Biofortified Calcium Phosphate Nanoparticles Elicit Secondary Metabolite Production in Carob Callus via Biosynthetic Pathway Activation
Abstract
1. Introduction
2. Results
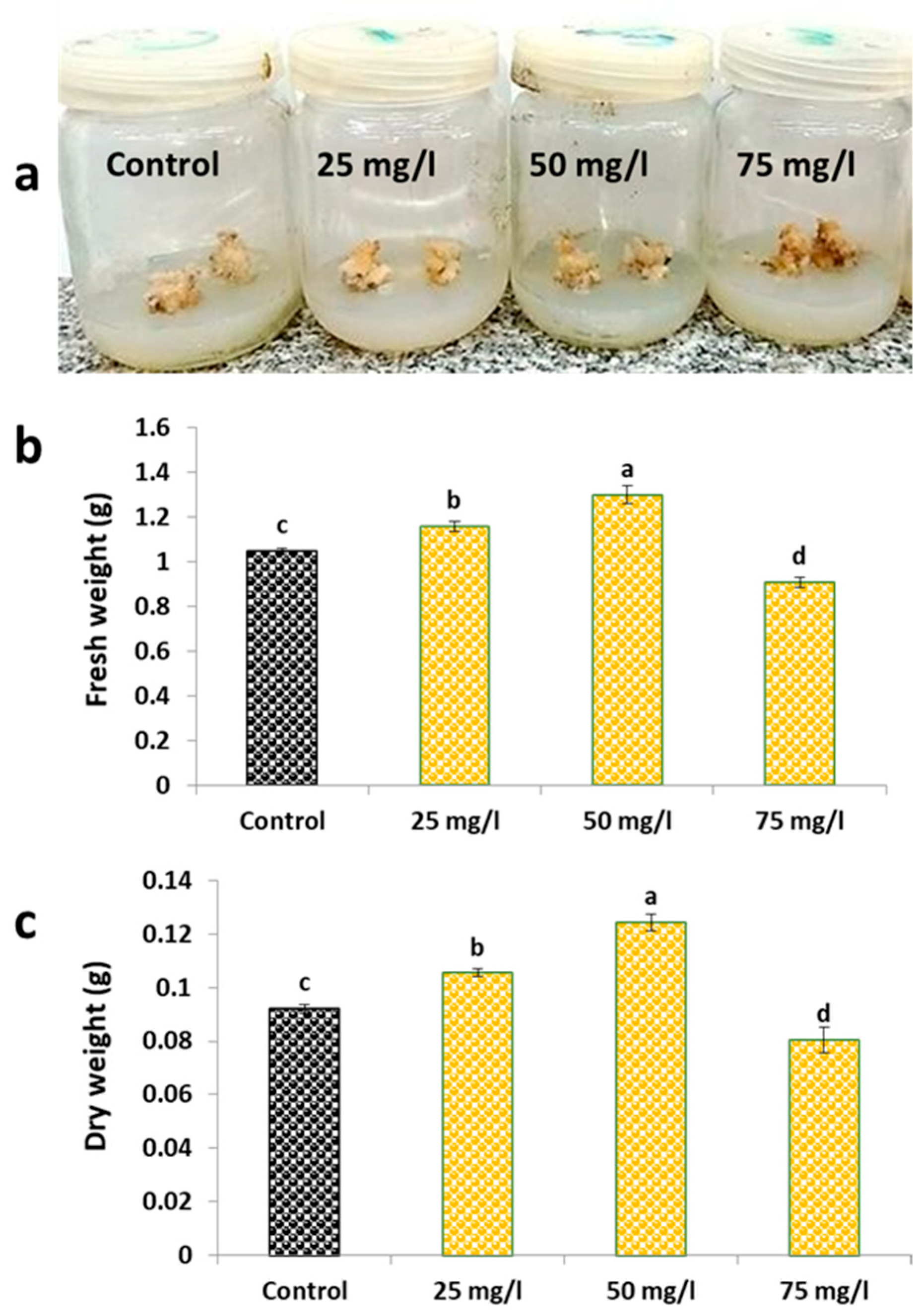
2.1. Growth Parameters and Morphological Observations
2.2. Secondary Metabolites and Total Antioxidant Capacity
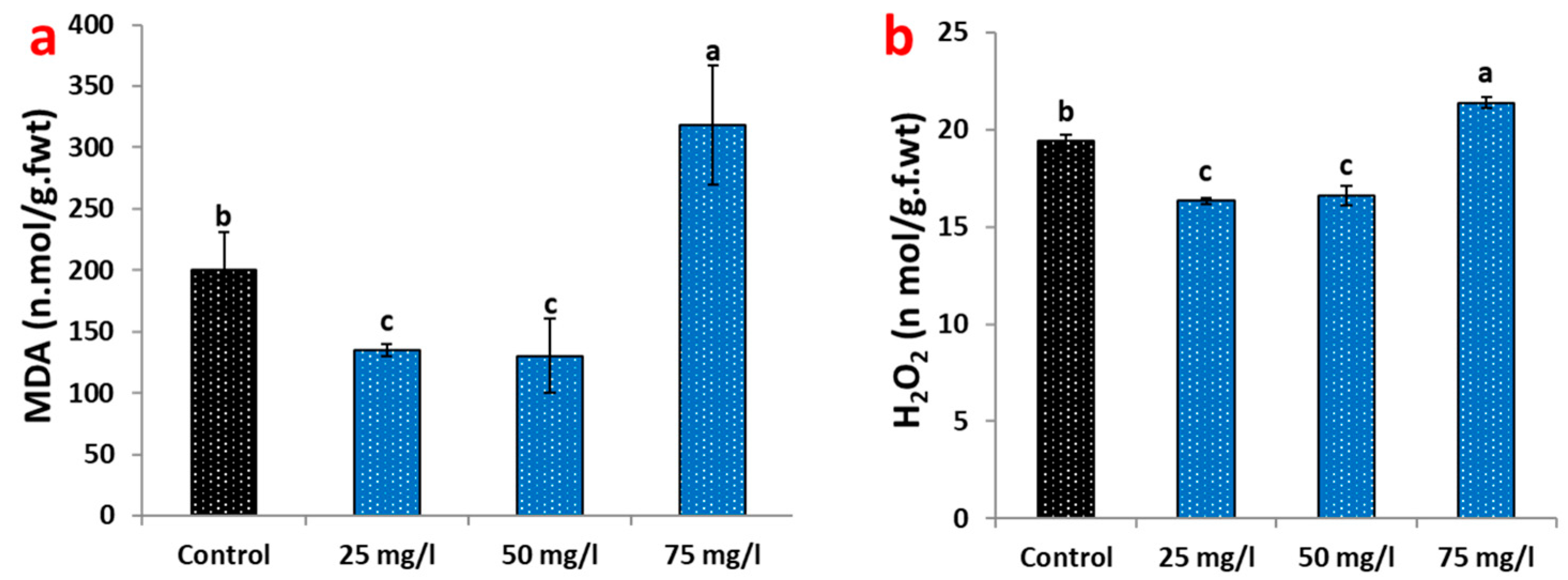
2.3. Oxidative Stress Responses to CaP-NP Treatment
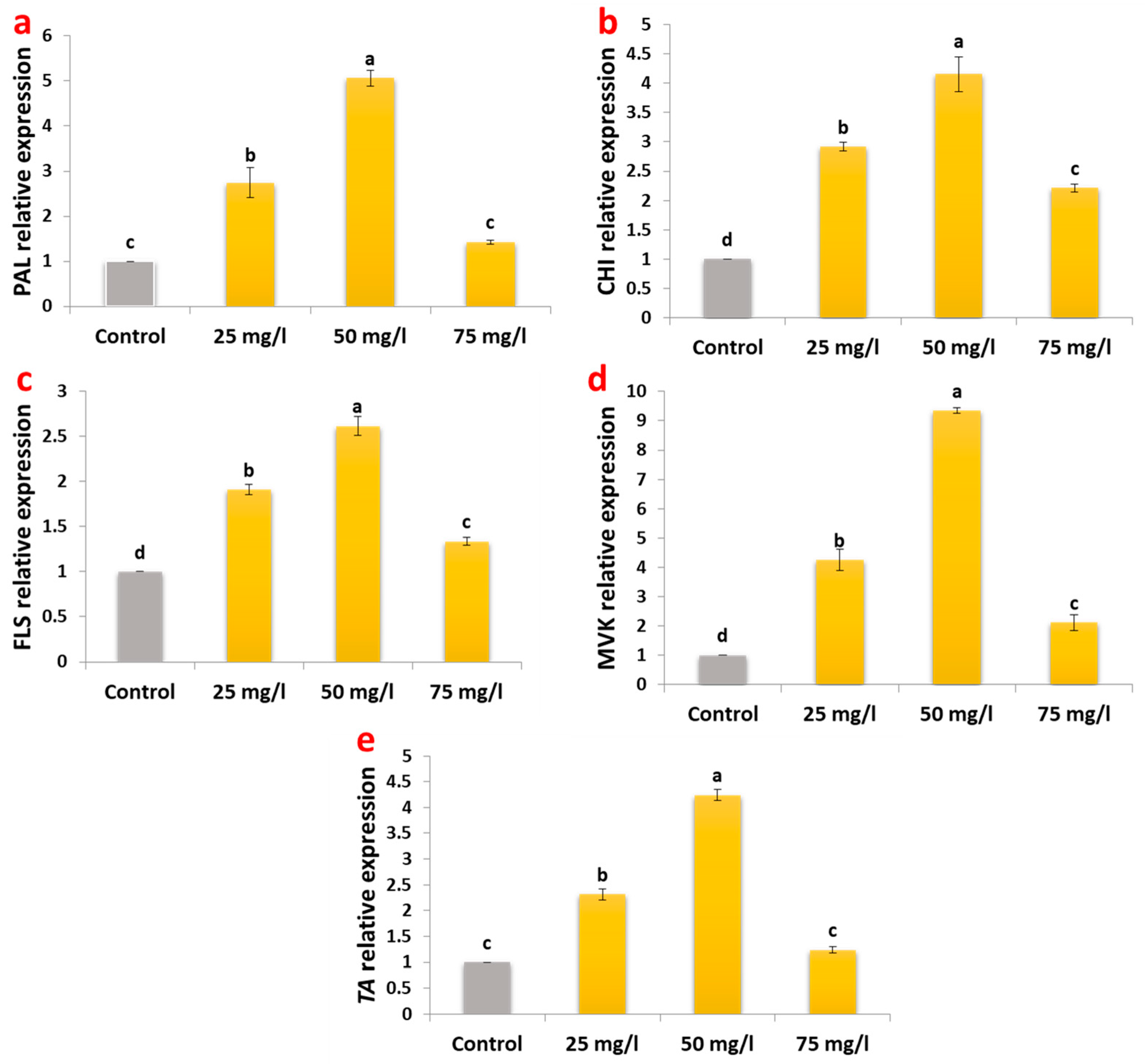
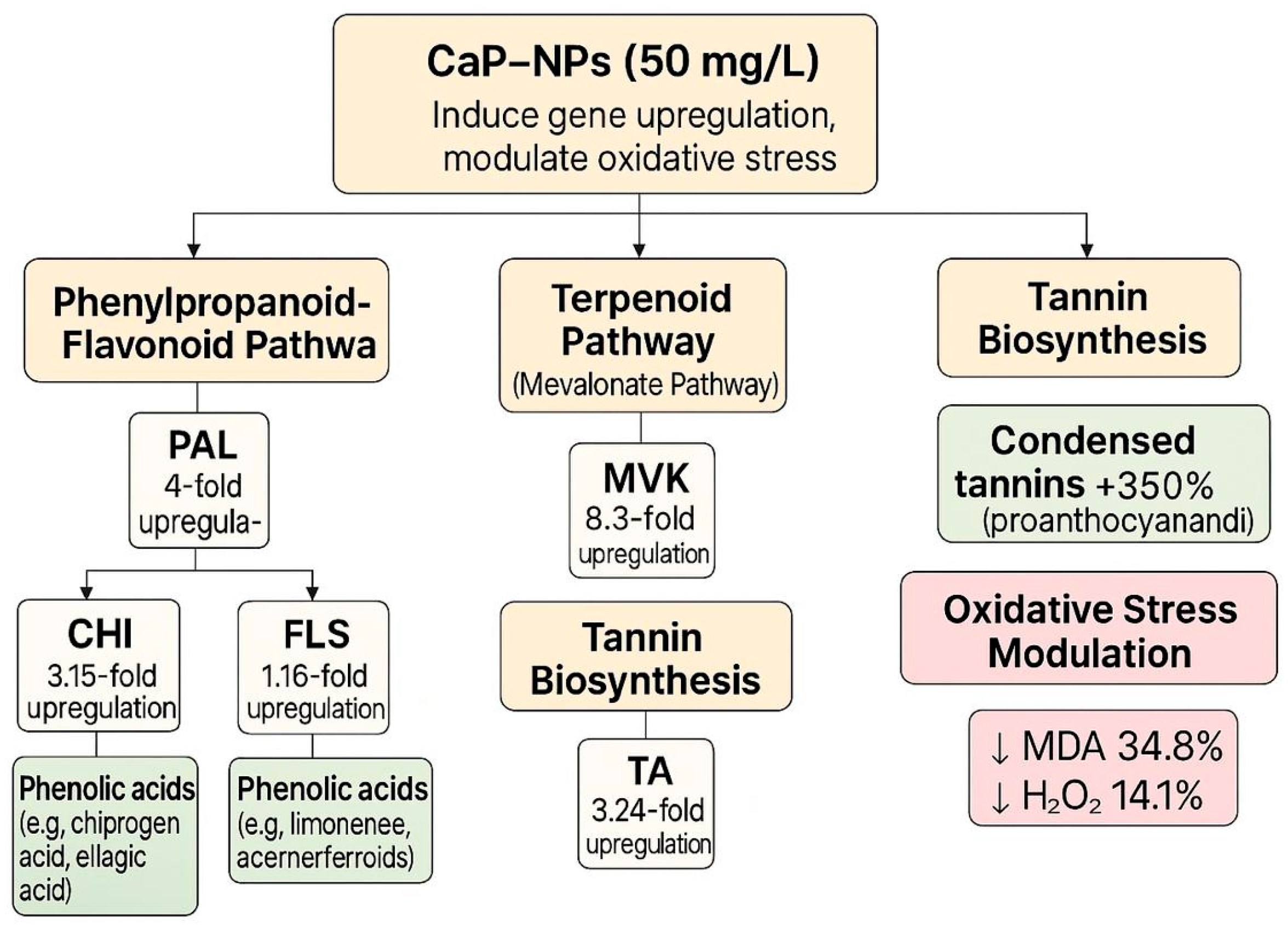
2.4. Gene Expression Modulation in Metabolic Pathways
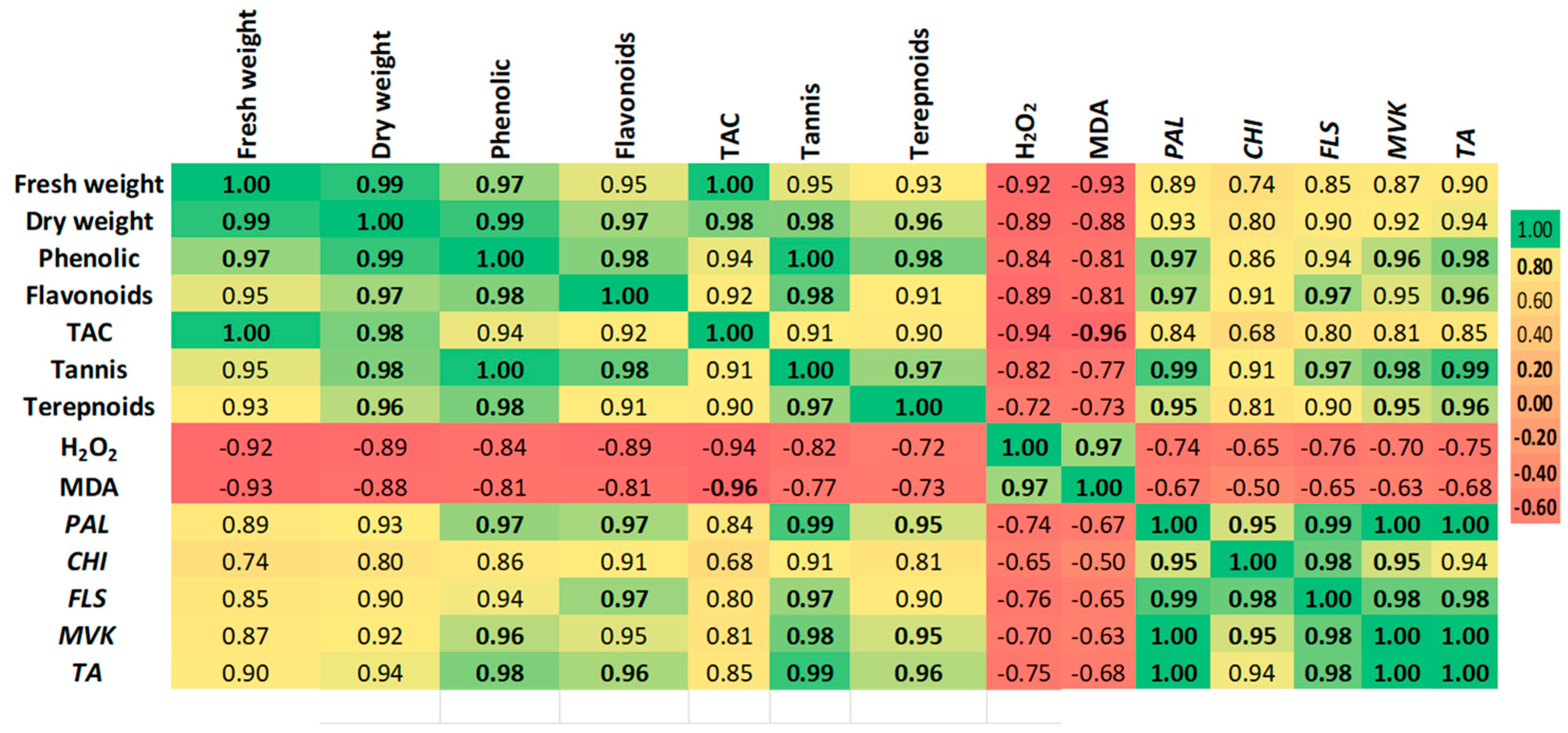
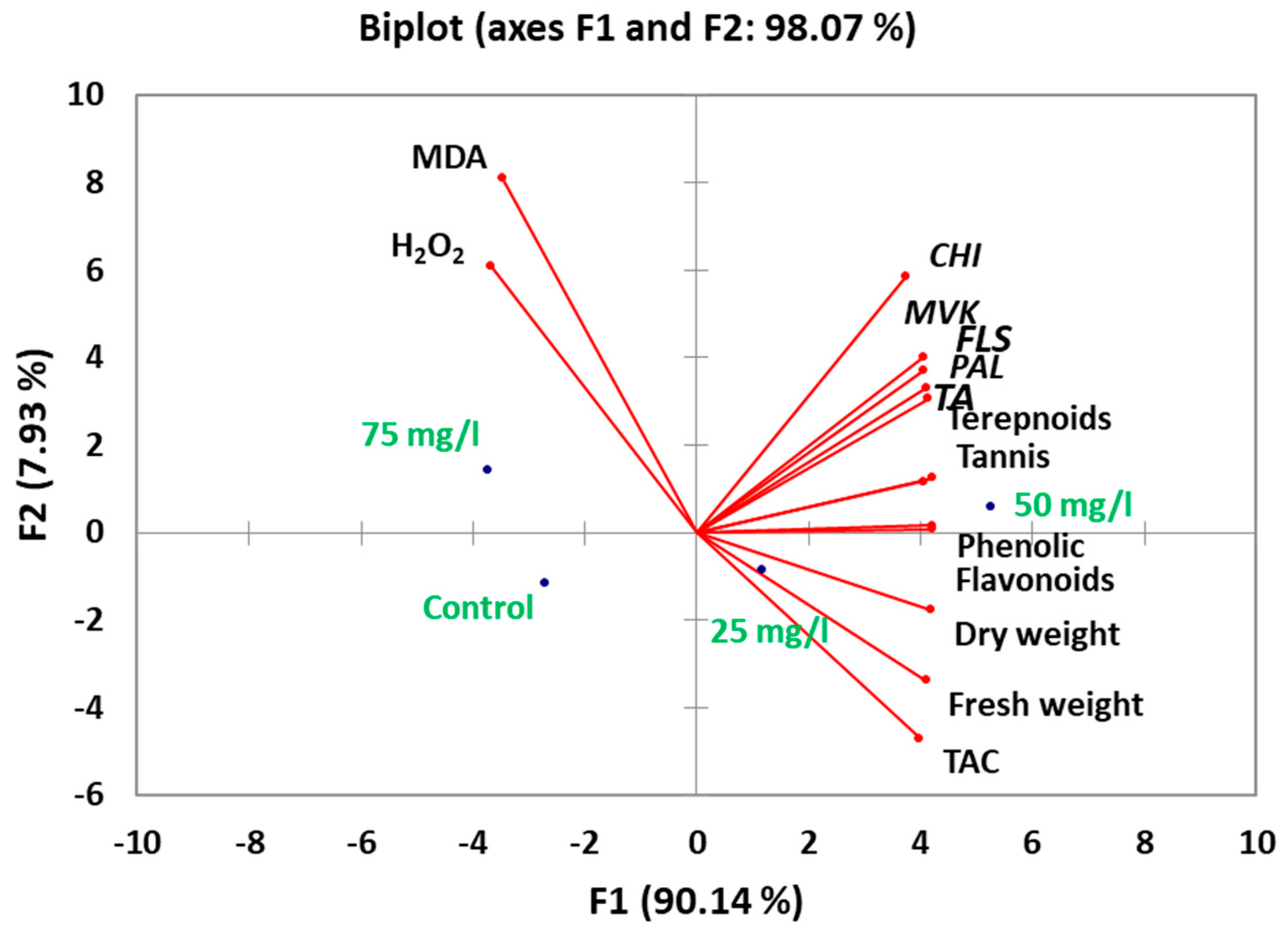
2.5. Correlation and Multivariate Analysis of Metabolic Responses
2.6. Quantitative Profiling of Phenolic and Flavonoid Compounds
2.7. Quantitative Analyses of Tannin
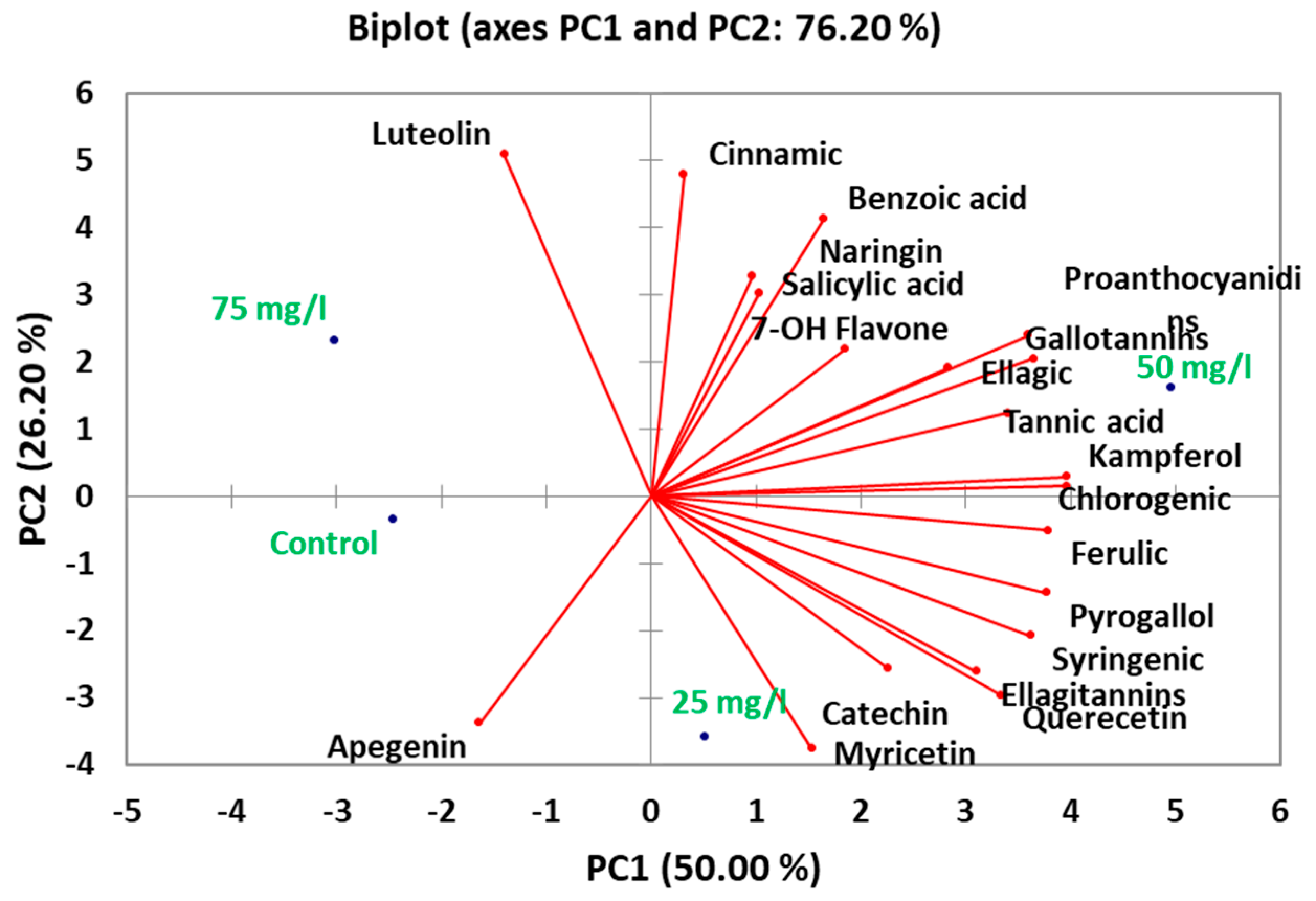
2.8. Principal Component Analysis of Phytochemical Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material and Callus Induction
4.2. Synthesis and Characterization of CaP-NPs
4.3. Culture Conditions and Nanoparticle Treatment
4.4. Biomass Assessment
4.5. Phytochemical Quantification
4.6. Antioxidant Capacity and Oxidative Stress Markers
4.7. Molecular Estimation
4.8. HPLC Conditions
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azab, A. Carob (Ceratonia siliqua): Health, medicine, chemistry. Eur. Chem. Bull. 2017, 6, 456. [Google Scholar] [CrossRef]
- Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring carob (Ceratonia siliqua L.): A comprehensive assessment of its characteristics, ethnomedicinal uses, phytochemical aspects, and pharmacological activities. Plants 2023, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Batlle, I.; Tous, J. Carob Tree: Ceratonia siliqua L. Promoting the Conservation and Use of Underutilized and Neglected Crops; Institute of Plant Genetics and Crop Plant Research: Rome, Italy; International Plant Genetic Resources Institute: Gatersleben, Germany, 1997; Volume 17, pp. 7–12. [Google Scholar]
- Deen, E.M.Z.; El-Sayed, O.M.; El-Rahman, A.; El-Sayed, I.; Hegazi, G.A.E.M. Studies on carob (Ceratonia siliqua L.) propagation. IOSR J. Agric. Vet. Sci. 2014, 7, 31–40. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Zunft, H.J.F.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Koebnick, C.; Grünwald, J. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef]
- Theophilou, I.C.; Neophytou, C.M.; Kakas, A.C.; Constantinou, A. Carob and Its Components in the Management of Gastrointestinal Disorders. J. Hepatol. Gastroenterol. 2017, 1, 5. [Google Scholar]
- Yousif, A.K.; Alghzawi, H. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume XI, p. 553. [Google Scholar] [CrossRef]
- Djebari, S.; Wrona, M.; Nerín, C.; Djaoudene, O.; Guemouni, S.; Boudria, A.; Madani, K. Phenolic compounds profile of macerates of different edible parts of carob tree (Ceratonia siliqua L.) using UPLC-ESI-Q-TOF-MSE: Phytochemical screening and biological activities. Fitoterapia 2024, 172, 105696. [Google Scholar] [CrossRef]
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef] [PubMed]
- Boliko, M.C. FAO and the situation of food security and nutrition in the world. J. Nutr. Sci. Vitaminol. 2019, 65, S4–S8. [Google Scholar] [CrossRef]
- Hssaini, L.; Meziani, R.; Razouk, R.; Irchad, A. Carob (Ceratonia siliqua L.) Cultivation, processing, and industrial applications: A review. Carob. Afr. Mediterr. Agric. J. Al Awamia 2024, 145, 87–107. [Google Scholar]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through vitro technologies-status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Elsherif, D.E.; Safhi, F.A.; Khalifa, A.M.; Ragab, G.A. Upregulated synthesis and production of bioactive compounds in Lotus arabicus L. by in vitro feeding with dried powder of date palm seeds. BMC Plant Biol. 2024, 24, 225. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Influence of exogenous elicitors on the production of secondary metabolites in plants: A review (“VSI: Secondary Metabolites”). Plant Stress 2023, 8, 100166. [Google Scholar] [CrossRef]
- Kalaba, M.H.; Moghannem, S.A.; El-Hawary, A.S.; Radwan, A.A.; Sharaf, M.H.; Shaban, A.S. Green synthesized ZnO nanoparticles mediated by streptomyces plicatus: Characterizations, antimicrobial and nematicidal activities and cytogenetic effects. Plants 2021, 10, 1760. [Google Scholar] [CrossRef]
- Naz, M.; Afzal, M.R.; Raza, M.A.; Pandey, S.; Qi, S.; Dai, Z.; Du, D. Calcium (Ca2+) signaling in plants: A plant stress perspective. S. Afr. J. Bot. 2024, 169, 464–485. [Google Scholar] [CrossRef]
- Brini, M.; Ottolini, D.; Calì, T.; Carafoli, E. Calcium in health and disease. Met. Ions Life Sci. 2013, 13, 81–137. [Google Scholar] [CrossRef] [PubMed]
- Mahundi, P.; Manwa, L. Energy nutrient metabolism: The inter-conversions and the chain reaction leading to oxidative-phosphorilation and production of adenosine tri-phosphate (ATP). J. Emerg. Trends Educ. Res. Policy Stud. 2015, 6, 383–390. [Google Scholar]
- Samal, D.P.K.; Sukla, L.B.; Bishoyi, A.K. Biosynthesis of phosphorus nanoparticles for sustainable agroecosystems: Next generation nanotechnology application for improved plant growth. ACS Omega 2025, 10, 14555–14565. [Google Scholar] [CrossRef]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.S.; Gabr, A.M.M.; Amin, M.A.; Taha, L.S. Biochemical Characterization of Micropropagated Ceratonia siliqua L. under Effect of Growth Regulators and Light Quality. Bull. Natl. Res. Cent. 2020, 44, 26. [Google Scholar] [CrossRef]
- Iqbal, Z.; Javad, S.; Naz, S.; Shah, A.A.; Shah, A.N.; Paray, B.A.; Gulnaz, A.; Abdelsalam, N.R. Elicitation of the in Vitro Cultures of Selected Varieties of Vigna radiata L. with Zinc Oxide and Copper Oxide Nanoparticles for Enhanced Phytochemicals Production. Front. Plant Sci. 2022, 13, 908532. [Google Scholar] [CrossRef]
- Elbanoby, N.E.; El-Settawy, A.A.A.; Mohamed, A.A.; Salem, M.Z.M. Phytochemicals Derived from Leucaena leucocephala (Lam.) de Wit (Fabaceae) Biomass and Their Antimicrobial and Antioxidant Activities: HPLC Analysis of Extracts. Biomass Convers. Biorefin. 2024, 14, 14593–14609. [Google Scholar] [CrossRef]
- Lankarani, S.M.J.; Karimi, J.; Rezaei, A. Impact of Zinc Oxide Nanoparticles and Iron on Stevia Rebaudiana Bertoni Growth, Nutrient Uptake, and Bioactive Compounds under in Vitro Conditions. Plant Cell Tissue Organ Cult. 2024, 159, 18. [Google Scholar] [CrossRef]
- Ramirez-Gil, J.G.; Lopera, A.A.; Garcia, C. Calcium Phosphate Nanoparticles Improve Growth Parameters and Mitigate Stress Associated with Climatic Variability in Avocado Fruit. Heliyon 2023, 9, e18658. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, N.; Islam, M.S.; Ahmad, M.A.; Ercisli, S.; Ullah, R.; Bari, A.; Munir, I. Rice Seeds Biofortification Using Biogenic Iron Oxide Nanoparticles Synthesized by Using Glycyrrhiza Glabra: A Study on Growth and Yield Improvement. Sci. Rep. 2024, 14, 12368. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Gezgin, S.; Athar, T.; Rajput, V.D.; Gupta, O.P.; Minkina, T. Insight into the Prospects for Nanotechnology in Wheat Biofortification. Biology 2021, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Mmereke, K.M.; Venkataraman, S.; Moiketsi, B.N.; Khan, M.R.; Hassan, S.H.; Rantong, G.; Masisi, K.; Kwape, T.E.; Gaobotse, G.; Zulfiqar, F.; et al. Nanoparticle Elicitation: A Promising Strategy to Modulate the Production of Bioactive Compounds in Hairy Roots. Food Res. Int. 2024, 178, 113910. [Google Scholar] [CrossRef]
- Darbahani, M.; Ghiyasi, M.R.; Rahaie, M. Nanoparticles as new elicitors for the production of bioactive and phytochemicals in vitro and in vivo plant culture. Phytochem. Rev. 2024, 1–24. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A. Plant Response to Engineered Metal Oxide Nanoparticles. Nanoscale Res. Lett. 2017, 12, 92. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An Overview on Manufactured Nanoparticles in Plants: Uptake, Translocation, Accumulation and Phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Zhang, X.P.; Ma, C.X.; Sun, L.R.; Hao, F.S. Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal. Behav. 2020, 15, 1748283. [Google Scholar] [CrossRef]
- Isidra-Arellano, M.C.; Delaux, P.-M.; Valdés-López, O. The phosphate starvation response system: Its role in the regulation of plant-microbe interactions. Plant Cell Physiol. 2021, 62, 392–400. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Begum, L.; Dey, B.; Nath, P.K.; Panda, S.K. Impact of calcium phosphate nanoparticles on rice plant. J. Plant Sci. Phytopathol. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Nasrallah, A.K.; Kheder, A.A.; Kord, M.A.; Fouad, A.S.; El-Mogy, M.M.; Atia, M.A.M. Mitigation of salinity stress effects on broad bean productivity using calcium phosphate nanoparticles application. Horticulturae 2022, 8, 75. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Oosalo, A.A.; Naseri, L.; Alirezalu, A.; Darvishzadeh, R.; Ebrahimi, S.N. Exogenous phenylalanine application effects on phytochemicals, antioxidant activity, hplc profiling, and pal and chs genes expression in table grapes (Vitis vinifera Cv. ‘Qzl Ouzum’). BMC Plant Biol. 2024, 24, 1216. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Liu, J.-M.; Lu, H.-Y.; Gao, S.-L. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep. 2009, 28, 1205–1213. [Google Scholar] [CrossRef]
- Park, C.H.; Xu, H.; Yeo, H.J.; Park, Y.E.; Hwang, G.-S.; Park, N.I.; Park, S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize LC and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021, 64, 64–73. [Google Scholar] [CrossRef]
- Al-Oubaidi, H.K.M.; Al-Khafagi, M.F.J. In vitro increasing medical compounds (tannins and phenols) of Punica granatum L. in callus using MgO NPs and CuO NPS. J. Pharm. Sci. Res. 2018, 10, 1085–1088. [Google Scholar]
- Wang, J.; Wang, K.; Lyu, S.; Huang, J.; Huang, C.; Xing, Y.; Wang, Y.; Xu, Y.; Li, P.; Hong, J.; et al. Genome-wide identification of tannase genes and their function inwound response and astringent substances accumulation in juglandaceae. Front. Plant Sci. 2021, 12, 664470. [Google Scholar] [CrossRef]
- Yang, Q.; Gu, C.; Zhang, X.; Hu, X.; Li, M.; Guan, W.; Kong, Y.; Gao, H. The research on metabolomics mechanism of calcium ion-induced whole black bean polyphenols and biological activities. Lebenson. Wiss. Technol. 2024, 195, 115851. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Lluch, M.A.; Masferrer, A.; Arró, M.; Boronat, A.; Ferrer, A. Molecular cloning and expression analysis of the mevalonate kinase gene from Arabidopsis thaliana. Plant Mol. Biol. 2000, 42, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of terpenoid compounds in food and pharmaceutical products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Silva, N.; Ivamoto-Suzuki, S.T.; Camargo, P.O.; Rosa, R.S.; Pereira, L.F.P.; Domingues, D.S. Low-copy genes in terpenoid metabolism: The evolution and expression of MVK and DXR genes in angiosperms. Plants 2020, 9, 525. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Breaking New ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr. Opin. Plant Biol. 2015, 25, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H. Therapeutic and biomedical potentialities of terpenoids-a review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Hadi, M.R.; Karimi, N. The role of calcium in plants’ salt tolerance. J. Plant Nutr. 2012, 35, 2037–2054. [Google Scholar] [CrossRef]
- Baskar, V.; Nayeem, S.; Kuppuraj, S.P.; Muthu, T.; Ramalingam, S. Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech 2018, 8, 362. [Google Scholar] [CrossRef]
- El-Esawy, M.A.; Elkhateeb, E.A.; Hassan, A.M.; Elsherif, D.E. Nanoparticle innovations: Impact of biogenic cap nanoparticles in mitigating the adverse effects of excessive nitrate application. Plant Soil 2025, 1–20. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Mutukwa, D.; Taziwa, R.T.; Khotseng, L. A review of plant-mediated Zno nanoparticles for photodegradation and antibacterial applications. Nanomaterials 2024, 14, 1182. [Google Scholar] [CrossRef] [PubMed]
- Bohdanovych, T.; Kuzema, P.; Anishchenko, V.; Duplij, V.; Kharchuk, M.; Lyzhniuk, V.; Shakhovsky, A.; Matvieieva, N. Comparison of silver and gold nanoparticles green synthesis by Artemisia annua hairy root extracts. Biol. Open 2025, 14, bio061739. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Razavi, S.M.; Khoshkam, M.; Asadi, A. Effects of green synthesis of sulfur nanoparticles from cinnamomum zeylanicum barks on physiological and biochemical factors of lettuce (Lactuca sativa). Physiol. Mol. Biol. Plants 2020, 26, 1055–1066. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jindal, K.K.; Singh, R.N. Phenolic content in male and female Carica papaya: A possible physiological marker for sex identification of vegetative seedlings. Physiol. Plant. 1975, 33, 104–107. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Ghorai, N.; Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoc. Exch. 2012, 5. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A.J.P.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Durgawale, T.P.; Durgawale, P.P.; Khanwelkar, C.C. Quantitative estimation of tannins by HPLC. Der Pharm. Lett. 2016, 8, 123–126. [Google Scholar]
- Negi, J.S.; Singh, P.; Pant, G.J.N.; Rawat, M.S.M. High-performance liquid chromatography analysis of plant saponins: An update 2005–2010. Pharmacogn. Rev. 2011, 5, 155–158. [Google Scholar] [CrossRef]

| No | Category | Compounds | 0 | 25 mg/L | 50 mg/L | 75 mg/L | RT |
|---|---|---|---|---|---|---|---|
| µg/mL | min | ||||||
| 1 | Phenolic | Chlorogenic acid | 6.03 ± 0.09 c | 8.24 ± 0.18 b | 14.02 ± 0.09 a | 4.27 ± 0.18 d | 3 |
| 2 | Ellagic acid | 10 ± 0.06 d | 18.54 ± 0.18 b | 25.05 ± 0.22 a | 20.10 ± 0.29 b | 5 | |
| 3 | Pyrogallol | 8.71 ± 0.15 c | 15.46 ± 0.38 b | 23.51 ± 0.58 a | ND | 6 | |
| 4 | Syringenic acid | 10.33 ± 0.60 | 13.72 ± 0.66 b | 15.92 ± 0.51 a | 6.28 ± 0.70 | 8 | |
| 5 | Ferulic acid | 4.95 ± 0.09 | 7.93 ± 0.02 b | 9.38 ± 0.35 a | 5.84 ± 0.08 | 13 | |
| 6 | Cinnamic acid | 6 ± 0.20 b | 2.01 ± 0.02 d | 6.48 ± 0.20 a | 5.53 ± 0.07 c | 14 | |
| 7 | Salicylic acid | 11.58 ± 0.17 a | 1.66 ± 0.11 d | 11.64 ± 0.12 a | 5.40 ± 0.13 b | 18.9 | |
| 8 | Benzoic acid | 5.31 ± 0.10 d | 7.51 ± 0.10 c | 15.27 ± 0.30 a | 14.55 ± 0.10 b | 15 | |
| 9 | Flavonoids | 7-OH flavone | 9.11 ± 0.08 b | 4.48 ± 0.21 d | 10 ± 0.01 a | 5.1 ± 0.06 c | 2 |
| 10 | Apegenin | 7.88 ± 0.16 a | 4.38 ± 0.13 b | ND | ND | 3.9 | |
| 11 | Naringin | 12.11 ± 0.18 a | ND | 12.39 ± 0.35 a | 5.29 ± 0.23 c | 9.1 | |
| 12 | Myricetin | 4.42 ± 0.36 d | 13.76 ± 0.34 a | 8.33 ± 0.16 b | 6.94 ± 0.07 c | 11 | |
| 13 | Catechin | 11.58 ± 0.17 a | 10.66 ± 0.11 b | 11.64 ± 0.12 a | 5.40 ± 0.13 c | 16.1 | |
| 14 | luteolin | 5.27 ± 0.18 c | ND | 6.15 ± 0.22 b | 12.12 ± 0.27 a | 20.8 | |
| 15 | Quercetin | 6.57 ± 0.54 b | 13.78 ± 0.34 a | 14.25 ± 0.46 a | ND | 7 | |
| 16 | Kaempferol | 8.48 ± 0.47 c | 15.7 ± 0.43 b | 24.35 ± 3.69 a | 9.91 ± 0.11 c | 17.16 | |
| Total content | 128.35 ± 3.6 c | 137.85 ± 2.78 b | 208.4 ± 2.8 a | 106.7 ± 2.42 d | |||
| No | Category | Compounds | 0 | 25 mg/L | 50 mg/L | 75 mg/L | RT |
|---|---|---|---|---|---|---|---|
| µg/mL | min | ||||||
| 1 | Tannins | Proanthocyanidins | 3.87 ± 0.26 c | 5.48 ± 0.57 b | 17.35 ± 0.55 a | 6.14 ± 0.20 b | 3.33 |
| 2 | Ellagitannins | 14.96 ± 0.31 c | 17.27 ± 0.42 b | 19.39 ± 0.54 a | 5.85 ± 0.32 d | 8 | |
| 3 | Gallotannins | 4.34 ± 0.54 c | 5.58 ± 0.64 bc | 8.82 ± 0.53 a | 5.56 ± 0.37 bc | 10.1 | |
| 4 | Tannic acid | 17.45 ± 0.57 c | 20.36 ± 0.40 b | 22.71 ± 0.54 a | 19.94 ± 0.26 b | 13.2 | |
| Total content | 40.62 ± 1.64 c | 48.69 ± 2.03 b | 68.27 ± 2.2 a | 37.49 ± 1.15 d | |||
| Gene | Primer Sequences 5′-3′ |
|---|---|
| Reference gene (ß-Actin) | F: 5′-GTGGGCCGCTCTAGGCACCAA-3′ R:5′-CTCTTTGATGTCACGCACGATTTC-3′ |
| Phenylalanine ammonia lyase (PAL) | F: 5′-GCAAGGAAAGCCCGAGTTTAC-3′ R: 5′-GGACCTTTTTGGCTACTTGGC-3′ |
| Chalcone isomerase (CHI) | F: 5′-TGGTGGCCTAGACAACGATGAGTT-3′ R: 5′-TCACACTCCCAACTTGGTTTCCCT-3′ |
| Flavonol synthase (FLS) | F: 5′-TTAAAGGAAGGTCTCGGTGGCGAA-3′ R: 5′-TCATTGGTGACGATGAGTGCGAGT-3′ |
| Mevalonate kinase (MVK) | F: 5′-TTATGTGTTGCGCTTTCAGC-3′ R: 5′-GAAGGCTTGCCATGAATGAT-3′ |
| Tannase enzyme (TA) | F: 5′-GCAGTGCGTTGGAGCAATGGTGGGC-3′ R: 5′-CCCCGATACAAATCTGGGATAAGTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsherif, D.E.; Safhi, F.A.; El-Esawy, M.A.; Mohammed, A.T.; Alaziz, O.A.; Subudhi, P.K.; Shaban, A.S. Biofortified Calcium Phosphate Nanoparticles Elicit Secondary Metabolite Production in Carob Callus via Biosynthetic Pathway Activation. Plants 2025, 14, 2093. https://doi.org/10.3390/plants14142093
Elsherif DE, Safhi FA, El-Esawy MA, Mohammed AT, Alaziz OA, Subudhi PK, Shaban AS. Biofortified Calcium Phosphate Nanoparticles Elicit Secondary Metabolite Production in Carob Callus via Biosynthetic Pathway Activation. Plants. 2025; 14(14):2093. https://doi.org/10.3390/plants14142093
Chicago/Turabian StyleElsherif, Doaa E., Fatmah A. Safhi, Mai A. El-Esawy, Alaa T. Mohammed, Osama A. Alaziz, Prasanta K. Subudhi, and Abdelghany S. Shaban. 2025. "Biofortified Calcium Phosphate Nanoparticles Elicit Secondary Metabolite Production in Carob Callus via Biosynthetic Pathway Activation" Plants 14, no. 14: 2093. https://doi.org/10.3390/plants14142093
APA StyleElsherif, D. E., Safhi, F. A., El-Esawy, M. A., Mohammed, A. T., Alaziz, O. A., Subudhi, P. K., & Shaban, A. S. (2025). Biofortified Calcium Phosphate Nanoparticles Elicit Secondary Metabolite Production in Carob Callus via Biosynthetic Pathway Activation. Plants, 14(14), 2093. https://doi.org/10.3390/plants14142093







