Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals
Abstract
1. Introduction
2. Review Methodology
3. Vetiver: From Countryside to Large Industries
3.1. Biotechnological Potential of Vetiver in Agribusiness
3.1.1. Vetiver for Bioremediation in Food Industries
3.1.2. Vetiver for Bioremediation in Chemical Industries
3.1.3. Vetiver for Bioremediation in Pharmaceutical Industries
3.1.4. Vetiver for Bioremediation in Textile Industries
3.1.5. Vetiver for Bioremediation in Mining Industries
3.1.6. Vetiver for Bioremediation in Nuclear Industries
3.1.7. Soil Stabilization and Residual Water Phytoremediation
3.1.8. Biorefineries Applied to the Production of Vetiver Derivatives
3.1.9. Patents Generation from Vetiver Plant Derivatives
Scope for Patents Using Vetiver
Look for Symbiotic Association from Vetiver Mycorrhiza to Other Green Plants
Developing the Organic Antibacterial Formulation
Nutraceutical from Vetiver as a Synergistic Combination
Formulation Based on Synergism for Cancer Treatment
Herbal-Based Drug to Overcome Various Drug-Resistant Bacteria
3.2. Phytochemical Studies Aimed at Prospecting Biomolecules
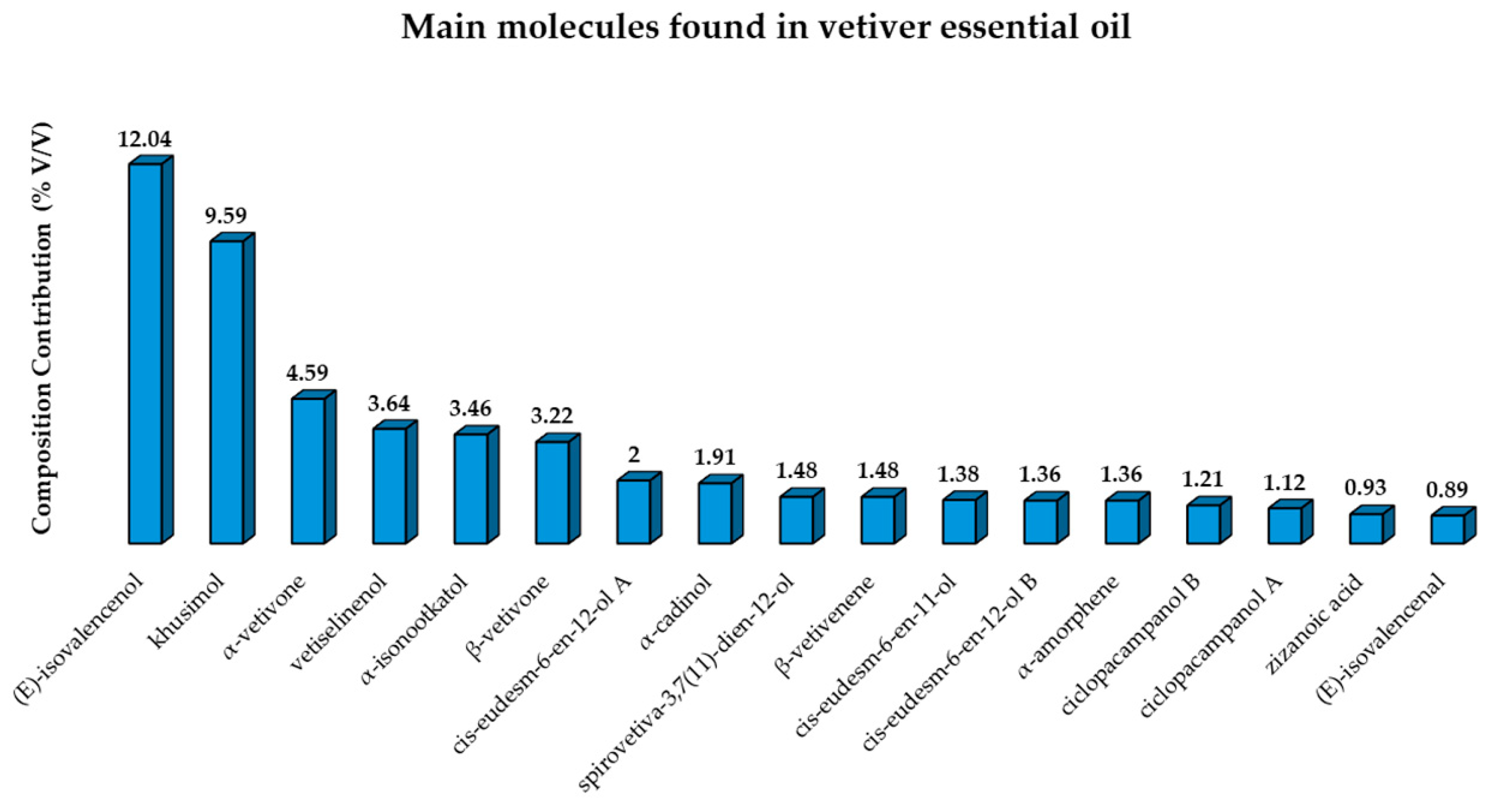
3.2.1. Main Phytochemical Differences Between Commercial Vetiver Essential Oils
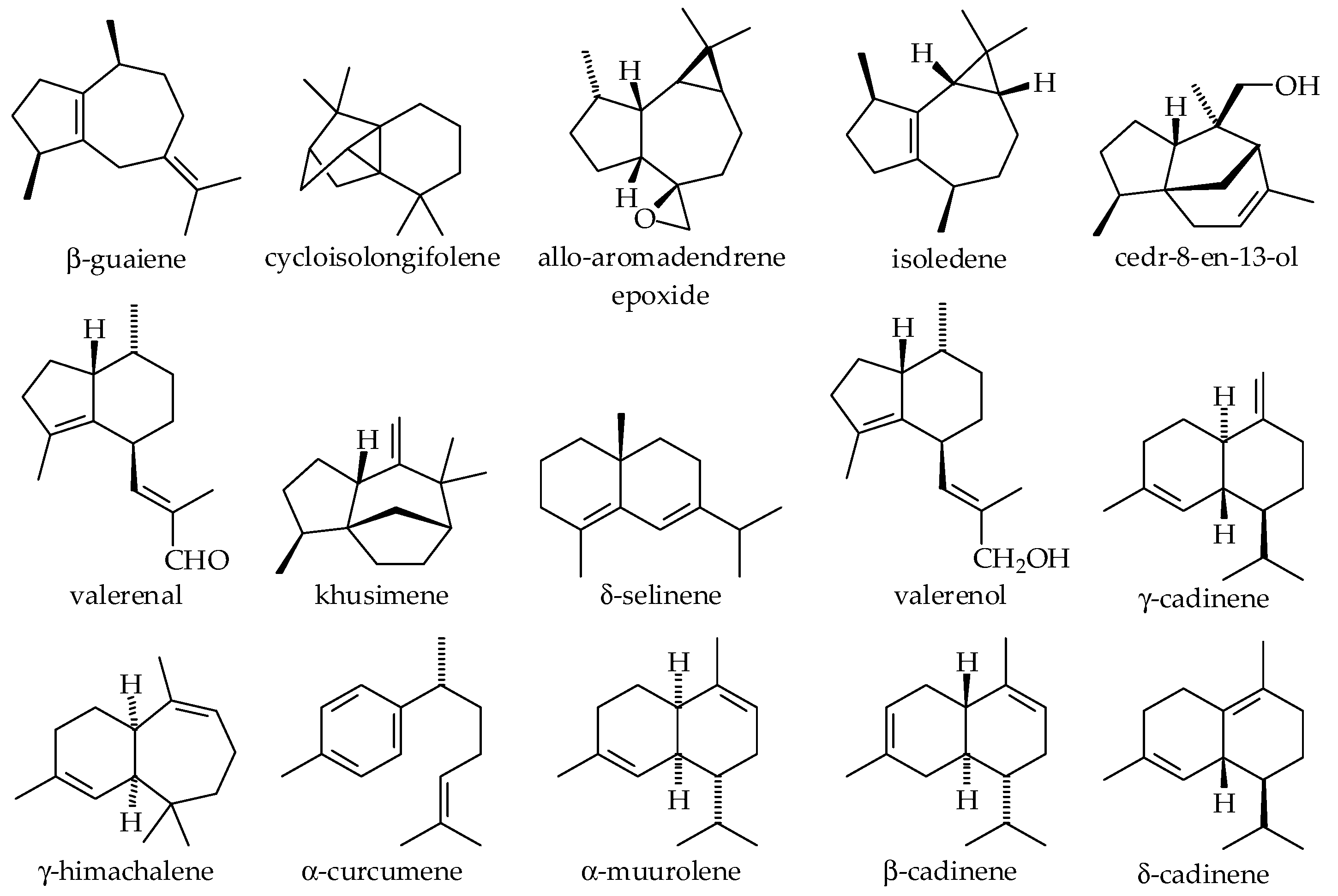
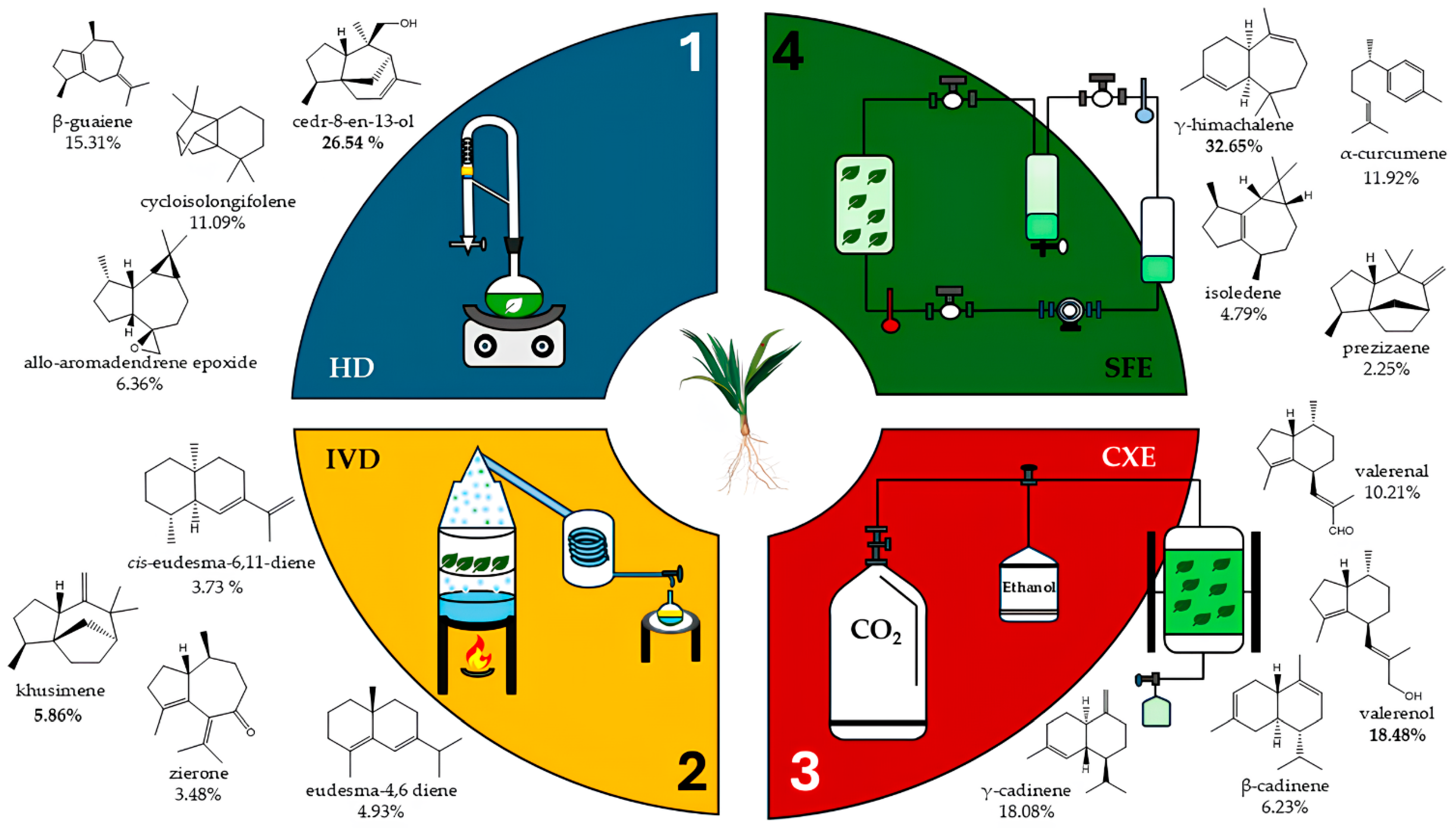
3.2.2. Technologies Used in Scale-Up Essential Oil Biomolecules Obtention
3.3. Vetiver Plant Derivatives and Their Potential Pharmacological Activities
3.3.1. Studies on the Biological Activities of Vetiver Essential Oil
3.3.2. Biological Activities of Vetiver Extracts and Components
3.3.3. Main Vetiver Vegetable Derivatives Diffused in the Pharmaceutical and Cosmetic Industries
3.4. Vetiver Essential Oil Biomolecules of Industrial Interest
Lignin Prospection from Vetiver Plant Derivatives
4. Conclusions
Funding
Conflicts of Interest
References
- Flora of Brazil 2020 under Construction, 2017. Botanical Garden of Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br (accessed on 12 August 2021).
- Verma, A.B. Vetiveria zizanioides (L.) Nash: A review of magic grass. J. Med. Plants Res. 2020, 8, 58–61. [Google Scholar]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical composition, antioxidant, and antimicrobial activities of Vetiveria zizanioides (L.) nash essential oil extracted by carbon dioxide expanded ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef] [PubMed]
- Kadarohman, A.; Dwiyanti, G.; Kadarusman, E. Quality and chemical composition of organic and non-organic vetiver oil. Indones. J. Chem. 2014, 14, 43–50. [Google Scholar] [CrossRef]
- Raman, J.K.; Gnansounou, E. A review on bioremediation potential of vetiver grass. In Waste Bioremediation; Springer: Singapore, 2018; pp. 127–140. [Google Scholar]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J. Rhizoremediation: A beneficial plant-microbe interaction. MPMI 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Gesesse, A.; Balemi, T.; Natarajan, P.; Amha, Y. Effect of vetiver grass headges in maintaining soil fertility and productivity at Anno Agro Industry Farm, Gobu Sayo District, Oromiya Region, Ethiopia. JSSD 2013, 1, 37–49. [Google Scholar]
- Suelee, A.L.; Hasan, S.N.; Kusin, F.M.; Yusuff, F.M.; Ibrahim, Z.Z. Phytoremediation potential of vetiver grass (Vetiveria zizanioides) for treatment of metal-contaminated water. Water Air Soil Pollut. 2017, 228, 158. [Google Scholar] [CrossRef]
- Dalton, P.A.; Smith, R.J.; Truong, P.N.V. Vetiver grass hedges for erosion control on a cropped flood plain: Hedge hydraulics. Agric. Water Manag. 1996, 31, 91–104. [Google Scholar] [CrossRef]
- Islam, M.P.; Bhuiyan, M.K.; Hossain, M.Z. Vetiver grass as a potential resource for rural development in Bangladesh. CIGR J. 2008, 1276, 1–8. [Google Scholar]
- Maiti, S.K.; Kumar, A. Energy plantation, medicinal and aromatic on contaminated soil. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 29–47. [Google Scholar]
- Fonseca Largo, K.M.; Ruiz Depablos, J.L.; Espitia-Sarmiento, E.F.; Llugsha Moreta, N.M. Artificial Floating Island with Vetiver for Treatment of Arsenic-Contaminated Water: A Real Scale Study in High-Andean Reservoir. Water 2020, 12, 3086. [Google Scholar] [CrossRef]
- Hailu, L.; Tesfaye, G.; Yaekob, T. Effect of vetiver grass (Vetiver zizanodes) hedgerows on selected soil properties and crop yield on farm land at Haru District, Western Ethiopia. Int. J. Res. Stud. Agric. Sci. 2020, 6, 35–41. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 11, 1571–1596. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Bioremediation of brewery wastewater using hydroponics planted with vetiver grass in Addis Ababa, Ethiopia. Bioresour. Bioprocess. 2018, 5, 1–2. [Google Scholar] [CrossRef]
- Marcacci, S.; Raveton, M.; Ravanel, P.; Schwitzguebel, J.P. Conjugation of atrazine in vetiver (Chrysopogon zizanioides Nash) grown in hydroponics. Environ. Exp. Bot. 2006, 56, 205–215. [Google Scholar] [CrossRef]
- Schwitzguebel, J.P.; Meyer, J.; Kidd, P. Pesticides removal using plants: Phytodegradation versus phytostimulation. In Phytoremediation Rhizoremediation; Springer: Dordrecht, The Netherlands, 2006; pp. 179–198. [Google Scholar]
- Gupta, S.; Schulin, R.; Kanekar, P.; Pakniker, K.M.; Schwitzguebel, J.P.; Raghu, K.; Kathrin, W. In-situ removal of Atrazine using synergy between Plant roots and rhizospheric organisms. Environ. Biotechnol. 2004, 1, 331. [Google Scholar]
- Datta, R.; Quispe, M.A.; Sarka, D. Greenhouse study on the phytoremediation potential of vetiver grass, Chrysopogon zizanioides L., in arsenic-contaminated soils. Bull. Environ. Contam. Toxicol. 2011, 86, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.X.; Li, Y.M.; Zheng, Y.; Hua, Y.; Datta, R.; Dan, Y.M.; Lv, P.; Sarkar, D. Uptake of 2, 4-bis (Isopropylamino)-6-methylthio-s-triazine by vetiver grass (Chrysopogon zizanioides L.) from hydroponic media. Bull. Environ. Contam. Toxicol. 2016, 96, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, Y.; Lv, P.; Punamiya, P.; Sarkar, D.; Dan, Y.; Ma, J.; Zheng, Y. Determination of prometryn in vetiver grass and water using gas chromatography–nitrogen chemiluminescence detection. J. Chromatogr. Sci. 2016, 54, 97–102. [Google Scholar] [CrossRef]
- Dousset, S.; Abaga, N.O.; Billet, D. Vetiver grass and micropollutant leaching through structured soil columns under outdoor conditions. Pedosphere 2016, 26, 522–532. [Google Scholar] [CrossRef]
- Abaga, N.O.; Dousset, S.; Munier-Lamy, C.; Billet, D. Effectiveness of vetiver grass (Vetiveria zizanioides L. Nash) for phytoremediation of endosulfan in two cotton soils from Burkina Faso. Int. J. Phytoremediation 2014, 16, 95–108. [Google Scholar] [CrossRef]
- Smolcz, S.U.; Cortés, V.G. Remediation of boron contaminated water and soil with vetiver phytoremediation technology in Northern Chile. In Proceedings of the 6th International Conference on Vetiver (ICV6), Da Nang, Vietnam, 5–8 May 2015. [Google Scholar]
- Angin, I.; Turan, M.; Ketterings, Q.M.; Cakici, A. Humic acid addition enhances B and Pb phytoextraction by vetiver grass (Vetiveria zizanioides (L.) Nash). Water Air Soil Pollut. 2008, 188, 335–343. [Google Scholar] [CrossRef]
- Xin, J.; Huang, B. Comparison of boron uptake, translocation, and accumulation in reed, cattail, and vetiver: An extremely boron-tolerant plant, vetiver. Plant Soil 2017, 416, 17–25. [Google Scholar] [CrossRef]
- Darajeh, N.; Idris, A.; Masoumi, H.R.; Nourani, A.; Truong, P.; Sairi, N.A. Modeling BOD and COD removal from palm oil mill secondary effluent in floating wetland by Chrysopogon zizanioides (L.) using response surface methodology. J. Environ. Manag. 2016, 181, 343–352. [Google Scholar] [CrossRef]
- Girija, N.; Pillai, S.S.; Koshy, M. Potential of vetiver for phytoremediation of waste in retting area. Ecoscan 2011, 1, 267–273. [Google Scholar]
- Singh, S.; Melo, J.S.; Eapen, S.; D’souza, S.F. Potential of vetiver (Vetiveria zizanoides L. Nash) for phytoremediation of phenol. Ecotoxicol. Environ. Saf. 2008, 71, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Saeb, K.; Khadami, R.; Khoramnejadian, S.; Abdollahi, E. Use of vetiver (Vetiveria zizanoides) in remediation of cyanide soil contamination. J. Biol. Today’s World 2015, 4, 150–155. [Google Scholar] [CrossRef]
- Ho, Y.N.; Mathew, D.C.; Hsiao, S.C.; Shih, C.H.; Chien, M.F.; Chiang, H.M.; Huang, C.C. Selection and application of endophytic bacterium Achromobacter xylosoxidans strain F3B for improving phytoremediation of phenolic pollutants. J. Hazard. Mater. 2012, 219, 43–49. [Google Scholar] [CrossRef]
- Das, P.; Datta, R.; Makris, K.C.; Sarkar, D. Vetiver grass is capable of removing TNT from soil in the presence of urea. Environ. Pollut. 2010, 158, 1980–1983. [Google Scholar] [CrossRef]
- Makris, K.C.; Shakya, K.M.; Datta, R.; Sarkar, D.; Pachanoor, D. High uptake of 2,4,6-trinitrotoluene by vetiver grass-potential for phytoremediation? Environ. Pollut. 2007, 146, 1–4. [Google Scholar] [CrossRef]
- Depledge, M. Pharmaceuticals: Reduce drug waste in the environment. Nature 2011, 478, 36. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Chen, J. Paracetamol in the environment and its degradation by microorganism. Appl. Microbiol. Biotechnol. 2012, 96, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszynska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Bacterial degradation of naproxen—Undisclosed pollutant in the environment. J. Environ. Manag. 2015, 145, 157–161. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Carminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Sarkar, D.; Datta, R. Removal of antibiotics and nutrients by Vetiver grass (Chrysopogon zizanioides) from secondary wastewater effluent. Int. J. Phytoremediation 2020, 22, 764–773. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Ziang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, D.; Das, P.; Panja, S.; Parikh, C.; Ramanathan, D.; Bagley, S.; Datta, R. Tetracycline uptake and metabolism by vetiver grass (Chrysopogon zizanioides L. Nash). Environ. Sci. Pollut. Res. 2016, 23, 24880–24889. [Google Scholar] [CrossRef]
- Datta, R.; Das, P.; Smith, S.; Punamiya, P.; Ramanathan, D.M.; Reddy, R.; Sarkar, D. Phytoremediation potential of vetiver grass [Chrysopogon zizanioides (L.)] for tetracycline. Int. J. Phytoremediation 2013, 15, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Marsidi, N.; Nye, C.K.; Abdullah, S.R.; Abu Hassan, H.; Halmi, M.I. Phytoremediation of naproxen in waste water using Vetiver zizaniodes. Int. J. Eng. Sci. Technol. 2016, 11, 1086–1097. [Google Scholar]
- Scott, A. Cutting out textile pollution. Chem. Eng. N. 2015, 93, 18–19. [Google Scholar] [CrossRef]
- Charoenlarp, K.; Surakul, K.; Winitkhetkamnoun, P.; Kanthupthim, P.; Panbumrung, P.; Udorn, S. Textile wastewater treatment using vetiver grass cultivated with floating platform technique. RMUTKJ 2016, 10, 51–57. [Google Scholar]
- Navarro, M.C.; Perez-Sirvent, C.; Martínez-Sanchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Pang, J.; Chan, G.S.; Zhang, J.; Liang, J.; Wong, M.H. Physiological aspects of vetiver grass for rehabilitation in abandoned metalliferous mine wastes. Chemosphere. 2003, 52, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Goswami, P.; Pathak, K.; Mukherjee, A. Vetiver grass: An environment clean-up tool for heavy metal contaminated iron ore mine-soil. Ecol. Eng. 2016, 90, 25–34. [Google Scholar] [CrossRef]
- Shu, W.S.; Xia, H.P.; Zhang, Z.Q.; Lan, C.Y.; Wong, M.H. Use of vetiver and three other grasses for revegetation of Pb/Zn mine tailings: Field experiment. Int. J. Phytoremediation 2002, 4, 47–57. [Google Scholar] [CrossRef]
- Shaw, G.; Bell, J.N. Competitive effects of potassium and ammonium on caesium uptake kinetics in wheat. J. Environ. Radioact. 1991, 13, 283–296. [Google Scholar] [CrossRef]
- Singh, S.; Eapen, S.; Thorat, V.; Kaushik, C.P.; Raj, K.; D’souza, S.F. Phytoremediation of 137cesium and 90strontium from solutions and low-level nuclear waste by Vetiveria zizanoides. Ecotoxicol. Environ. Saf. 2008, 69, 306–311. [Google Scholar] [CrossRef]
- Grimshaw, R.G.; Helfer, L. Vetiver Grass for Soil and Water Conservation, Land Rehabilitation, and Embankment Stabilization; World Bank Technical paper no. 273; The World Bank: Washington, DC, USA, 1995. [Google Scholar]
- Hung, L.V.; Maslov, O.D.; Thu My, T.T.; Ho, P.K.; Nhan, D.D. Uranium uptake of Vetiveria zizanioides (L.) Nash. Technical Report INR-E-18-2010-71; Joint Institute for Nuclear Research: Dubna, Russia, 2010. [Google Scholar]
- Chusreeaeom, K.; Roongtanakiat, N. Selection of vetiver grass based on growth and nutrient content under saline water irrigation and waterlogging prior to mutagenesis. Songklanakarin J. Sci. Technol. 2020, 42, 1. [Google Scholar]
- Holanda, F.S.R.; Dias, K.L.L.D.; Santos, L.D.V.; Brito, C.R.D.M.; Melo, J.C.R.D.; Santos, L.S. Development and morphometric characteristics of vetiver grass under different doses of organic fertilizer. Rev. Caatinga 2021, 34, 20–30. [Google Scholar] [CrossRef]
- Zago, V.C.P.; das Dores, N.C.; Watts, B.A. Strategy for phytomanagement in an area affected by iron ore dam rupture: A study case in Minas Gerais State, Brazil. Environ. Pollut. 2019, 249, 1029–1037. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Li, R.; Chang, J.; Fu, J. Influence of Vetiver Root System on Mechanical Performance of Expansive Soil: Experimental Studies. Adv. Civ. Eng. 2020, 11, 2027172. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Gholami, S.; Ehteshami, M.; Salari, M. Evaluation of Phytoremediation Potential of Vetiver Grass (Chrysopogon zizanioides (L.) Roberty) for Wastewater Treatment. Adv. Mater. Sci. Eng. 2021, 12, 3059983. [Google Scholar] [CrossRef]
- Siyar, R.; Ardejani, F.D.; Farahbakhsh, M.; Norouzi, P.; Yavarzadeh, M.; Maghsoudy, S. Potential of Vetiver grass for the phytoremediation of a real multi-contaminated soil, assisted by electrokinetic. Chemosphere 2020, 246, 125802. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Boyce, A.N.; Abas, M.R.; Mahmood, N.Z.; Han, F. Evaluation of Vetiver grass uptake efficiency in single and mixed heavy metal contaminated soil. Environ. Process. 2020, 7, 207–226. [Google Scholar] [CrossRef]
- Davamani, V.; Parameshwari, C.I.; Arulmani, S.; John, J.E.; Poornima, R. Hydroponic phytoremediation of paperboard mill wastewater by using vetiver (Chrysopogon zizanioides). J. Environ. Chem. Eng. 2021, 9, 105528. [Google Scholar] [CrossRef]
- Raman, J.K.; Alves, C.M.; Gnansounou, E. A review on moringa tree and vetiver grass–Potential biorefinery feedstocks. Bioresour. Technol. 2018, 249, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Gnansounou, E.; Alves, C.M.; Raman, J.K. Multiple applications of vetiver grass—A review. Int. J. Educ. 2017, 2, 125–141. [Google Scholar]
- Natarajan, S.; Thamba, N.B.; Nandi, S.; Kavitha, K.V.N.; Ayyagari, S. Effect of fiber orientation on the tensile and flexural properties of vetiverfiber reinforced epoxy composites. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1123, 012048. [Google Scholar] [CrossRef]
- Shakthivel, S.; Kurmapu, S. Characteristic investigation on vetiver fibre reinforced polyethylene composites. AIP Conf. Proc. 2021, 2317, 040014. [Google Scholar]
- Raman, J.K.; Gnansounou, E. Furfural production from empty fruit bunch—A biorefinery approach. Ind. Crops Prod. 2015, 69, 371–377. [Google Scholar] [CrossRef]
- Soegoto, E.S.; Jumansyah, R.I.Z.K.Y.; Luckyardi, S.E.N.N.Y. Vetiver Waste Fertilizer as A Growth Catalyst on Mung Bean. Int. J. Eng. Sci. Technol. 2021, 16, 165–175. [Google Scholar]
- Santana, P.H.L.; Burak, D.L.; Thiengo, C.C.; Peçanha, A.L.; Neves, M.A.; de Sá Mendonça, E. Jack beans and vetiver grass growth on iron ore tailing sediments from the Doce River dam disaster in Brazil: Plant growth regulator effects under different edaphic conditions. J. Soils Sediments 2020, 20, 4103–4110. [Google Scholar] [CrossRef]
- Notar Francesco, I.; Filippi, J.J.; Antoniotti, S. Sustainable Manufacture of a Valuable Fragrance Ingredient: Lipase-Catalyzed Acylation of Vetiver Essential Oil and Chemoselectivity between Sesquiterpene Alcohols. ChemPlusChem 2017, 82, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Raharjo, T.T.; Ambarwati, Y. Isolation and Identification of Terpenoid Compound from Vetiver Grass-Root (Vetiveria zizanioides Stapf) as a Repellent against Termite (Cyrptotermes sp.) through Bioactivity Assay. J. Phys. Conf. Ser. 2021, 1751, 012101. [Google Scholar] [CrossRef]
- Arokyaraj, D.M.; Rathiga, G. Phytochemicals and pharmacological activities of kabhasura kudineer, nilavembu kudineer, adathodai kudineer-a review. Int. J. Trans. Res. Ind. Med. 2020, 2, 41–51. [Google Scholar]
- Grover, M.; Behl, T.; Bungau, S.; Aleya, L. Potential therapeutic effect of Chrysopogon zizanioides (Vetiver) as an anti-inflammatory agent. Environ. Sci. Pollut. Res. 2021, 28, 15597–15606. [Google Scholar] [CrossRef]
- Ali, S.A.; Nayak, A.K.; Banerjee, S.; Sen, K.K. In silico molecular docking of Vetiver oil and formulation of Vetiver oil-Encapsulated gellan gum-based Microcapsules for Antidepressant activity. Res. J. Pharm. Technol. 2020, 13, 3135–3142. [Google Scholar] [CrossRef]
- Lia, G.; Fangb, X.; Lib, J.; Wub, N.; Wangb, H.; Wanga, X. Preparation of super activated carbon of vetiver root: Optimization by response surface method and study of adsorption behavior on bisphenol A in solution. In Proceedings of the 11th International Conference on Challenges in Environmental Science & Engineering, Bangkok, Thailand, 4–8 November 2018. [Google Scholar]
- Mao, X.; Kang, Q.; Liu, Y.; Siyal, A.A.; Ao, W.; Ran, C.; Dai, J. Microwave-assisted pyrolysis of furfural residue in a continuously operated auger reactor: Biochar characterization and analysis. Energy 2019, 168, 573–584. [Google Scholar] [CrossRef]
- Joshi, D.D.; Sharma, R.K. Herbal Drugs in Patent Regime, DM Verlag, Saarbrücken, Germany Dr. Müller: 2011; ISBN-13: 978-3639321821. ISBN-10: 3639321820.
- Chahal, K.K.; Bhardwaj, U.; Kaushal, S.; Sandhu, A.K. Chemical composition and biological properties of Chrysopogon zizanioides (L.) Roberty syn. Vetiveria zizanioides (L.) Nash—A Review. Indian J. Nat. Prod. Resour. 2015, 6, 251–260. [Google Scholar]
- Adams, R.P.; Habte, M.; Park, S.; Dafforn, M.R. Preliminary comparison of vetiver root essential oils from cleansed (bacteria- and fungus-free) versus non-cleansed (normal) vetiver plants. Biochem. Syst. Ecol. 2004, 32, 1137–1144. [Google Scholar] [CrossRef]
- Srivastava, J.; Chandra, H.; Singh, N. Allelopathic response of Vetiveria zizanioides (L.) Nash on members of the family Enterobacteriaceae and Pseudomonas spp. Environmentalist 2007, 27, 253–260. [Google Scholar] [CrossRef]
- Glory, J.I.; Amutha, A.; Rahman, F.; Kumaravelu, P.; Muthiah, N.S. Comparative study of Vetiveria zizanioides and Foeniculum vulgare extracts on behavioral despair of wistar albino rats. J. Chem. Pharm. Res. 2015, 7, 729–734. [Google Scholar]
- Chen, F.; Wang, X.; Kim, H. Antioxidant, Anticarcinogenic and Termiticidal Activities of Vetiver Oil. 2003. Available online: http://www.vetiver.org/ICV3-Proceedings/USA_medicinal.pdf (accessed on 12 October 2021).
- Rao, R.R.; Suseela, M.R. Vetiveria zizaniodes (Linn.) Nash. A multipurpose ecofriendly grass of India. In Proceedings of the Second International Conference on Vetiver, Office of the Royal Development Projects Board, Bangkok, Thailand, 18–20 January 2000; pp. 444–448. [Google Scholar]
- Blanchard, J.S. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu. Rev. Biochem. 1996, 65, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Paillat, L.; Périchet, C.; Pierrat, J.P.; Lavoine, S.; Filippi, J.J.; Meierhenrich, U.; Fernandez, X. Purification of vetiver alcohols and esters for quantitative high-performance thin-layer chromatography determination in Haitian vetiver essential oils and vetiver acetates. J. Chromatogr. A 2012, 1241, 103–111. [Google Scholar] [CrossRef]
- Champagnat, P.; Figueredo, G.; Chalchat, J.C.; Carnat, A.P.; Bessière, J.M. A study on the composition of commercial Vetiveria zizanioides oils from different geographical origins. J. Essent. Oil Res. 2006, 18, 416–422. [Google Scholar] [CrossRef]
- Filippi, J.J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2013, 1288, 127–148. [Google Scholar] [CrossRef]
- Martinez, J.; Rosa, P.T.V.; Menut, C.; Leydet, A.; Brat, P.; Pallet, D.; Meireles, M.A.A. Valorization of Brazilian Vetiver (Vetiveria zizanioides (L.) Nash ex Small) Oil. J. Agric. Food Chem. 2004, 52, 6578–6584. [Google Scholar] [CrossRef]
- Danh, L.T.; Mammucari, R.; Truong, P.; Foster, N. Response surface method applied to supercritical carbon dioxide extraction of Vetiveria zizanioides essential oil. J. Chem. Eng. 2009, 155, 617–626. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lai, C.C.; Lin, C.C.; Chou, S.T. Effect of Vetiveria zizanioides essential oil on melanogenesis in melanoma cells: Downregulation of tyrosinase expression and suppression of oxidative stress. Sci. World J. 2014, 2014, 213013. [Google Scholar] [CrossRef]
- Danh, L.T.; Truong, P.; Mammucari, R.; Foster, N. Extraction of vetiver essential oil by ethanol-modified supercritical carbon dioxide. J. Chem. Eng. 2010, 165, 26–34. [Google Scholar] [CrossRef]
- Massardo, D.R.; Senatore, F.; Alifano, P.; Del Giudice, L.; Pontieri, P. Vetiver oil production correlates with early root growth. Biochem. Syst. Ecol. 2006, 34, 376–382. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Wongpornchai, S.; Promsiri, A. Highly volatile constituents of Vetiveria zizanioides roots grown under different cultivation conditions. Molecules 2006, 11, 817–826. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Suyono, H.; Susanti, D. The Medical Benefits of Vetiver Essential Oil. In Proceedings of the 2nd International Conference of Essential Oils—ICEO, Banda Aceh, Indonesia, 29–30 October 2019; pp. 9–12, ISBN 978-989-758-456-5. [Google Scholar]
- Grover, M.; Behl, T.; Virmani, T.; Bhatia, S.; Al-Harrasi, A.; Aleya, L. Chrysopogon zizanioides—A Review on Its Pharmacognosy, Chemical Composition and Pharmacological Activities. Environ. Sci. Pollut. Res. 2021, 28, 56752–56767. [Google Scholar] [CrossRef] [PubMed]
- International Trade in Goods and Services visualization. Available online: https://www.bea.gov/data/intl-trade-investment/international-trade-goods-and-services (accessed on 12 August 2021).
- Jain, S.K. Dictionary of Indian Folk Medicine and Ethnobotany; Deep Publ.: New Delhi, India, 1991. [Google Scholar]
- Singh, K.K.; and Maheshwari, J.K. Traditional phytotherapy amongst the tribals of Varanasri district, U.P. J. Econ. Taxon. Bot. 1983, 4, 829–838. [Google Scholar]
- Khan, U.G.; Saeed, A.; Alam, M.T. Indusyunic Medicine: Traditional Medicine of Herbal, Animal and Mineral Origin in Pakistan; Department of Pharmacognosy, Faculty of Pharmacy, University of Karachi: Karachi, Pakistan, 1997. [Google Scholar]
- The Vetiver Network International. Patron - H.R.H Princess Maha Chakri Sirindhorn of Thailand. Dick G. Socio-Economic Dimensions Of Vetiver In Rainfed Areas Of Karnataka (India). Available online: https://www.vetiver.org/document-folders/socio-economic-dimensions-of-vetiver-in-rainfed-areas-of-karnataka-india/ (accessed on 12 August 2021).
- Narong, C.; Keith, C. Other Uses, and Utilization of Vetiver. AU J. T. 2003, 7, 81–91. [Google Scholar]
- Lingga, P. Recipes of Traditional Medicine, 19th ed.; Penebar Swadaya: Jakarta, Indonesia, 2001. (In Indonesian) [Google Scholar]
- Shealy, C.N. The Illustrated Encyclopedia of Healing Remedies; Element: Shaftesbury, UK, 1998. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisous Plants of Southern and Eastern Africa, 2nd ed.; Livingstone: London, UK, 1962. [Google Scholar]
- Gurib-Fakim, A.; Sewraj, M.; Gueho, J.; Dulloo, E. Medical Ethnobotany and some weeds of Mauritius and Rodrigues. J. Ethnopharmacol. 1993, 39, 175–185. [Google Scholar] [CrossRef]
- Anon. Medicinal Plants of Nepal; Department of Medicinal Plants: Kathmandu, Nepal, 1970. [Google Scholar]
- Su-Tze, C.; Chia-Pei, L.; Chih-Chien, L.; Shih, Y. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chem. 2012, 134, 262–268. [Google Scholar]
- Amarasiri, S.S.; Attanayake, A.P.; Arawwawala, L.D.A.M.; Jayatilaka, K.A.P.W.; Mudduwa, L.K.B. Nephroprotective activity of Vetiveria zizanioides (L.) Nash supplement in doxorubicin-induced nephrotoxicity model of Wistar rats. J. Food Biochem. 2021, 45, e13901. [Google Scholar]
- Luqman, S.; Kumar, R.; Kaushik, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian. J. Biochem. Biophys. Methods 2009, 46, 122–125. [Google Scholar]
- Kim, H.J.; Chen, F.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J. Agric. Food. Chem. 2005, 53, 7691–7695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.C.R.; Henderson, G.; Chen, F.; Fei, H.; Laine, R.A. Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. J. Chem. Ecol. 2001, 27, 1617–1625. [Google Scholar] [CrossRef]
- Lima, G.M.; Quintans-Júnior, L.J.; Thomazzi, S.M.; Almeida, E.M.S.A.; Melo, M.S.; Serafini, M.R.; Cavalcanti, S.C.H.; Gelain, D.P.; Santos, J.P.A.S.; Blank, A.F.; et al. Phytochemical screening, antinociceptive and anti-inflammatory activities of Chrysopogon zizanioides essential oil. Braz. J. Pharm. 2012, 22, 443–450. [Google Scholar] [CrossRef]
- Zahoor, S.; Shahid, S.; Fatima, U. Review of Pharmacological Activities of Vetiveria zizanioides (Linn) Nash. J. Basic Appl. Sci. 2018, 14, 235–238. [Google Scholar] [CrossRef][Green Version]
- Balasankar, D.; Vanilarasu, K.; Preetha P., S.; Umadevi, S.R.M.; Bhowmik, D. Traditional and Medicinal Uses of Vetiver. J. Med. Plants Stud. 2013, 1, 191–200. [Google Scholar]
- Burger, P.; Pandreau, A.; Watson, M.; Janci, O.; Cassisa, V.; Kempf, M.; Azoulay, S.; Fernandez, X. Vetiver Essential Oil in Cosmetics: What Is New? Medicines 2017, 16, 41. [Google Scholar] [CrossRef]
- Nararak, J.; Sanguanpong, U.; Sukkanon, C.; Manguin, S.; Chareonviriyaphap, T. Synergistic Repellent and Irritant Effects of a Mixture of β-Caryophyllene Oxide and Vetiver Oil against Mosquito Vectors. Insects 2023, 14, 773. [Google Scholar] [CrossRef]
- Mao, L.; Henderson, G.; Bourgeois, W.J.; Vaughn, J.A.; Laine, R.A. Vetiver oil and nootkatone effects on the growth of pea and citrus. Ind. Crops Prod. 2013, 23, 327–332. [Google Scholar] [CrossRef]
- Zhu, B.C.; Henderson, G.; Chen, F.; Maistrello, L.; Laine, R.A. Nootkatone is a repellent for Formosan subterranean termite (Coptotermes formosanus). J. Chem. Ecol. 2001, 27, 523–531. [Google Scholar] [CrossRef]
- Orchard, A.; Van Vuuren, S.F.; Viljoen, A.M.; Kamatou, G. The in vitro antimicrobial evaluation of commercially essential oils and their combinations against acne. Int. J. Cosmet. Sci. 2018, 40, 226–243. [Google Scholar] [CrossRef]
- Chaumont, J.P.; Bardey, I. The In Vitro Antifungal Activities of Seven Essential Oils. Fitoterapia. 1989, 60, 263–266. [Google Scholar]
- Viollon, C.; Chaumont, J.P. Antifungal Properties of Essential Oils and Their Compounds upon of Cryptococcus neoformans. Mycopatholgia 1994, 128, 151–153. [Google Scholar] [CrossRef]
- Semde, Z.; Koudou, J.; Zongo, C.; Figueredo, G.; Somda, M.K.; Ganou, L.; Traore, A.S. Chemical composition, antioxidant and antimicrobial activities of the essential oil of Vetiveria nigritana (Benth.) Stapf roots from Burkina Faso. J. Aappl. Biol. Biotechnol. 2017, 5, 29–36. [Google Scholar]
- Viollon, C.; Mandin, D.; Chaumont, J.P. Antagonistic Activities, In Vitro, of Some Essential Oils and Natural Volatile Compounds in Relation to the Growth of Trichomonas vaginalis. Fitoterapia 1996, 67, 279–281. [Google Scholar]
- Jindapunnapat, K.; Reetz, N.D.; MacDonald, M.H.; Bhagavathy, G.; Chinnasri, B.; Soonthornchareonnon, N.; Saanarukkit, A.; Chauhan, K.R.; Chitwood, D.J.; Meyer, S.L.F. Activity of Vetiver Extracts and Essential Oil against Meloidogyne incognita. J. Nematol. 2018, 50, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.R.; Gupta, S.; Roy, S.; Kalani, K.; Pal, A.; Thakur, J.P.; Saikia, D.; Sharma, A.; Darmwal, N.S.; Darokar, M.P.; et al. Tricyclic sesquiterpenes from Vetiveria zizanoides (L.) Nash as antimycobacterial agents. Chem. Biol. Drug Des. 2013, 82, 587–594. [Google Scholar] [CrossRef]
- Atif, M.; Ilavenil, S.; Devanesan, S.; AlSalhi, M.S.; Choi, K.C.; Vijayaraghavan, P.; Alfuraydi, A.A.; Alanazi, N.F. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2020, 147, 112239. [Google Scholar] [CrossRef]
- Anyango, J.J.; Bautze, D.; Fiaboe, K.K.M.; Lagat, Z.O.; Muriuki, A.W.; Stöckli, S.; Onyambu, G.K.; Musyoka, M.W.; Karanja, E.N.; Adamtey, N. Termite-Induced Injuries to Maize and Baby Corn under Organic and Conventional Farming Systems in the Central Highlands of Kenya. Insects 2019, 22, 367. [Google Scholar] [CrossRef]
- Dubey, N.; Raghav, C.S.; Gupta, R.L.; Chhonkar, S.S. Chemical composition and antifungal activity of vetiver oil of North and South India against Rhizoctonia solani. Pest Res. J. 2010, 22, 63–67. [Google Scholar]
- Soidrou, S.H.; Farah, A.; Satrani, B.; Ghanmi, M.; Jennan, S.; Hassane, S.O.S.; Lachkar, M.; El Abed, S.; Ibnsouda Koraichi, S.; Bousta, D. Fungicidal activity of four essential oils from Piper capense, Piper borbonense and Vetiveria zizanoides growing in Comoros against fungi decay wood. J. Essent. Oil Res. 2013, 25, 216–223. [Google Scholar] [CrossRef]
- Stevanovic, D.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Parveen, S.; Gupta, V.K.; Luqman, S. Anti-tuberculosis activity of Indian grass KHUS (Vetiveria zizanioides L. Nash). Complement. Ther. Med. 2012, 20, 434–436. [Google Scholar] [CrossRef]
- Soni, A.; Dahiya, P. Screening of phytochemicals and antimicrobial potential of extracts of Vetiveria zizanioides and Phragmites karka against clinical isolates. Int. J. Appl. Pharm. 2015, 7, 22–24. [Google Scholar]
- Krishnaveni, V.; Amphrita, S. Analysis of phytochemical and anti-microbial activity on Vetiveria zizanioides ethanolic extract for healthcare applications. Int. J. Pharma Bio Sci. 2016, 7, 758–765. [Google Scholar]
- Snigdha, M.; Kumar, S.S.; Sharmistha, M.; Deepa, C. An Overview on Vetiveria zizanioides. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 777. [Google Scholar]
- Karan, S.K.; Mishra, S.K.; Pal, D. Antihyperglycemic Effect of Vetivera zizanoidies (L.) Nash Root Extract in Alloxan Induced Diabetic Rats. J. Pharm. Sci. Innov. 2012, 1, 35–38. [Google Scholar]
- Rajeswari, G.; Rajagopalan, V. Evaluation of Anti-Diabetic Effects of Chrysopogon zizanioides L. in Root Extracts in Streptozotocin Induced Diabeteic Wistar Rats. J. Sci. Innov. Res. 2013, 2, 555–574. [Google Scholar]
- Chomchalow, N. The Utilization of Vetiver as Medicinal and Aromatic Plants with Special References to Thailand; Tech. Bull. No. 2001/1; PVRN/ORDPB: Bangkok, Thailand, 2000. [Google Scholar]
- MacDonald, D. Emotions and Essential Oils. A Modern Resource for Healing Emotional Reference Guide. In Enlighten Alternative Healing, 2nd ed.; Enlighten: Wexford, Ireland, 2013. [Google Scholar]
- Gupta, R.; Sharma, K.K.; Afzal, M.; Damanhouri, Z.A.; Ali, B.; Kaur, R.; Kazmi, I.; Anwar, F. Anticonvulsant activity of ethanol extracts of Vetiveria zizanioides roots in experimental mice. Pharm. Biol. 2013, 51, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Saiyudthong, S.; Pongmayteegul, S.; Marsden, C.A.; Phansuwan-Pujito, P. Anxiety-like Behaviour and c-fos Expression in Rats that Inhaled Vetiver Essential Oil. Nat. Prod. Res. 2015, 29, 2141–2144. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Styal, P.; Setzer, W.N. Antifungal and Cytotoxicity Activities of Sixty Comercially-Available Essential Oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety. A Guide for Health Care Professional, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hanifa, M.; Wulandari, R.; Zulfin, U.M.; Nugroho, E.P.; Haryanti, S.; Meiyanto, E. Chemopreventive property of vetiver oil targets CNR2. Asian Pac. J. Cancer Prev. 2022, 23, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Shah, P.; Thakkar, V.; Al-Rejaie, S.; Gandhi, T. Hepatoprotective Potential of Methanolic Extract of Vetiveria Zizanioides Roots against Carbon Tetrachlorideinduced acute Liver Damage in Rats. Digest J. Nanomater. Biostruct. 2013, 8, 835–844. [Google Scholar]
- Bhardwaj, U.; Chahal, K.K.; Dhillon, N.K.; Kaur, R. Nematicidal Activity of Vetiver Essential Oil and Its Fractions Against Meloidogyne incognita. Indian J. Nematol. 2017, 43, 43–50. [Google Scholar]
- Zhu, B.C.; Henderson, G.; Sauer, A.M.; Yu, Y.; Crowe, W.; Laine, R.A. Structure-activity of valencenoid derivatives and their repellence to the Formosan subterranean termite. J. Chem. Ecol. 2003, 29, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, S. Essential Oil and its Insecticidal Activity of Medicinal Aromatic Plant Vetiveria zizanioides (L.) Against the Red Flour Beetle Tribolium castaneum (Herbst). Asian J. Agric. Sci. 2010, 2, 84–88. [Google Scholar]
- Atta, B.; Rizwan, M.; Sabir, A.M. Damage potential of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) on wheat grains stored in hermetic and non-hermetic storage bags. Int. J. Trop. Insect Sci. 2020, 40, 27–37. [Google Scholar] [CrossRef]
- Jain, S.C.; Nowicki, T.E.S.; Meinwald, J. Insect Repellents from Vetiver Oil: I. Zizanal and Epizizanal. Tet. Lett. 1982, 23, 4639–4642. [Google Scholar] [CrossRef]
- Henderson, G.; Heumann, D.O.; Laine, R.A.; Maistrello, L.; Zhu, B.C.; Chen, F. Extracts of Vetiver Oil as Repellent and Toxicant to Ants, Ticks, and Cockroaches. Priority to US09/932,555. U.S. Patent 6906108B2, 17 August 2001. [Google Scholar]
- Urvashi, K.K.; Ramandeep Kaur, C.; Kaur, J. Evaluation of Vetiver Oil against Selected Rice Pathogens. Natural Resource Management: Ecological Perspectives Indian Ecological Society. In Proceedings of the International Conference-2016, SKUAST, Jammu, India, 18–20 February 2016. [Google Scholar]
- Yan-Hui, L.; Xu-Song, Z.; Zhong-Xian, L. Application of vetiver grass Vetiveria zizanioides: Poaceae (L.) as a trap plant for rice stem borer Chilo suppressalis: Crambidae (Walker) in the paddy fields. J. Integr. Agric. 2019, 18, 797–804. [Google Scholar]
- Barbieri, C.; Borsotto, P. Essential oils: Market and legislation. Potential Essent. Oils 2018, 107–127. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Lunz, K.; Stappen, I. Back to the Roots. An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules. 2021, 26, 3155. [Google Scholar] [CrossRef]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.M.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.; Drechsler, M. Fabrication of alginate-based O/W nanoemulsions for transdermal drug delivery of lidocaine: Influence of the oil phase and surfactant. Molecules 2021, 26, 2556. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A novel drug delivery approach for enhancement of bioavailability. Recent Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Al-Khalifa, A.R.; Aouf, A.; Boukhebti, H.; Farouk, A. Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil. Sci. Rep. 2020, 10, 2812. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First ‘Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Strzemski, M. Toxicity of carlina oxide—A natural polyacetylene from the Carlina acaulis roots—In vitro and in vivo study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lei, L.; Qian, Y.; Xie, A.; Qiu, X. Biomass lignin stabilized anti-UV high internal phase emulsions: Preparation, rheology, and application as carrier materials. ACS Sustain. Chem. Eng. 2018, 7, 810–818. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Ago, M.; Tardy, B.L.; Wang, L.; Guo, J.; Khakalo, A.; Rojas, O.J. Supramolecular assemblies of lignin into nano-and microparticles. MRS Bull. 2017, 42, 371–378. [Google Scholar] [CrossRef]

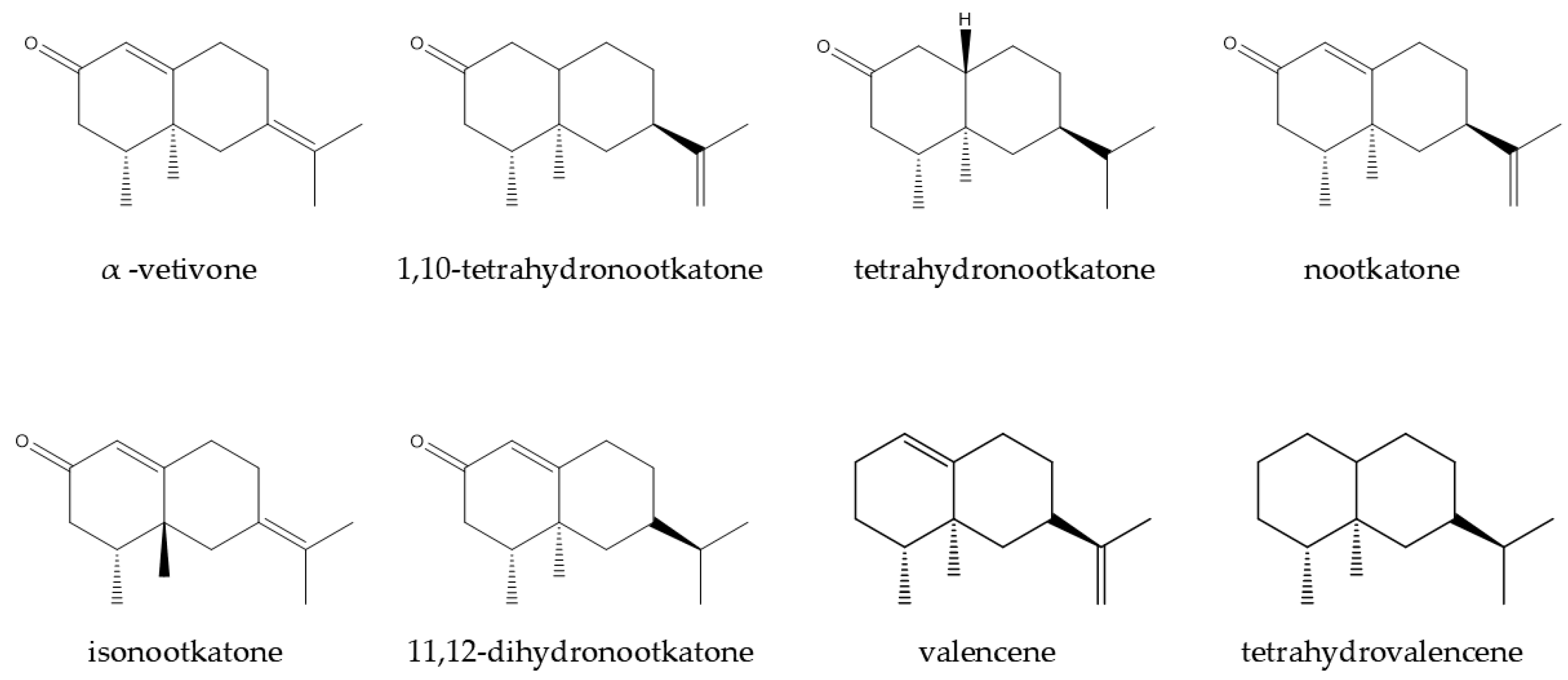
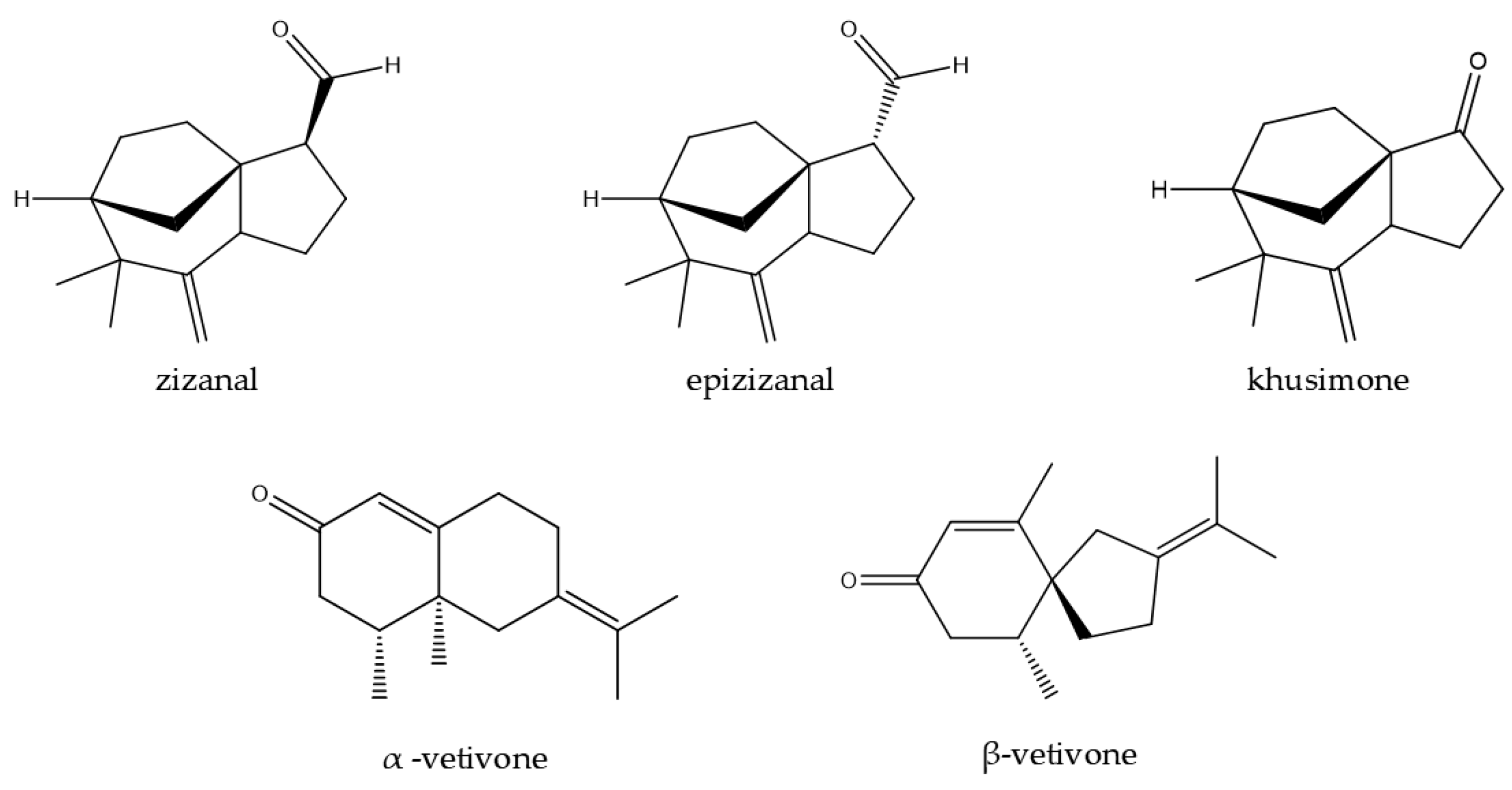


| Component | Target | Reference |
|---|---|---|
| Leaf | Paper pulp | [64] |
| Textile fibers | [65,66] | |
| Furfural | [64,67] | |
| Biofertilizer | [68,69] | |
| Root oil | Aroma | [70] |
| Termite-repellent terpenoid | [71] | |
| Anti-hypertensive and anti-spasmodic | [72] | |
| Anti-inflammatory agent | [73] | |
| Gum microcapsules with antidepressant activity | [74] | |
| Oil Free Root | Activated charcoal | [75] |
| Countries | Compounds (%) | ||||||
|---|---|---|---|---|---|---|---|
| (E)-Isovalencenol | Khusimol | α-Vetivone | β-Vetivone | Vetiselinenol | β-Vetivenene | Zizanoic Acid | |
| Madagascar | 3.3 | 6.93 | 5.73 | 11.46 | 2.75 | 4.5 | 5.16 |
| India | 2.4 | 26.7 | 2.6 | 2.8 | 1.7 | 12.2 | 1.0 |
| Indonesia | 2.78 | 10.66 | 4,25 | 7.31 | 0.42 | 7.13 | 1.84 |
| Filipinas | 4.2 | 22.08 | 2.85 | 7.24 | 2.15 | 2.01 | 1.55 |
| Guatemala | 7.1 | 20.3 | 2.7 | 0.6 | 1.6 | 2.1 | 27.2 |
| Haiti | 14.62 | 11.57 | 6.28 | 4.45 | 5.79 | 2.03 | 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcellos-Silva, I.G.C.; dos Santos, F.K.F.; Kharkwal, H.; Chander, S.; Kharkwal, A.C.; Awasthi, R.; Dhiman, N.; Sharma, B.; Kulkarni, G.T.; Larssen, H.; et al. Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals. Plants 2025, 14, 1435. https://doi.org/10.3390/plants14101435
Barcellos-Silva IGC, dos Santos FKF, Kharkwal H, Chander S, Kharkwal AC, Awasthi R, Dhiman N, Sharma B, Kulkarni GT, Larssen H, et al. Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals. Plants. 2025; 14(10):1435. https://doi.org/10.3390/plants14101435
Chicago/Turabian StyleBarcellos-Silva, Ian G. C., Filipe K. F. dos Santos, Harsha Kharkwal, Subhash Chander, Amit C. Kharkwal, Rajendra Awasthi, Neerupma Dhiman, Bhupesh Sharma, Giriraj T. Kulkarni, Harold Larssen, and et al. 2025. "Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals" Plants 14, no. 10: 1435. https://doi.org/10.3390/plants14101435
APA StyleBarcellos-Silva, I. G. C., dos Santos, F. K. F., Kharkwal, H., Chander, S., Kharkwal, A. C., Awasthi, R., Dhiman, N., Sharma, B., Kulkarni, G. T., Larssen, H., Silva, J. M. L., Souza, M. A. d., Setzer, W. N., & Veiga-Junior, V. F. (2025). Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, Biorefineries, and the Production of Volatile Phytochemicals. Plants, 14(10), 1435. https://doi.org/10.3390/plants14101435












