Chemical Profiling of Polyphenolic Fraction of Cannabis sativa L. vr. Kompolti Industrial Inflorescences: Insights into Cannabidiol Neuroprotective Effects in a Cellular Model of Parkinson’s Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Major Phytochemical Components in Kompolti Water Infusion
Identification of Major Components in C. sativa by LC-ESI-HR-MS/MS Analysis
2.2. Phenolic Components and In Vitro Antioxidant Activity
2.3. Correlation Between Phenolic Compounds and Antioxidant Activity of Extracts
2.4. Mineral Composition of the Extracts
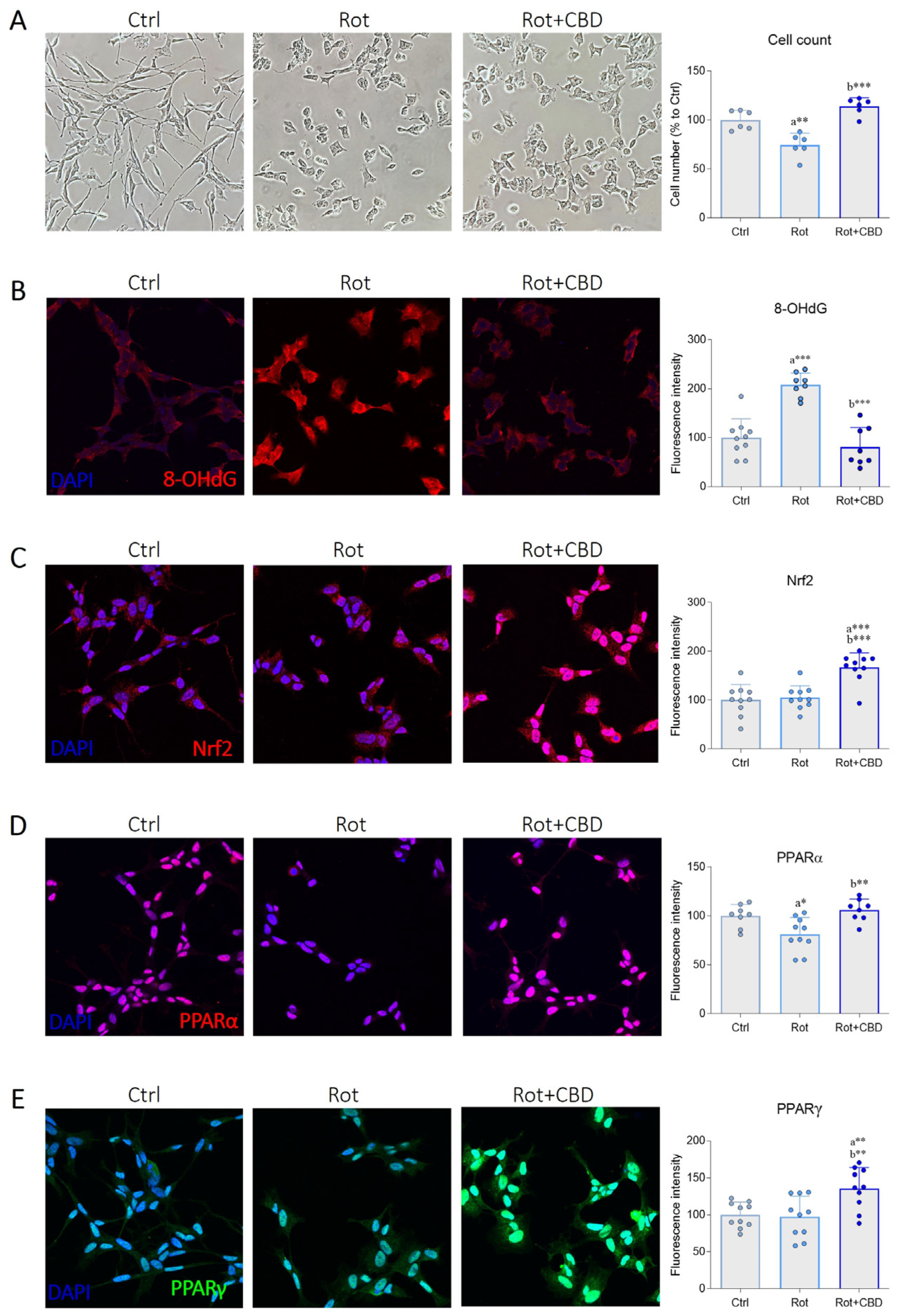
2.5. Effects of CBD from C. sativa L. in a Cellular Model of Parkinson’s Disease
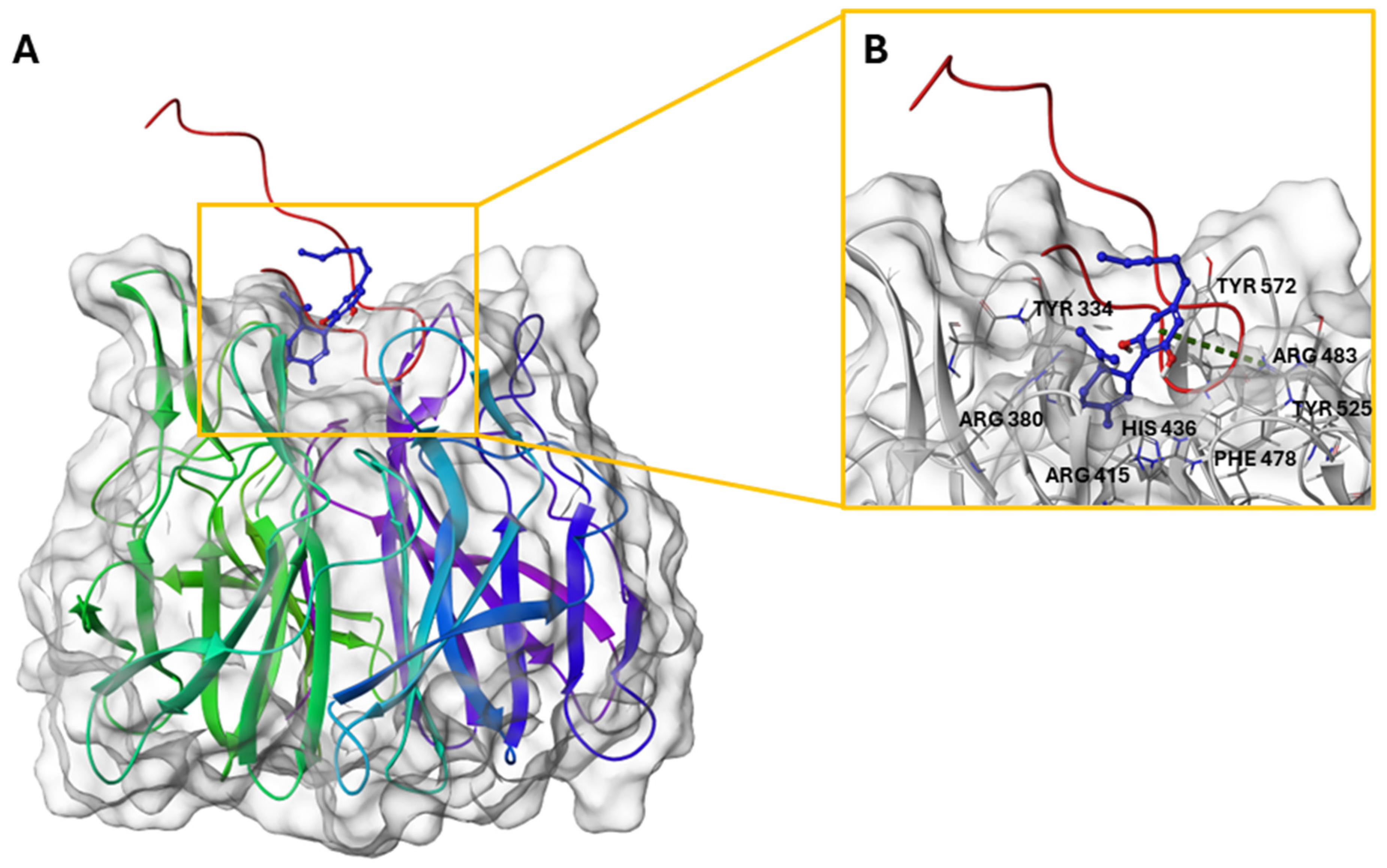
2.6. CBD/Nrf2 Complex: Molecular Docking Studies
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Water Infusion (WI) Preparation of Kompolti Variety
3.4. Sample Preparation and NMR Analyses
3.5. Metabolites Identification in the Different Extracts of Kompolti Hemp Variety by LC-ESI-Q-Exactive MS/MS Experiments
3.6. Determination of Total Phenolic Content and Flavonoid Content
3.6.1. Extraction Procedure (HEC)
3.6.2. Folin-Ciocalteu Assay
3.6.3. Total Flavonoid Content (TFC)
3.7. Antioxidant Capacity
3.7.1. DPPH Radical Scavenging Activity
3.7.2. FRAP Assay
3.8. Macro and Microelements Content Determination
3.9. Determination of Biological Activity
3.9.1. Cell Culture
3.9.2. Immunofluorescence
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Gąssowska-Dobrowolska, M.; Chlubek, M.; Kolasa, A.; Tomasiak, P.; Korbecki, J.; Skowrońska, K.; Tarnowski, M.; Masztalewicz, M.; Baranowska-Bosiacka, I. Microglia and Astroglia—The Potential Role in Neuroinflammation Induced by Pre- and Neonatal Exposure to Lead (Pb). Int. J. Mol. Sci. 2023, 24, 9903. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2022, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- AmeliMojarad, M.; AmeliMojarad, M. The neuroinflammatory role of microglia in Alzheimer’s disease and their associated therapeutic targets. CNS Neurosci. Ther. 2024, 30, e14856. [Google Scholar] [CrossRef] [PubMed]
- Morén, C.; deSouza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef]
- Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. Quantitative Determination of Cannabinoids in Individual Glandular Trichomes of Cannabis sativa L. (Cannabaceae). Am. J. Bot. 1978, 65, 1103–1106. [Google Scholar] [CrossRef]
- Alexander, S.P.H. Therapeutic potential of cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Mendivil, E.J.; Romano, M.; García, M.; Martínez, M.E. A Systematic Review of Medical Cannabinoids Dosing in Human. Clin. Ther. 2022, 44, e39–e58. [Google Scholar] [CrossRef] [PubMed]
- Sunda, F.; Arowolo, A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J. 2020, 34, 14083–14092. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Kozela, E.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol—Studies in BV-2 microglia and encephalitogenic T cells. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 289–296. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Front. Immunol. 2021, 11, 613613. [Google Scholar] [CrossRef]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In Vitro and In Vivo Efficacy of Non-Psychoactive Cannabidiol in Neuroblastoma. Curr. Oncol. 2016, 23, 15–22. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Lehmann, C.; Fisher, N.B.; Tugwell, B.; Szczesniak, A.; Kelly, M.; Zhou, J. Experimental cannabidiol treatment reduces early pancreatic inflammation in type 1 diabetes. Clin. Hemorheol. Microcirc. 2017, 64, 655–662. [Google Scholar] [CrossRef]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2019, 68, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Batista, J.; Viana, R.; Baetas, A.; Orestes, E.; Andrade, M.; Honório, K.; Da Silva, A. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef]
- Machado Bergamaschi, M.; Helena Costa Queiroz, R.; Waldo Zuardi, A.; Alexandre, S.; Crippa, J. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Pereira, S.R.; Tello Velasquez, J.; Duggan, S.; Ivanisevic, B.; McKenna, J.P.; McCreary, C.; Downer, E.J. Recent advances in the understanding of the aetiology and therapeutic strategies in burning mouth syndrome: Focus on the actions of cannabinoids. Eur. J. Neurosci. 2020, 55, 1032–1050. [Google Scholar] [CrossRef] [PubMed]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Critical Role of Mast Cells and Peroxisome Proliferator–Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 2015, 194, 5211–5222. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Prashantha Kumar, B.R.; Kumar, A.P.; Jose, J.A.; Prabitha, P.; Yuvaraj, S.; Chipurupalli, S.; Jeyarani, V.; Manisha, C.; Banerjee, S.; Jeyabalan, J.B.; et al. Minutes of PPAR-γ agonism and neuroprotection. Neurochem. Int. 2020, 140, 104814. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 31 March 2025).
- Yangsud, J.; Ahkkarachinoreh, P.; Santasanasuwan, S.; Suksaeree, J.; Songsak, T.; Maha, A.; Madaka, F.; Monton, C. Effect of vegetable oil types on the stability of cannabinoids in cannabis sublingual drops. J. Curr. Sci. Technol. 2021, 11, 15–23. [Google Scholar] [CrossRef]
- Marino, S.; Festa, C.; Zollo, F.; Iorizzi, M. Novel Steroidal Components from the Underground Parts of Ruscus aculeatus L. Molecules 2012, 17, 14002–14014. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.). Molecules 2018, 24, 83. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Knapsack. Available online: https://www.knapsackfamily.com/knapsack_core/top.php (accessed on 1 February 2025).
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef]
- Choi, Y.H.; Hazekamp, A.; Peltenburg-Looman, A.M.G.; Frédérich, M.; Erkelens, C.; Lefeber, A.W.M.; Verpoorte, R. NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa. Phytochem. Anal. 2004, 15, 345–354. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef]
- Brkljača, N.; Đurović, S.; Milošević, S.; Gašić, U.; Panković, D.; Zeković, Z.; Pavlić, B. Sequential extraction approach for sustainable recovery of various hemp (Cannabis sativa L.) bioactive compounds. Sustain. Chem. Pharm. 2023, 35, 101213. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Li, C.-R.; Yang, L.-X.; Guo, Z.-F.; Yang, H.; Zhang, Y.; Wang, Y.-M.; Zhang, G.-Z.; Li, P.; Gao, W. LC-MS-based untargeted metabolomics reveals chemical differences of Cannabis leaves from different regions of China. Ind. Crops Prod. 2022, 176, 114411. [Google Scholar] [CrossRef]
- Gong, F.; Fung, Y.-S.; Liang, Y.-Z. Determination of Volatile Components in Ginger Using Gas Chromatography−Mass Spectrometry with Resolution Improved by Data Processing Techniques. J. Agric. Food Chem. 2004, 52, 6378–6383. [Google Scholar] [CrossRef]
- Aresta, A.; Damascelli, A.; De Vietro, N.; Zambonin, C. Measurement of squalene in olive oil by fractional crystallization or headspace solid phase microextraction coupled with gas chromatography. Int. J. Food Prop. 2020, 23, 1845–1853. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yang, Y. Hot Water Extraction of Antioxidants from Tea Leaves—Optimization of Brewing Conditions for Preparing Antioxidant-Rich Tea Drinks. Molecules 2023, 28, 3030. [Google Scholar] [CrossRef]
- Tzimas, P.S.; Petrakis, E.A.; Halabalaki, M.; Skaltsounis, L.A. Extraction solvent selection for Cannabis sativa L. by efficient exploration of cannabinoid selectivity and phytochemical diversity. Phytochem. Anal. 2023, 35, 163–183. [Google Scholar] [CrossRef]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Leaner and greener analysis of cannabinoids. Anal. Bioanal. Chem. 2017, 409, 3153–3163. [Google Scholar] [CrossRef]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Machera, K. Macro and Trace Elements in Hemp (Cannabis sativa L.) Cultivated in Greece: Risk Assessment of Toxic Elements. Front. Chem. 2021, 9, 654308. [Google Scholar] [CrossRef]
- Olivari, I.; Paz, S.; Gutiérrez, Á.J.; González-Weller, D.; Hardisson, A.; Sagratini, G.; Rubio, C. Macroelement, trace element, and toxic metal levels in leaves and infusions of yerba mate (Ilex paraguariensis). Environ. Sci. Pollut. Res. 2020, 27, 21341–21352. [Google Scholar] [CrossRef]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The suitability of the simplified method of the analysis of coffee infusions on the content of Ca, Cu, Fe, Mg, Mn and Zn and the study of the effect of preparation conditions on the leachability of elements into the coffee brew. Food Chem. 2013, 141, 1956–1961. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Zambon, L.M.; Motta, A.C.V.; Wendling, I. Composition, Hot-Water Solubility of Elements and Nutritional Value of Fruits And. Ciência E Agrotecnologia 2015, 39, 593–603. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.-F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Behl, T.; Madaan, P.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Chigurupati, S.; Alrashdi, I.; Bungau, S.G. Elucidating the Neuroprotective Role of PPARs in Parkinson’s Disease: A Neoteric and Prospective Target. Int. J. Mol. Sci. 2021, 22, 10161. [Google Scholar] [CrossRef]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol goes nuclear: The role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- Katoh, Y.; Iida, K.; Kang, M.-I.L.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Abed, D.A.; Goldstein, M.; Albanyan, H.; Jin, H.; Hu, L. Discovery of direct inhibitors of Keap1–Nrf2 protein–protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B 2015, 5, 285–299. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Lo, S.-C.; Li, X.; Henzl, M.T.; Beamer, L.J.; Hannink, M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006, 25, 3605–3617. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity of Phaseolus vulgaris L. Dry Beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Menezes, I.M.N.R.; Nascimento, P.d.A.; Yamamoto, C.I.; Oliveira, A. Evaluation of trace elements in cannabis products. J. Food Compos. Anal. 2022, 113, 104721. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput.—Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger. Release 2021-1: Protein Preparation Wizard; Epik; Schrödinger, LLC: New York, NY, USA; Impact; Schrödinger, LLC: New York, NY, USA; Prime; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger. Release 2021-1; Maestro; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger. Release 2021-1; LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger. Release 2021-1; Glide; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, K.; Repasky, M.P.; Leswing, K.; Abel, R.; Shoichet, B.K.; Jerome, S.V. Efficient Exploration of Chemical Space with Docking and Deep Learning. J. Chem. Theory Comput. 2021, 17, 7106–7119. [Google Scholar] [CrossRef]
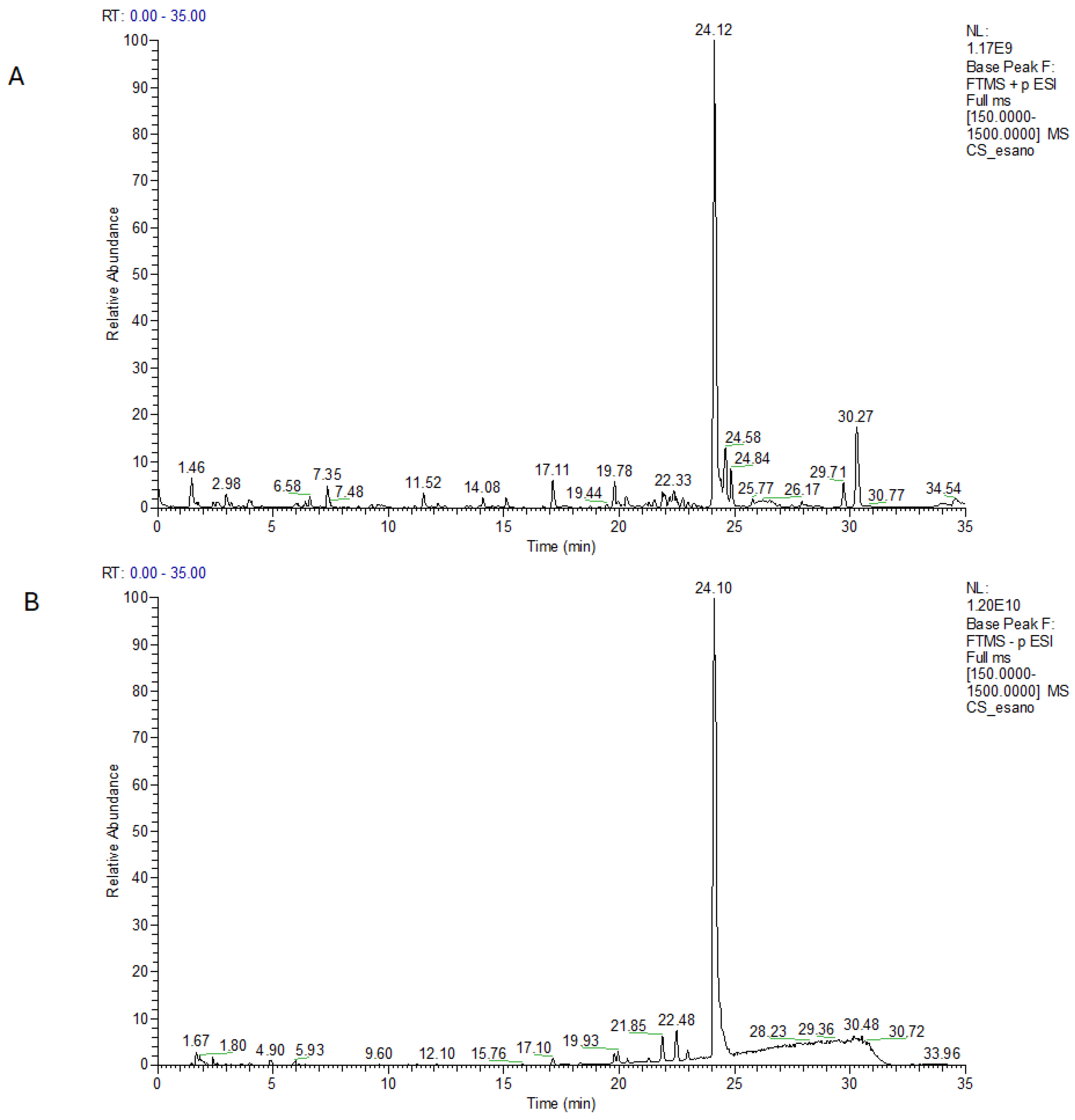



| Positive Ion Mode | |||||
|---|---|---|---|---|---|
| Name | Formula | ppm | [M + H]+ | Rt | MS/MS |
| (−)-Caryophyllene oxide | C15H24O | −0.7 | 203.1793 | 14.2 | 147.1166/133.1012/121.1011/105.0701/95.0858 |
| Unsaturated cannabispirol | C15H18O3 | −1.4 | 247.1325 | 15.08 | 211.1113/169.1008/183.1165 |
| 11-nor-9-carboxy-Δ9-tetrahydrocannabinol | C21H28O4 | −0.6 | 345.2058 | 19.2 | 327.1587/285.1121/205.0488 |
| Δ9-THCA derivative (C4) | C21H28O4 | −0.8 | 345.2057 | 22.9 | 327.1592/193.1218/205.0858 |
| Cannabidiolic acid (CBDA) | C22H30O4 | −3.1 | 359.2206 | 24.1 | 219.1012/261.1479/285.1484 |
| Cannabidiol (CBD) | C21H30O2 | −1.5 | 315.2312 | 24.8 | 259.1689/193.1221/135.1168/181.1219 |
| Octadecanamide | C18H37NO | −1.6 | 284.2943 | 34.2 | 141.1274 |
| Negative Ion Mode | |||||
| Name | Formula | ppm | [M − H]− | Rt | MS/MS |
| 9-OxoODE | C18H30O3 | −1.3 | 295.2264 | 22.4 | 277.2168/195.1383 |
| Positive Ion Mode | |||||
|---|---|---|---|---|---|
| Name | Formula | ppm | [M + H]+ | Rt | MS/MS |
| Hedione | C13H22O3 | −0.88 | 209.1534 | 5.22 | 125.0960/153.0908 |
| 5-(1-Butyl-1-hydroxypentyl)-1.3-benzenediol | C15H24O3 | −1.9 | 253.1793 | 5.55 | 217.1583/159.1166 |
| 2,6-Dimethoxy-4-propylphenol | C11H16O3 | −3.04 | 197.1168 | 6.95 | 176.1064/161.0960/135.1168 |
| 5-(1-Butyl-1-penten-1-yl)-1,3-benzenediol | C15H22O2 | −0.55 | 235.1691 | 7.75 | 186.1852 |
| β-cannabispiranol | C15H20O3 | −0.2 | 249.1483 | 9.05 | 231.1375/213.1871/189.1272 |
| Diosmetin | C16H12O6 | −0.56 | 301.0705 | 12.34 | 286.0469/258.0522 |
| α-cannabispiranol | C15H20O3 | 0.8 | 249.1482 | 13.51 | 231.1387/189.0909/163.0751 |
| Humulene | C15H24 | −0.57 | 205.195 | 14.14 | 121.0648 |
| 6-Gingerol | C17H26O4 | −0.81 | 277.1796 | 15.49 | 304.6685/310.8908 |
| Cannabielsoic acid A | C22H30O5 | 1.3 | 375.2152 | 17.0 | 357.2057/339.1952/275.1275 |
| Cannabicoumarononic acid A | C22H28O5 | −0.61 | 373.2007 | 17.83 | 355.1899/337.1791/331.1793 |
| Cannabidiolic acid (CBDA) | C22H30O4 | −0.27 | 359.2204 | 19.65 | 341.2105/261.1480/219.1014 |
| Epoxyhexahydrocannabinol acetate | C23H32O4 | −0.90 | 373.2370 | 21.80 | 355.1899/337.1791/331.1793 |
| Δ9-THCA derivative (C1) | C18H23O4 | −1.8 | 303.1585 | 19.8 | 285.1479/205.0857/163.0387 |
| Cannabichromenic acid (CBCA) | C22H30O4 | −0.31 | 359.2206 | 24.3 | 341.2105/261.1480/219.1014 |
| Cannabidiol (CBD) | C21H30O2 | −1.41 | 315.2314 | 25.1 | 259.1688/193.1222 |
| Palmitic amide | C16H33NO | −2.03 | 256.263 | 29.8 | 88.0761 |
| Negative Ion Mode | |||||
| Name | Formula | ppm | [M − H]− | Rt | MS/MS |
| 15,16-DiHODE | C18H32O4 | −1.09 | 311.2224 | 16.3 | 201.1124/223.1697 |
| 6,7-Epoxy-CBGA | C22H30O5 | 1.6 | 375.2172 | 19.6 | 357.1796/331.1902/313.2167/261.1493/203.1069 |
| 11-Hydroxy-Δ9-THC | C21H30O3 | −0.27 | 329.2121 | 20.2 | 311.2017/271.1700 |
| Cannabielsoic acid A | C22H30O5 | 1.3 | 373.2017 | 22.6 | 357.2057/339.1952/275.1275 |
| 16-Hydroxyhexadecanoic acid | C16H32O3 | 0.02 | 271.2279 | 27.0 | 225.2219/253.2170 |
| Positive Ion Mode | |||||
|---|---|---|---|---|---|
| Name | Formula | (ppm) | [M + H]+ | Rt | MS/MS |
| Phenylalanylthreonine | C13H18N2O4 | −1.2 | 267.1336 | 2.88 | 121.0648 |
| D-(+)-Tryptophan | C11H12N2O2 | −0.6 | 205.097 | 4.91 | 146.0599/188.0704 |
| L-Phenylalanine | C9H11NO2 | 0.02 | 166.0863 | 6.11 | 105.0337/149.0598 |
| Kaempferol 3-O-rutinoside | C27H30O15 | −1.0 | 595.165 | 11.3 | 329.0651/287.0548/433.1829 |
| Orientin | C21H20O11 | −2.5 | 449.1067 | 11.62 | 287.0923 |
| Apigenin 7-O-rutinoside | C27H30O14 | −2.8 | 579.1691 | 11.9 | 433.1125/271.0605/313.0703/397.0918 |
| Vitexin | C21H20O10 | −0.5 | 433.1127 | 12.2 | 313.0703/397.0914/271.0612 |
| Luteolin 7-O-glucuronide | C21H18O12 | −0.3 | 463.087 | 12.7 | 287.0545 |
| Apigenin 7-glucuronide | C21H18O11 | −2.7 | 447.0909 | 13.72 | 271.0596 |
| Hispidulin 7-glucuronide | C22H20O12 | −0.3 | 477.1026 | 13.95 | 301.0703 |
| αlpha-Cannabispiranol | C15H20O3 | −1.9 | 249.1480 | 16.14 | 231.1375/163.0750/137.0596 |
| Luteolin | C15H10O6 | −0.8 | 287.0548 | 16.4 | 153.0182/173.1322 |
| Andrographolide | C20H30O5 | 351.214 | 17.1 | 281.0828/221.0796 | |
| Apigenin | C15H10O5 | −0.61 | 271.0599 | 17.83 | 153.0186 |
| Cannabidiolic acid (CBDA) | C22H30O4 | −0.36 | 359.2216 | 22.86 | 261.1482 |
| (9Z)-2-Oxo-9-octadecenamide | C18H33NO2 | −1.59 | 296.2579 | 26.02 | 279.2316/261.2209/233.2260 |
| Cannabichromenic acid (CBCA) | C22H30O4 | −2.3 | 359.2216 | 27.318 | 219.1014/261.1482 |
| Tetrahydrocannabinol | C21H30O2 | −2.28 | 315.2311 | 27.87 | 259.1689/193.1222 |
| Palmitic amide | C16H33NO | −2.38 | 256.2629 | 32.17 | |
| Octadeca-9,12-dienal | C18H32O | −1.13 | 265.2523 | 32.57 | 207.1236 |
| Positive Ion Mode | |||||
|---|---|---|---|---|---|
| Name | Formula | ppm | [M + H]+ | Rt | MS/MS |
| N6,N6,N6-Trimethyl-L-lysine | C9H20N2O2 | 0.3 | 189.1598 | 1.69 | |
| L-(+)-Arginine | C6H14N4O2 | −1.5 | 175.1187 | 1.75 | 146.9600/118.9649 |
| N-(1-Deoxy-1-fructosyl)proline | C11H19NO7 | −0.8 | 278.1232 | 2.28 | 260.1125/242.1017/116.0707 |
| Citric acid | C6H8O7 | −1.2 | 210.0606 | 2.65 | |
| Leucylproline | C11H20N2O3 | 0.3 | 229.1548 | 3.94 | |
| Guanosine | C10H13N5O5 | −0.05 | 284.099 | 3.97 | 152.0565 |
| Kaempferol 3-galactoside-7-rhamnoside | C27H30O15 | −0.06 | 595.1657 | 11.60 | 287.0544 |
| Apigenin 7-O-rutinoside | C27H32O15 | −0.11 | 579.1706 | 11.84 | 433.1125/313.0702/271.0602 |
| Luteolin glucuronide | C21H18O12 | −0.41 | 463.0869 | 12.74 | 287.0547 |
| Apigenin 7-O-glucuronide | C21H18O11 | −3.16 | 447.0919 | 13.59 | 271.0596 |
| Octadeca-9,12-dienal | C18H32O | −1.93 | 265.2520 | 32.65 | 207.1238 |
| Negative Ion Mode | |||||
| Name | Formula | (ppm) | [M − H]− | Rt (min) | MS/MS |
| Galactonic acid | C6H12O7 | −4.28 | 195.0502 | 1.81 | 129.0180/75.0074 |
| Galactaric acid | C6H10O8 | −3.21 | 209.0296 | 1.82 | |
| L-Gulonolactone | C6H10O6 | −3.25 | 223.0454 | 1.85 | |
| Citric acid | C6H8O7 | −4.75 | 191.0188 | 2.07 | |
| Guanosine | C10H13N5O5 | −0.36 | 282.0843 | 3.98 | 150.0409 |
| Protocatechuic acid hexoside | C13H16O9 | 0.64 | 315.0724 | 6.76 | 153.0183/109.0283 |
| Tuberonic acid glucoside | C18H28O9 | 3.3 | 387.1662 | 10.16 | 207.1018/163.1117 |
| Sinapoylglucose | C17H22O10 | −4.19 | 385.1137 | 10.33 | 223.0605/208.0381/179.0703 |
| Apigenin 7-O-rutinoside | C27H30O14 | −1.74 | 577.1563 | 11.84 | 269.0453/413.0869 |
| Coutaric acid | C13H12O8 | −1.69 | 295.0454 | 12.80 | 173.0082/111.0074/85.0281 |
| 11-hydroxy THCA-2-glucuronic acid | C28H38O11 | 1.76 | 549.234 | 13.91 | 343.1913/189.0923 |
| Sample | Total Phenolic | Flavonoids | DPPH | FRAP |
|---|---|---|---|---|
| (mg g−1 GAE d.w.) | (mg g−1 QUE d.w.) | (mg Kg−1 TE d.w.) | (mmol Kg−1 TE d.w.) | |
| HEC | 3.29 ± 0.22 | 1.39 ± 0.3 | 58.87 ± 0.54 | 2.76 ± 0.03 |
| WI | 1.00 ± 0.13 | 0.48 ± 0.1 | 11.28 ± 0.04 | 1.49 ± 0.01 |
| Inflorescence MCI | Infusion WI | ||
|---|---|---|---|
| Microelements (mg Kg−1) | Wavelength (nm) | Mean ± SD | |
| Cu | 327.395 | 7.99 ± 0.59 | 3.43 ± 0.21 |
| Fe | 238.204 | 365.39 ± 37.03 | 2.86 ± 0.14 |
| Ni | 231.604 | 1.12 ± 0.29 | 0.57 ± 0.04 |
| Ba | 455.403 | 33.94 ± 1.12 | 1.00 ± 0.07 |
| Co | 230.786 | 0.24 ± 0.07 | <0.07 |
| Cr | 267.716 | 0.79 ± 0.11 | 0.02 ± 0.01 |
| Pb | 220.353 | 1.43 ± 0.26 | <0.14 |
| V | 292.401 | 0.61 ± 0.12 | <0.14 |
| Zn | 213.857 | 58.41 ± 0.82 | 19.00 ± 0.11 |
| B | 249.772 | 66.91 ± 1.96 | 8.57 ± 0.08 |
| Macroelements (g Kg−1) | |||
| Ca | 393.366 | 50.40 ± 0.24 | 5.34 ± 0.09 |
| K | 766.491 | 12.65 ± 0.38 | 11.16 ± 0.17 |
| Mg | 279.553 | 2.92 ± 0.12 | 1.17 ± 0.08 |
| P | 214.914 | 2.77 ± 0.16 | 0.53 ± 0.07 |
| S | 181.972 | 1.71 ± 0.09 | 0.72 ± 0.01 |
| Si | 251.611 | 2.20 ± 0.11 | 0.27 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantasma, F.; D’Urso, G.; Martella, N.; Capuano, A.; Boccia, E.; Samukha, V.; De Felice, V.; Saviano, G.; Trombetta, F.; Lauro, G.; et al. Chemical Profiling of Polyphenolic Fraction of Cannabis sativa L. vr. Kompolti Industrial Inflorescences: Insights into Cannabidiol Neuroprotective Effects in a Cellular Model of Parkinson’s Disease. Plants 2025, 14, 1473. https://doi.org/10.3390/plants14101473
Fantasma F, D’Urso G, Martella N, Capuano A, Boccia E, Samukha V, De Felice V, Saviano G, Trombetta F, Lauro G, et al. Chemical Profiling of Polyphenolic Fraction of Cannabis sativa L. vr. Kompolti Industrial Inflorescences: Insights into Cannabidiol Neuroprotective Effects in a Cellular Model of Parkinson’s Disease. Plants. 2025; 14(10):1473. https://doi.org/10.3390/plants14101473
Chicago/Turabian StyleFantasma, Francesca, Gilda D’Urso, Noemi Martella, Alessandra Capuano, Eleonora Boccia, Vadym Samukha, Vincenzo De Felice, Gabriella Saviano, Federico Trombetta, Gianluigi Lauro, and et al. 2025. "Chemical Profiling of Polyphenolic Fraction of Cannabis sativa L. vr. Kompolti Industrial Inflorescences: Insights into Cannabidiol Neuroprotective Effects in a Cellular Model of Parkinson’s Disease" Plants 14, no. 10: 1473. https://doi.org/10.3390/plants14101473
APA StyleFantasma, F., D’Urso, G., Martella, N., Capuano, A., Boccia, E., Samukha, V., De Felice, V., Saviano, G., Trombetta, F., Lauro, G., Segatto, M., Chini, M. G., Bifulco, G., Casapullo, A., & Iorizzi, M. (2025). Chemical Profiling of Polyphenolic Fraction of Cannabis sativa L. vr. Kompolti Industrial Inflorescences: Insights into Cannabidiol Neuroprotective Effects in a Cellular Model of Parkinson’s Disease. Plants, 14(10), 1473. https://doi.org/10.3390/plants14101473













