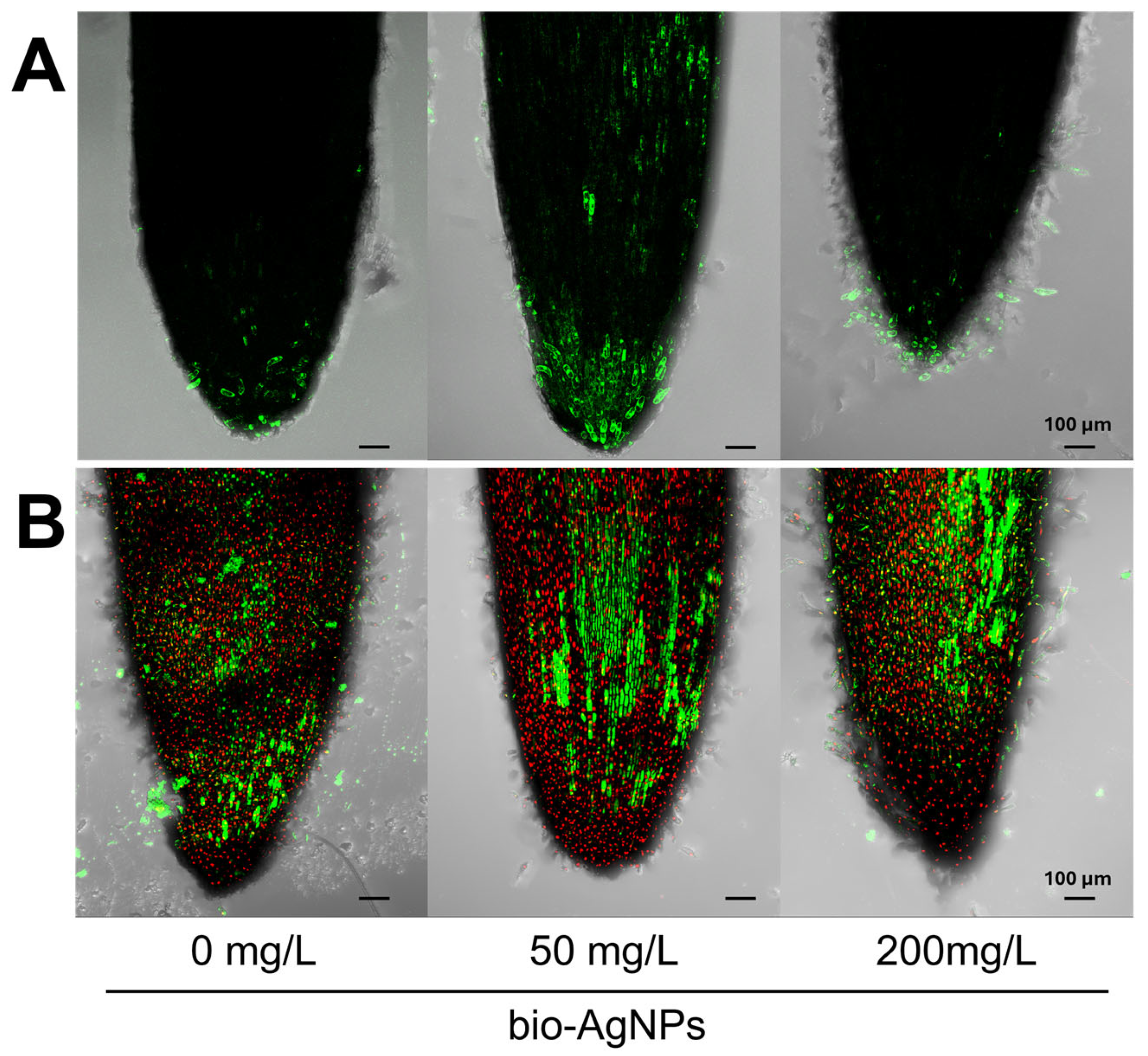


| bio-AgNPs | ||||
|---|---|---|---|---|
| 0 mg/L | 50 mg/L | 200 mg/L | ||
| Length (mm) | R | 24.2 ± 3.5 a | 28.2 ± 2.01 a | 27.9 ± 1.8 a |
| E | 9.8 ± 1.4 a | 9.0 ± 1.1 a | 9.5 ± 0.8 a | |
| Fresh weight (mg) | R | 43.8 ± 2.8 a | 45.2 ± 4.8 a | 48.5 ± 4.0 a |
| E | 36.2 ± 5.4 a | 37.5 ± 2.4 a | 37.6 ± 2.1 a | |
| C | 279.3 ± 8.4 a | 291.6 ± 6.9 a | 283.9 ± 9.7 a | |
| Dry weight (mg) | R | 3.5 ± 0.4 a | 3.9 ± 0.6 a | 4.4 ± 0.4 a |
| E | 3.7 ± 0.4 a | 3.7 ± 0.3 a | 3.9 ± 0.5 a | |
| C | 123.2 ± 0.9 a | 123.6 ± 1.3 a | 113.9 ± 3.6 b | |
| Germinability (%) | 92.2 ± 6.2 a | 93.6 ± 5.6 a | 95.6 ± 3.2 a | |
| bio-AgNPs | ||||
|---|---|---|---|---|
| 0 mg/L | 50 mg/L | 200 mg/L | ||
| CAT (µmol H2O2 min−1 mg−1 protein) | Root | 9.61 ± 0.34 a | 11.29 ± 1.58 a | 10.49 ± 0.33 a |
| Epicotyl | 10.16 ± 1.79 a | 8.58 ± 0.11 a | 9.14 ± 0.77 a | |
| Cotyledons | 2.79 ± 0.18 a | 1.98 ± 0.06 b | 2.03 ± 0.17 b | |
| APX (µmol ASC min−1 mg−1 protein) | Root | 0.16 ± 0.00 b | 0.19 ± 0.00 a | 0.19 ± 0.01 a |
| Epicotyl | 0.21 ± 0.00 a | 0.18 ± 0.00 b | 0.19 ± 0.00 b | |
| Cotyledons | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | |
| GPOX (µmol H2O2 min−1 mg−1 protein) | Root | 0.15 ± 0.01 a | 0.15 ± 0.00 a | 0.16 ± 0.01 b |
| Epicotyl | 0.29 ± 0.00 a | 0.27 ± 0.00 c | 0.28 ± 0.00 b | |
| Cotyledons | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 ab | |
| SOD (U mg−1 protein) | Root | 11.33 ± 0.56 a | 12.90 ± 0.67 a | 11.57 ± 0.70 a |
| Epicotyl | 9.68 ± 1.07 a | 9.59 ± 0.18 a | 10.39 ± 0.16 a | |
| Cotyledons | 2.25 ± 0.09 b | 2.54 ± 0.09 a | 2.20 ± 0.11 b | |
| TAC (µM TEAC g−1 FW) | Root | 1.58 ± 0.06 a | 1.68 ± 0.04 a | 1.60 ± 0.06 a |
| Epicotyl | 2.11 ± 0.02 b | 2.21 ± 0.05 a | 2.20 ± 0.10 ab | |
| Cotyledons | 1.55 ± 0.09 a | 1.74 ± 0.07 a | 1.82 ± 0.11 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stałanowska, K.; Głowacka, K.; Buszewski, B.; Lahuta, L.B. The Resistance of Germinating Pea (Pisum sativum L.) Seeds to Silver Nanoparticles. Plants 2025, 14, 1594. https://doi.org/10.3390/plants14111594
Stałanowska K, Głowacka K, Buszewski B, Lahuta LB. The Resistance of Germinating Pea (Pisum sativum L.) Seeds to Silver Nanoparticles. Plants. 2025; 14(11):1594. https://doi.org/10.3390/plants14111594
Chicago/Turabian StyleStałanowska, Karolina, Katarzyna Głowacka, Bogusław Buszewski, and Lesław Bernard Lahuta. 2025. "The Resistance of Germinating Pea (Pisum sativum L.) Seeds to Silver Nanoparticles" Plants 14, no. 11: 1594. https://doi.org/10.3390/plants14111594
APA StyleStałanowska, K., Głowacka, K., Buszewski, B., & Lahuta, L. B. (2025). The Resistance of Germinating Pea (Pisum sativum L.) Seeds to Silver Nanoparticles. Plants, 14(11), 1594. https://doi.org/10.3390/plants14111594







