Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates
Abstract
1. Introduction
2. Results and Discussion
2.1. Salt-Reduced Kelp Powder for Fermentation via the Boiling Process
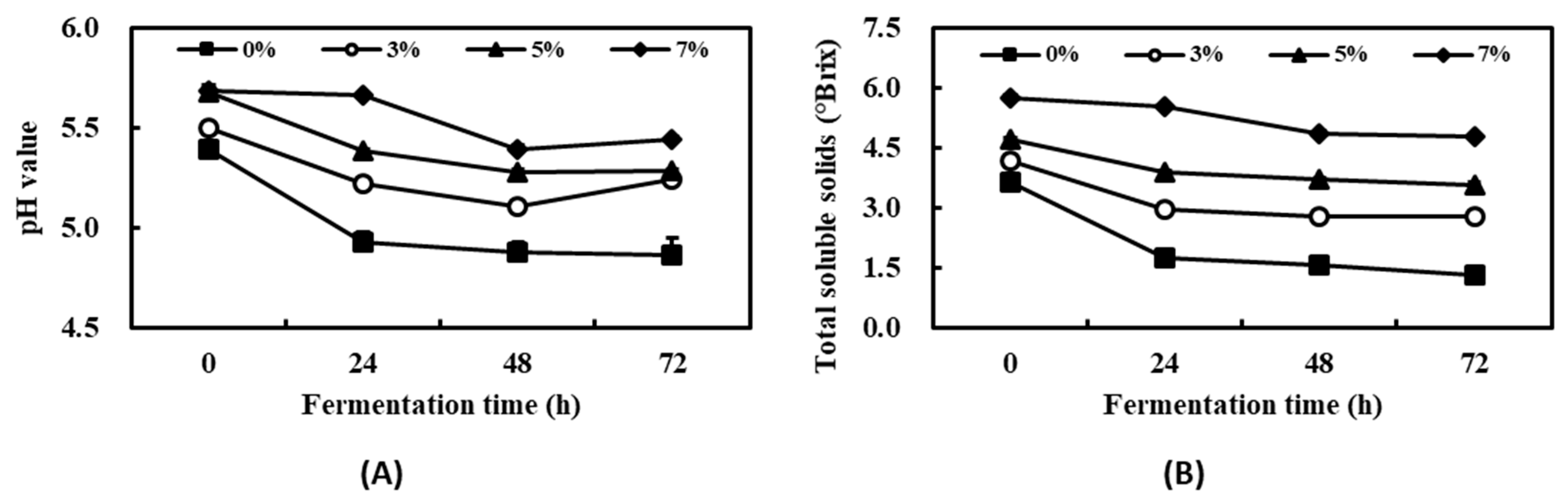
2.2. Changes to Physicochemical Properties during the Production of Fermented Kelp for Hydroponics
2.3. Changes to Amino Acid Contents in Fermented Kelp for Hydroponics
2.4. Total Phenolic Content and Total Flavonoid Content in the Extracts from Ginseng Sprouts Cultivated with Fermented Kelp
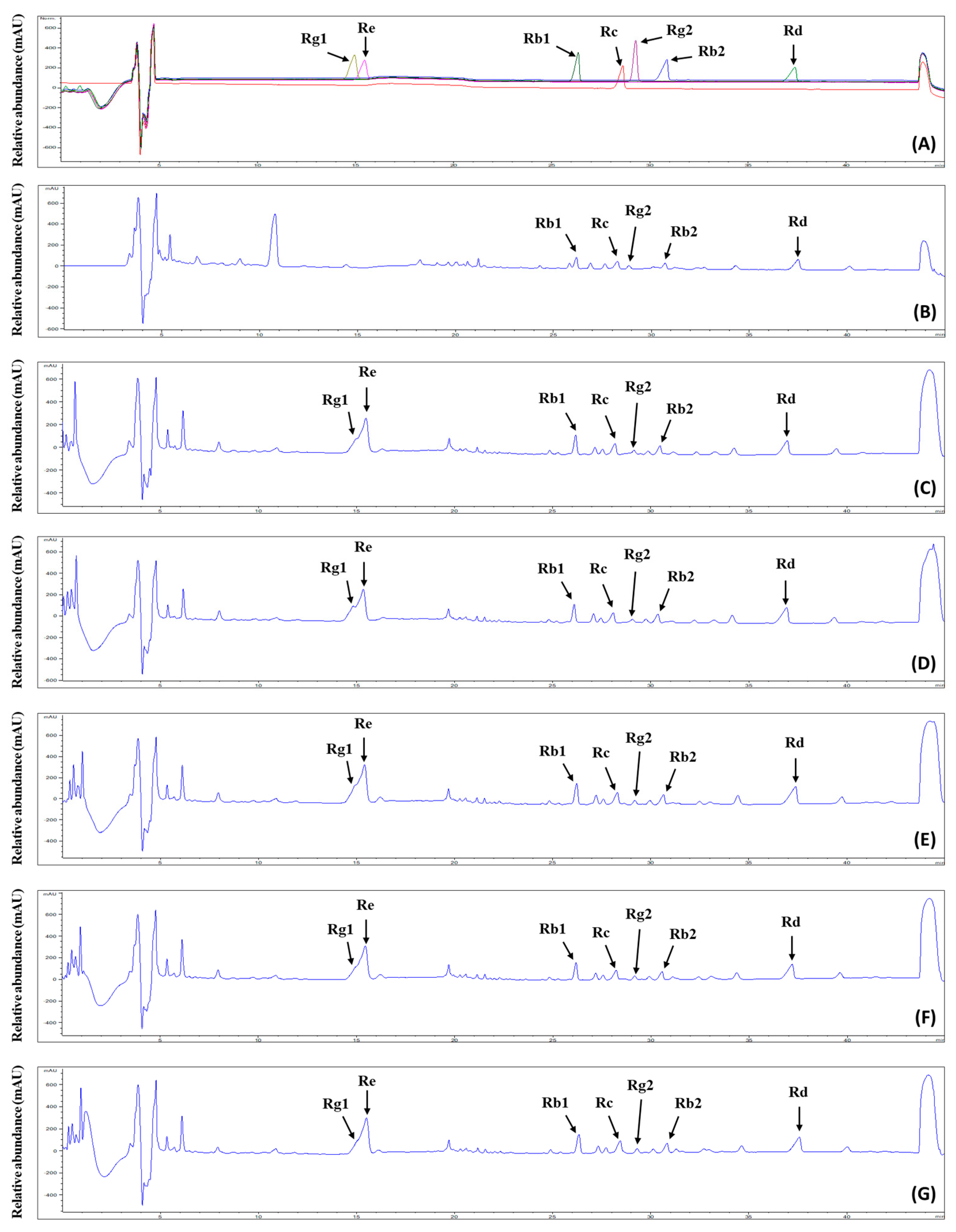
2.5. Ginsenosides in Extracts from Ginseng Sprouts Treated with Fermented Kelp
3. Materials and Methods
3.1. Materials
3.2. Preparation of Kelp Powder
3.3. Salinity, pH, and Total Soluble Solids Determination
3.4. Production of Fermented Kelp for Hydroponics
3.5. Cultivation of Ginseng Sprouts with Fermented Kelp in a Smart-Farm System
3.6. Analysis of Free and Protein-Bound Amino Acids in Fermented Kelp
3.7. Preparation of Ginseng Sprout Extracts
3.8. Preparation of the Ginsenoside-Enriched Fraction
3.9. Total Phenolic Contents
3.10. Total Flavonoid Contents
3.11. Identification and Quantification of Ginsenosides in Ginseng Sprout Extracts
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Jang, S.N.; Lee, G.O.; Sim, H.S.; Kang, M.J.; et al. Comprehensive comparison of chemical composition and antioxidant activity of Panax ginseng sprouts by different cultivation systems in a plant factory. Plants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Fu, F.; Lai, Q.; Zhang, L.; Liu, T.; Yu, B.; Kou, J.; Li, F. Cardioprotective effect of ginsenoside Rb1 via regulating metabolomics profiling and AMP-activated protein kinase-dependent mitophagy. J. Ginseng Res. 2022, 46, 255–265. [Google Scholar] [CrossRef]
- Du, Y.; Fu, M.; Wang, Y.T.; Dong, Z. Neuroprotective effects of ginsenoside Rf on amyloid-β-induced neurotoxicity in vitro and in vivo. J. Alzheimer’s Dis. 2018, 64, 309–322. [Google Scholar] [CrossRef]
- Wang, M.; Yan, S.J.; Zhang, H.T.; Li, N.; Liu, T.; Zhang, Y.L.; Li, X.X.; Ma, Q.; Qiu, X.C.; Fan, Q.Y.; et al. Ginsenoside Rh2 enhances the antitumor immunological response of a melanoma mice model. Oncol. Lett. 2017, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fu, W.; Xu, H.; Li, S.; Yang, X.; Yang, W.; Sui, D.; Wang, Q. Ginsenoside Rc attenuates myocardial ischaemic injury through antioxidative and anti-inflammatory effects. Pharm. Biol. 2022, 60, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Yang, L.; Zhang, L.; Liu, C.; Han, M. Effects of cultivation ages and modes on microbial diversity in the rhizosphere soil of Panax ginseng. J. Ginseng Res. 2016, 40, 28–37. [Google Scholar] [CrossRef]
- Song, Y.N.; Hong, H.G.; Son, J.S.; Kwon, Y.O.; Lee, H.H.; Kim, H.J.; Park, J.H.; Son, M.J.; Oh, J.G.; Yoon, M.H. Investigation of ginsenosides and antioxidant activities in the roots, leaves, and stems of hydroponic-cultured ginseng (Panax ginseng Meyer). Prev. Nutr. Food Sci. 2019, 24, 283–292. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.J.; O’Hare, G.M.P. Modelling the smart farm. Inf. Process. Agric. 2017, 4, 179–187. [Google Scholar] [CrossRef]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y.H. Silicon as a smart fertilizer for sustainability and crop improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Mafra, S.; Teixeira, E.; Figueiredo, F. Smart strawberry farming using edge computing and IoT. Sensors 2022, 22, 5866. [Google Scholar] [CrossRef]
- Modarelli, G.C.; Vanacore, L.; Rouphael, Y.; Langellotti, A.L.; Masi, P.; De Pascale, S.; Cirilli, C. Hydroponic and aquaponics floating raft systems elicit differential growth and quality responses to consecutive cuts of basil crop. Plants 2023, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.H.; Kim, E.J.; Lee, J.M.; Park, J.W.; Kim, Y.S.; Kim, G.R.; Lee, J.S.; Lee, E.P.; You, Y.H. White LED lighting increases the root productivity of Panax ginseng C. A. Meyer in a hydroponic cultivation system of a plant factory. Biology 2023, 12, 1052. [Google Scholar] [CrossRef]
- Song, J.S.; Jung, S.K.; Jee, S.H.; Yoon, J.W.; Byeon, Y.S.; Park, S.I.; Kim, S.B. Growth and bioactive phytochemicals of Panax ginseng sprouts grown in an aeroponic system using plasma-treated water as the nitrogen source. Sci. Rep. 2021, 11, 2924. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jeong, E.B.; Oh, D.K. Complete bioconversion of protopanaxadiol-type ginsenosides to compound K by extracellular enzymes from the isolated strain Aspergillus tubingensis. J. Agric. Food Chem. 2021, 69, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lee, S.J.; Yuan, Q.P.; Im, W.T.; Kim, S.C.; Han, N.S. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis. J. Ginseng Res. 2018, 42, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Hwang, H.L.; Lee, J.N.; Sohn, S.O.; Lee, S.H.; Jung, M.Y.; Lim, H.I.; Park, H.W.; Lee, J.H. Evaluation of ginsenoside bioconversion of lactic acid bacteria isolated from kimchi. J. Ginseng Res. 2017, 41, 524–530. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461. [Google Scholar] [CrossRef]
- Yuan, Y.; Chu, D.; Fan, J.; Zou, P.; Qin, Y.; Geng, Y.; Cui, Z.; Wang, X.; Zhang, C.; Li, X.; et al. Ecofriendly conversion of algal waste into valuable plant growth-promoting rhizobacteria (PGPR) biomass. Waste Manag. 2021, 120, 576–584. [Google Scholar] [CrossRef]
- Kim, E.J.; Fathoni, A.; Jeong, G.T.; Jeong, H.D.; Nam, T.J.; Kong, I.S.; Kim, J.K. Microbacterium oxydans, a novel alginate- and laminarin-degrading bacterium for the reutilization of brown-seaweed waste. J. Environ. Manag. 2013, 130, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Jurković, N.; Kolb, N.; Colić, I. Nutritive value of marine algae Laminaria japonica and Undaria pinnatifida. Die Nahr. 1995, 39, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L.; El-Swaify, S.A.; Evensen, C.I.; Habte, M.; Ho, M.C.; Huang, X.; Hue, N.V.; Ikawa, H.; Kirby, R.; Schmitt, D.P.; et al. Plant Nutrient Management in Hawaii’s Soils, 1st ed.; The College of Tropical Agriculture and Human Resources: Honolulu, HI, USA, 2000; pp. 31–55. [Google Scholar]
- Zheng, S.; Jiang, J.; He, M.; Zou, S.; Wang, C. Effect of kelp waste extracts on the growth and development of pakchoi (Brassica chinensis L.). Sci. Rep. 2016, 6, 38683. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Stirk, W.A.; Placková, L.; Kulkarni, M.G.; Dolezal, K.; Staden, J.V. Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Kim, S.H.; Ellinger, J.E.; Markley, J.L. Dosage effects of salt and pH stresses on Saccharomyces cerevisiae as monitored via metabolites by using two dimensional NMR spectroscopy. Bull. Korean Chem. Soc. 2013, 34, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Correira, H.; Soares, C.; Morais, S.; Pinto, E.; Marques, A.; Nunes, M.L.; Almeida, A.; Delerue-Matos, C. Seaweeds rehydration and boiling: Impact on iodine, sodium, potassium, selenium, and total arsenic contents and health benefits for consumption. Food Chem. Toxicol. 2021, 155, 112385. [Google Scholar] [CrossRef] [PubMed]
- Deolu-Ajayi, A.O.; Meer, I.M.; Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef] [PubMed]
- Perata, O.; Alpi, A.; LoSchiavo, F. Influence of ethanol on plant cells and tissues. J. Plant Physiol. 1986, 126, 181–188. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.D.; Won, Y.S.; Hong, S.M.; Moon, K.D.; Seo, K.I. Anti-fatigue effect of Prunus mume vinegar in high-intensity exercised rats. Nutrients 2020, 12, 1205. [Google Scholar] [CrossRef]
- Park, W.L.; Cho, H.D.; Kim, J.H.; Min, H.J.; Seo, K.I. Antioxidant activity and blood alcohol concentration lowering effect of fermented Hovenia dulcis fruit vinegar. Food Sci. Biotechnol. 2022, 32, 299–308. [Google Scholar] [CrossRef]
- Vallejo, B.; Matallana, E.; Aranda, A. Saccharomyces cerevisiae nutrient signaling pathways show an unexpected early activation pattern during winemaking. Microb. Cell Fact. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Geem, K.R.; Kim, J.W.; Bae, W.S.; Jee, M.G.; Yu, J.; Jang, I.B.; Lee, D.Y.; Hong, C.P.; Shim, D.H.; Ryu, H.J. Nitrate enhances the secondary growth of storage roots. J. Ginseng Res. 2023, 47, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Ubeda, C.; Hidalgo, C.; Mas, A.; Troncoso, A.M.; Morales, M.L. Changes on free amino acids during the alcoholic fermentation of strawberry and persimmon. Int. J. Food Sci. Technol. 2015, 50, 48–54. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Jones, L.J.; Craven, G.S.; Somerset, S.M.; Palmer, C. Amino acid profiles of kinema, a soybean-fermented food. Food Chem. 1997, 59, 69–75. [Google Scholar] [CrossRef]
- Baik, I.H.; Kim, K.H.; Lee, K.A. Antioxidant, anti-inflammatory and antithrombotic effects of ginsenoside compound K enriched extract derived from ginseng sprouts. Molecules 2021, 26, 4102. [Google Scholar] [CrossRef]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advencas in saponin diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Lam, V.P.; Lee, M.H.; Park, J.S. Optimization of indole-3-acetic acid concentration in a nutrient solution for increasing bioactive compound accumulation and production of Agastache rugosa in a plant factory. Agriculture 2020, 10, 343. [Google Scholar] [CrossRef]
- Kim, G.S.; Lee, S.E.; Noh, H.J.; Kwon, H.; Lee, S.W.; Kim, S.Y.; Kim, Y.B. Effects of natural bioactive products on the growth and ginsenoside contents of Panax ginseng cultured in an aeroponic system. J. Ginseng Res. 2012, 34, 430–441. [Google Scholar] [CrossRef]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum biostimulant improves the growth of Zea mays grown under phosphorus impoverished conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef]
- Marino, R.; Iammarino, M.; Santillo, A.; Muscarella, M.; Caroprese, M.; Albenzio, M. Rapid method for determination of amino acids in milk. J. Dairy Sci. 2010, 93, 2367–2370. [Google Scholar] [CrossRef]
- Shehzad, O.; Ha, I.J.; Park, Y.M.; Ha, Y.W.; Kim, Y.S. Development of a rapid and convenient method to separate eight ginsenosides from Panax ginseng by high-speed countercurrent chromatography coupled with evaporative light scattering detection. J. Sep. Sci. 2011, 34, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.I.; Ryu, J.H.; Seo, K.S.; Kang, K.Y.; Park, S.H.; Ha, T.H.; Ahn, J.W.; Kang, S.Y. Comparative study on phenolic compounds and antioxidant activities of hop (Humulus lupulus L.) strobile extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]

| Contents | Raw Kelp | Boiled Kelp |
|---|---|---|
| Salinity (%) | 1.97 ± 0.01 | 0.43 ± 0.01 |
| Contents (mg/100 g) | Initial Broth | FK |
|---|---|---|
| Free amino acids | ||
| Aspartic acid | 11.45 ± 0.56 a | 6.59 ± 0.02 b |
| Glutamic acid | 28.17 ± 1.54 a | 12.59 ± 0.06 b |
| Serine | 0.58 ± 0.04 a | 0.41 ± 0.05 b |
| Histidine | 0.67 ± 0.03 a | 0.48 ± 0.01 b |
| Glycine | 0.52 ± 0.02 a | 0.45 ± 0.02 b |
| Threonine | 0.31 ± 0.02 a | 0.22 ± 0.01 b |
| Arginine | 2.30 ± 0.09 a | 0.79 ± 0.02 b |
| Alanine | 3.72 ± 0.18 a | 1.92 ± 0.08 b |
| Tyrosine | 0.40 ± 0.02 a | 0.15 ± 0.01 b |
| Phenylalanine | 0.54 ± 0.03 a | 0.16 ± 0.01 b |
| Lysine | 0.36 ± 0.01 b | 0.76 ± 0.01 a |
| Total | 50.77 ± 2.66 | 24.55 ± 0.31 |
| Protein-bound amino acids | ||
| Aspartic acid | 55.34 ± 1.59 a | 59.19 ± 2.61 a |
| Glutamic acid | 76.62 ± 4.78 a | 74.53 ± 3.38 a |
| Serine | 11.22 ± 0.82 a | 15.66 ± 2.72 a |
| Glycine | 14.13 ± 0.38 b | 17.70 ± 1.97 a |
| Threonine | 11.25 ± 0.98 b | 15.40 ± 0.98 a |
| Arginine | 13.94 ± 0.64 b | 17.50 ± 1.04 a |
| Alanine | 17.21 ± 0.33 b | 20.91 ± 0.86 a |
| Valine | 11.91 ± 0.91 b | 17.13 ± 2.22 a |
| Leucine | 12.82 ± 0.82 b | 19.23 ± 0.39 a |
| Lysine | 17.56 ± 1.45 b | 23.79 ± 0.67 a |
| Total | 241.65 ± 12.43 | 281.04 ± 16.48 |
| Contents | Control | Concentrations of FK Treatment | ||||
|---|---|---|---|---|---|---|
| 0% | 10% | 25% | 50% | 100% | ||
| TPC (mg GAE/g) | 11.62 ± 0.25 c | 16.86 ± 0.55 a | 15.58 ± 0.21 b | 15.42 ± 0.15 b | 15.49 ± 0.41 b | 15.16 ± 0.21 b |
| TFC (mg QE/g) | 63.76 ± 1.36 b | 70.31 ± 0.52 a | 69.71 ± 1.55 a | 58.71 ± 2.73 c | 62.57 ± 2.36 b | 63.17 ± 2.73 b |
| Ginsenosides | Retention Time (min) | Calibration Curve | Correlation Coefficient (r2) | Test Range (mg/mL) | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|---|
| Rg1 | 14.95 | y = 6.3944x − 3.3708 | 0.9997 | 0.0125–1.000 | 1.63 | 16.27 |
| Re | 15.45 | y = 4.399x − 168.34 | 0.9991 | 0.0125–1.000 | 5.62 | 56.23 |
| Rb1 | 26.36 | y = 4.2953x − 8.8375 | 0.9995 | 0.0125–1.000 | 6.90 | 69.01 |
| Rc | 28.62 | y = 4.2794x + 63.467 | 0.9992 | 0.0125–1.000 | 3.27 | 32.67 |
| Rg2 | 29.27 | y = 6.7113x + 45.192 | 1.0000 | 0.0125–1.000 | 2.11 | 21.08 |
| Rb2 | 30.86 | y = 4.9103x + 47.146 | 0.9998 | 0.0125–1.000 | 3.97 | 39.74 |
| Rd | 37.38 | y = 4.3557x + 21.278 | 0.9998 | 0.0125–1.000 | 4.34 | 43.38 |
| Ginsenosides (μg/g) | Control | FK-Treatment Concentrations | ||||
|---|---|---|---|---|---|---|
| 0% | 10% | 25% | 50% | 100% | ||
| Rg1 | N.D. | 494.62 ± 2.72 b | 465.92 ± 30.23 b | 633.29 ± 33.05 a | 501.27 ± 77.82 b | 499.92 ± 112.21 b |
| Re | N.D. | 1514.57 ± 53.92 b | 1607.49 ± 43.14 b | 1891.27 ± 1.11 a | 1499.75 ± 78.87 b | 1520.45 ± 140.77 b |
| Rb1 | 330.46 ± 71.94 c | 427.81 ± 0.44 b | 418.82 ± 7.27 b | 518.23 ± 14.38 a | 400.43 ± 0.83 b | 503.58 ± 18.10 a |
| Rc | 244.36 ± 2.84 d | 359.61 ± 79.64 ab | 324.59 ± 44.01 bc | 419.88 ± 31.98 a | 292.23 ± 11.12 bd | 387.26 ± 70.88 ab |
| Rg2 | 54.28 ± 1.59 b | 63.29 ± 0.89 a | 56.29 ± 9.09 b | 66.94 ± 2.28 a | 62.60 ± 1.78 a | 65.60 ± 7.37 a |
| Rb2 | 144.40 ± 3.13 e | 197.98 ± 3.91 d | 206.64 ± 0.51 c | 264.91 ± 2.41 a | 195.43 ± 2.83 d | 239.43 ± 7.37 b |
| Rd | 461.08 ± 2.11 e | 599.19 ± 1.31 d | 757.27 ± 11.07 b | 913.37 ± 22.32 a | 681.76 ± 3.05 c | 687.55 ± 10.11 c |
| Factors | Control | Concentrations of FK | ||||
|---|---|---|---|---|---|---|
| 0% | 10% | 25% | 50% | 100% | ||
| FK soaking | − | − | + | + | + | + |
| Aeroponic medium | Tap water | 0.2% FK | 0.2% FK | 0.2% FK | 0.2% FK | 0.2% FK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-W.; Kim, J.-H.; Jeong, B.-G.; Park, J.-K.; Jang, H.-Y.; Oh, Y.-S.; Kang, K.-Y. Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates. Plants 2024, 13, 463. https://doi.org/10.3390/plants13030463
Park K-W, Kim J-H, Jeong B-G, Park J-K, Jang H-Y, Oh Y-S, Kang K-Y. Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates. Plants. 2024; 13(3):463. https://doi.org/10.3390/plants13030463
Chicago/Turabian StylePark, Kyung-Wuk, Jeong-Ho Kim, Beom-Gyun Jeong, Jun-Ki Park, Ho-Yeol Jang, Yun-Seo Oh, and Kyung-Yun Kang. 2024. "Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates" Plants 13, no. 3: 463. https://doi.org/10.3390/plants13030463
APA StylePark, K.-W., Kim, J.-H., Jeong, B.-G., Park, J.-K., Jang, H.-Y., Oh, Y.-S., & Kang, K.-Y. (2024). Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates. Plants, 13(3), 463. https://doi.org/10.3390/plants13030463







