Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence
Abstract
1. Introduction
2. Results
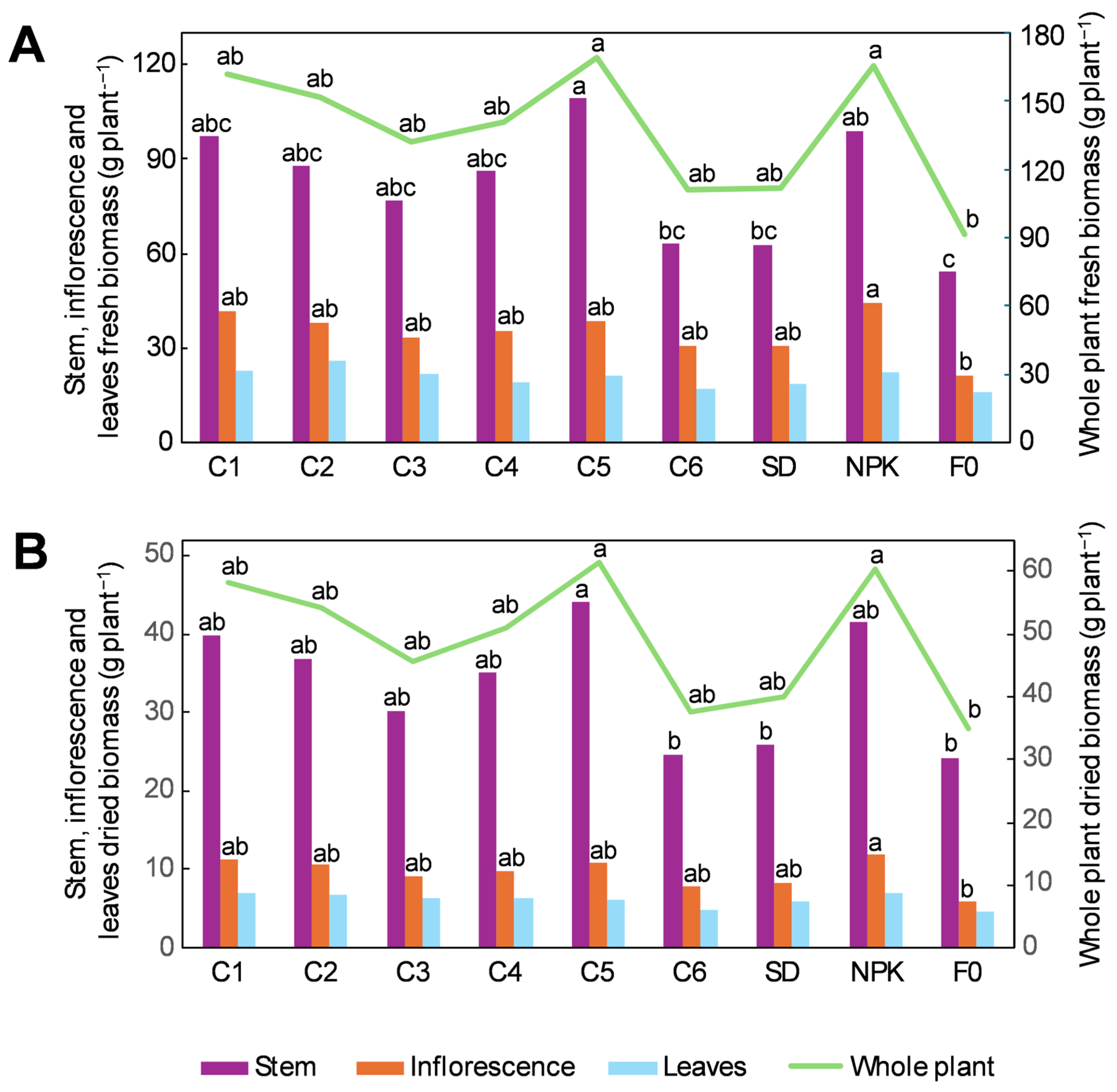
2.1. Morphological Traits and Biomass Accumulation in Hemp Plants Grown Under the Different Fertilization Treatments
2.2. Phytochemical Content in the Inflorescences of Hemp Plants Grown Under the Different Fertilization Treatments
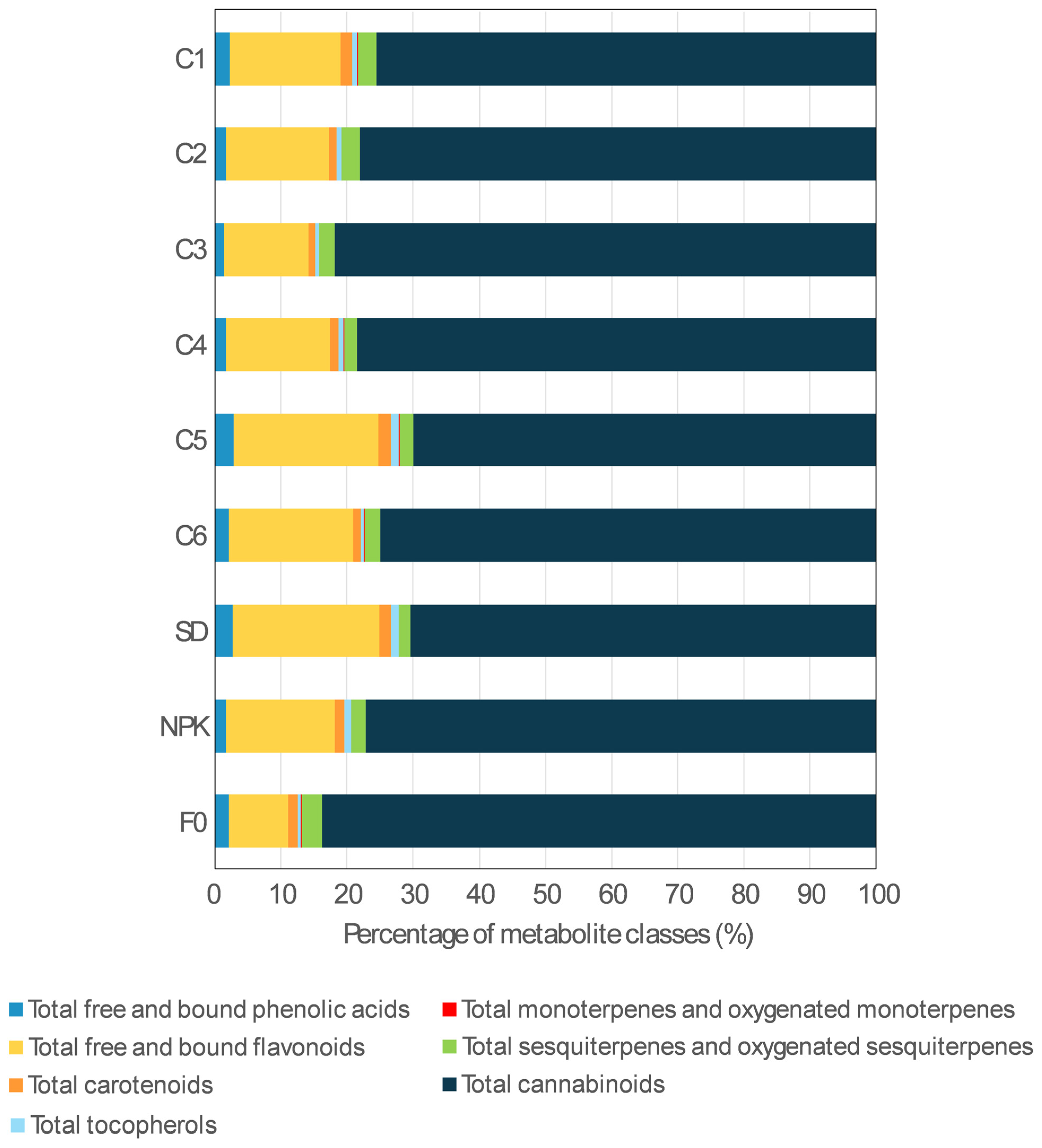
2.2.1. Phytochemical Profile Composition
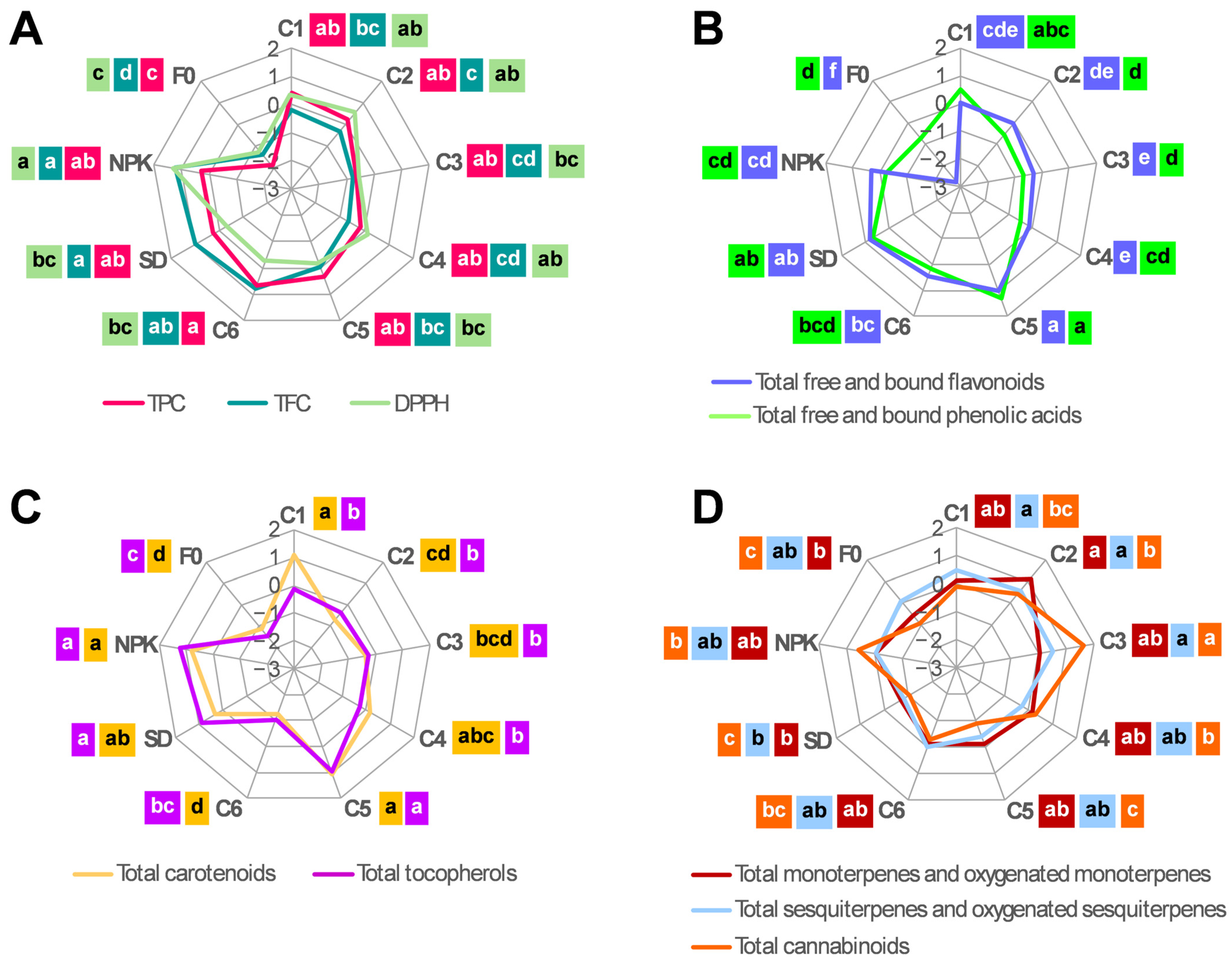
2.2.2. Phytochemical Content
2.3. Phytochemical Yield in Hemp Plants Grown Under the Different Fertilization Treatments
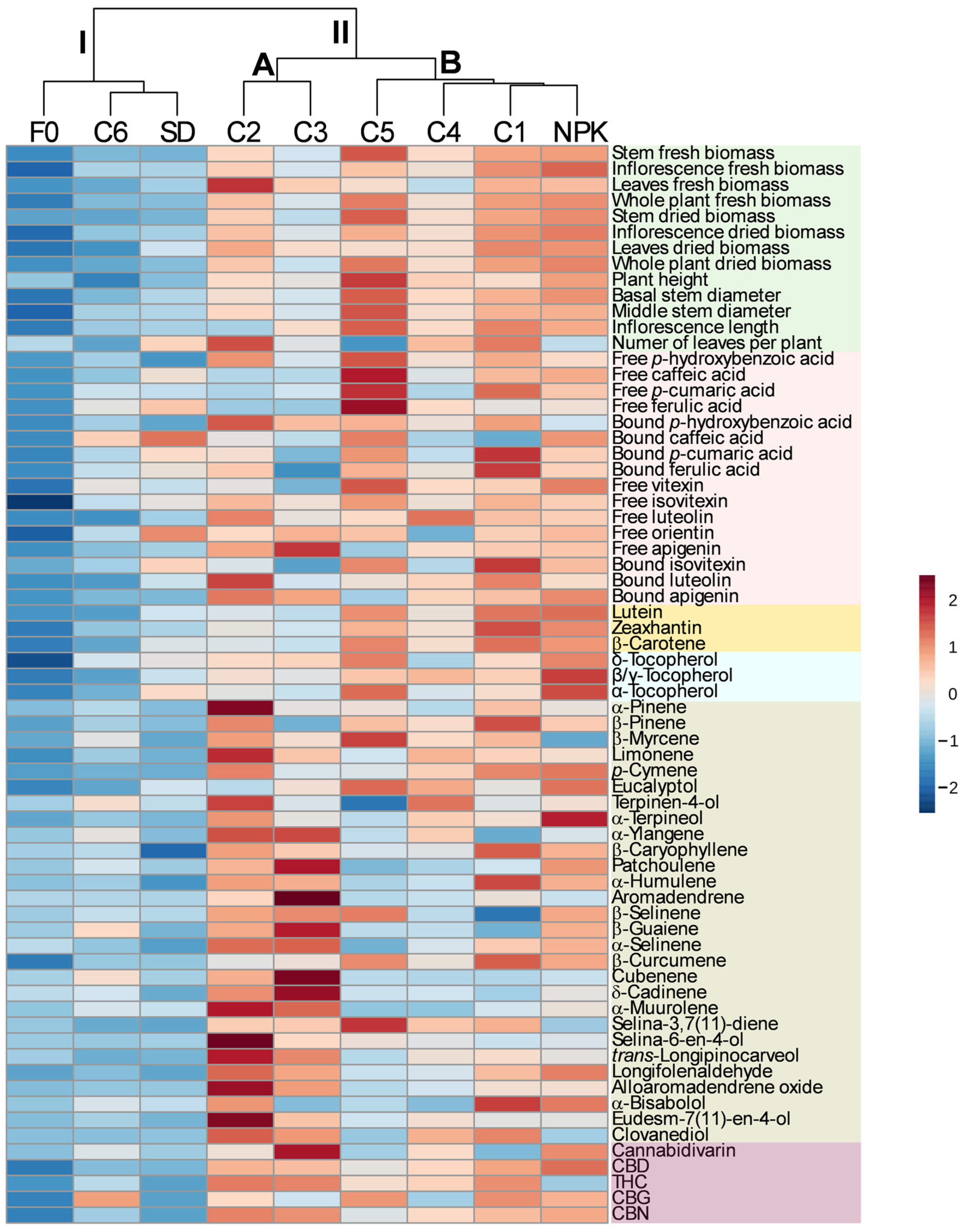
2.4. Multivariate Analysis
3. Discussion
3.1. Effect of the Different Treatments on the Growth and Biomass Accumulation of Hemp Plants
3.2. Effect of the Different Treatments on the Phytochemical Accumulation and Yield in the Inflorescences of Hemp Plants
4. Materials and Methods
4.1. Preparation and Analysis of the Six Composts and the Digestate
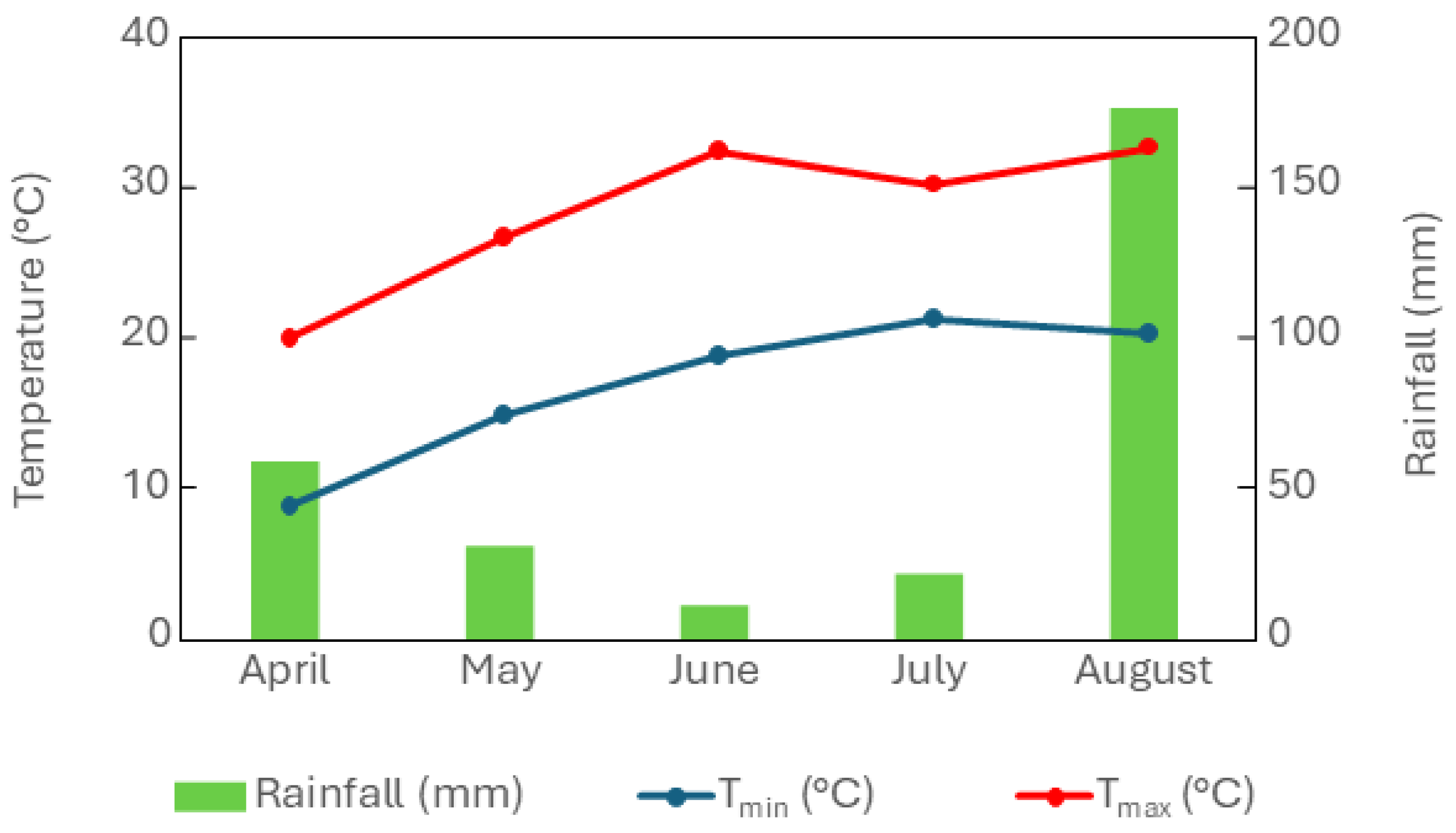
4.2. Plant Material and Experimental Design
4.3. Determination of Morphological Parameters and Biomass
4.4. Spectrophotometric Measurements
4.4.1. Preparation of the Extract
4.4.2. Determination of the Total Phenolic Content and Total Flavonoid Content
4.4.3. Determination of the Total Antioxidant Activity
4.5. HPLC Measurements
4.5.1. Determinations of Free and Bound Phenolic Compounds
4.5.2. Determination of Carotenoids
4.5.3. Determination of Tocopherols
4.6. Determination of Terpenes and Cannabinoids by GC-MS
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialog. Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–474. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial hemp (Cannabis sativa L.) agronomy and utilization: A review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Nath, M.K. Benefits of cultivating industrial hemp (Cannabis sativa ssp. sativa)—A versatile plant for a sustainable future. Chem. Proc. 2022, 10, 14. [Google Scholar]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Trono, D. Elicitation as a tool to improve the accumulation of secondary metabolites in Cannabis sativa. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Ho, T.T.K.; Tra, V.T.; Le, T.H.; Nguyen, N.-K.-Q.; Tran, C.-S.; Nguyen, P.-T.; Vo, T.-D.-H.; Thai, V.-N.; Bui, X.-T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Gurmessa, B.; Cocco, S.; Ashworth, A.J.; Udawatta, R.P.; Cardelli, V.; Ilari, A.; Serrani, D.; Fornasier, F.; Del Gatto, A.; Pedretti, E.F.; et al. Short term effects of digestate and composted digestate on soil health and crop yield: Implications for sustainable biowaste management in the bioenergy sector. Sci. Total Environ. 2024, 906, 167208. [Google Scholar] [CrossRef] [PubMed]
- Oldani, E.; Cabianca, A.; Dahlin, P.; Ruthes, A.C. Biogas digestate as potential source for nematicides. Environ. Technol. Innov. 2023, 29, 103025. [Google Scholar] [CrossRef]
- Bouchtaoui, E.M.; Haouas, A.; Dababat, A.A.; Lahlali, R.; Benali, A.; Fahr, M.; Smouni, A.; Azim, K.; Liu, Z.; Li, J.; et al. Exploring mechanisms of compost-mediated suppression of plant pathogens: A critical review. Appl. Soil Ecol. 2024, 203, 105644. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility, A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Wortman, S.E.; Holmes, A.A.; Miernicki, E.; Knoche, K.; Pittelkow, C.M. First-season crop yield response to organic soil amendments: A meta-analysis. Agron. J. 2017, 109, 1210–1217. [Google Scholar] [CrossRef]
- Oyetunji, O.; Bolan, N.; Hancock, G. A comprehensive review on enhancing nutrient use efficiency and productivity of broadacre (arable) crops with the combined utilization of compost and fertilizers. J. Environ. Manag. 2022, 317, 115395. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidou, C.; Mola, M.; Papakostas, S.; Aschonitis, V.G.; Monokrousos, N.; Kougias, P.G. The effect of anaerobic digestate as an organic soil fertilizer on the diversity and structure of the indigenous soil microbial and nematode communities. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Ronga, D.; Pellati, F.; Brighenti, V.; Laudicella, K.; Laviano, L.; Fedailaine, M.; Benvenuti, S.; Pecchioni, N.; Francia, E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha x piperita L.) in hydroponics. J. Appl. Res. Med. Aromat. Plants 2018, 11, 18–26. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Younis, A.; Abideen, Z.; Naveed, M.; Koyro, H.-W.; Siddique, K.H.M. Biochar, compost, and biochar–compost blend applications modulate growth, photosynthesis, osmolytes, and antioxidant system of medicinal plant Alpinia zerumbet. Front. Plant Sci. 2021, 12, 707061. [Google Scholar] [CrossRef] [PubMed]
- Khater, R.M.R.; Sabry, R.M.; Pistelli, L.; Abd-ElGawad, A.M.; Soufan, W.; El-Gendy, A.N.G. Effect of compost and titanium dioxide application on the vegetative yield and essential oil composition of coriander. Sustainability 2022, 14, 322. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Velechovský, J.; Malík, M.; Kaplan, L.; Tlustoš, P. Application of individual digestate forms for the improvement of hemp production. Agriculture 2021, 11, 1137. [Google Scholar] [CrossRef]
- Bruce, D.; Connelly, G.; Ellison, S. Different fertility approaches in organic hemp (Cannabis sativa L.) production alter floral biomass yield but not CBD:THC ratio. Sustainability 2022, 14, 6222. [Google Scholar] [CrossRef]
- Kosmidis, S.; Stavropoulos, P.; Kakabouki, I.; Papastylianou, P.; Roussis, I.; Mavroeidis, A.; Beslemes, D.; Bilalis, D. Combined effect of biocompost and biostimulant on root characteristics of Cannabis sativa L. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2023, 80, 47–53. [Google Scholar] [CrossRef]
- Finnan, J.; Burke, B. Potassium fertilization of hemp (Cannabis sativa). Ind. Crop. Prod. 2013, 41, 419–422. [Google Scholar] [CrossRef]
- Deng, G.; Du, G.; Yang, Y.; Bao, Y.; Liu, F. Planting density and fertilization evidently influence the fiber yield of hemp (Cannabis sativa L.). Agronomy 2019, 9, 368. [Google Scholar] [CrossRef]
- Atoloye, I.A.; Adesina, I.; Shahbazi, A.; Bhowmik, A. Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization. Open Agric. 2022, 7, 373–381. [Google Scholar] [CrossRef]
- James, M.S.; Vann, M.C.; Suchoff, D.H.; McGinnis, M.; Whipker, B.E.; Edmisten, K.L.; Gatiboni, L.C. Hemp yield and cannabinoid concentrations under variable nitrogen and potassium fertilizer rates. Crop Sci. 2023, 63, 1555–1565. [Google Scholar] [CrossRef]
- Duong, T.; Penfold, C.; Marschner, P. Amending soils of different texture with six compost types: Impact on soil nutrient availability, plant growth and nutrient uptake. Plant Soil 2012, 354, 197–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, Y.; Hu, Y.; Christie, P.; Zhang, J.; Li, X. Maize yield and soil fertility with combined use of compost and inorganic fertilizers on a calcareous soil on the North China Plain. Soil Tillage Res. 2016, 155, 85–94. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Towards a more sustainable fertilization: Combined use of compost and inorganic fertilization for tomato cultivation. Agric. Ecosyst. Environ. 2014, 196, 178–184. [Google Scholar] [CrossRef]
- Biswas, S.S.; Ghosh, A.; Singhal, S.K.; Biswas, D.R.; Roy, T.; Sarkar, A.; Das, D. Phosphorus enriched organic amendments can increase nitrogen use efficiency in wheat. Commun. Soil Sci. Plant Anal. 2019, 50, 1178–1191. [Google Scholar] [CrossRef]
- Sieracka, D.; Frankowski, J.; Wacławek, S.; Czekała, W. Hemp biomass as a raw material for sustainable development. Appl. Sci. 2023, 13, 9733. [Google Scholar] [CrossRef]
- Chaoui, H.I.; Zibilske, L.M.; Ohno, T. Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biol. Biochem. 2003, 35, 295–302. [Google Scholar] [CrossRef]
- Butler, T.J.; Han, K.J.; Muir, J.P.; Weindorf, D.C.; Lastly, L. Dairy manure compost effects on corn silage production and soil properties. Agron. J. 2008, 100, 1541–1545. [Google Scholar] [CrossRef]
- van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; de Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Skibsted, L.H. Vitamin and non-vitamin antioxidants and their interaction in food. J. Food Drug Anal. 2012, 20, 66. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crop. Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Dalavaye, N.; Nicholas, M.; Pillai, M.; Erridge, S.; Sodergren, M.H. The clinical translation of α-humulene—A scoping review. Planta Med. 2024, 90, 664–674. [Google Scholar] [CrossRef] [PubMed]
- FDA. Substances Added to Food (Formerly EAFUS). Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances (accessed on 7 March 2025).
- O’Sullivan, S.E.; Jensen, S.S.; Nikolajsen, G.N.; Bruun, H.Z.; Bhuller, R.; Hoeng, J. The therapeutic potential of purified cannabidiol. J. Cannabis Res. 2023, 5, 21. [Google Scholar] [CrossRef]
- Springer, U.; Klee, J. Prüfung der leistungsfähigkeit von einigen wichtigeren verfahren zur bestimmung des kohlenstoffs mittels chromschwefelsaure sowie vorschlag einer neuen schnellmethode. J. Plant Nutr. Soil Sci. 1954, 64, 1–26. [Google Scholar] [CrossRef]
- Technicon Industrial Systems, 1973. Ammonium in water and waste water. In Industrial Method; No. 90-70W-B; Revised January 1978; Technicon Industrial Systems: Tarrytown, NY, USA, 1978. [Google Scholar]
- Gazzetta Ufficiale-Serie Generale n. 121 del 26-5-2010. Supplemento Ordinario n. 106. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2010-05-26&atto.codiceRedazionale=010G0096&elenco30giorni=false (accessed on 25 March 2025).
- Mishchenko, S.; Mokher, J.; Laiko, I.; Burbulis, N.; Kyrychenko, H.; Dudukova, S. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemės Ūkio Moksl. 2017, 24, 31–36. [Google Scholar] [CrossRef]
- Iannucci, A.; Beleggia, R.; Galletti, S.; Pecchioni, N.; Trono, D. Impact of seed inoculation with trichoderma afroharzianum strains on plant growth, root morphology, and leaf phenolic content in hemp (Cannabis sativa L.) at early growth stages. Agriculture 2024, 14, 511. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Muy-Rangel, D.; Urías-Orona, V. Chickpea (Cicer arietinum) and soybean (Glycine max) hulls: Byproducts with potential use as a source of high value-added food products. Waste Biomass Valor. 2017, 8, 1199–1203. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsa, R.; Yang, R.; Cui, W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Beleggia, R.; Iannucci, A.; Menga, V.; Quitadamo, F.; Suriano, S.; Citti, C.; Pecchioni, N.; Trono, D. Impact of chitosan-based foliar application on the phytochemical content and the antioxidant activity in hemp (Cannabis sativa L.) inflorescences. Plants 2023, 12, 3692. [Google Scholar] [CrossRef]
- Beleggia, R.; Menga, V.; Fulvio, F.; Fares, C.; Trono, D. Effect of genotype, year, and their interaction on the accumulation of bioactive compounds and the antioxidant activity in industrial hemp (Cannabis sativa L.) inflorescences. Int. J. Mol. Sci. 2023, 24, 8969. [Google Scholar] [CrossRef]
| Treatment a | Plant Height (cm) | Basal Stem Diameter (mm) | Middle Stem Diameter (mm) | Inflorescence Length (cm) | Number of Leavesper Plant (n) |
|---|---|---|---|---|---|
| C1 | 223.6 abc | 12.2 ab | 10.0 ab | 55.9 a | 17.9 |
| C2 | 224.3 abc | 11.6 abc | 9.6 ab | 45.1 ab | 18.3 |
| C3 | 218.3 abc | 11.2 abc | 9.2 abc | 50.3 ab | 16.7 |
| C4 | 227.1 ab | 11.8 abc | 9.5 ab | 50.7 ab | 17.3 |
| C5 | 254.7 a | 13.0 a | 10.8 a | 57.8 a | 15.4 |
| C6 | 184.5 c | 10.4 bc | 8.8 bc | 44.4 ab | 15.6 |
| SD | 199.2 bc | 10.9 abc | 8.9 abc | 45.1 ab | 17.1 |
| NPK | 237.8 a | 12.5 ab | 10.1 ab | 53.8 a | 16.3 |
| F0 | 201.4 bc | 9.6 c | 7.6 c | 38.5 b | 16.3 |
| Class | Metabolite b | Treatment a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | SD | NPK | F0 | ||
| Flavonoids | Free vitexin | 1435.1 cd | 1279.8 d | 821.9 e | 1627.0 bc | 2112.3 a | 1747.2 b | 1383.7 cd | 1825.9 b | 449.3 f |
| Free isovitexin | 1202.0 ab | 1283.5 ab | 1273.5 ab | 1181.7 ab | 1321.9 a | 1253.7 ab | 1346.8 a | 1078.9 b | 405.4 c | |
| Free luteolin | 88.1 b | 116.9 a | 84.0 b | 128.2 a | 84.6 b | 47.6 c | 66.4 bc | 81.0 b | 46.4 c | |
| Free orientin | 985.1 c | 1009.1 c | 1269.8 b | 657.0 d | 1040.3 c | 1051.6 c | 1576.8 a | 1017.0 c | 569.2 d | |
| Free apigenin | 58.1 | 68.3 | 82.5 | 57.9 | 35.5 | 45.5 | 45.7 | 49.8 | 41.6 | |
| Bound isovitexin | 48.2 a | 29.5 cd | 18.0 d | 26.8 cd | 44.5 ab | 32.5 bc | 47.0 a | 35.1 abc | 32.5 bc | |
| Bound luteolin | 14.2 ab | 17.9 a | 10.4 bc | 12.8 b | 10.2 bc | 6.3 c | 11.4 b | 9.6 bc | 6.5 c | |
| Bound apigenin | 8.5 bcd | 11.3 ab | 12.1 a | 9.5 abc | 6.0 d | 6.0 d | 6.8 cd | 9.1 abcd | 6.8 cd | |
| Phenolic acids | Free p-hydroxybenzoic acid | 80.5 | 91.3 | 85.5 | 82.8 | 93.8 | 84.4 | 64.6 | 71.5 | 91.5 |
| Free caffeic acid | 37.9 | 28.0 | 35.2 | 29.6 | 50.7 | 32.9 | 43.0 | 37.0 | 49.0 | |
| Free p-cumaric acid | 152.6 ab | 95.0 e | 121.8 cd | 102.2 de | 172.5 a | 135.5 bc | 132.7 bc | 119.7 cd | 117.5 cde | |
| Free ferulic acid | 39.8 ab | 27.1 b | 36.6 ab | 58.3 ab | 105.2 a | 65.5 ab | 98.4 ab | 53.3 ab | 18.6 b | |
| Bound p-hydroxybenzoic acid | 9.2 | 11.3 | 10.1 | 8.7 | 9.3 | 9.2 | 7.4 | 6.9 | 9.2 | |
| Bound caffeic acid | 3.0 d | 8.7 c | 8.0 c | 6.3 cd | 13.7 b | 15.9 ab | 19.0 a | 13.1 b | 2.3 d | |
| Bound p-cumaric acid | 175.9 a | 114.4 c | 76.9 d | 98.2 cd | 149.0 ab | 122.3 bc | 160.5 a | 114.3 c | 74.7 d | |
| Bound ferulic acid | 28.2 a | 22.6 a | 11.3 b | 21.1 a | 24.2 a | 22.4 a | 26.0 a | 20.4 ab | 19.2 ab | |
| Carotenoids | Lutein | 212.2 ab | 148.7 de | 158.7 cde | 177.2 bcd | 214.4 a | 124.4 e | 185.5 abc | 210.6 ab | 152.6 cde |
| Zeaxhantin | 43.9 | 33.8 | 36.1 | 37.1 | 38.4 | 34.5 | 35.0 | 38.7 | 32.6 | |
| β-Carotene | 141.5 a | 96.6 cde | 104.8 bcd | 121.0 abc | 143.9 a | 81.9 de | 127.4 abc | 129.9 ab | 64.9 e | |
| Tocopherols | δ-Tocopherol | 1.3 | 1.3 | 1.6 | 1.1 | 1.5 | 1.6 | 1.7 | 1.5 | 1.2 |
| β + γ-Tocopherol | 19.0 | 18.7 | 19.5 | 22.7 | 19.2 | 17.7 | 20.9 | 24.2 | 18.3 | |
| α-Tocopherol | 153.6 b | 138.8 b | 144.3 b | 142.7 b | 227.8 a | 94.3 bc | 221.8 a | 238.2 a | 64.8 c | |
| Monoterpenes | α-Pinene | 6.1 ab | 13.3 a | 5.8 ab | 3.4 b | 5.6 ab | 5.3 ab | 1.7 b | 4.5 b | 2.3 b |
| β-Pinene | 2.1 | 2.3 | 0.6 | 1.8 | 1.9 | 1.2 | 0.7 | 1.6 | 0.6 | |
| β-Myrcene | 0.7 a | 0.9 a | 0.7 a | 0.7 a | 1.2 a | 0.7 a | 0.0 b | 0.0 b | 0.0 b | |
| Limonene | 1.0 abc | 1.7 a | 1.4 ab | 1.5 ab | 0.9 bc | 0.9 bc | 0.6 c | 0.9 bc | 0.6 c | |
| p-Cymene | 5.8 | 6.6 | 5.6 | 6.2 | 4.7 | 4.7 | 4.3 | 5.9 | 5.3 | |
| Oxygenated monoterpenes | Eucalyptol | 2.0 | 1.6 | 2.6 | 2.7 | 2.8 | 1.8 | 2.4 | 2.5 | 1.9 |
| Terpinen-4-ol | 1.0 bc | 2.1 a | 1.3 ab | 2.1 a | 0.0 c | 1.7 ab | 1.0 b | 1.0 b | 1.3 ab | |
| α-Terpineol | 0.8 | 1.1 | 1.0 | 1.1 | 0.7 | 0.8 | 0.7 | 1.2 | 0.8 | |
| Sesquiterpenes | α-Ylangene | 0.6 c | 2.4 ab | 2.7 a | 1.9 abc | 1.1 bc | 1.9 abc | 1.0 bc | 1.0 bc | 1.5 abc |
| β-Caryophyllene | 156.5 | 147.0 | 158.7 | 127.0 | 107.9 | 126.9 | 52.6 | 116.0 | 160.2 | |
| Patchoulene | 1.4 b | 3.2 ab | 5.4 a | 1.1 b | 0.5 b | 1.9 b | 1.2 b | 2.7 ab | 1.5 b | |
| α-Humulene | 137.2 | 128.2 | 154.7 | 110.6 | 88.6 | 118.9 | 75.5 | 103.1 | 145.5 | |
| Aromadendrene | 4.4 bc | 6.1 b | 19.9 a | 3.0 cd | 2.0 d | 2.5 cd | 2.1 d | 2.6 cd | 2.8 cd | |
| β-Selinene | 0.5 | 1.8 | 2.2 | 1.4 | 1.9 | 1.7 | 1.3 | 1.5 | 1.9 | |
| β-Guaiene | 1.4 b | 6.0 ab | 8.4 a | 3.3 b | 2.6 b | 6.6 ab | 2.1 b | 4.4 ab | 3.9 b | |
| α-Selinene | 7.7 | 13.1 | 13.7 | 9.0 | 4.6 | 7.7 | 4.9 | 8.9 | 10.3 | |
| β-Curcumene | 128.9 | 109.6 | 125.6 | 118.2 | 126.9 | 118.5 | 114.5 | 111.9 | 122.5 | |
| Cubenene | 2.6 b | 8.5 ab | 15.4 a | 3.0 b | 3.0 b | 8.1 ab | 3.1 b | 3.1 b | 4.4 b | |
| δ-Cadinene | 5.0 b | 13.0 ab | 18.7 a | 7.8 b | 6.5 b | 10.4 ab | 4.5 b | 6.8 b | 10.2 ab | |
| α-Muurolene | 7.0 bc | 33.5 a | 27.5 a | 0.0 c | 0.0 c | 11.5 b | 6.8 bc | 8.8 bc | 0.0 c | |
| Selina-3,7(11)-diene | 23.7 abc | 23.1 abcd | 28.5 abc | 30.0 ab | 40.3 a | 11.2 bcd | 8.8 d | 9.8 cd | 26.9 abcd | |
| Oxygenated sesquiterpenes | Selina-6-en-4-ol | 2.6 b | 10.9 a | 5.3 ab | 3.9 b | 3.9 b | 2.7 b | 2.4 b | 2.8 b | 2.8 b |
| trans-Longipinocarveol | 8.6 abc | 16.3 a | 14.2 ab | 10.1 abc | 7.0 bc | 7.3 bc | 5.7 c | 7.2 bc | 10.0 abc | |
| Longifolenaldehyde | 5.8 ab | 7.8 a | 7.5 a | 4.5 bc | 3.9 bc | 4.3 bc | 2.7 c | 6.4 a | 3.9 bc | |
| Alloaromadendrene oxide | 5.1 b | 10.7 a | 8.7 a | 4.4 b | 3.4 b | 3.7 b | 3.1 b | 4.5 b | 3.7 b | |
| α-Bisabolol | 137.9 a | 118.3 ab | 22.4 d | 21.8 d | 43.2 d | 87.9 bc | 53.5 cd | 115.6 ab | 46.8 d | |
| Eudesm-7(11)-en-4-ol | 4.2 bc | 12.8 a | 7.5 b | 6.6 b | 4.3 bc | 4.6 bc | 2.2 c | 4.4 bc | 3.6 bc | |
| Clovanediol | 5.2 a | 6.2 a | 5.9 a | 4.3 a | 0.5 b | 0.5 b | 0.5 b | 0.7 b | 0.5 b | |
| Cannabinoids | Cannabidivarin | 510.8 d | 1432.8 bcd | 3521.7 abc | 1664.2 bc | 725.4 d | 1730.4 bc | 950.9 cd | 1904.9 b | 1136.6 bcd |
| Cannabidiol (CBD) | 15,793.4 ab | 16,370.8 a | 18,099.5 a | 15,953.4 ab | 13,197.5 c | 13,958.8 bc | 12,770.6 c | 16,669.4 a | 13,004.1 c | |
| ∆9-Tetrahydrocannabinol (THC) | 392.1 ab | 497.3 a | 555.9 a | 401.2 ab | 327.7 bc | 330.0 bc | 192.2 c | 184.8 c | 192.4 c | |
| Cannabigerol (CBG) | 312.0 a | 260.6 ab | 238.0 ab | 159.9 bc | 337.7 a | 369.5 a | 95.4 c | 290.6 ab | 51.9 c | |
| Cannabinol (CBN) | 357.9 | 456.0 | 515.3 | 418.7 | 302.1 | 356.1 | 253.6 | 383.9 | 258.9 | |
| Class of Metabolites a | Treatment b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | SD | NPK | F0 | |
| Total free and bound phenolic acids | 5.96 ab | 4.20 c | 3.39 cd | 4.05 c | 6.73 a | 3.77 c | 4.32 bc | 4.99 bc | 2.13 d |
| Total free and bound flavonoids | 43.44 abc | 40.21 abc | 33.21 c | 35.83 bc | 50.82 a | 32.74 c | 37.23 bc | 47.64 ab | 9.49 d |
| Total carotenoids | 4.57 a | 3.01 bc | 2.73 bcd | 3.28 ab | 4.30 a | 1.84 cd | 2.88 bc | 4.43 a | 1.50 d |
| Total tocopherols | 1.93 b | 1.72 b | 1.49 bc | 1.64 b | 2.78 a | 0.88 cd | 1.93 bc | 3.04 a | 0.45 d |
| Total monoterpenes and oxygenated monoterpenes | 0.25 ab | 0.34 a | 0.17 bcde | 0.19 bcd | 0.19 bcd | 0.12 cde | 0.09 de | 0.22 bc | 0.08 e |
| Total sesquiterpenes and oxygenated sesquiterpenes | 7.69 a | 7.34 ab | 6.07 abc | 4.48 cd | 4.86 bcd | 4.17 cd | 3.01 d | 6.62 abc | 3.33 d |
| Total cannabinoids | 196.16 abc | 200.26 ab | 213.04 ab | 183.05 abc | 163.35 bcd | 128.63 cde | 116.44 de | 229.61 a | 84.90 e |
| Raw Material | Compost Composition (% w/w) | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | |
| Solid digestate from buffalo effluent | 50 | 50 | 50 | 67 | 75 | 84 |
| Cardoon-based spent mushroom substrate | 50 | — | — | — | — | — |
| Straw-based spent mushroom substrate | — | 50 | — | — | — | — |
| Cardoon waste | — | — | 50 | 33 | 25 | 16 |
| Amendment | Property a | |||
|---|---|---|---|---|
| pH | EC (dS m−1) | OC (g Kg−1) | N (g Kg−1) | |
| C1 | 8.1 ± 0.00 | 1.11 ± 0.02 | 187.0 ± 0.03 | 18.4 ± 0.50 |
| C2 | 8.0 ± 0.00 | 1.20 ± 0.07 | 190.8 ± 0.25 | 16.8 ± 0.50 |
| C3 | 7.9 ± 0.07 | 0.84 ± 0.03 | 211.2 ± 0.13 | 6.4 ± 0.42 |
| C4 | 7.9 ± 0.07 | 0.79 ± 0.01 | 214.6 ± 2.13 | 18.1 ± 0.96 |
| C5 | 7.6 ± 0.07 | 0.96 ± 0.09 | 296.8 ± 4.98 | 21.6 ± 4.96 |
| C6 | 7.8 ± 0.07 | 0.97 ± 0.08 | 282.2 ± 6.28 | 21.9 ± 1.70 |
| SD | 8.9 ± 0.07 | 1.11 ± 0.02 | 409.1 ± 1.34 | 17.6 ± 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicignano, M.; Beleggia, R.; del Piano, L.; Enotrio, T.; Suriano, S.; Raimo, F.; Trono, D. Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence. Plants 2025, 14, 1519. https://doi.org/10.3390/plants14101519
Sicignano M, Beleggia R, del Piano L, Enotrio T, Suriano S, Raimo F, Trono D. Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence. Plants. 2025; 14(10):1519. https://doi.org/10.3390/plants14101519
Chicago/Turabian StyleSicignano, Mariarosaria, Romina Beleggia, Luisa del Piano, Tommaso Enotrio, Serafino Suriano, Francesco Raimo, and Daniela Trono. 2025. "Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence" Plants 14, no. 10: 1519. https://doi.org/10.3390/plants14101519
APA StyleSicignano, M., Beleggia, R., del Piano, L., Enotrio, T., Suriano, S., Raimo, F., & Trono, D. (2025). Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence. Plants, 14(10), 1519. https://doi.org/10.3390/plants14101519







