Synergistic Alleviation of Saline–Alkali Stress and Enhancement of Selenium Nutrition in Rice by ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase-Producing Serratia liquefaciens and Biogenically Synthesized Nano-Selenium
Abstract
1. Introduction
2. Results
2.1. Screening and Identification of ACC Deaminase-Producing Strain and Biosynthesis of Nano-Selenium
2.2. Physicochemical Characterization of Biogenic Nano-Selenium
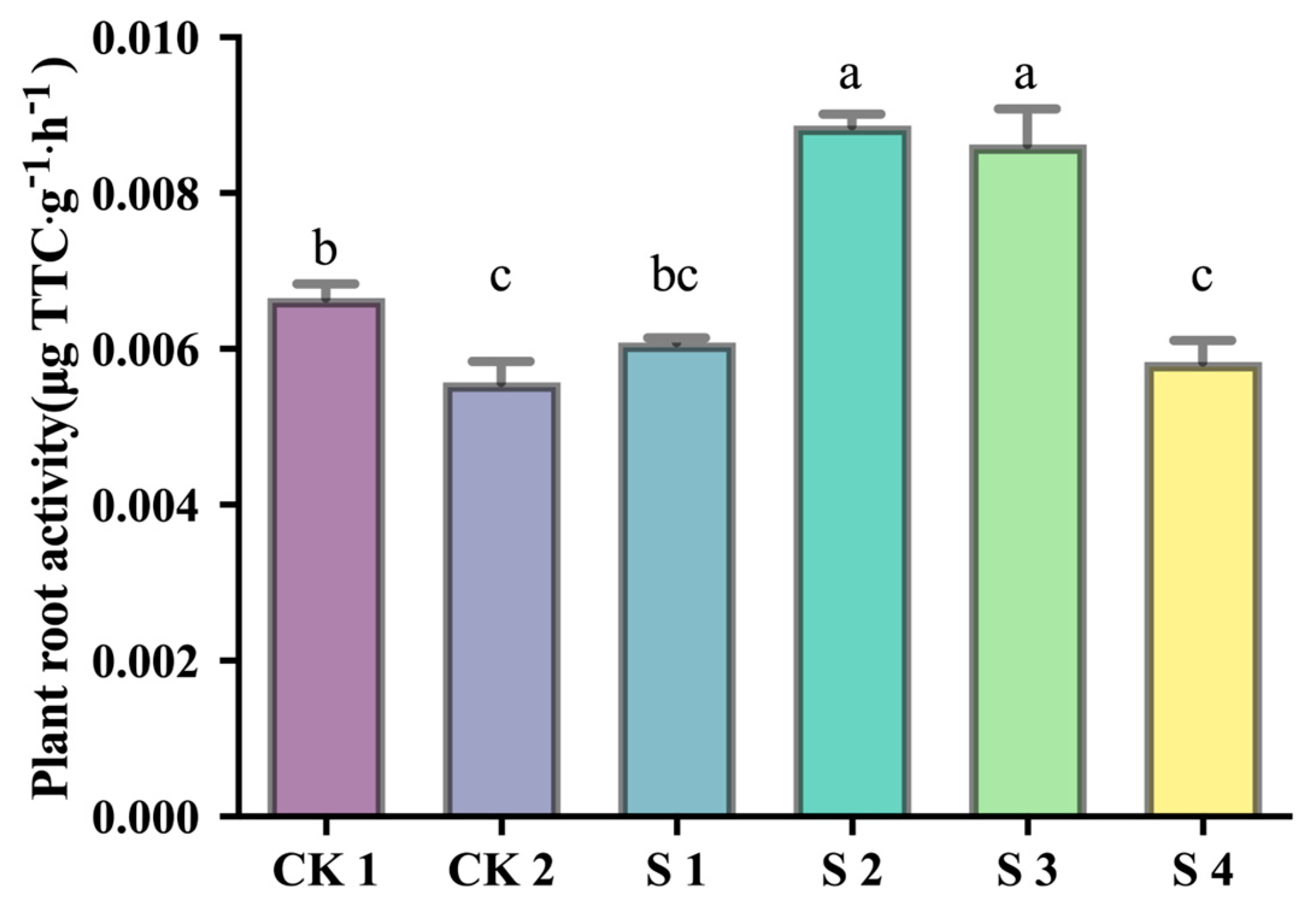
2.3. Effects of Composite Inoculant on Rice Seedling Growth Under Saline–Alkali Stress
2.4. Effects of Compound Fungicide on Rice Yield and Selenium Content in Grains
2.5. Effects of Compound Fungicides on Nutrient Availability in Rhizosphere Soil
2.6. Selenium Accumulation and Translocation in Rice
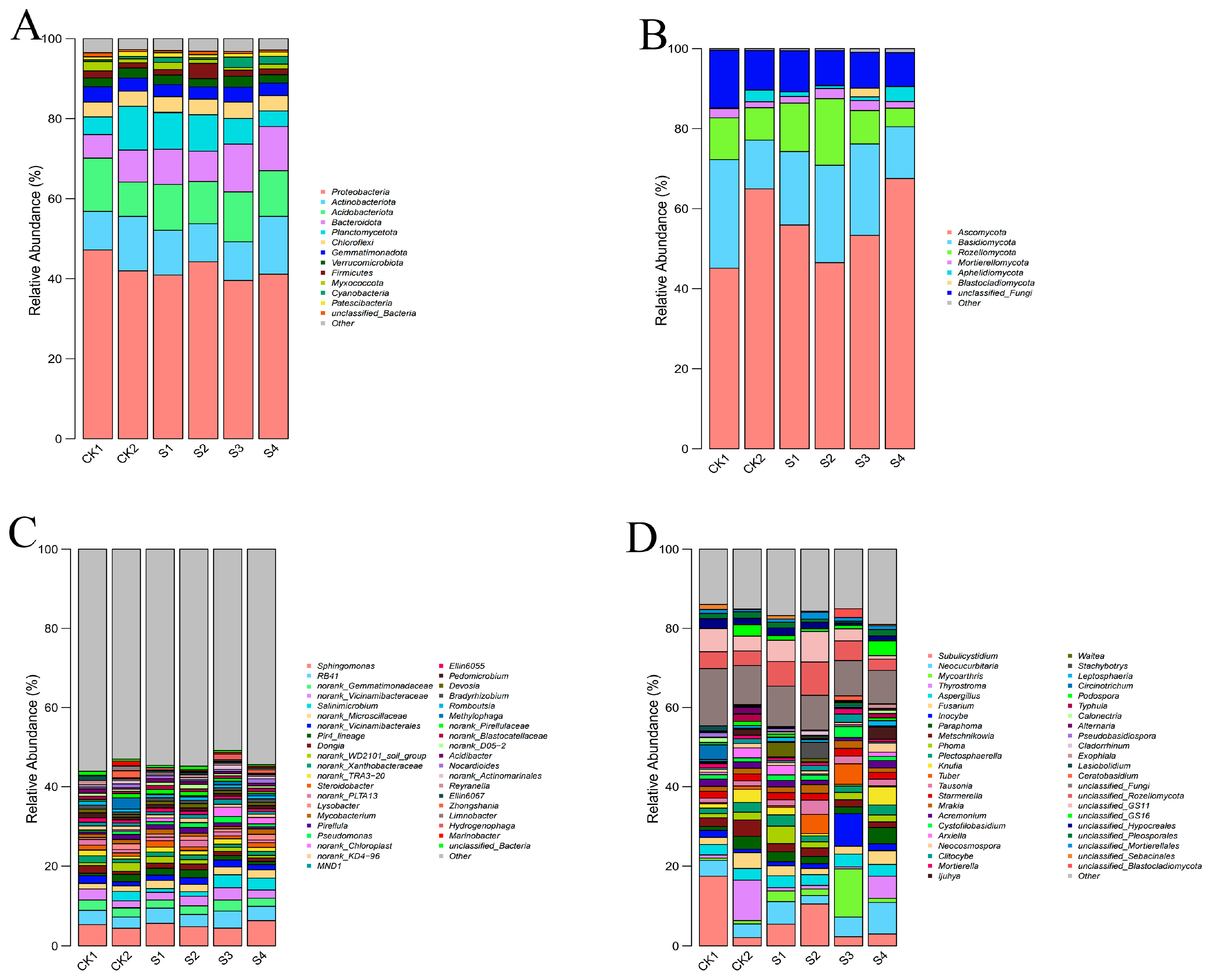
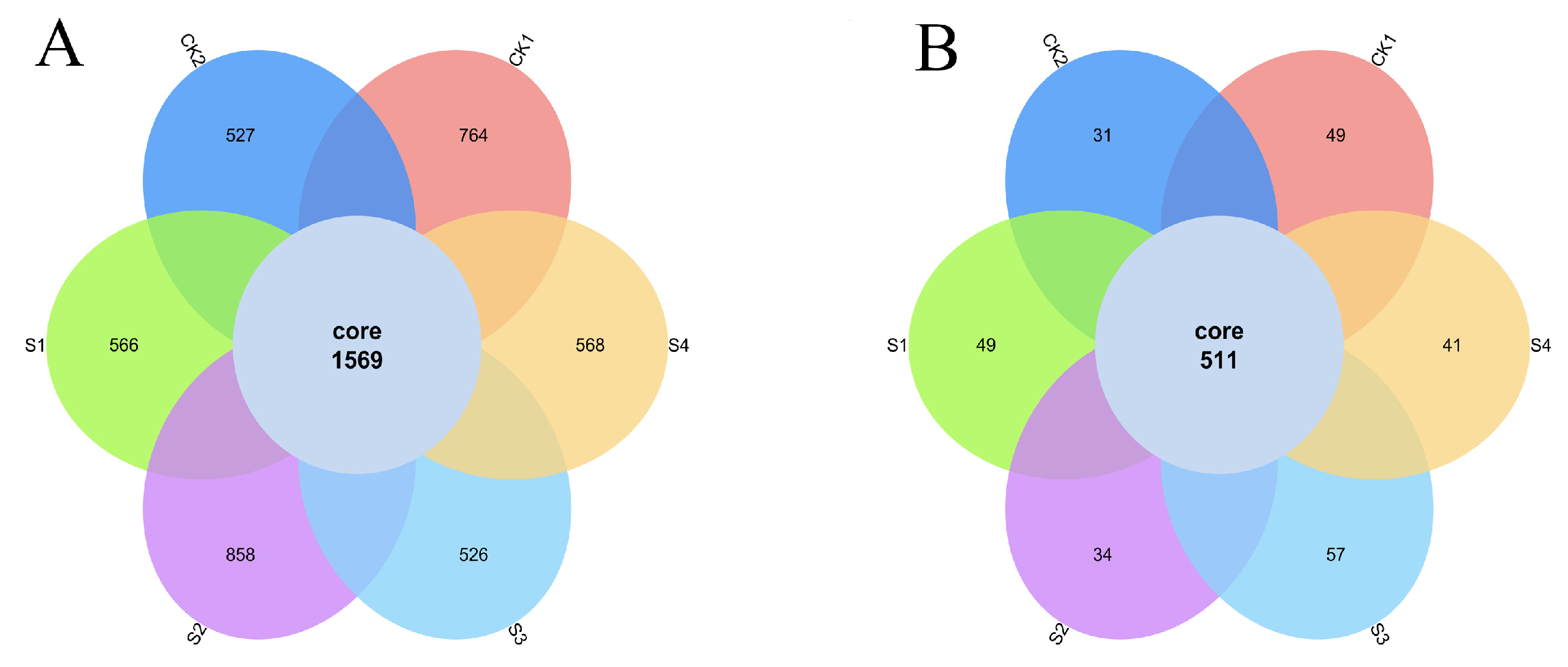
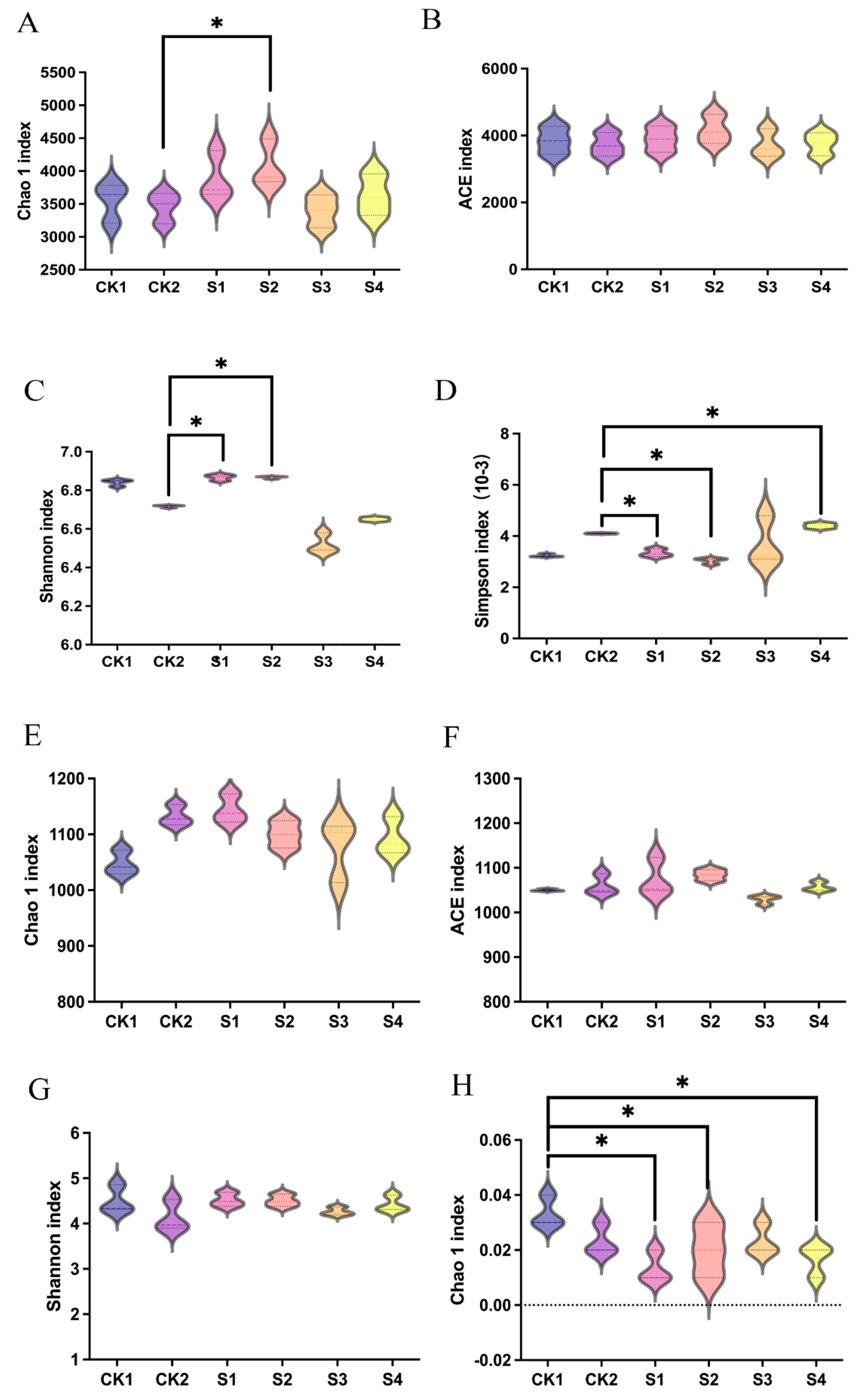
2.7. Effects of Compound Fungicides on Rhizosphere Microbial Community Structure
3. Discussion
3.1. Alleviation of Saline–Alkali Stress by ACC Deaminase Activity of Serratia liquefaciens
3.2. Characteristics of Biogenic Nano-Selenium and Its Synergistic Effect with S. liquefaciens
3.3. Composite Inoculant Enables Safe and Effective Selenium Biofortification in Rice
3.4. Positive Regulation of Soil Nutrients and Microbial Communities by the Composite Inoculant
3.5. Limitations
4. Materials and Methods
4.1. Plant Materials and Soil Preparation
4.2. Screening and Identification of ACC Deaminase-Producing Bacteria
4.2.1. Enrichment and Isolation of Bacterial Strains
4.2.2. Determination of ACC Deaminase Activity
4.2.3. Molecular Identification of the Isolate
4.3. Biosynthesis and Characterization of Nano-Selenium
4.3.1. Biosynthesis of SeNPs
4.3.2. Physicochemical Characterization of SeNPs
4.4. Pot Experiment and Bioformulation
4.4.1. Preparation of Composite Bioinoculant
4.4.2. Pot Trial Design
4.5. Measurement Parameters and Methods
4.5.1. Rice Growth and Yield Measurements
4.5.2. Selenium Content in Plant Tissues
4.5.3. Soil Physicochemical Analysis
4.5.4. Rhizosphere Microbial Community Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Van Der Straeten, D. 1-Aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, Y. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Roy Choudhury, A.; Trivedi, P.; Madhaiyan, M.; Choi, J.; Choi, W.; Park, J.-H.; Walitang, D.I.; Sa, T. ACC deaminase producing endophytic bacteria enhances cell viability of rice (Oryza sativa L.) under salt stress by regulating ethylene emission pathway. Environ. Exp. Bot. 2023, 213, 105411. [Google Scholar] [CrossRef]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing Soil Health and Plant Growth through Microbial Fertilizers: Mechanisms, Benefits, and Sustainable Agricultural Practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Tripathi, A.; Pandey, S.S.; Chanotiya, C.S.; Kalra, A. ACC-Deaminase-Producing Endophyte Brachybacterium paraconglomeratum Strain SMR20 Ameliorates Chlorophytum Salinity Stress via Altering Phytohormone Generation. J. Plant Growth Regul. 2015, 35, 553–564. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.-H.; Zhong, C.; Zhu, L.-F.; Cao, X.-C.; Yu, S.-M.; Allen Bohr, J.; Hu, J.-J.; Jin, Q.-Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef]
- Evans, R.D.; Grochowina, N.M.; Basu, N.; O’Connor, E.M.; Hickie, B.E.; Rouvinen-Watt, K.; Evans, H.E.; Chan, H.M. Uptake of selenium and mercury by captive mink: Results of a controlled feeding experiment. Chemosphere 2016, 144, 1582–1588. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Saxena, S.; Carlson, D.; Billington, R.; Orley, J. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Qual. Life Res. 2001, 10, 711–721. [Google Scholar] [CrossRef]
- Girling, C.A. Selenium in agriculture and the environment. Agric. Ecosyst. Environ. 1984, 11, 37–65. [Google Scholar] [CrossRef]
- Tabibi, M.; Aghaei, S.; Amoozegar, M.A.; Nazari, R.; Zolfaghari, M.R. Characterization of green synthesized selenium nanoparticles (SeNPs) in two different indigenous halophilic bacteria. BMC Chem. 2023, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Lens, P.N.L. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Karle, J.A.; Shrift, A. Use of selenite, selenide, and selenocysteine for the synthesis of formate dehydrogenase by a cysteine-requiring mutant ofEscherichia coli K-12. Biol. Trace Elem. Res. 1986, 11, 27–35. [Google Scholar] [CrossRef]
- von Wintzingerode, F.; Schattke, A.; Siddiqui, R.A.; Rösick, U.; Göbel, U.B.; Gross, R. Bordetella petrii sp. nov., isolated from an anaerobic bioreactor, and emended description of the genus Bordetella. Int. J. Syst. Evol. Microbiol. 2001, 51, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Yadav, S.; Sharma, M.M.M.; Sharma, P. Interaction Between Metal Nanoparticles and PGPR on the Plant Growth and Development. In Nanomaterials and Nanocomposites Exposures to Plants: Response, Interaction, Phytotoxicity and Defense Mechanisms; Springer: Singapore, 2023. [Google Scholar]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; van Diggelen, J.M.H.; Op den Camp, H.J.M.; Visser, E.J.W.; Lucassen, E.C.H.E.T.; Vile, M.A.; Jetten, M.S.M.; Smolders, A.J.P.; Roelofs, J.G.M. Microbial Transformations of Nitrogen, Sulfur, and Iron Dictate Vegetation Composition in Wetlands: A Review. Front. Microbiol. 2012, 3, 156. [Google Scholar] [CrossRef]
- Chawngthu, L.; Hnamte, R.; Lalfakzuala, R. Isolation and Characterization of Rhizospheric Phosphate Solubilizing Bacteria from Wetland Paddy Field of Mizoram, India. Geomicrobiol. J. 2020, 37, 366–375. [Google Scholar] [CrossRef]
- Welsh, D.T. Nitrogen fixation in seagrass meadows: Regulation, plant–bacteria interactions and significance to primary productivity. Ecol. Lett. 2002, 3, 58–71. [Google Scholar] [CrossRef]
- Sheirdil, R.A.; Hayat, R.; Zhang, X.-X.; Abbasi, N.A.; Ali, S.; Ahmed, M.; Khattak, J.Z.K.; Ahmad, S. Exploring Potential Soil Bacteria for Sustainable Wheat (Triticum aestivum L.) Production. Sustainability 2019, 11, 3361. [Google Scholar] [CrossRef]
- Huang, S.-W.; Chen, X.; Wang, D.-D.; Jia, H.-L.; Wu, L. Bio-reduction and synchronous removal of hexavalent chromium from aqueous solutions using novel microbial cell/algal-derived biochar particles: Turning an environmental problem into an opportunity. Bioresour. Technol. 2020, 309, 123304. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Alharbi, K.; Hafez, E.M.; Omara, A.E.-D.; Rashwan, E.; Alshaal, T. Zinc oxide nanoparticles and PGPR strengthen salinity tolerance and productivity of wheat irrigated with saline water in sodic-saline soil. Plant Soil 2023, 493, 475–495. [Google Scholar] [CrossRef]
- Xia, I.F.; Kong, H.-K.; Wu, M.M.H.; Lu, Y.; Wong, K.-H.; Kwok, K.W.H. Selenium Nanoparticles (SeNPs) Immunomodulation Is More Than Redox Improvement: Serum Proteomics and Transcriptomic Analyses. Antioxidants 2022, 11, 964. [Google Scholar] [CrossRef]
- Tang, C.; Li, S.; Zhang, K.; Li, J.; Han, Y.; Zhan, T.; Zhao, Q.; Guo, X.; Zhang, J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Li, Y.; Lin, C.; Yi, D.; Heng, W.; Li, Q.; Tao, L.; Hongjun, Y.; Weijie, J. Selenium in plants: A nexus of growth, antioxidants, and phytohormones. J. Plant Physiol. 2024, 296, 154237. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-Aminocyclopropane-1-carboxylic Acid. Agric. Biol. Chem. 2014, 42, 1825–1831. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ruf, M.; Brunner, I. Vitality of tree fine roots: Reevaluation of the tetrazolium test. Tree Physiol. 2003, 23, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Belzile, N. High performance liquid chromatography coupled to atomic fluorescence spectrometry for the speciation of the hydride and chemical vapour-forming elements As, Se, Sb and Hg: A critical review. Anal. Chim. Acta 2010, 671, 9–26. [Google Scholar] [CrossRef] [PubMed]
- GB/T 22923-2008; Determination of Nitrogen Phosphorus Potassium for Fertilizers by Auto Analyzer. State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine: Beijing, China; Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Agronomy Monographs; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 5. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium, and potassium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 9. [Google Scholar]



| Treatment | Shoot Fresh Weight (g/plant) | Shoot Dry Weight (g/plant) | Plant Height (cm) |
|---|---|---|---|
| CK1 | 0.22 ± 0.02 a | 0.04 ± 0.00 ab | 12.53 ± 0.52 ab |
| CK2 | 0.11 ± 0.01 c | 0.02 ± 0.01 c | 9.93 ± 0.15 e |
| S1 | 0.14 ± 0.01 b | 0.03 ± 0.00 b | 11.50 ± 0.30 cd |
| S2 | 0.18 ± 0.02 b | 0.04 ± 0.00 b | 12.07 ± 0.32 bc |
| S3 | 0.23 ± 0.03 a | 0.05 ± 0.01 a | 13.07 ± 0.57 a |
| S4 | 0.16 ± 0.01 b | 0.04 ± 0.00 b | 10.80 ± 0.62 d |
| Treatment | Panicle Weight (g) | Filled Grains/Panicle | Spikelet Sterility (%) |
|---|---|---|---|
| CK1 | 2.45 ± 0.73 b | 85 ± 18.25 b | 3.18 |
| CK2 | 0.79 ± 0.18 d | 28 ± 7.08 d | 4.21 |
| S1 | 1.42 ± 0.35 c | 48 ± 15.06 c | 2.43 |
| S2 | 2.48 ± 0.80 b | 83 ± 21.14 b | 3.07 |
| S3 | 3.42 ± 0.34 a | 115 ± 13.82 a | 2.93 |
| S4 | 1.09 ± 0.37 cd | 39 ± 15.76 cd | 1.53 |
| Treatment | TN (g/kg) | TP (g/kg) | TK (g/kg) | HN (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|---|
| CK1 | 3.05 ± 0.06 bc | 0.95 ± 0.06 a | 4.21 ± 0.03 c | 279.90 ± 2.02 a | 30.40 ± 0.38 d | 78.17 ± 2.75 ab |
| CK2 | 2.95 ± 0.06 c | 0.81 ± 0.08 b | 1.15 ± 0.04 f | 209.58 ± 0.62 f | 27.00 ± 0.37 e | 74.07 ± 4.25 b |
| S1 | 3.11 ± 0.04 b | 0.85 ± 0.05 ab | 2.33 ± 0.04 d | 234.44 ± 0.75 e | 31.80 ± 1.02 c | 79.03 ± 2.66 ab |
| S2 | 3.07 ± 0.10 bc | 0.86 ± 0.03 ab | 2.04 ± 0.04 e | 259.41 ± 1.55 c | 36.20 ± 0.71 a | 81.01 ± 2.64 a |
| S3 | 3.15 ± 0.04 ab | 0.87 ± 0.04 ab | 4.79 ± 0.07 a | 264.29 ± 0.33 b | 33.40 ± 0.69 b | 75.02 ± 2.00 b |
| S4 | 3.24 ± 0.09 a | 0.79 ± 0.04 b | 4.68 ± 0.05 b | 244.68 ± 1.37 d | 32.00 ± 0.48 c | 75.00 ± 1.00 b |
| Treatment | Root | Stem | Leaf | Grain |
|---|---|---|---|---|
| CK1 | 0.35 ± 0.06 | 0.03 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CK2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| S1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| S2 | 988.76 ± 29.14 | 552.93 ± 10.22 | 394.77 ± 9.37 | 205.04 ± 9.31 |
| S3 | 1636.28 ± 35.85 | 908.02 ± 13.57 | 391.81 ± 8.78 | 234.13 ± 6.61 |
| S4 | 3319.26 ± 8.52 | 1806.10 ± 17.38 | 996.18 ± 12.02 | 628.79 ± 8.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Wei, X.; Pan, X.; Xie, B.; Xin, S.; Song, K. Synergistic Alleviation of Saline–Alkali Stress and Enhancement of Selenium Nutrition in Rice by ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase-Producing Serratia liquefaciens and Biogenically Synthesized Nano-Selenium. Plants 2025, 14, 2376. https://doi.org/10.3390/plants14152376
Zhu N, Wei X, Pan X, Xie B, Xin S, Song K. Synergistic Alleviation of Saline–Alkali Stress and Enhancement of Selenium Nutrition in Rice by ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase-Producing Serratia liquefaciens and Biogenically Synthesized Nano-Selenium. Plants. 2025; 14(15):2376. https://doi.org/10.3390/plants14152376
Chicago/Turabian StyleZhu, Nina, Xinpei Wei, Xingye Pan, Benkang Xie, Shuquan Xin, and Kai Song. 2025. "Synergistic Alleviation of Saline–Alkali Stress and Enhancement of Selenium Nutrition in Rice by ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase-Producing Serratia liquefaciens and Biogenically Synthesized Nano-Selenium" Plants 14, no. 15: 2376. https://doi.org/10.3390/plants14152376
APA StyleZhu, N., Wei, X., Pan, X., Xie, B., Xin, S., & Song, K. (2025). Synergistic Alleviation of Saline–Alkali Stress and Enhancement of Selenium Nutrition in Rice by ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase-Producing Serratia liquefaciens and Biogenically Synthesized Nano-Selenium. Plants, 14(15), 2376. https://doi.org/10.3390/plants14152376






