Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential
Abstract
1. Introduction
2. Generalities and Classification of Cannabis Metabolites
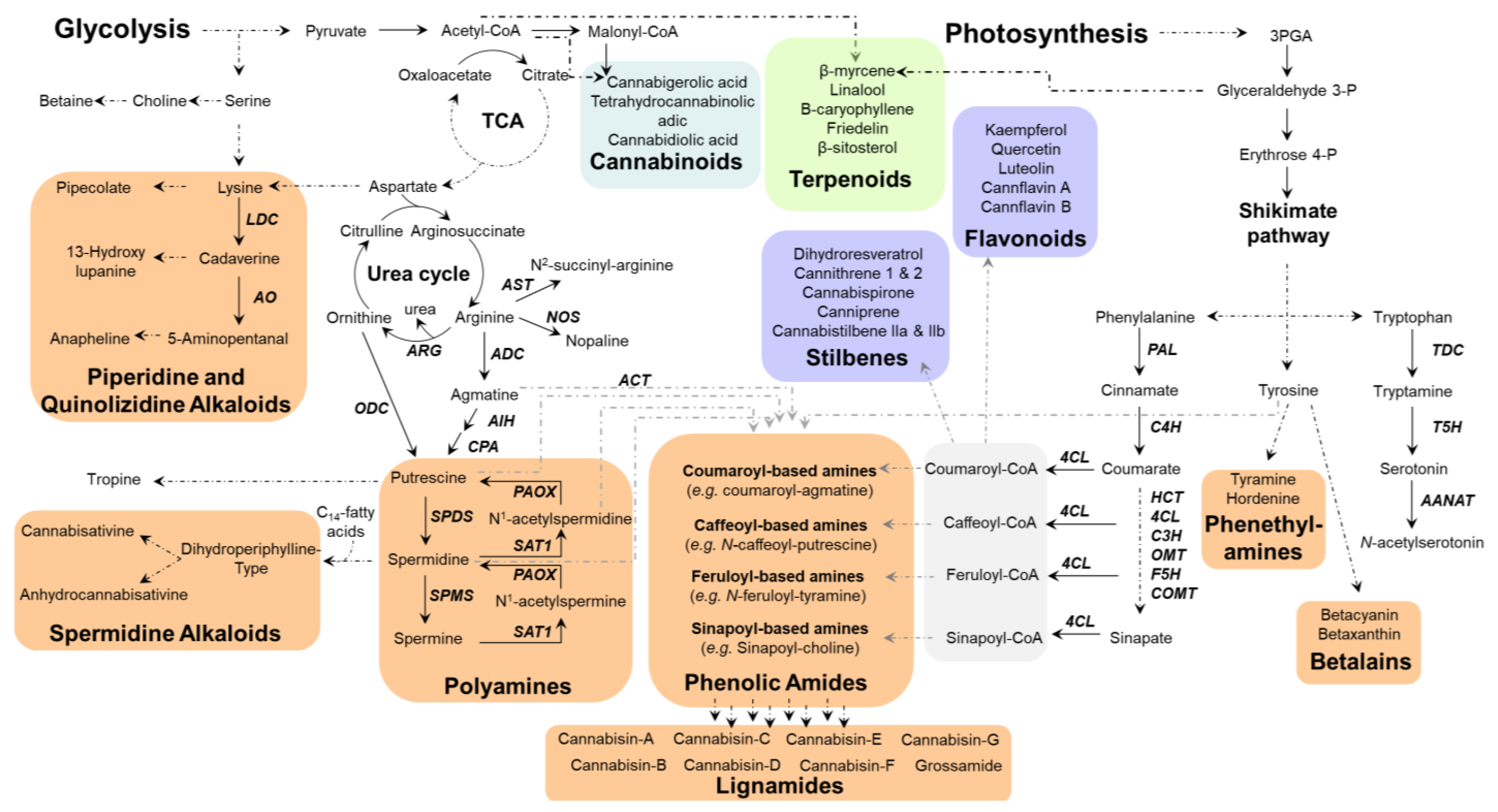
2.1. Phenolic Compounds of Cannabis
2.2. Alkaloids of Cannabis
2.3. Specialized Metabolites of Cannabis
3. Biosynthetic Routes to Nitrogen-Containing and Phenolic Metabolites in Cannabis
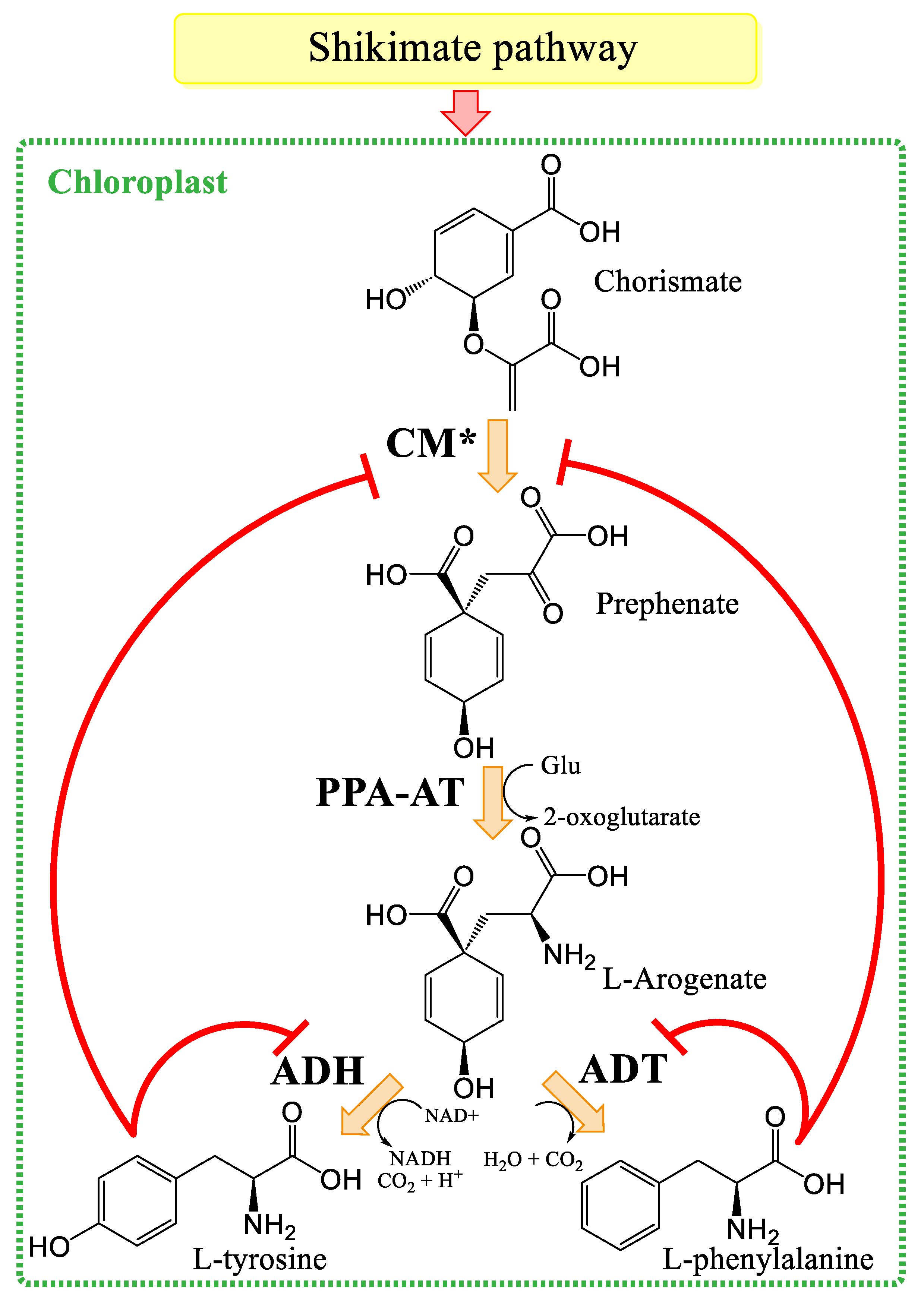
3.1. Plant Aromatic Amino Acid Biosynthesis
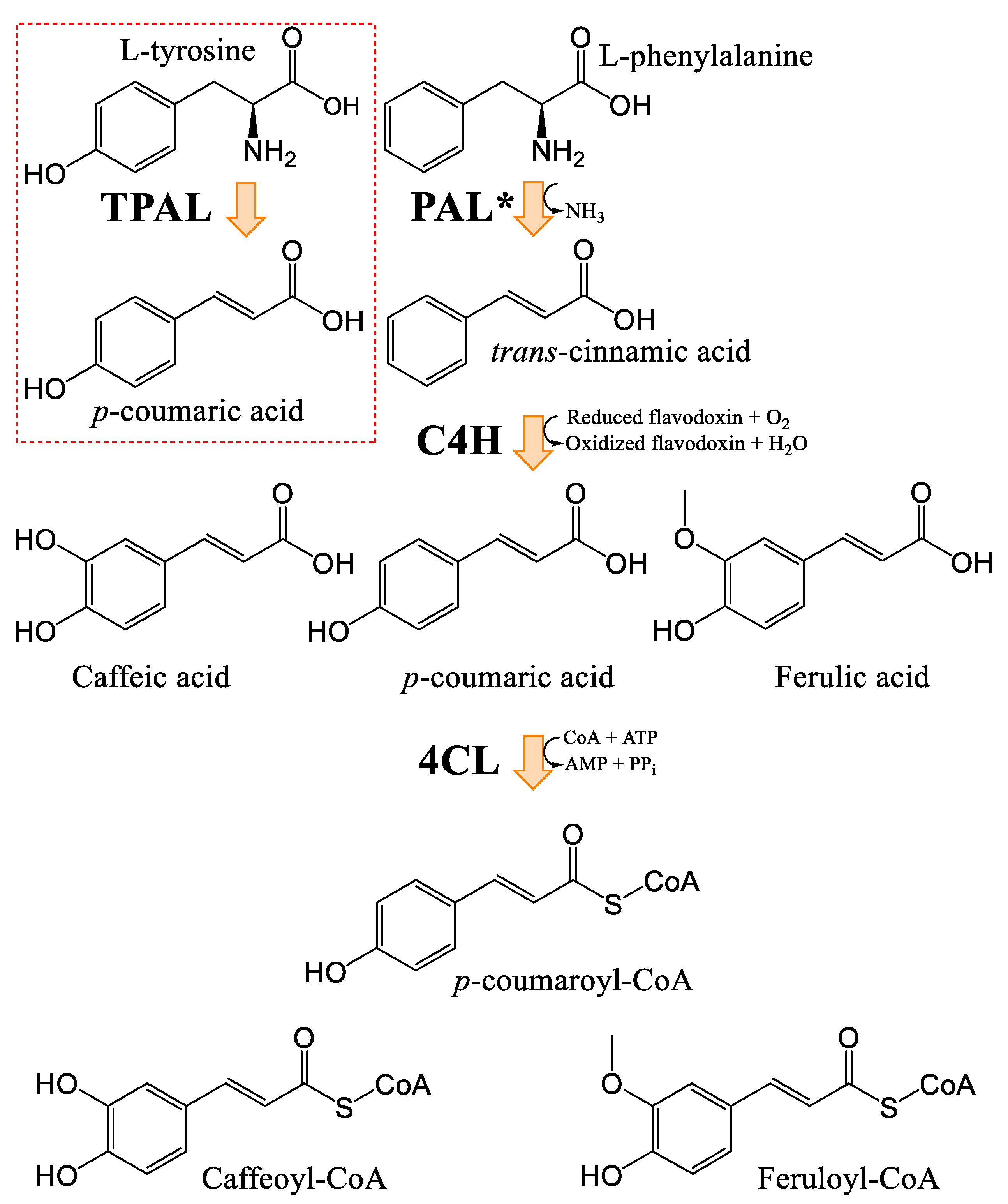
3.2. General Phenylpropanoids and Phenolic Acids Biosynthesis
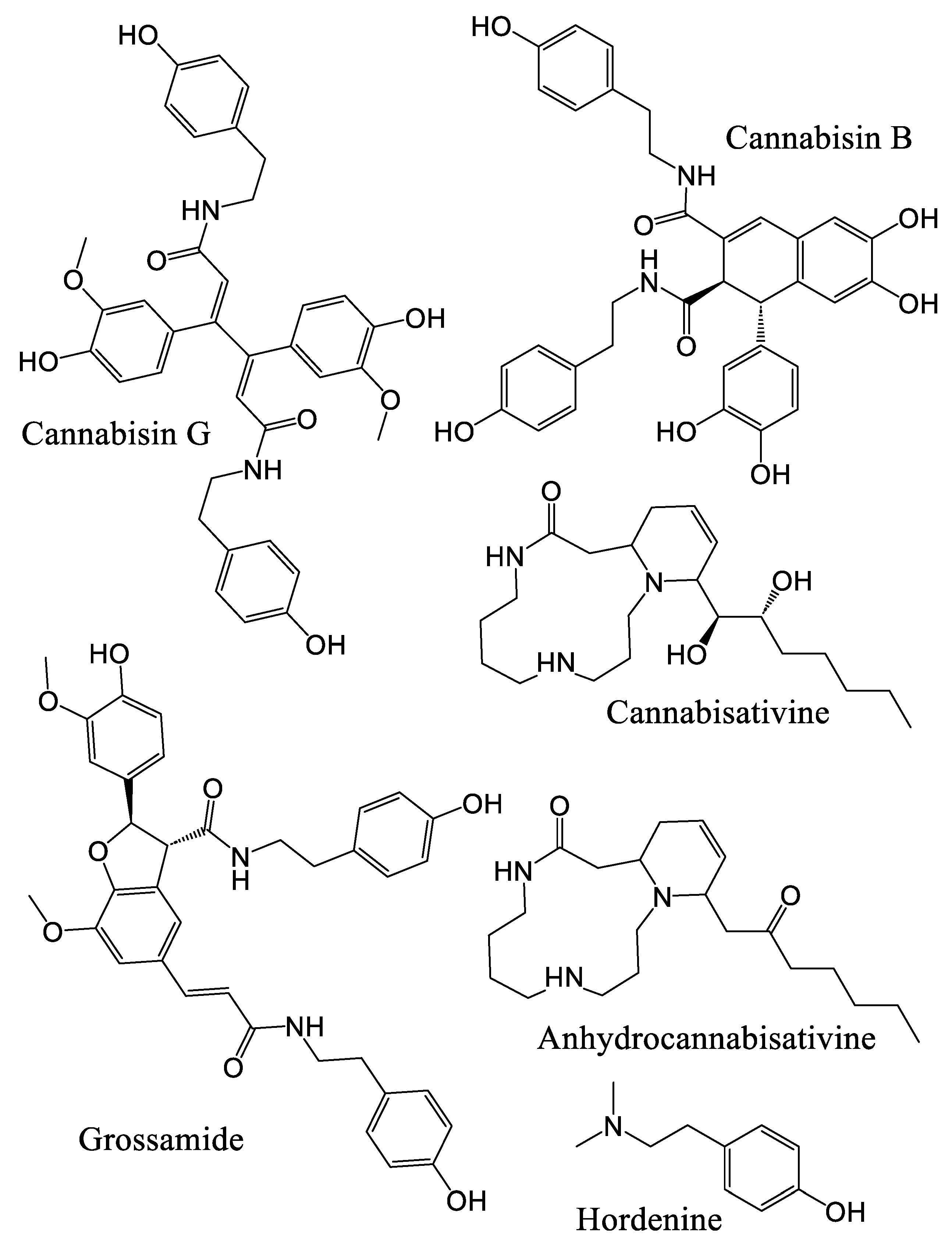
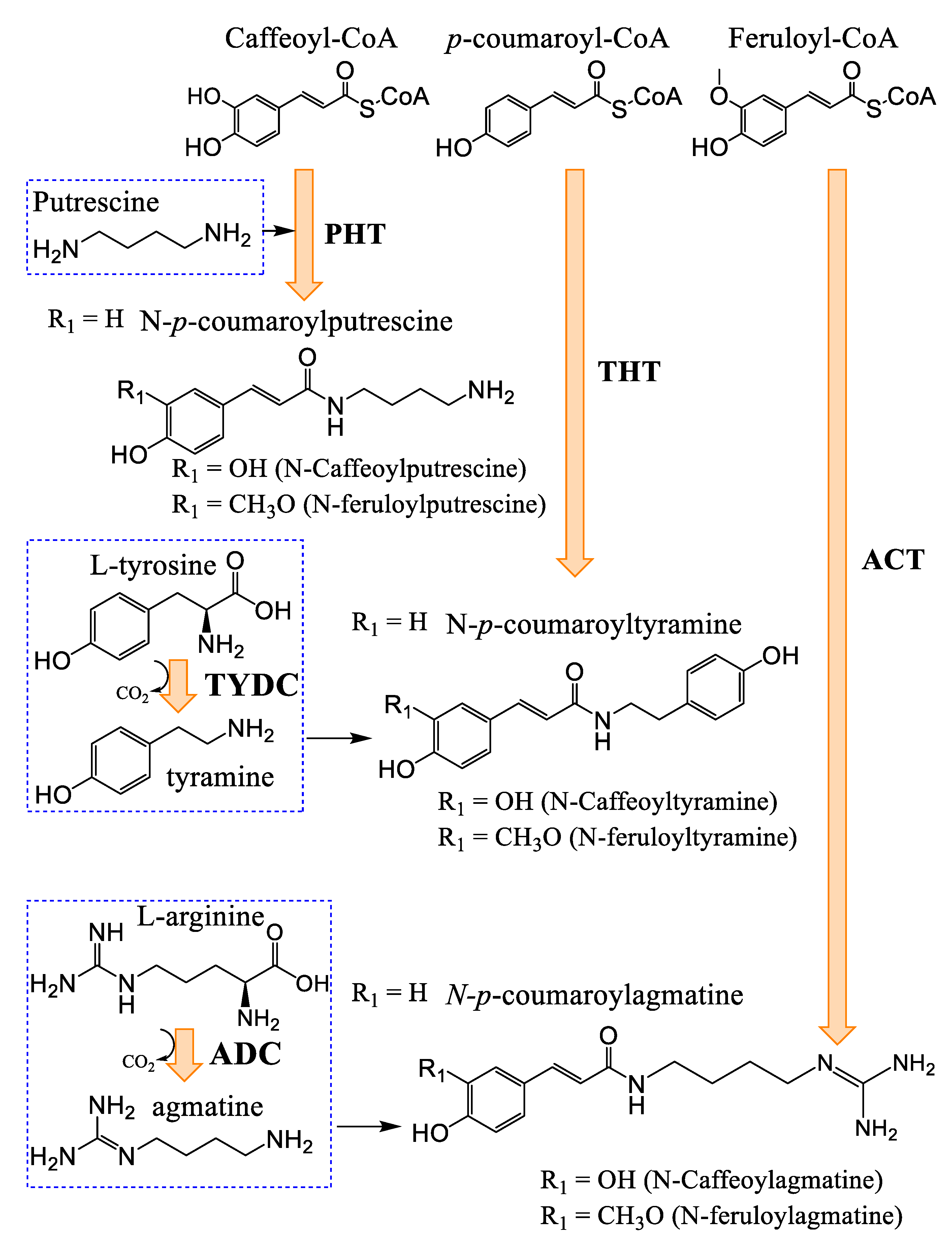
3.3. Hydroxycinnamic Acids and Lignanamides Biosynthesis
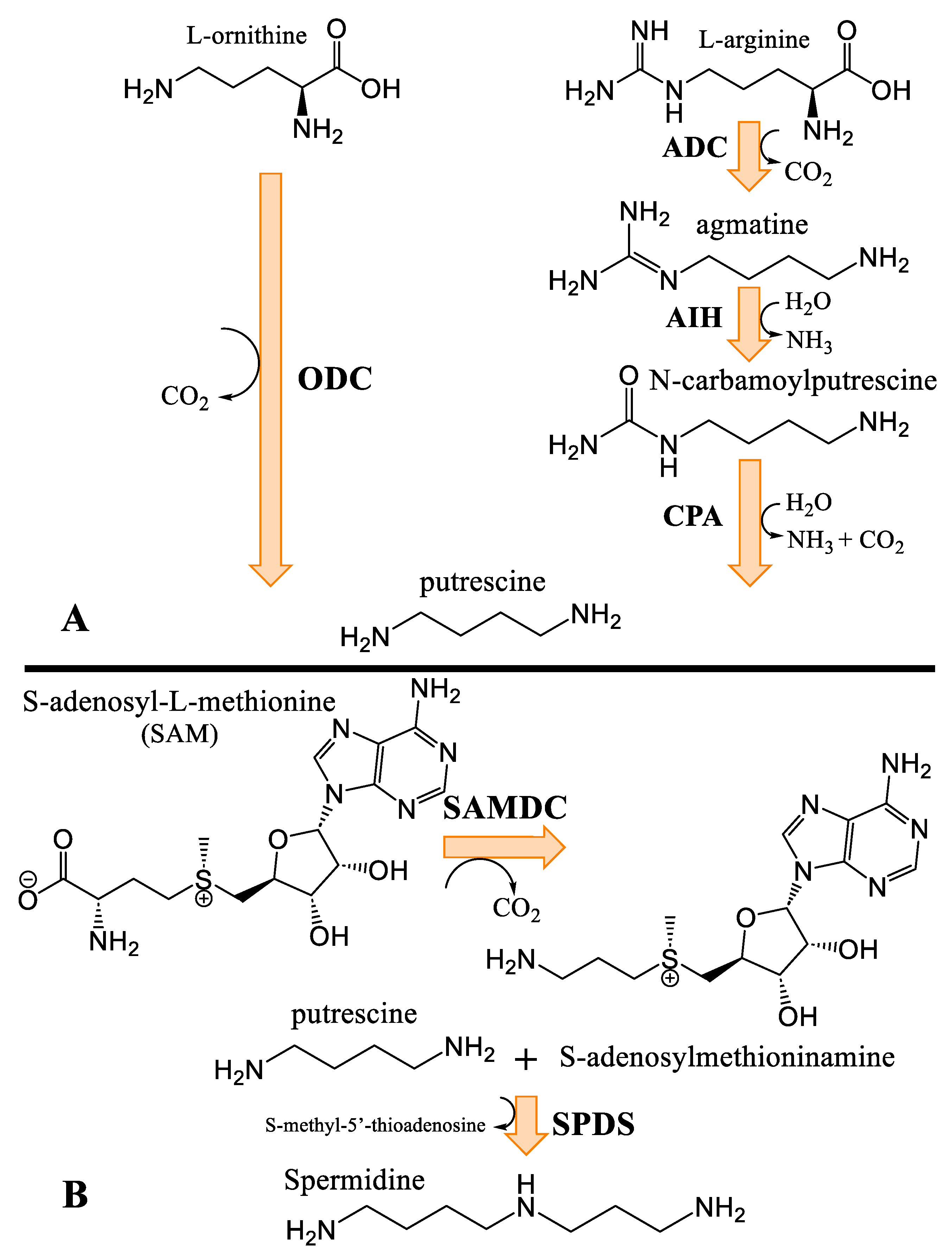
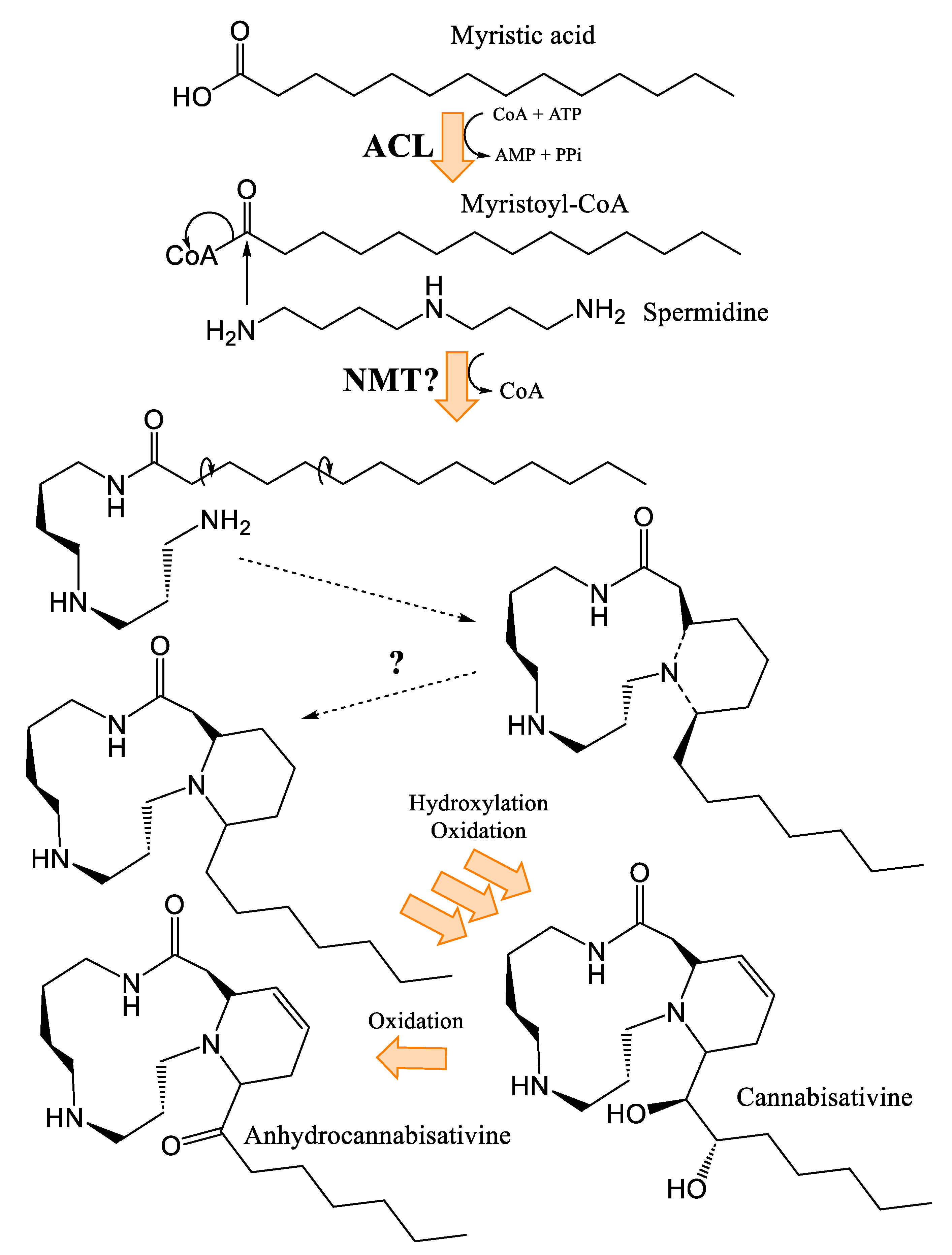
3.4. Biosynthesis of Spermidine and Spermidine Alkaloids
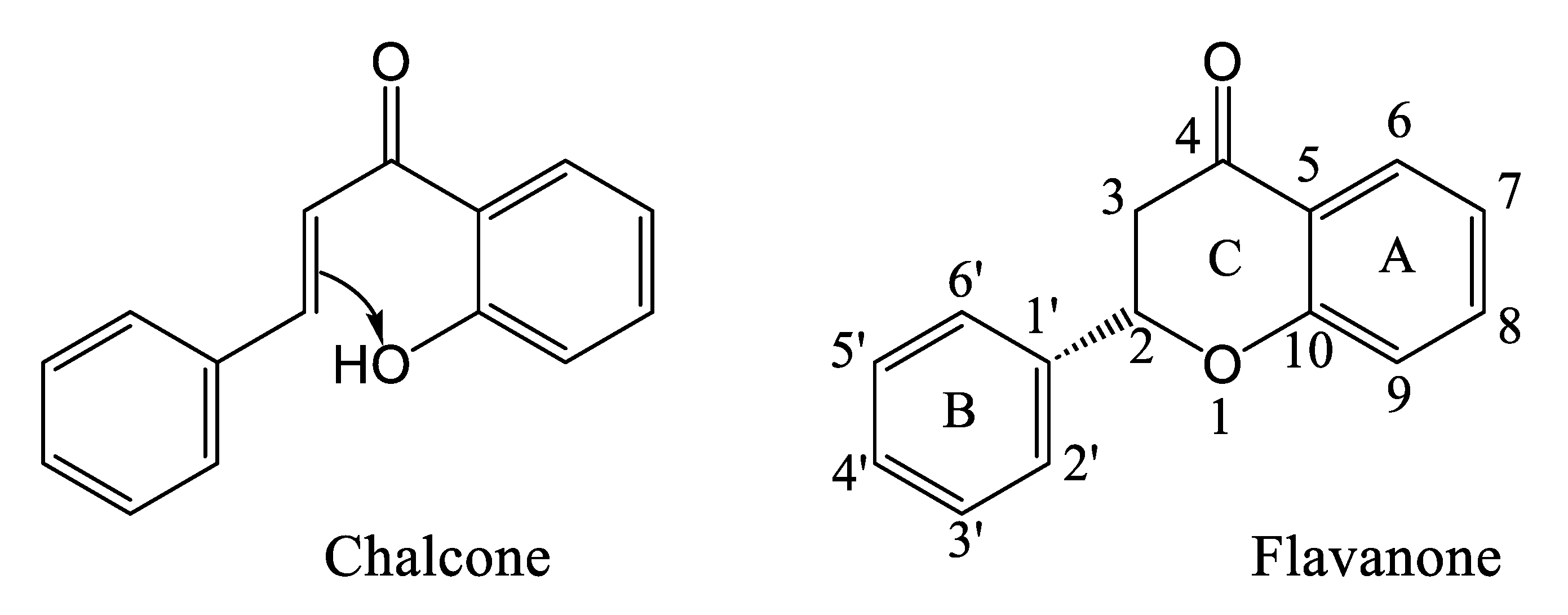
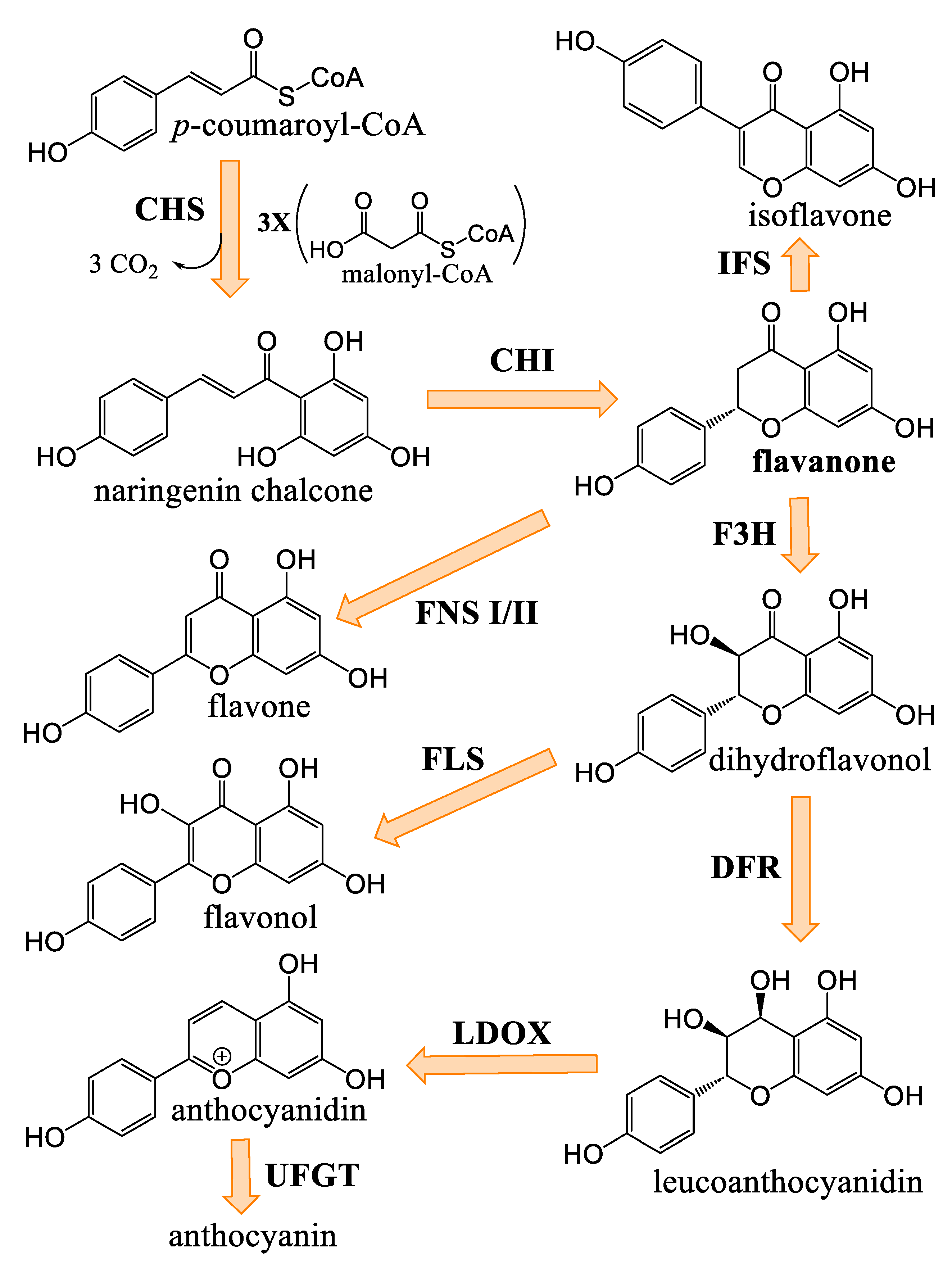
3.5. Flavonoids of Cannabis
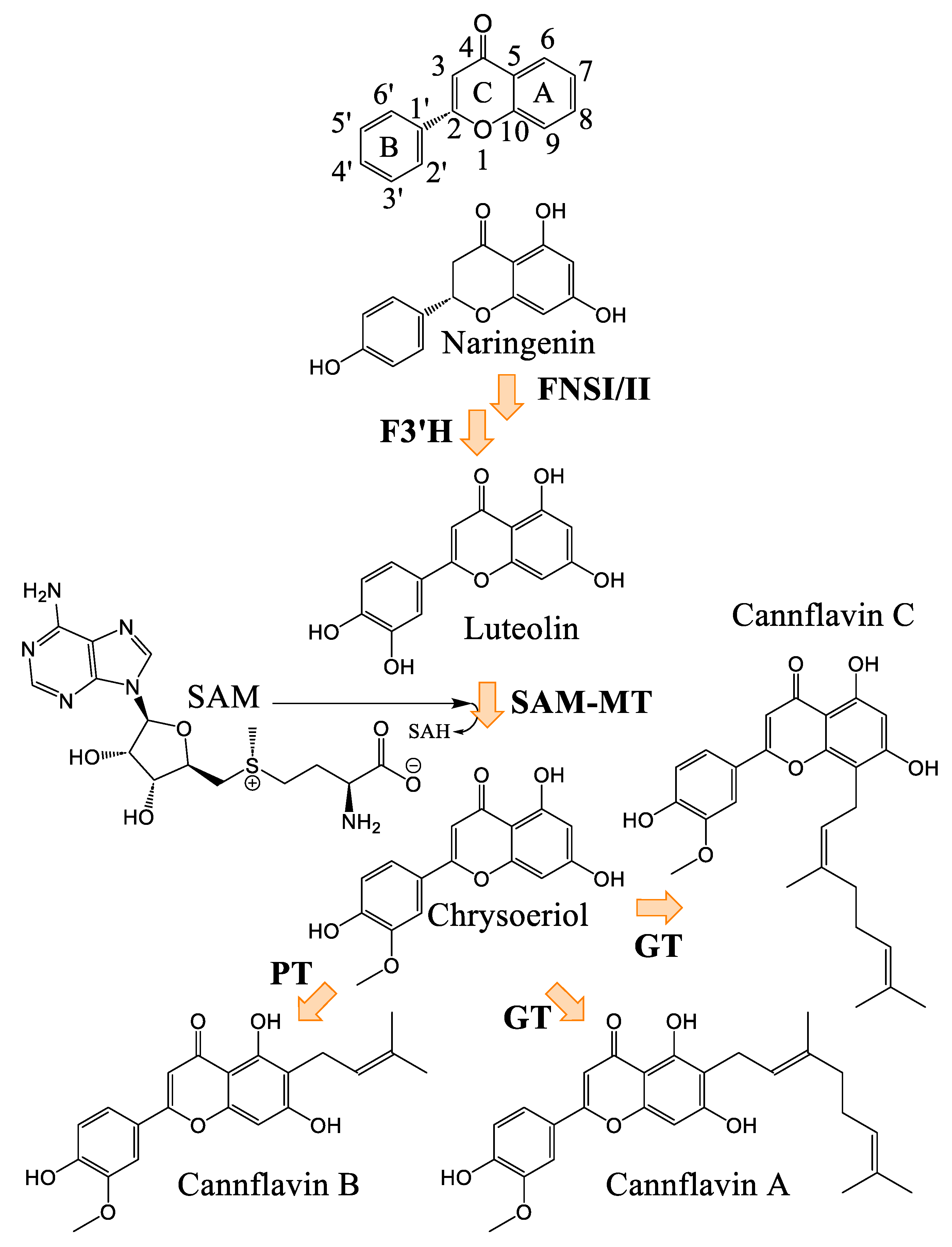
Biosynthesis of Cannflavins
3.6. Stilbenes of Cannabis
Biosynthesis of Cannabis Stilbenes
4. Biological Activity of Cannabis Metabolites
4.1. Polyamines and Their Physiological Roles
4.2. Biological Activity of Cannabis Alkaloids and Other Nitrogen-Containing Compounds
4.2.1. In Vitro Activity of Cannabis Nitrogen-Containing Compounds
HCAA Activity In Vitro
Lignanamide Activity In Vitro
4.2.2. In Vivo Activity of Cannabis Nitrogen-Containing Compounds
Cannabis HCAA In Vivo Activity
4.3. Biological Activity of Cannabis Flavonoids
4.3.1. Cannabis Flavonoids In Vitro Activity
4.3.2. In Silico Predicted Activity of Cannabis Flavonoids
4.4. Biological Activity of Cannabis Stilbenes
4.4.1. In Vitro Activity of Cannabis Stilbenes
4.4.2. In Vivo Activity of Cannabis Stilbenes
5. Regulatory Considerations for Non-Cannabinoid Compounds
6. Future Research Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Enzyme | Name | Gene ID | Chromosome | mRNA | Protein | Isoform |
|---|---|---|---|---|---|---|
| Chorismate mutase | CM1 | LOC115700776 | 8 | XM_030628416.2 | XP_030484276.1 | X1 |
| XM_030628417.2 | XP_030484277.1 | X2 | ||||
| CM2 | LOC115699048 | 8 | XM_030626256.2 | XP_030482116.2 | - | |
| LOC115694782 | X | XM_030621875.2 | XP_030477735.2 | X1 | ||
| XM_030621874.2 | XP_030477734.2 | X2 | ||||
| Prephenate amino-transferase | PPA-AT | LOC115712503 | 4 | XM_030640790.2 | XP_030496650.2 | - |
| LOC115712504 | 4 | XM_030640791.2 | XP_030496651.2 | - | ||
| Arogenate dehydratase | ADT1 | LOC115698788 | 8 | XM_061102953.1 | XP_060958936.1 | X1 |
| XM_030625964.2 | XP_030481824.2 | X2 | ||||
| XM_061102954.1 | XP_060958937.1 | X3 | ||||
| XM_061102955.1 | XP_060958938.1 | X4 | ||||
| XM_061102956.1 | XP_060958939.1 | X5 | ||||
| ADT2 | LOC115721090 | 2 | XM_030650332.2 | XP_030506192.1 | X1 | |
| XM_030650334.2 | XP_030506194.1 | X2 | ||||
| XM_030650336.2 | XP_030506196.1 | X3 | ||||
| ADT3 | LOC115719371 | 2 | XM_030648387.2 | XP_030504247.1 | - | |
| Arogenate dehydrogenase | ADH1 | LOC115703598 | 1 | XM_030630828.2 | XP_030486688.2 | - |
| LOC133033635 | 1 | XM_061108628.1 | XP_060964611.1 | - | ||
| LOC115725067 | 6 | XM_030654482.2 | XP_030510342.1 | - | ||
| ADH2 | LOC115707881 | 1 | XM_030635972.2 | XP_030491832.2 | - |
| Enzyme | Name | Gene ID | Chromosome | mRNA | Protein |
|---|---|---|---|---|---|
| Phenylalanine ammonia-lyase | PAL1 | LOC115706668 | 1 | XM_030634382.2 | XP_030490242.2 |
| PAL | LOC115709862 | 3 | XM_030638093.2 | XP_030493953.1 | |
| LOC115709383 | 3 | XM_030637471.2 | XP_030493331.2 | ||
| Cinnamate 4-hydroxylase | C4H | LOC115719463 | 2 | XM_030648524.2 | XP_030504384.1 |
| CYP73A100 | LOC115709609 | 3 | XM_030637745.2 | XP_030493605.2 | |
| 4-Coumarate-CoA ligase | 4CL1 | LOC115699364 | 8 | XM_030626724.2 | XP_030482584.2 |
| 4CL2 | LOC115717276 | 5 | XM_030646244.2 | XP_030502104.2 | |
| CCL1 | LOC115694777 | 6 | XM_061116895.1 | XP_060972878.1 | |
| XM_030621866.2 | XP_030477726.2 | ||||
| Caffeoyl-CoA-O-methyltransferase | CCOMT | LOC115702947 | X | XM_030630419.2 | XP_030486279.1 |
| Caffeate 3-O-methyltransferase | COMT | LOC115716906 | 5 | XM_030645827.2 | XP_030501687.2 |
| LOC115716367 | XM_030645149.2 | XP_030501009.2 | |||
| LOC115697697 | 7 | XM_030624792.2 | XP_030480652.2 | ||
| LOC115721930 | 9 | XM_030651036.2 | XP_030506896.2 | ||
| LOC115723302 | XM_030652742.2 | XP_030508602.2 | |||
| Tyrosine decarboxylase | TYDC | LOC115696858 | 7 | XM_030623740.2 | XP_030479600.1 |
| LOC115721923 | 9 | XM_030651029.2 | XP_030506889.2 | ||
| TYDC1 | LOC115721880 | 9 | XM_061105012.1 | XP_060960995.1 | |
| Spermidine hydroxycinnamoyl transferase | SHCT | LOC115716016 | 5 | XM_030644703.2 | XP_030500563.2 |
| LOC115695248 | 6 | XM_030622328.2 | XP_030478188.2 | ||
| LOC115696820 | 7 | XM_030623703.2 | XP_030479563.2 | ||
| Spermidine hydroxycinnamoyl Transferase-like | SHCT-L | LOC115712649 | 4 | XM_030640956.2 | XP_030496816.2 |
| LOC115716993 | 5 | XM_030645919.2 | XP_030501779.2 | ||
| LOC115716541 | XM_030645353.2 | XP_030501213.2 | |||
| Hydroxycinnamoyl transferase | HCT | LOC115721023 | 2 | XM_030650250.2 | XP_030506110.2 |
| Enzyme | Name | Gene ID | Chromosome | mRNA | Protéine | Isoform |
|---|---|---|---|---|---|---|
| Ornithine decarboxylase | ODC | LOC115714249 | X | XM_030642880.2 | XP_030498740.1 | - |
| Arginine decarboxylase | ADC | LOC115725364 | 6 | XM_030654854.2 | XP_030510714.2 | - |
| Agmatine Imino-hydrolase | AIH | LOC115710158 | 3 | XM_030638493.2 | XP_030494353.2 | - |
| N-carbamoyl-putrescine amidase | CPA | LOC115721138 | 2 | XM_030650390.2 | XP_030506250.2 | - |
| S-adenosyl-methionine synthase | SAMS1 | LOC115711364 | X | XM_030639731.2 | XP_030495591.1 | - |
| LOC115719259 | 2 | XM_061108853.1 | XP_060964836.1 | X1 | ||
| XM_061108854.1 | XP_060964837.1 | X2 | ||||
| XM_030648224.2 | XP_030504084.1 | X3 | ||||
| SAMS3 | LOC115711343 | 3 | XM_030639680.2 | XP_030495540.1 | - | |
| SAM decarboxylase proenzyme | SAMDC | LOC115725611 | 6 | XM_030655195.2 | XP_030511055.2 | - |
| SAMDC4 | LOC115721913 | 9 | XM_030651018.2 | XP_030506878.1 | - | |
| Spermidine synthase | SPDS | LOC115716589 | 4 | XM_030641228.2 | XP_030497088.2 | - |
| Spermine synthase | SPS | LOC115716589 | 5 | XM_030645419.2 | XP_030501279.2 | X1.1 |
| XM_061115311.1 | XP_060971294.1 | X1.2 | ||||
| XM_061115312.1 | XP_060971295.1 | X2.1 | ||||
| XM_030645418.2 | XP_030501278.2 | X2.2 | ||||
| N-myristoyl-transferase | NMT | LOC115707577 | 1 | XM_030635591.2 | XP_030491451.1 | - |
| Enzyme | Name | Gene ID | Chromosome | mRNA | Protein | Isoform |
|---|---|---|---|---|---|---|
| Chalcone synthase | CHS | LOC115724170 | 9 | NM_001397940.1 | NP_001384869.1 | - |
| Chalcone isomerase | CHI | LOC115716382 | 5 | XM_030645165.2 | XP_030501025.2 | X1 |
| XM_061115784.1 | XP_060971767.1 | X2 | ||||
| XM_061115783.1 | XP_060971766.1 | X3 | ||||
| XM_061115782.1 | XP_060971765.1 | X4 | ||||
| Flavanone 3-dioxygenase | F3H | LOC115702709 | - | XM_030630171.2 | XP_030486031.2 | - |
| Flavonol synthase | FLS | LOC115717395 | 5 | XM_030646361.2 | XP_030502221.2 | - |
| Dihydroflavonol 4-reductase | DFR | LOC115710150 | 3 | XM_030638485.2 | XP_030494345.2 | - |
| Leucoantho-cyanidin dioxygenase | LDOX | LOC115716756 | 5 | XM_030645652.2 | XP_030501512.2 | - |
| UDP-glucose–flavonol O-glucosyl-transferase | UFGT | LOC115703032 | X | XM_061106990.1 | XP_060962973.1 | - |
| LOC115704110 | X | XM_030631332.2 | XP_030487192.2 | - | ||
| LOC115704783 | X | XM_061106847.1 | XP_060962830.1 | - | ||
| LOC115704809 | 1 | XM_030632016.2 | XP_030487876.2 | - | ||
| Flavonoid 3′-hydroxylase | F3′H | LOC115715620 | 5 | XM_030644276.2 | XP_030500136.2 | - |
| SAM-dependent-methyltransferase | SAM-MT | LOC115724992 | 6 | XM_030654382.2 | XP_030510242.1 | X1 |
| XM_061117310.1 | XP_060973293.1 | X2 |
| Enzyme | Name | Gene ID | Chromosome | mRNA | Protein | Isoform |
|---|---|---|---|---|---|---|
| 2 alcenal reductase | DBR | LOC115708061 | 1 | XM_030636233.2 | XP_030492093.2 | - |
| LOC115697048 | 7 | XM_030623951.2 | XP_030479811.1 | - | ||
| LOC115697049 | XM_030623952.2 | XP_030479812.1 | - | |||
| LOC115698188 | XM_030625376.2 | XP_030481236.1 | - | |||
| LOC115696848 | XM_030623733.2 | XP_030479593.1 | - | |||
| LOC115697314 | XM_030624260.2 | XP_030480120.1 | X1 | |||
| XM_061118868.1 | XP_060974851.1 | X2 | ||||
| XM_061118869.1 | XP_060974852.1 | X3 | ||||
| Bibenzyl synthase | BBS | LOC115713254 | X | XM_030641739.2 | XP_030497599.2 | - |
References
- ElSohly, M.A. Marijuana and the Cannabinoids; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Blatt-Janmaat, K.; Qu, Y. The Biochemistry of Phytocannabinoids and Metabolic Engineering of Their Production in Heterologous Systems. Int. J. Mol. Sci. 2021, 22, 2454. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef]
- Brickell, C.D.; Alexander, C.; Cubey, J.J.; David, J.C.; Hoffman, M.H.A.; Leslie, A.C.; Malécot, V.; Jin, X.B. International Code of Nomenclature for Cultivated Plants (ICNCP or Cultivated Plant Code), Incorporating the Rules and Recommendations for Naming Plants in Cultivation Adopted by the International Union of Biological Sciences and the International Commission for the Nomenclature of Cultivated Plants, 9th ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2016; Volume 18, pp. 1–190. Available online: https://www.ishs.org/sites/default/files/static/ScriptaHorticulturae_18.pdf (accessed on 27 April 2025).
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Ross, S.; EISohly, M. Constituents of Cannabis sativa L. XXVIII. A review of the natural constituents: 1980–1994. Zagazig J. Pharm. Sci. 1995, 4, 150–160. [Google Scholar] [CrossRef]
- Balant, M.; Gras, A.; Ruz, M.; Valles, J.; Vitales, D.; Garnatje, T. Traditional uses of Cannabis: An analysis of the CANNUSE database. J. Ethnopharmacol. 2021, 279, 114362. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Gagné, V.; Boucher, N.; Desgagné-Penix, I. Cannabis Roots: Therapeutic, Biotechnological and Environmental Aspects. Cannabis Cannabinoid Res. 2024, 9, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Gagné, V.; Merindol, N.; Boucher, R.; Boucher, N.; Desgagné-Penix, I. Rooted in therapeutics: Comprehensive analyses of Cannabis sativa root extracts reveals potent antioxidant, anti-inflammatory, and bactericidal properties. Front. Pharmacol. 2024, 15, 1465136. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. Pharma Nutr. 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Radwan, M.M.; Metwaly, A.M.; Eissa, I.H.; Hazekamp, A.; Sohly, M.A. Chemistry and Biological Activities of Cannflavins of the Cannabis Plant. Cannabis Cannabinoid Res. 2023, 8, 974–985. [Google Scholar] [CrossRef]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From plant to patient: A scoping review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef] [PubMed]
- O’Croinin, C.; Garcia Guerra, A.; Doschak, M.R.; Löbenberg, R.; Davies, N.M. Therapeutic Potential and Predictive Pharmaceutical Modeling of Stilbenes in Cannabis sativa. Pharmaceutics 2023, 15, 1941. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Q.; Hou, P.; Li, F.; Guo, S.; Song, W.; Zhang, H.; Liu, X.; Zhang, S.; Zhang, J.; et al. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018, 9, 6608–6617. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis Roots: A Traditional Therapy with Future Potential for Treating Inflammation and Pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Terui, Y.; Yoshida, T.; Sakamoto, A.; Saito, D.; Oshima, T.; Kawazoe, M.; Yokoyama, S.; Igarashi, K.; Kashiwagi, K. Polyamines protect nucleic acids against depurination. Int. J. Biochem. Cell Biol. 2018, 99, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laido, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- González Mera, I.F.; González Falconí, D.E.; Morera Córdova, V. Secondary metabolites in plants: Main classes, phytochemical analysis and pharmacological activities. Bionatura 2019, 4, 1000–1009. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-Cannabinoid Metabolites of Cannabis sativa L. with Therapeutic Potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Sun, W.; Shen, H.; Xu, H.; Tang, X.; Tang, M.; Ju, Z.; Yi, Y. Chalcone Isomerase a Key Enzyme for Anthocyanin Biosynthesis in Ophiorrhiza japonica. Front. Plant Sci. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Deya, P.; Kumarb, A.; Guptac, M.; Leea, B.M.; Bhaktad, T.; Dashd, S.; Kima, H.S. Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity-An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
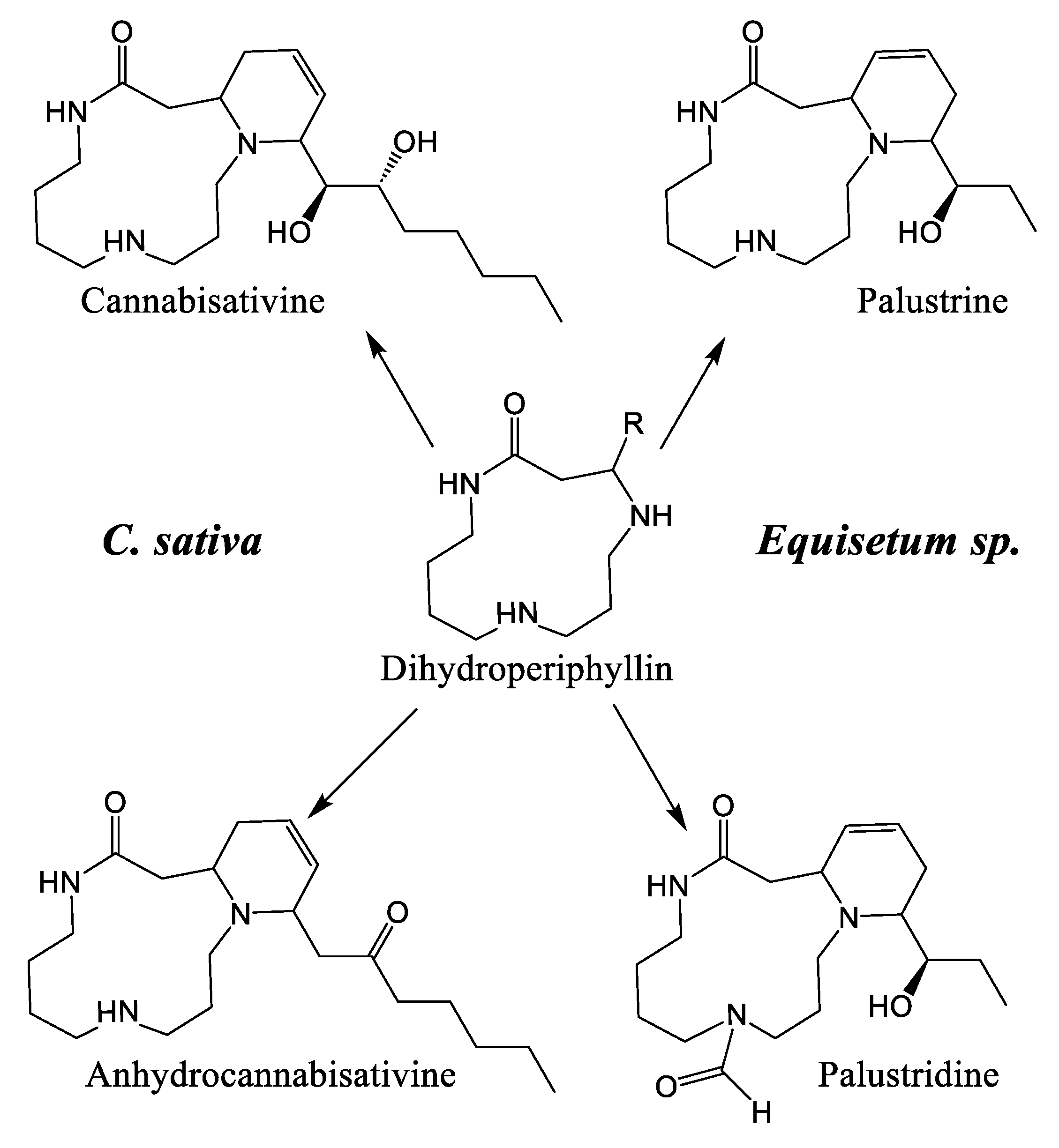
- Elsohly, M.A.; Turner, C.E.; Phoebe, C.H., Jr.; Knapp, J.E.; Schiff, P.L., Jr.; Slatkin, D.J. Anhydrocannabisativine, a new alkaloid from Cannabis sativa L. J. Pharm. Sci. 1978, 67, 124. [Google Scholar] [CrossRef]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simoes-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of Lignanamides from Hemp (Cannabis sativa L.) Seed and Their Antioxidant and Acetylcholinesterase Inhibitory Activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.L.; Scutt, A.M.; Evans, F.J. Cannflavin A and B, prenylated flavones from Cannabis sativa L. Experientia 1986, 42, 452–453. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Gonzalez-Paramas, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.R.; Hodgins, D.S.; Abell, C.W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J. Biol. Chem. 1976, 251, 4646–4650. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Bassard, J.E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef]
- Wuddineh, W.; Minocha, R.; Minocha, S.C. Polyamines in the Context of Metabolic Networks. Methods Mol. Biol. 2018, 1694, 1–23. [Google Scholar] [PubMed]
- Izzo, L.; Castaldo, L.; Narvaez, A.; Graziani, G.; Gaspari, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Anarat-Cappillino, G.; Sattely, E.S. The chemical logic of plant natural product biosynthesis. Curr. Opin. Plant Biol. 2014, 19, 51–58. [Google Scholar] [CrossRef]
- Piovesana, S.; Aita, S.E.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Guarnaccia, P.; Montone, C.M.; Lagana, A. In-depth cannabis fatty acid profiling by ultra-high performance liquid chromatography coupled to high resolution mass spectrometry. Talanta 2021, 228, 122249. [Google Scholar] [CrossRef]
- Wang, B.; Dai, T.; Sun, W.; Wei, Y.; Ren, J.; Zhang, L.; Zhang, M.; Zhou, F. Protein N-myristoylation: Functions and mechanisms in control of innate immunity. Cell. Mol. Immunol. 2021, 18, 878–888. [Google Scholar] [CrossRef]
- Latter, H.L.; Abraham, D.J.; Turner, C.E.; Knapp, J.E.; Schiff, P.L.; Slatkin, D.J. Cannabisativine, a new alkaloid from Cannabis sativa L. root. Tetrahedron Lett. 1975, 16, 2815–2818. [Google Scholar] [CrossRef]
- Martens, S.; Preuss, A.; Matern, U. Multifunctional flavonoid dioxygenases: Flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Allegrone, G.; Pollastro, F.; Magagnini, G.; Taglialatela-Scafati, O.; Seegers, J.; Koeberle, A.; Werz, O.; Appendino, G. The Bibenzyl Canniprene Inhibits the Production of Pro-Inflammatory Eicosanoids and Selectively Accumulates in Some Cannabis sativa Strains. J. Nat. Prod. 2017, 80, 731–734. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Yamamoto, R.; Kojima, N.; Yahata, M.; Kato, M. Molecular characterization of a flavanone 3-hydroxylase gene from citrus fruit reveals its crucial roles in anthocyanin accumulation. BMC Plant Biol. 2023, 23, 233. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Boddington, K.F.; Soubeyrand, E.; Van Gelder, K.; Casaretto, J.A.; Perrin, C.; Forrester, T.J.B.; Parry, C.; Al-Abdul-Wahid, M.S.; Jentsch, N.G.; Magolan, J.; et al. Bibenzyl synthesis in Cannabis sativa L. Plant J. 2022, 109, 693–707. [Google Scholar] [CrossRef]
- Pal, M.; Janda, T. Role of polyamine metabolism in plant pathogen interactions. J. Plant Sci. Phytopathol. 2017, 1, 95–100. [Google Scholar]
- Zhou, J.W.; Luo, H.Z.; Jiang, H.; Jian, T.K.; Chen, Z.Q.; Jia, A.Q. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.W.; Ruan, L.Y.; Chen, H.J.; Luo, H.Z.; Jiang, H.; Wang, J.S.; Jia, A.Q. Inhibition of Quorum Sensing and Virulence in Serratia marcescens by Hordenine. J. Agric. Food Chem. 2019, 67, 784–795. [Google Scholar] [CrossRef]
- Chikazawa, M.; Sato, R. Identification of Functional Food Factors as β2-Adrenergic Receptor Agonists and Their Potential Roles in Skeletal Muscle. J. Nutr. Sci. Vitaminol. 2018, 64, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Schoene, N. Synthesis and Characterization of N-Coumaroyltyramine as a Potent Phytochemical Which Arrests Human Transformed Cells via Inhibiting Protein Tyrosine Kinases. Biochem. Biophys. Res. Commun. 2002, 292, 1104–1110. [Google Scholar] [CrossRef]
- Masi, M.; Koirala, M.; Delicato, A.; Di Lecce, R.; Merindol, N.; Ka, S.; Seck, M.; Tuzi, A.; Desgagne-Penix, I.; Calabro, V.; et al. Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb. an Amaryllidaceae Species Collected in Senegal. Biomolecules 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Wongsakul, A.; Lertnitikul, N.; Suttisri, R.; Jianmongkol, S. N-Trans-p-Coumaroyltyramine Enhances Indomethacin- and Diclofenac-induced Apoptosis Through Endoplasmic Reticulum Stress-dependent Mechanism in MCF-7 Cells. Anticancer Res. 2022, 42, 1833–1844. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Chen, Z.; Chen, Y.; Santhanam, R.K.; Xue, Z.; Ma, Q.; Guo, Q.; Liu, W.; Zhang, M.; et al. Effects of N-trans-feruloyltyramine isolated from laba garlic on antioxidant, cytotoxic activities and H2O2-induced oxidative damage in HepG2 and L02 cells. Food Chem. Toxicol. 2019, 130, 130–141. [Google Scholar] [CrossRef]
- Badawy, A.M.; Eltamany, E.E.; Hussien, R.M.; Mohamed, O.G.; El-Ayouty, M.M.; Nafie, M.S.; Tripathi, A.; Ahmed, S.A. Cornulacin: A new isoflavone from Cornulaca monacantha and its isolation, structure elucidation and cytotoxicity through EGFR-mediated apoptosis. RSC Med. Chem. 2024, 15, 3228–3238. [Google Scholar] [CrossRef]
- Zhou, K.; Han, L.; Li, W.; Liu, S.; Chen, T.; Chen, J.; Lv, J.; Zhou, X.; Li, Q.; Meng, X.; et al. Pipersarmenoids, new amide alkaloids from Piper sarmentosum. Fitoterapia 2024, 177, 106090. [Google Scholar] [CrossRef]
- Rea Martinez, J.; Selo, G.; Fernandez-Arche, M.A.; Bermudez, B.; Garcia-Gimenez, M.D. Dual Role of Phenyl Amides from Hempseed on BACE 1, PPARgamma, and PGC-1alpha in N2a-APP Cells. J. Nat. Prod. 2021, 84, 2447–2453. [Google Scholar] [CrossRef]
- Othman, A.; Sayed, A.M.; Amen, Y.; Shimizu, K. Possible neuroprotective effects of amide alkaloids from Bassia indica and Agathophora alopecuroides: In vitro and in silico investigations. RSC Adv. 2022, 12, 18746–18758. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Neuroprotective effect of trans-N-caffeoyltyramine from Lycium chinense against H2O2 induced cytotoxicity in PC12 cells by attenuating oxidative stress. Biomed. Pharmacother. 2017, 93, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef]
- Ayanlowo, A.G.; Garadi, Z.; Boldizsar, I.; Darcsi, A.; Nedves, A.N.; Varjas, B.; Simon, A.; Alberti, A.; Riethmuller, E. UHPLC-DPPH method reveals antioxidant tyramine and octopamine derivatives in Celtis occidentalis. J. Pharm. Biomed. Anal. 2020, 191, 113612. [Google Scholar] [CrossRef]
- Bakrim, S.; Elouafy, Y.; Touhtouh, J.; Aanniz, T.; El Kadri, K.; Khalid, A.; Fawzy, S.; Mesaik, M.A.; Lee, L.H.; Chamkhi, I.; et al. Exploring the chemistry, biological effects, and mechanism insights of natural coumaroyltyramine: First report. Fitoterapia 2024, 178, 106182. [Google Scholar] [CrossRef]
- Xie, L.W.; Atanasov, A.G.; Guo, D.A.; Malainer, C.; Zhang, J.X.; Zehl, M.; Guan, S.H.; Heiss, E.H.; Urban, E.; Dirsch, V.M.; et al. Activity-guided isolation of NF-kappaB inhibitors and PPARgamma agonists from the root bark of Lycium chinense Miller. J. Ethnopharmacol. 2014, 152, 470–477. [Google Scholar] [CrossRef]
- Doan, T.P.; Zhang, M.; An, J.P.; Ponce-Zea, J.E.; Mai, V.H.; Ryu, B.; Park, E.J.; Oh, W.K. Metabolite Profiling of Allium hookeri Leaves Using UHPLC-qTOF-MS/MS and the Senomorphic Activity of Phenolamides. Nutrients 2023, 15, 5109. [Google Scholar] [CrossRef]
- Lee, S.H.; Veeriah, V.; Levine, F. Liver fat storage is controlled by HNF4alpha through induction of lipophagy and is reversed by a potent HNF4alpha agonist. Cell Death Dis. 2021, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Okpala, E.O.; Onocha, P.A.; Ali, M.S.; Ur-Rehmen, S.Z.; Lateef, M. Zenkeramide: A new iso-benzofuranone propanamide and urease inhibitory constituents of Celtis zenkeri Engl stem bark (Ulmaceae). Nat. Prod. Res. 2023, 37, 93–98. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Y.; Wang, M.; Lin, Y.; Zeng, B.; Zheng, X.; Song, Y.; Zeng, R. Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa). Int. J. Mol. Sci. 2024, 25, 5946. [Google Scholar] [CrossRef]
- Kim, J.K.; Heo, H.Y.; Park, S.; Kim, H.; Oh, J.J.; Sohn, E.H.; Jung, S.H.; Lee, K. Characterization of Phenethyl Cinnamamide Compounds from Hemp Seed and Determination of Their Melanogenesis Inhibitory Activity. ACS Omega 2021, 6, 31945–31954. [Google Scholar] [CrossRef]
- Tomosaka, H.; Chin, Y.W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kinghorn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry). Phytother. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Chen, T.; Hao, J.; He, J.; Zhang, J.; Li, Y.; Liu, R.; Li, L. Cannabisin B induces autophagic cell death by inhibiting the AKT/mTOR pathway and S phase cell cycle arrest in HepG2 cells. Food Chem. 2013, 138, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Q.; Fan, P. Cannabisin F from Hemp (Cannabis sativa) Seed Suppresses Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglia as SIRT1 Modulator. Int. J. Mol. Sci. 2019, 20, 507. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yan, X.; Bobrovskaya, L.; Ji, M.; Yuan, H.; Lou, H.; Fan, P. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-kappaB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol. Cell. Biochem. 2017, 428, 129–137. [Google Scholar] [CrossRef]
- Li, C.X.; Song, X.Y.; Zhao, W.Y.; Yao, G.D.; Lin, B.; Huang, X.X.; Li, L.Z.; Song, S.J. Characterization of enantiomeric lignanamides from Solanum nigrum L. and their neuroprotective effects against MPP+-induced SH-SY5Y cells injury. Phytochemistry 2019, 161, 163–171. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Brieger, L.; Strohmann, C.; Spiteller, M. Anti-inflammatory steroidal sapogenins and a conjugated chalcone-stilbene from Dracaena usambarensis Engl. Fitoterapia 2020, 146, 104717. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, K.S.; Lee, E.S.; Kim, Y.G.; Kim, Y.I.; Cheon, J.Y.; Jung, H.J.; Kim, K.M.; Lee, I.S. Inhibitory effects of phenylpropionamides from the hemp seed husk on tyrosinase. Int. J. Biol. Macromol. 2024, 282 Pt 2, 136939. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Ding, C.; Wen, F.; Sun, F.; Liu, Y.; Tao, C.; Yao, J. Beneficial Effects of Hordenine on a Model of Ulcerative Colitis. Molecules 2023, 28, 2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, R.Z.; Ma, L.; Ding, Q.Y.; Meng, J.H.; Chen, Y.G.; Wu, J.H. Antiprolactinoma Effect of Hordenine by Inhibiting MAPK Signaling Pathway Activation in Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 3107290. [Google Scholar] [CrossRef]
- Zhang, X.; Du, L.; Zhang, J.; Li, C.; Zhang, J.; Lv, X. Hordenine Protects Against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Inflammation. Front. Pharmacol. 2021, 12, 712232. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; He, D.; Hu, G.; Wang, H.; Ye, B.; He, Y.; Gao, X.; Liu, D. Hordenine inhibits neuroinflammation and exerts neuroprotective effects via inhibiting NF-kappaB and MAPK signaling pathways in vivo and in vitro. Int. Immunopharmacol. 2022, 108, 108694. [Google Scholar] [CrossRef]
- Agrawal, M.; Singhal, M.; Semwal, B.C.; Arora, S.; Singh, B.; Sikarwar, V.; Sethi, P.; Chaudhary, H.; Akram, W.; Bhargva, S.; et al. Neuroprotective action of hordenine against the Aluminium Chloride (AlCl3) induced Alzheimer’s diseases & associated memory impairment in experimental rats. Pharmacol. Res.-Mod. Chin. Med. 2024, 12, 100492. [Google Scholar]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive phenolic amides from Celtis africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Barrett, M.L.; Gordon, D.; Evans, F.J. Isolation from Cannabis sativa L. of cannflavin--a novel inhibitor of prostaglandin production. Biochem. Pharmacol. 1985, 34, 2019–2024. [Google Scholar] [CrossRef]
- Fitzpatrick, J.K.; O’Riordan, D.; Downer, E.J. Cannflavin A inhibits TLR4-induced chemokine and cytokine expression in human macrophages. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Radwan, M.M.; Elsohly, M.A.; Slade, D.; Ahmed, S.A.; Wilson, L.; El-Alfy, A.T.; Khan, I.A.; Ross, S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry 2008, 69, 2627–2633. [Google Scholar] [CrossRef]
- Salem, M.M.; Capers, J.; Rito, S.; Werbovetz, K.A. Antiparasitic activity of C-geranyl flavonoids from Mimulus bigelovii. Phytother. Res. 2011, 25, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; Fujitani, M.; Kato, R.; Smid, S. Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid beta-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone. Biochem. Pharmacol. 2019, 169, 113609. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Dupre, D.J. Anti-cancer properties of cannflavin A and potential synergistic effects with gemcitabine, cisplatin, and cannabinoids in bladder cancer. J. Cannabis Res. 2022, 4, 41. [Google Scholar] [CrossRef]
- Puopolo, T.; Chang, T.; Liu, C.; Li, H.; Liu, X.; Wu, X.; Ma, H.; Seeram, N.P. Gram-Scale Preparation of Cannflavin A from Hemp (Cannabis sativa L.) and Its Inhibitory Effect on Tryptophan Catabolism Enzyme Kynurenine-3-Monooxygenase. Biology 2022, 11, 1416. [Google Scholar] [CrossRef]
- Li, H.; Deng, N.; Puopolo, T.; Jiang, X.; Seeram, N.P.; Liu, C.; Ma, H. Cannflavins A and B with Anti-Ferroptosis, Anti-Glycation, and Antioxidant Activities Protect Human Keratinocytes in a Cell Death Model with Erastin and Reactive Carbonyl Species. Nutrients 2023, 15, 4565. [Google Scholar] [CrossRef]
- Lahaise, M.; Boujenoui, F.; Beaudry, F. Cannflavins isolated from Cannabis sativa impede Caenorhabditis elegans response to noxious heat. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Hussain, W.; Akhtar, A. Probing the Pharmacological Binding Properties, and Reactivity of Selective Phytochemicals as Potential HIV-1 protease Inhibitors. Univ. Sci. 2019, 24, 441–464. [Google Scholar]
- Powers, C.N.; Setzer, W.N. An In-Silico Investigation of Phytochemicals as Antiviral Agents Against Dengue Fever. Comb. Chem. High Throughput Screen 2016, 19, 516–536. [Google Scholar] [CrossRef]
- Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. In-silico screening for anti-Zika virus phytochemicals. J. Mol. Graph. Model. 2016, 69, 78–91. [Google Scholar] [CrossRef]
- Kuo, C.T.; Hsu, M.J.; Chen, B.C.; Chen, C.C.; Teng, C.M.; Pan, S.L.; Lin, C.H. Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol. Lett. 2008, 177, 48–58. [Google Scholar] [CrossRef]
- Chen, T.H.; Pan, S.L.; Guh, J.H.; Chen, C.C.; Huang, Y.T.; Pai, H.C.; Teng, C.M. Denbinobin induces apoptosis by apoptosis-inducing factor releasing and DNA damage in human colorectal cancer HCT-116 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 447–457. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Calzado, M.A.; de Vinuesa, A.G.; Caballero, F.J.; Ech-Chahad, A.; Appendino, G.; Krohn, K.; Fiebich, B.L.; Munoz, E. Denbinobin, a naturally occurring 1,4-phenanthrenequinone, inhibits HIV-1 replication through an NF-kappaB-dependent pathway. Biochem. Pharmacol. 2008, 76, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Song, J.I.; Kang, Y.J.; Yong, H.Y.; Kim, Y.C.; Moon, A. Denbinobin, a phenanthrene from dendrobium nobile, inhibits invasion and induces apoptosis in SNU-484 human gastric cancer cells. Oncol. Rep. 2012, 27, 813–818. [Google Scholar]
- Yang, H.; Lee, P.J.; Jeong, E.J.; Kim, H.P.; Kim, Y.C. Selective apoptosis in hepatic stellate cells mediates the antifibrotic effect of phenanthrenes from Dendrobium nobile. Phytother. Res. 2012, 26, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Hsu, M.J.; Chen, C.C.; Chen, B.C.; Hong, C.Y.; Teng, C.M.; Pan, S.L.; Chiu, W.T.; Lin, C.H. Denbinobin induces human glioblastoma multiforme cell apoptosis through the IKKalpha-Akt-FKHR signaling cascade. Eur. J. Pharmacol. 2013, 698, 103–109. [Google Scholar] [CrossRef]
- Lu, T.L.; Han, C.K.; Chang, Y.S.; Lu, T.J.; Huang, H.C.; Bao, B.Y.; Wu, H.Y.; Huang, C.H.; Li, C.Y.; Wu, T.S. Denbinobin, a phenanthrene from Dendrobium nobile, impairs prostate cancer migration by inhibiting Rac1 activity. Am. J. Chin. Med. 2014, 42, 1539–1554. [Google Scholar] [CrossRef]
- Liu, H.E.; Chang, A.S.; Teng, C.M.; Chen, C.C.; Tsai, A.C.; Yang, C.R. Potent anti-inflammatory effects of denbinobin mediated by dual inhibition of expression of inducible no synthase and cyclooxygenase 2. Shock 2011, 35, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Liu, X.; Zhao, C.; Yin, J.; Li, X.; Zhang, X.; Wang, J.; Wang, S. Distinctive anti-inflammatory effects of resveratrol, dihydroresveratrol, and 3-(4-hydroxyphenyl)-propionic acid on DSS-induced colitis in pseudo-germ-free mice. Food Chem. 2023, 400, 133904. [Google Scholar] [CrossRef]
- Lam, C.S.; Xia, Y.X.; Chen, B.S.; Du, Y.X.; Liu, K.L.; Zhang, H.J. Dihydro-Resveratrol Attenuates Oxidative Stress, Adipogenesis and Insulin Resistance in In Vitro Models and High-Fat Diet-Induced Mouse Model via AMPK Activation. Nutrients 2023, 15, 3006. [Google Scholar] [CrossRef]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol Metabolites Are Able to Reduce Steatosis in Cultured Hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Chen, P.H.; Peng, C.Y.; Pai, H.C.; Teng, C.M.; Chen, C.C.; Yang, C.R. Denbinobin suppresses breast cancer metastasis through the inhibition of Src-mediated signaling pathways. J. Nutr. Biochem. 2011, 22, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Pan, S.L.; Lai, C.Y.; Wang, C.Y.; Chen, C.C.; Shen, C.C.; Teng, C.M. The inhibition of angiogenesis and tumor growth by denbinobin is associated with the blocking of insulin-like growth factor-1 receptor signaling. J. Nutr. Biochem. 2011, 22, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.S.; Ku, C.F.; Guan, Y.F.; Xiao, H.T.; Shi, X.K.; Wang, H.Q.; Bian, Z.X.; Tsang, S.W.; Zhang, H.J. Dihydro-Resveratrol Ameliorates Lung Injury in Rats with Cerulein-Induced Acute Pancreatitis. Phytother. Res. 2016, 30, 663–670. [Google Scholar] [CrossRef]
- European Medicines Agency. European Medicines Agency (EMA)-Guideline on Good Agricultural and Collection Practice (GACP) for Starting Materials of Herbal Origin; European Medicines Agency: Amsterdam, The Netherlands, 2024. [Google Scholar]
- U.S. Food and Drug Administration. Food and Drug Administration (FDA)-Botanical Drug Development Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2016.
- Health Canada. Health Canada-Guidance Document—Good Manufacturing Practices for Cannabis; Government of Canada: Ottawa, ON, Canada, 2019.
- Gertsch, J.; Pertwee, R.G.; Di Marzo, V. Phytocannabinoids beyond the Cannabis plant—Do they exist? Br. J. Pharmacol. 2010, 160, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Mihai, M.; Dulf, F.; Ona, A.; Muntean, L.; Ranga, F.; Urda, C.; Pop, D.; Mihaiescu, T.; Marza, S.M.; et al. Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats. Nutrients 2023, 15, 2944. [Google Scholar] [CrossRef]
- NAS National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. Challenges and Barriers in Conducting Cannabis Research. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academic Press: Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425757/ (accessed on 27 April 2025).
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef]
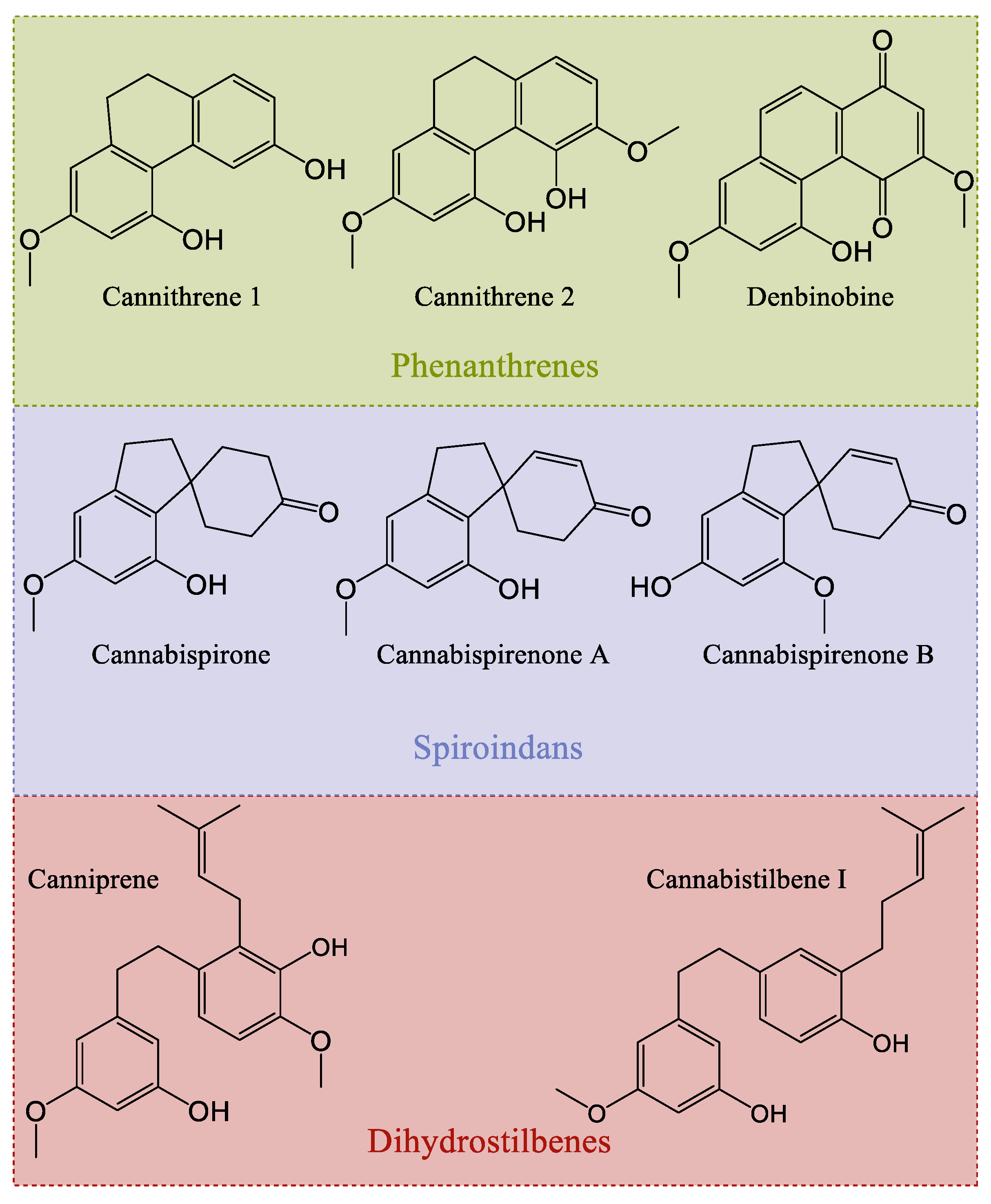
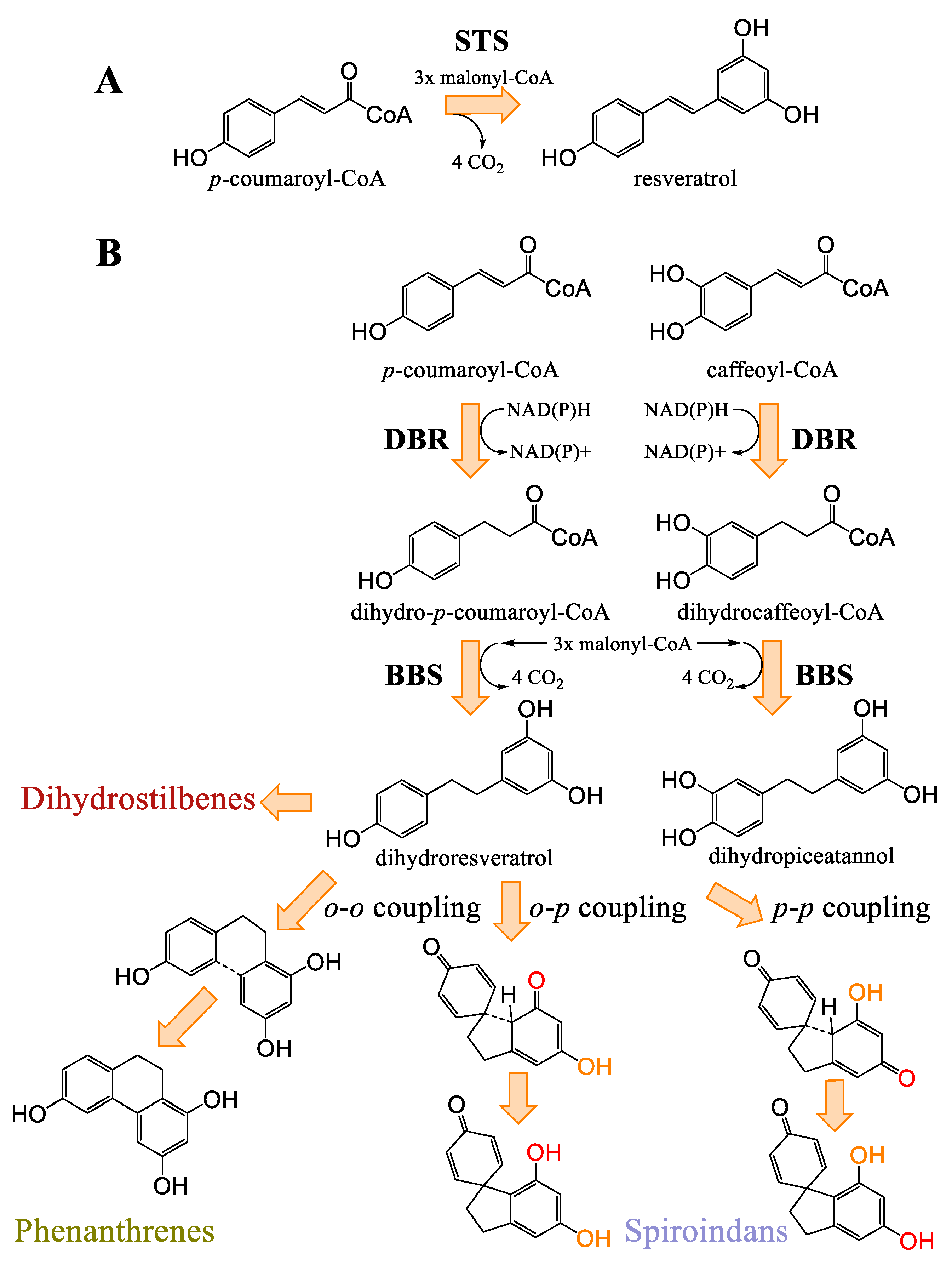
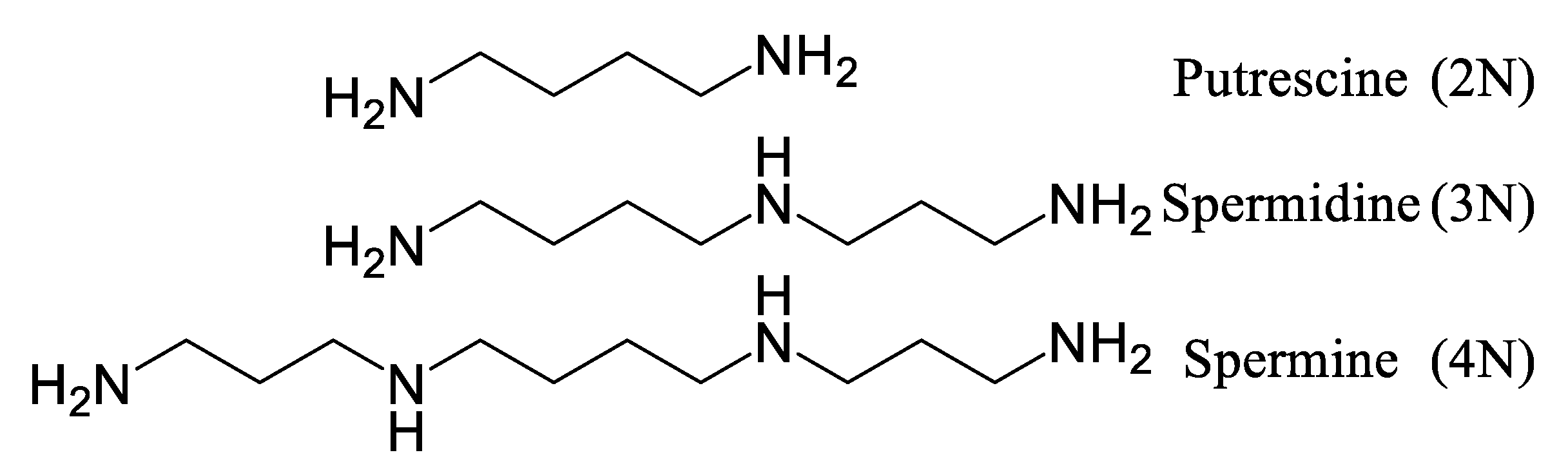
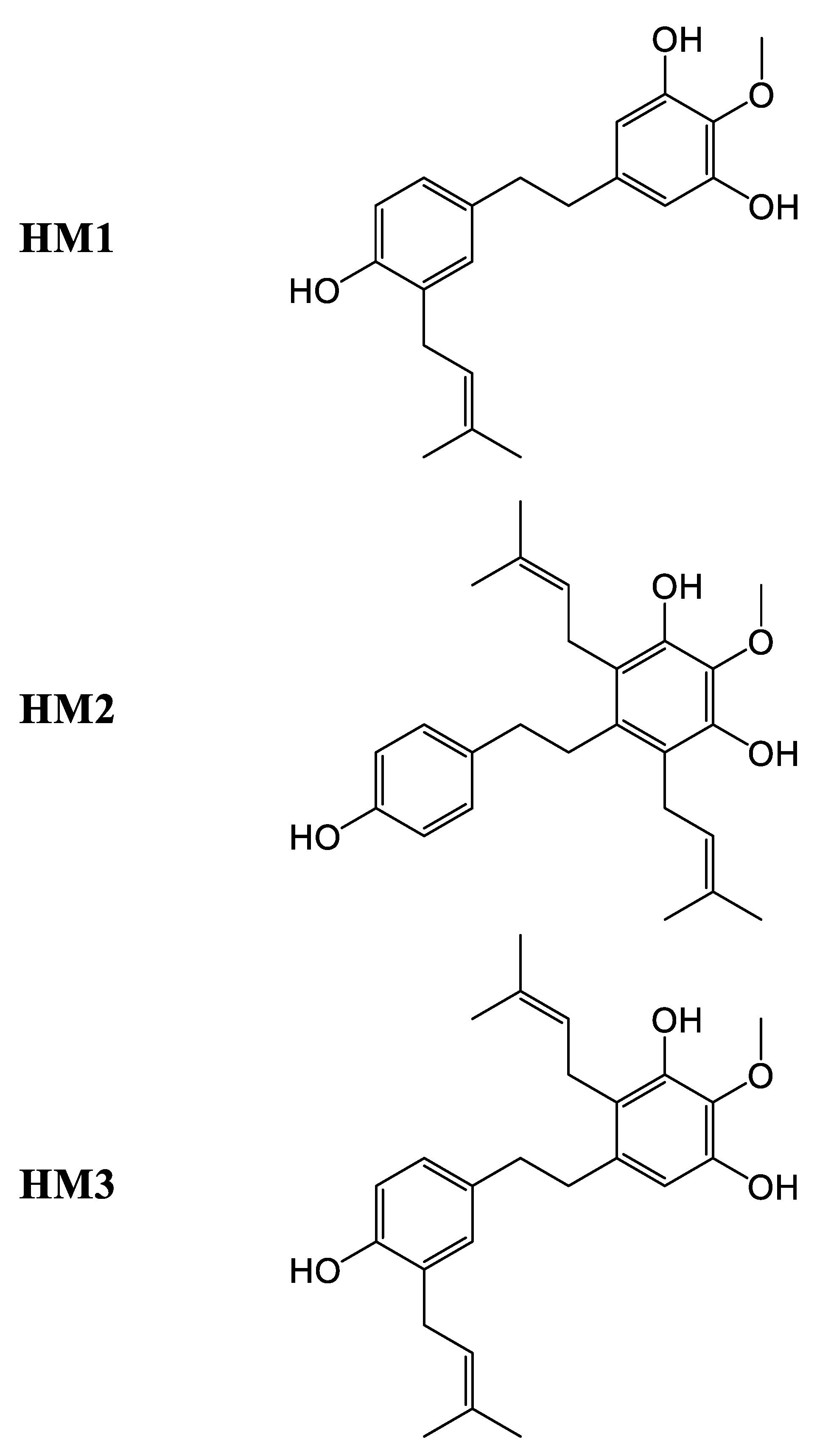
| Type | Example | Structure |
|---|---|---|
| Simple phenols | Eugenol | 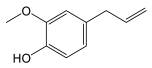 |
| Phenolic acids | Protocatechuic acid |  |
| Cinnamic acids | p-coumaric acid | 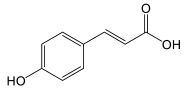 |
| Flavones | Cannflavin B |  |
| Flavanones | Naringenin |  |
| Anthocyanins | Cyanidine 3-O-rutinoside |  |
| Stilbenes | Dihydroresveratrol | 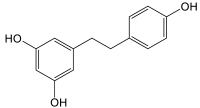 |
| Type | Precursor | Chemical | Example | Structure |
|---|---|---|---|---|
| Piperidine | Aspartate/ ornithine | Heterocyclic | Piperidine | 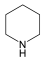 |
| Pyrrolidine | Arginine/ ornithine | Heterocyclic | Pyrrolidine | 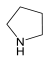 |
| Spermidine | Arginine/ ornithine | Heterocyclic | Cannabisativine | 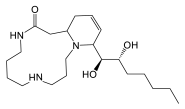 |
| Pyridine | Tryptophan | Heterocyclic | Trigonelline | 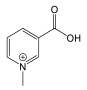 |
| Phenylethyl-amine | Tyrosine/ Phenylalanine | Aliphatic | Hordenine | 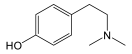 |
| Compound | Range Levels (mg/kg) | Mean Levels (mg/kg) | Cultivar for Quantification | Tissue | Pharmacological Significance | Ref. |
|---|---|---|---|---|---|---|
| Cannabisativine | - | 2.5 | Root, leaf | - | ||
| Anhydrocannabisativine | - | 0.3 | Root, leaf | - | [21] | |
| Hordenine | - | - | Root | Antibacterial, anti-asthmatic, smooth muscle relaxation, anti-inflammatory, muscle hypertrophy stimulation, anti-prolactinoma, neuroprotection, cognitive dysfunction attenuation | ||
| N-p-trans-coumaroyltyramine | 7.6–19.8 - | 11.8 196.9 | High THC variety High CBD variety Intermediate variety Zenit | Root, seed, leaf | Antiproliferative, tyrosine kinase inhibition, antiproliferative drug sensitivity enhancer, acetylcholinesterase inhibition, antioxidant, senomorphic activity, antibacterial, insect deterrent, anti-melanogenesis | [82,131] |
| N-caffeoyltyramine | 0.1–76.2 - | 23.7 339.0 | Antal Carmagnola Kompolti Tiborszallasi Zenit | Inflorescence, seed, root | Antiproliferative, tyrosine kinase inhibition, neuroprotective, antioxidant, anti-inflammatory, lipophagic agent, antifungal, anti-melanogenesis | [51,131] |
| N-feruloyltyramine | - | 278.8 | Zenit | Seed, root, leaf | Antiproliferative, tyrosine kinase inhibition, neuroprotective, antioxidant, anti-inflammatory, senomorphic activity, lipophagic agent, insect deterrent, anti-melanogenesis | [131] |
| Grossamide | - | 830.0 | Zenit | Seed, root | Anti-inflammatory, neuroprotective | [131] |
| Cannabisin A | 0.005–2.9 - | 1.0 82.7 | Antal Carmagnola Kompolti Tiborszallasi Zenit | Inflorescence, seed | Antioxidant, tyrosinase inhibition | [51,131] |
| Cannabisin B | 0.02–1.1 - | 0.5 130.0 | Antal Carmagnola Kompolti Tiborszallasi Zenit | Inflorescence, seed, root | Antioxidant, antiproliferative, tyrosinase inhibition | [51,131] |
| Cannabisin C | 0.003–0.4 - | 0.1 179.7 | Antal Carmagnola Kompolti Tiborszallasi Zenit | Inflorescence, seed, root | - | [51,131] |
| Cannabisin D | - | 444.4 | Zenit | Seed | Antioxidant, anti-inflammatory | [131] |
| Cannabisin F | - | 471.0 | Zenit | Seed | Anti-inflammatory, neuroprotective | [131] |
| Cannabisin G | - | - | Root | Antioxidant | ||
| Cannabisin Q | - | 201.3 | Zenit | Seed | - | [131] |
| Cannflavin A | 19.6–130.0 21–280 | 61.8 97 | Antal Carmagnola Kompolti Tiborszallasi Ermo Carma THC-3 MH-WU-112 | Inflorescence, leaf | Anti-inflammatory, anti-leishmanial, anti β-amyloid, antiproliferative, KMO inhibition, lipid peroxidation inhibition, anti-nociceptive, potential anti-viral activity | [51,57] |
| Cannflavin B | 11.9–215.5 9–106 | 84.5 43 | Antal Carmagnola Kompolti Tiborszallasi Ermo Carma THC-3 MH-WU-112 | Inflorescence, leaf | Lipid peroxidation inhibition, anti-nociceptive | [51,57] |
| Cannflavin C | - | - | Leaf | Anti-leishmanial | ||
| Denbinobin | - | - | Leaf | Antiproliferative, anti-metastatic, anti-inflammatory | ||
| Canniprene | 4–2085 | 433 | Ermo Carma Carmagnola THC-3 MH-WU-112 | Leaf | Anti-inflammatory, antiproliferative, potential anti-viral activity | [57] |
| Cannabispirenone | - | - | Leaf | Anti-inflammatory | ||
| HM1 | - | - | Leaf | Antiproliferative, cholesterol transport | ||
| HM2 | - | - | Leaf | Antiproliferative, cholesterol transport | ||
| HM3 | - | - | Leaf | Antiproliferative, cholesterol transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucher, R.; Germain, H.; Desgagné-Penix, I. Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential. Plants 2025, 14, 1372. https://doi.org/10.3390/plants14091372
Boucher R, Germain H, Desgagné-Penix I. Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential. Plants. 2025; 14(9):1372. https://doi.org/10.3390/plants14091372
Chicago/Turabian StyleBoucher, Raphaël, Hugo Germain, and Isabel Desgagné-Penix. 2025. "Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential" Plants 14, no. 9: 1372. https://doi.org/10.3390/plants14091372
APA StyleBoucher, R., Germain, H., & Desgagné-Penix, I. (2025). Exploring the Lesser-Known Bioactive Natural Products of Plant Species of the Genus Cannabis L.: Alkaloids, Phenolic Compounds, and Their Therapeutic Potential. Plants, 14(9), 1372. https://doi.org/10.3390/plants14091372









