Effects of Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of EOs
2.2. Bioassays with S. frugiperda
2.2.1. Acute Toxicity of EOs to S. frugiperda in a Topical Application Trial
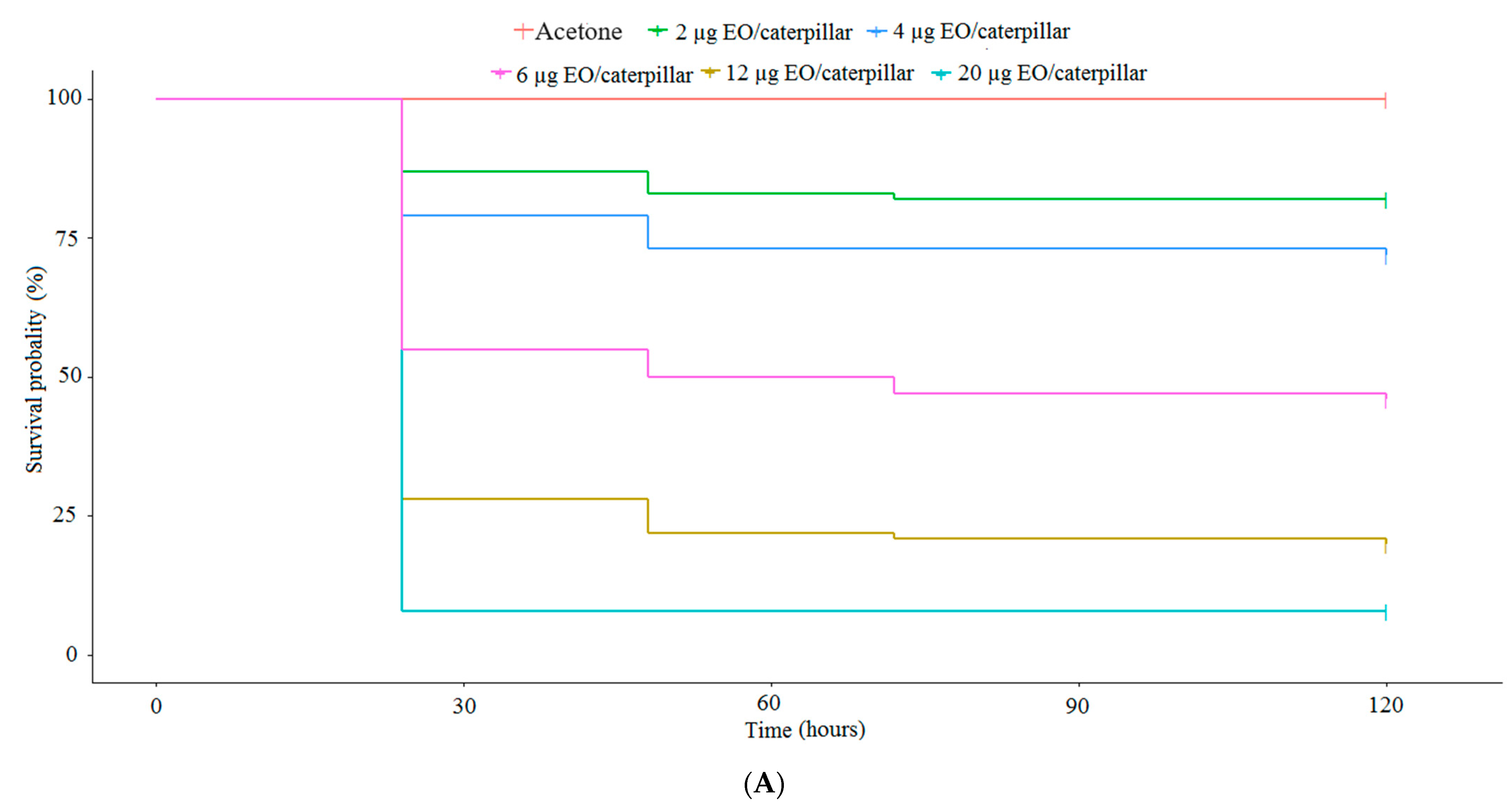
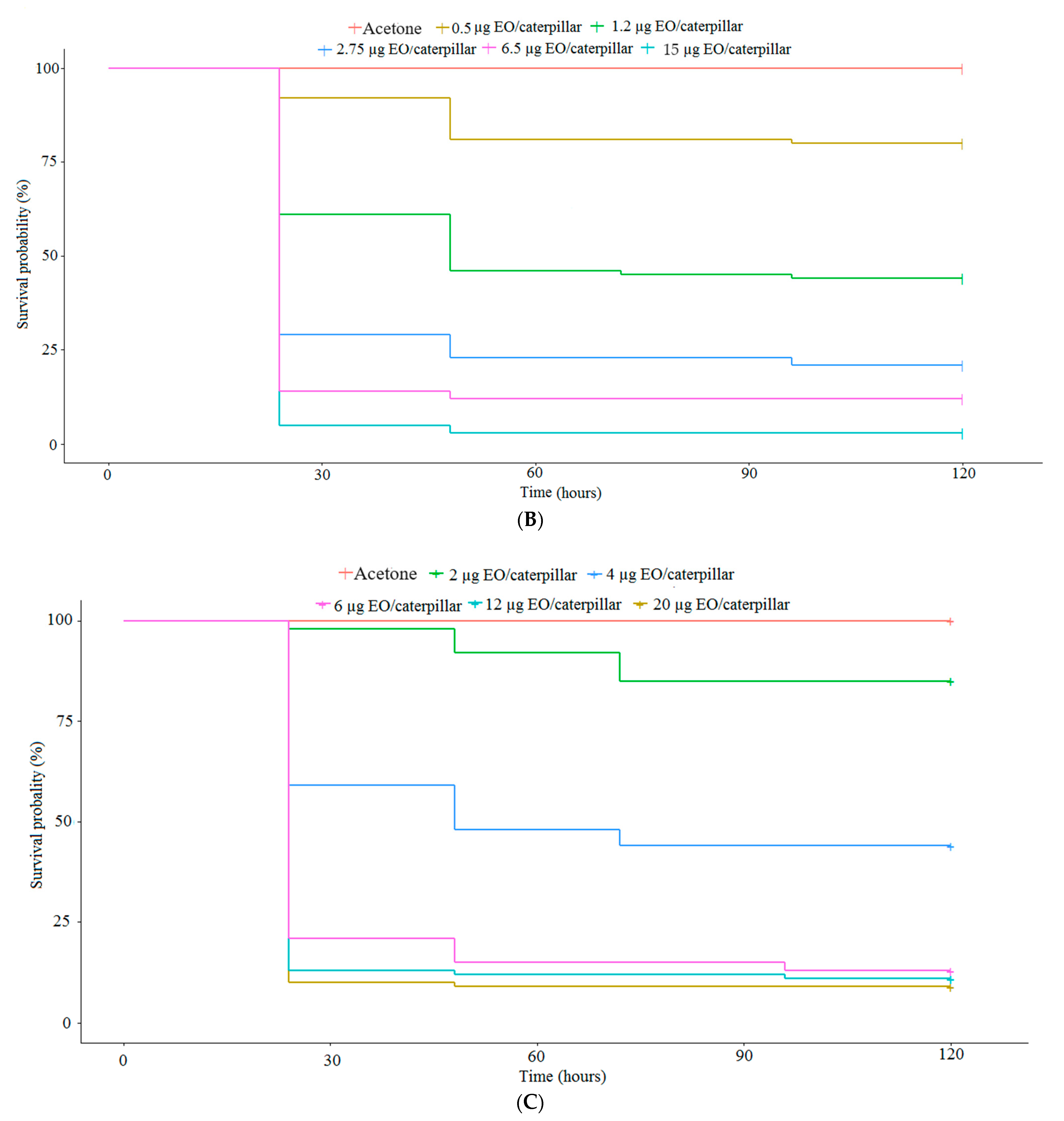
2.2.2. Chronic Toxicity of EOs to S. frugiperda in an Ingestion Trial
2.2.3. Determination of Dose-Response and Time-Response Curves
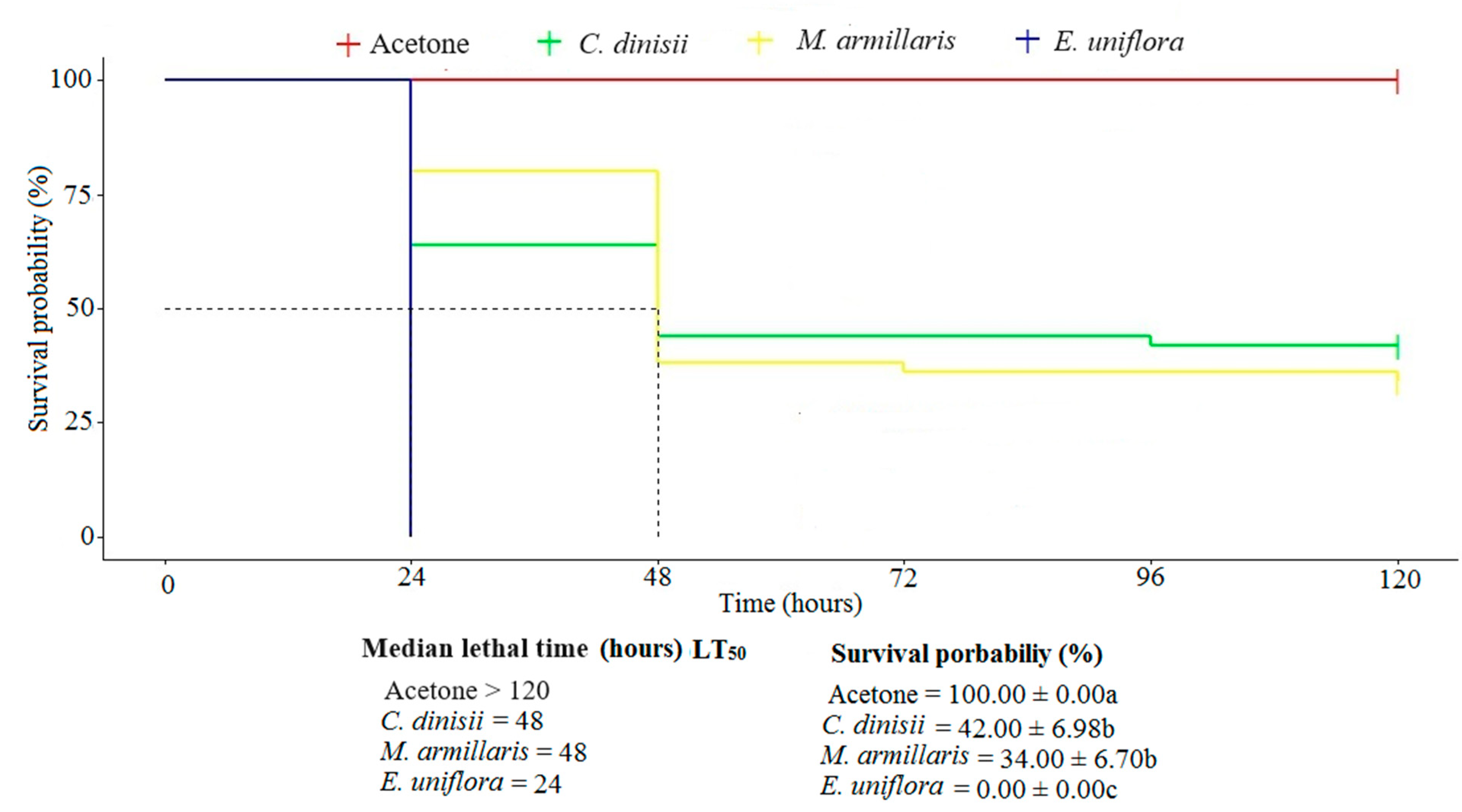
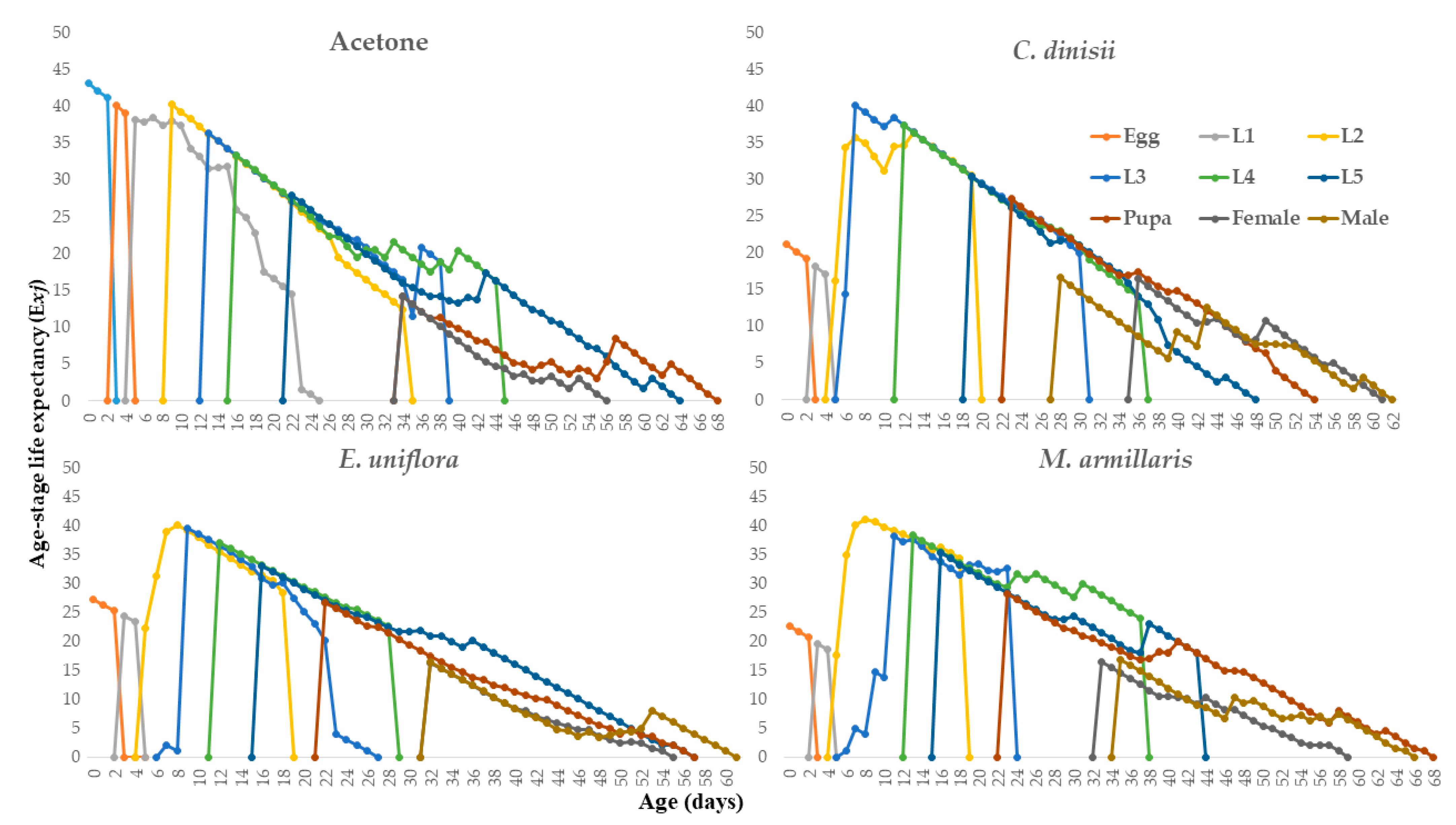
2.2.4. Life History and Demographic Parameters of S. frugiperda Caterpillar Treated with the Essential Oils of C. dinissi, E. uniflora and M. armillaris
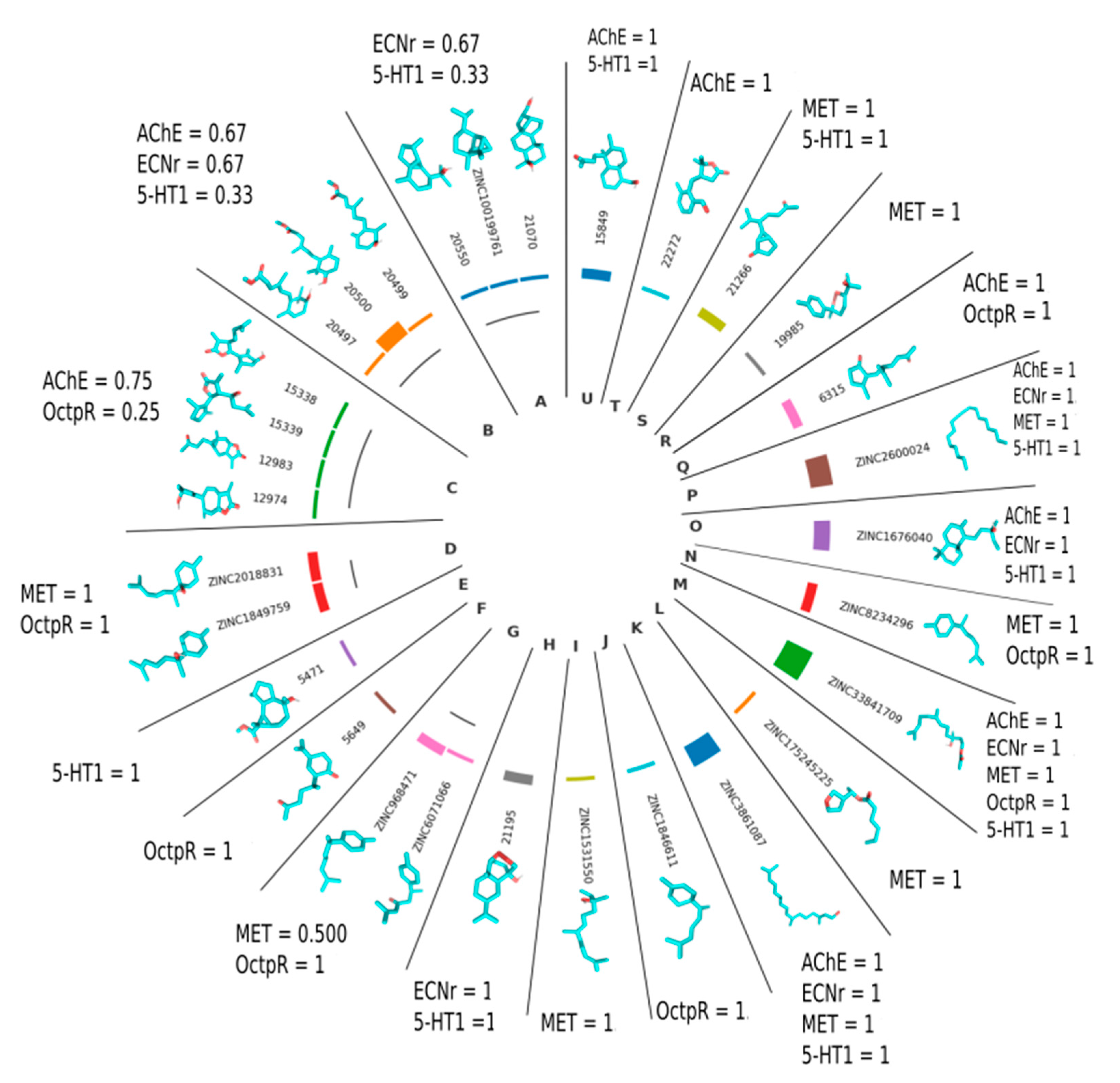
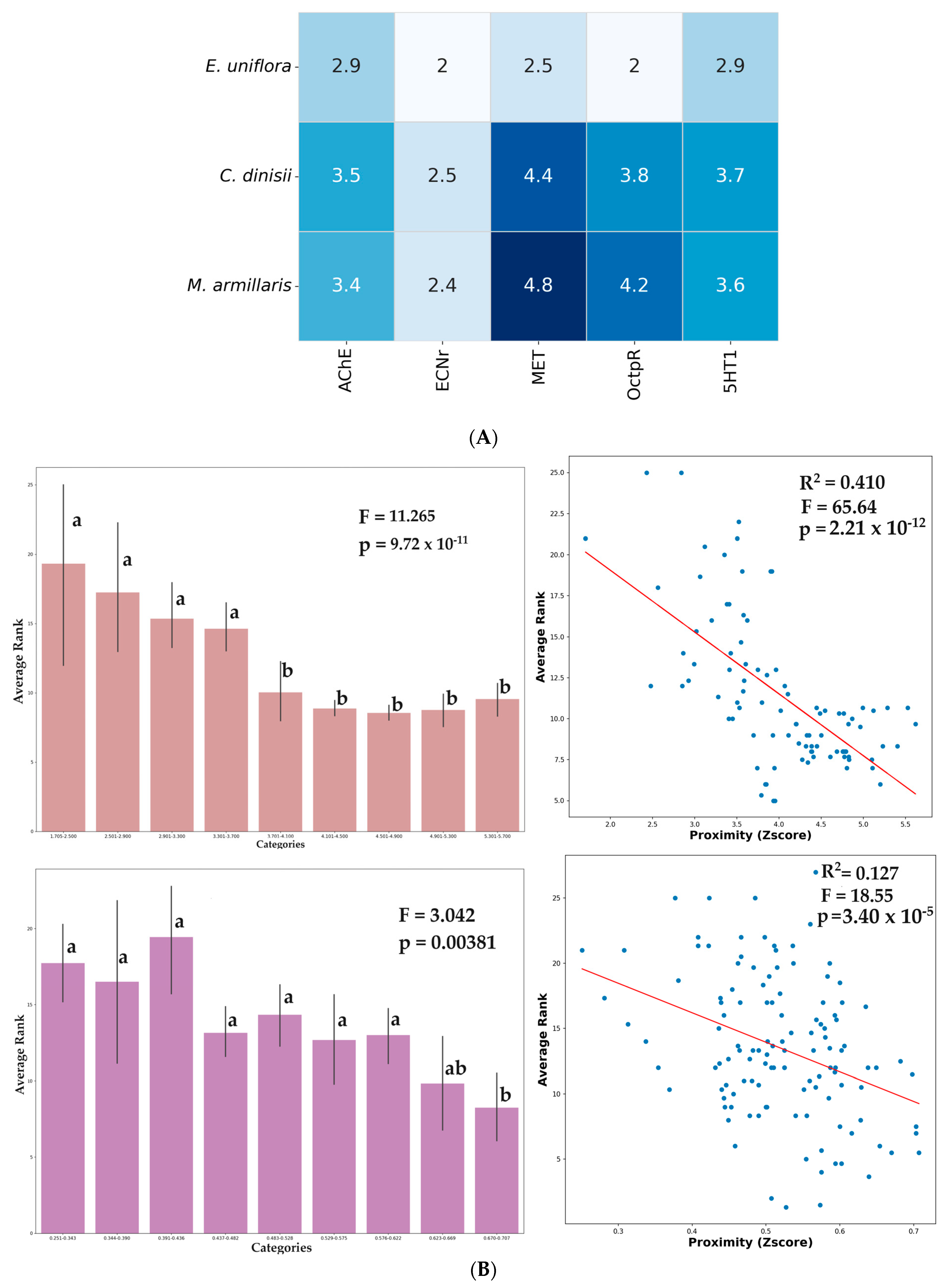
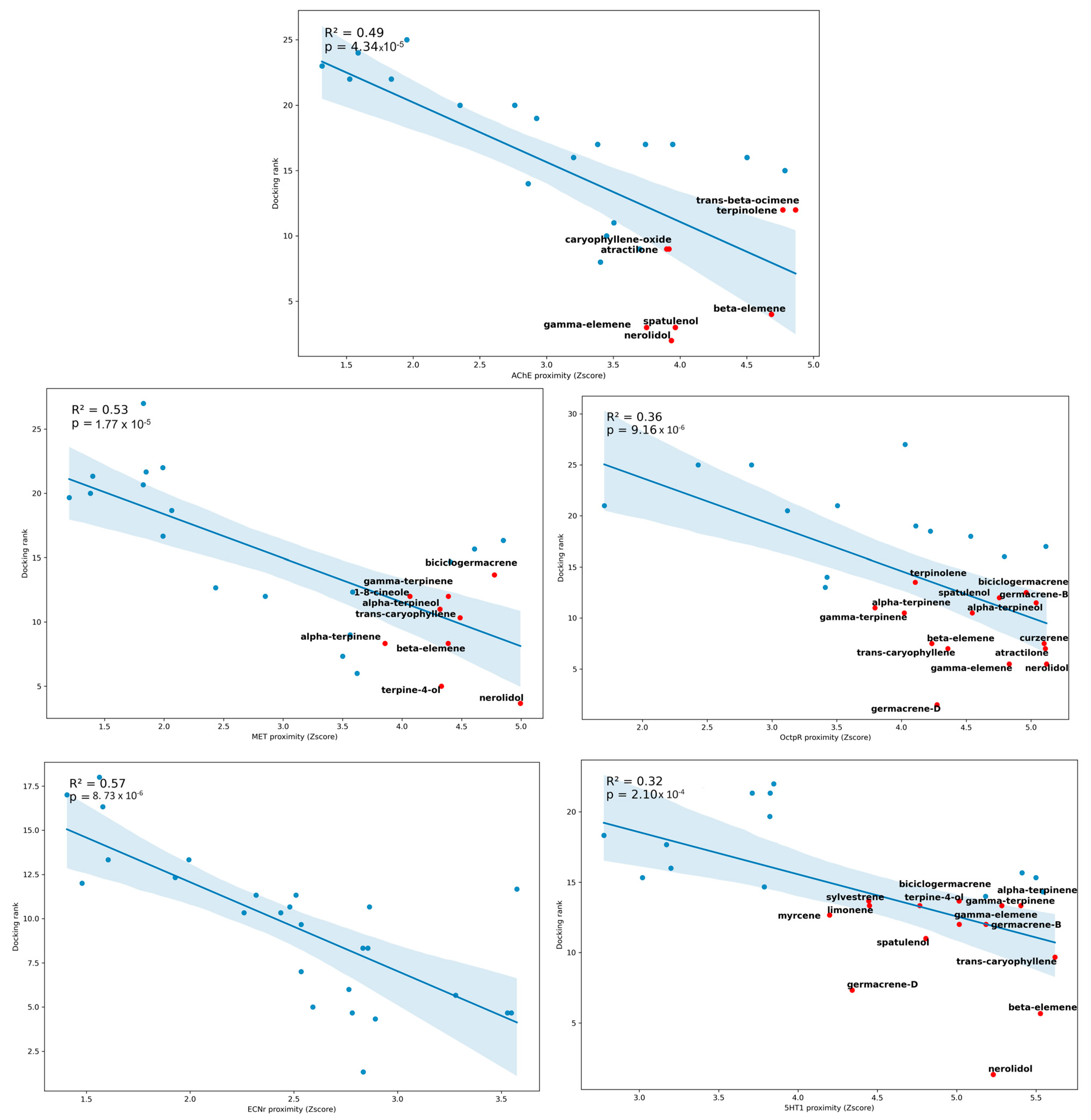
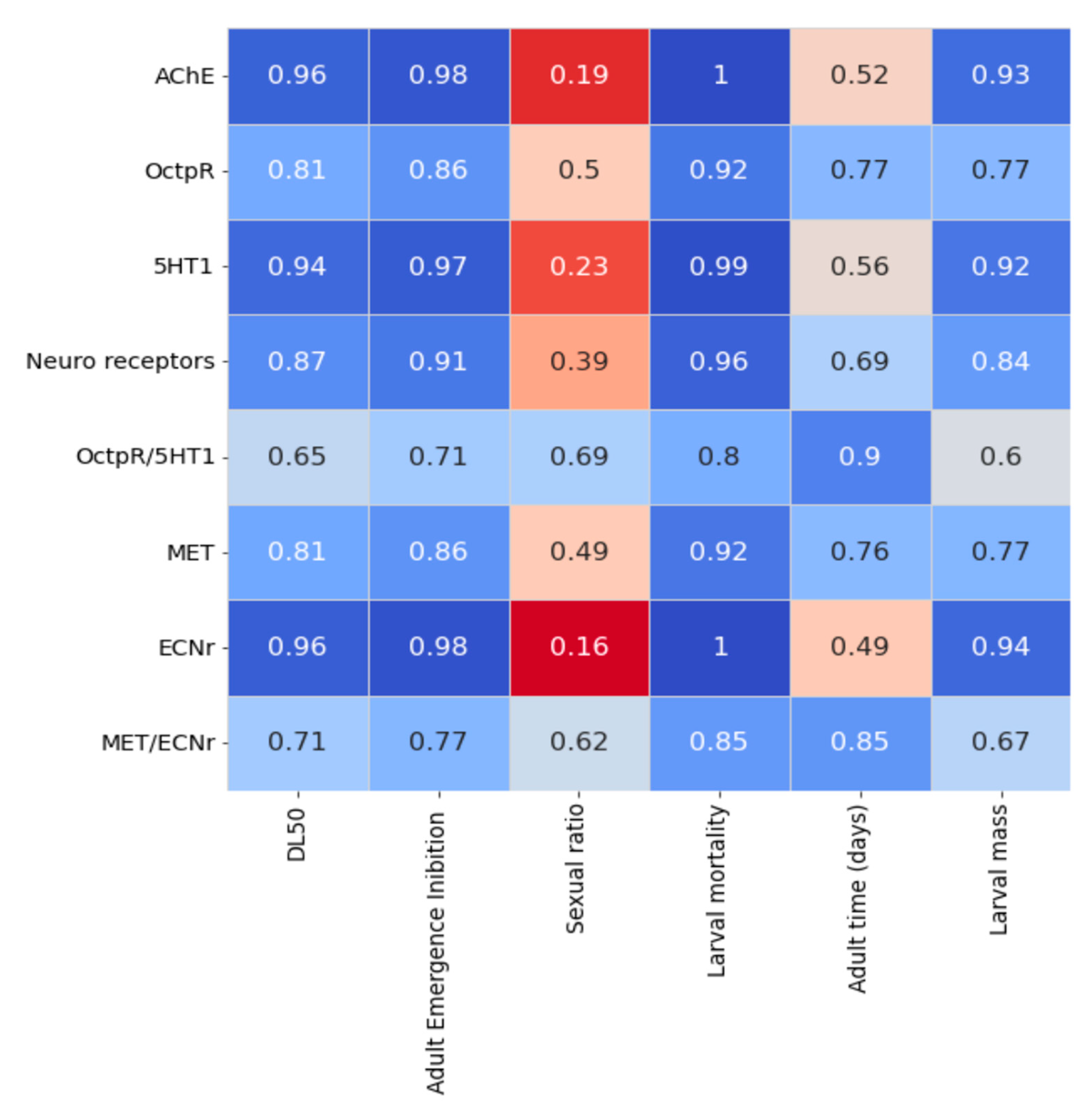
2.3. Virtual Screening and Computational Analysis
3. Discussion
4. Materials and Methods
4.1. EOs Production and Chemical Characterization
4.2. Rearing of S. frugiperda
4.3. Bioassays with S. frugiperda
4.3.1. Acute Toxicity of EOs to S. frugiperda in a Topical Application Trial
4.3.2. Chronic Toxicity of EOs to S. frugiperda in an Ingestion Trial
4.3.3. Determination of Dose-Response and Time-Response Curves
4.3.4. Life History Table and Demographic Parameters of S. frugiperda Caterpillar Treated with the EOs of C. dinisii, E. uniflora and M. armillaris
4.4. Statistical Analyses
4.5. Virtual Screening and Computational Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sombra, K.E.S.; Aguiar, C.V.S.; Oliveira, S.J.; Barbosa, M.G.; Zocolo, G.J.; Pastori, P.L. Potencial pesticide of three EOs against Spodoptera frugiperda (J.E. Smith) (Lepdoptera:Noctuidae). Chil. J. Agric. Res. 2020, 80, 617–628. [Google Scholar] [CrossRef]
- Sun, X.-x.; Hu, C.-x.; Jia, H.-r.; Wu, Q.-l.; Shen, X.-j.; Zhao, S.-y.; Jiang, Y.-y.; Wu, K.-m. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Shylesha, A.N.; Jalali, S.K.; Gupta, A.; Varshney, R.; Venkatesan, T.; Shetty, P.; Ojha, R.; Ganiger, P.C.; Navik, O.; Subaharan, K.; et al. Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control 2018, 32, 145–151. [Google Scholar] [CrossRef]
- Rane, R.; Walsh, T.K.; Lenancker, P.; Gock, A.; Dao, T.H.; Nguyen, V.L.; Khin, T.N.; Amalin, D.; Chittarath, K.; Fahem, M.; et al. Complex multiple introductions drive fall armyworm invasions into Asia and Australia. Sci. Rep. 2023, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Tek, T.W.; Lastus, K.; William, J.; Thomas, W. Confirmation of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Papua New Guinea by molecular diagnostics of mitochondrial DNA COI gene. BioInvasions Rec. 2023, 12, 103–116. [Google Scholar]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Days, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Nebo, L.; Matos, A.P.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F.; Rodrigues, R.R. Atividade inseticida dos frutos de Trichilia claussenii (Meliaceae) sobre Spodoptera frugiperda. Quim. Nova 2010, 33, 1849–1852. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of resistance to organophosphates and pyrethroids in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef]
- Vélez, A.M.; Vellichirammal, N.N.; Jurat-Fuentes, J.L.; Siegfried, B.D. Cry1F resistance among lepidopteran pests: A model for improved resistance management? Curr. Opin. Insect Sci. 2016, 15, 116–124. [Google Scholar] [CrossRef]
- Alves, D.S.; Carvalho, G.A.; Oliveira, D.F.; Corrêa, A.D. Screening of Brazilian plant extracts as candidates for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2018, 44, 32–38. [Google Scholar] [CrossRef]
- Figueiredo, C.S.; Lemes, A.R.N.; Sebastião, I.; Desidério, J.A. Synergism of the Bacillus thuringiensis Cry1, Cry2, and Vip3 proteins in Spodoptera frugiperda control. Appl. Biochem. Biotechnol. 2019, 188, 798–809. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Ni, H.; Guo, D.; Yang, F.; Wang, X.; Wu, S.; Gao, C. Susceptibility of fall armyworm, Spodoptera frugiperda (J.E. Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.S.A.; Guidolin, A.S.; Salmeron, E.; Kanno, R.H.; Padovez, F.E.O.; Fatoretto, J.C.; Omoto, C. Geographical distribution of Vip3Aa20 resistance allele frequencies in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Pest Manag. Sci. 2020, 76, 169–178. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.O.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef]
- Souza, J.R.; Carvalho, G.A.; Moura, A.P.; Couto, M.H.G.; Maia, J.B. Toxicity of some insecticides used in maize crop on Trichogramma pretiosum (Hymenoptera, Trichogrammatidae) immature stages. Chil. J. Agric. Res. 2014, 74, 234–239. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. EOs as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Seide, V.E.; Bernardes, R.C.; Pereira, E.J.G.; Lima, M.A.P. Glyphosate is lethal and Cry toxins alter the development of the stingless bee Melipona quadrifasciata. Environ. Pollut. 2018, 243, 1854–1860. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indc. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. EOs and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.A.; Sanchez-Teyer, L.; Vázquez-Flota, F.; Monforte-González, M.; Tamayo-Ordóñez, Y.; Tamayo-Ordóñez, M.C.; Rivera, G. Biological effects of natural products against Spodoptera spp. Crop. Protect. 2018, 114, 195–207. [Google Scholar] [CrossRef]
- Menezes, E.L.A. Inseticidas Botânicos: Seus Princípios Ativos, Modo de Ação e Uso Agrícola; Embrapa Agrobiologia, Seropédica: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Rizzo, R.; Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Fofie, N.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four EOs in protein baits on Bactrocera oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Oliveira, J.A.C.; Ferreira, L.S.; Garcia, I.P.; Santos, H.L.; Ferreira, G.S.; Rocha, J.P.M.; Nunes, S.A.; Carvalho, A.A.; Pinto, J.E.B.P.; Betolucci, S.K.V. Eugenia uniflora, Melaleuca armillaris and Schinus molle EOs to manage larvae of the filarial vector Culex quinquefasciatus (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2022, 29, 34749–34758. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.C.; Garcia, I.P.; Corrêa, E.J.A.; Lima, L.H.F.; Santos, H.L.; Assis, R.M.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Larvicidal susceptibility of EOs from Cinnamodendron dinisii, Callistemon viminalis and Myrcia tomentosa against Culex quinquefasciatus (Say) (Diptera: Culicidae). J. S. Afr. Bot. 2023, 163, 95–104. [Google Scholar] [CrossRef]
- Vedovatto, F.; Valério Júnior, C.; Astolfi, V.; Mielniczki, P.A.A.; Romano, S.S.; Paroul, N.; Cansiano, R.L. Essential oil of Cinnamodendron dinisii Schwake for the control of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Rev. Bras. Plantas Med. 2015, 17, 1055–1060. [Google Scholar] [CrossRef][Green Version]
- Carvalho, N.R.; Rodrigues, N.R.; Macedo, G.E.; Bristot, I.J.; Boligon, A.A.; De Campos, M.M.; Cunha, F.A.; Coutinho, H.D.; Klamt, F.; Merritt, T.J.S.; et al. Eugenia uniflora leaf essential oil promotes mitochondrial dysfunction in Drosophila melanogaster through the inhibition of oxidative phosphorylation. Toxicol. Res. 2017, 6, 526–534. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, B.; Khaled, J.M.; Benelli, G. Curzerene, trans-β-elemenone, and γ-elemene as effective larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus: Toxicity on non-target aquatic predators. Environ. Sci. Pollut. Res. 2018, 25, 10272–10282. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.P.; Figueiredo, K.G.; Alves, D.S.; Oliveira, D.F.; Silva, G.H.; Silva, G.T.S.; Oliveira, M.S.; Biondi, A.; Carvalho, G.A. Survival and demography of the tomato borer (Tuta absoluta) exposed to citrus essential oils and major compounds. Agriculture 2023, 13, 538. [Google Scholar] [CrossRef]
- Corrêa, E.J.A.; Carvalho, F.C.; Oliveira, J.A.C.; Bertolucci, S.K.V.; Scotti, M.T.; Silveira, C.H.; Guedes, F.C.; Melo, J.O.F.; Melo-Minardi, R.C.; Lima, L.H.F. Elucidating the molecular mechanisms of EOs’ insecticidal action using a novel cheminformatics protocol. Sci. Rep. 2023, 13, 4598. [Google Scholar] [CrossRef]
- Cruz, G.S.; Wanderley-Teixeira, V.; Oliveira, J.V.; Lopes, F.S.C.; Barbosa, D.R.S.; Breda, M.O.; Dutra, K.A.; Guedes, C.A.; Navarro, D.M.A.F.; Teixeira, A.A.C. Efeitos subletais de óleos essenciais de Eucalyptus staigeriana (Myrtales: Myrtaceae), Ocimum gratissimum (Lamiales: Laminaceae) e Foeniculum vulgare (Apiales: Apiaceae) na Biologia de Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2016, 109, 660–666. [Google Scholar]
- Negrini, M.; Fidelis, E.G.; Schurt, D.A.; Pereira, R.S.; Bizzo, H.R. Insecticidal activity of essential oils in controlling fall armyworm, Spodoptera frugiperda. Plant Parasitol. 2019, 86, e1112018. [Google Scholar] [CrossRef]
- Melo, J.P.R.; Câmara, C.A.G.; Moraes, M.M. Bioactivity of formulas containing EOs from the family Myrtaceae for the management of deltamethrin-resistant Plutella xylostella (L.) (Lepitoptera: Plutellidae). Phytoparasitica 2023, 51, 305–321. [Google Scholar] [CrossRef]
- Lobo, A.P.; Câmara, C.A.G.; Melo, J.P.R.; Moraes, M.M. Chemical composition and repellent activity of EOs from the leaves of Cinnamomum zeylanicum and Eugenia uniflora agaist Diaphania hyalinata L. (Lepidoptera: Crambidae). J. Plant Dis. Prot. 2019, 126, 79–87. [Google Scholar] [CrossRef]
- Lima, A.P.S.; Santana, E.D.R.; Santos, A.C.C.; Silva, J.E.; Ribeiro, G.T.; Pinheiro, A.M.; Santos, I.T.B.F.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Insecticide activity of botanical compounds against Spodoptera frugiperda and selectivity to the predatory bug Podisus nigrispinus. Crop Protect. 2020, 136, 105230. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Bertolucci, S.K.V.; Carvalho, G.A. Toxicity of EOs and pure compounds of Lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Protect. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Alves, A.C.L.; Silva, T.I.; Batista, J.L.; Galvão, J.C.C. Insecticidal activity of EOs on Spodoptera frugiperda and selectivity to Euborellia annulipes. Braz. J. Biol. 2024, 84, e260522. [Google Scholar] [CrossRef]
- Cruz, G.S.; Wanderley-Teixeira, V.; Oliveira, J.V.; D’Assunção, C.G.; Cunha, F.M.; Teixeira, Á.A.C.; Guedes, C.A.; Dutra, K.A.; Barbosa, D.R.S.; Breda, M.O. Effect of trans-anethole, limonene and their combination on nutritional components and their impact on reproductive parameters and testicular aptosis in Spodoptera frugiperda (Lepidoptera: Noctuidae). Chem. Biol. Interact. 2016, 263, 74–80. [Google Scholar] [CrossRef]
- Buteler, M.; Stadler, T. A Review on the mode of action and current use of petroleum distillate spray oils. In Pesticides in the Modern World—Pesticies Use and Management; IntechOpen: London, UK, 2011. [Google Scholar]
- Lima, V.L.S.; Celestino, F.N.; Pratissoli, D.; Dalvi, L.P.; Carvalho, J.R.E.; Paes, J.P.P. Atividade inseticida do óleo de mamona sobre Diaphania nitidalis (Stoll) (Lepidoptera: Pyralidae). Rev. Bras. Cienc. Agrar. 2015, 10, 347–351. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Haddi, K.; Ribeiro, B.M.; Corrêia, R.F.T.; Tomé, H.V.V.; Santos-Amaya, O.; Pereira, E.J.G.; Guedes, R.N.C.; Santos, G.R.; Oliveira, E.E.; et al. Essential oil of Siparuna guianensis as an alternative tool for improved lepidopter an control and resistance management practices. Sci. Rep. 2018, 8, 7215. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of EOs in the insect nervous system a review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with multifaceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Landi, M.; Misra, B.B.; Muto, A.; Bruno, L.; Araniti, F. Phytotoxicity, morphological, and metabolic effects of the sesquiterpenoid nerolidol on Arabidopsis thaliana seedling roots. Plants 2020, 9, 1347. [Google Scholar] [CrossRef]
- Ghoneim, K.; Hamadah, K.H.; Selim, S.H.; Waheeb, H. Biopesticide potential of Nerolidol, a sesquiterpene compound, and its drastic impact on the growth and metamorphosis of the cotton plant Spodoptera littoralis (Lepidoptera: Noctuidae). Sch. Acad. J. Biosci. 2021, 9, 36–57. [Google Scholar]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- Thompson, C.S.; Yagi, K.J.; Chen, Z.F.; Tobe, S.S. The effects of octopamine on juvenile hormone biosynthesis, electrophysiology, and cAMP content of the corpora allata of the cockroach Diploptera punctata. J. Comp. Physiol. B 1990, 160, 241–249. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Liu, S. Juvenile hormone studies in Drosophila melanogaster. Front. Physiol. 2022, 12, 785320. [Google Scholar] [CrossRef]
- King, J.E.; Bennett, G.W. Mortality and developmental abnormalities induced by two juvenile hormone analogs on nymphal german cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 1988, 81, 225–227. [Google Scholar] [CrossRef]
- Guillette, R. Evolution and function in serotonergic systems. Integr. Comp. Biol. 2006, 46, 838–846. [Google Scholar] [CrossRef]
- Blenau, W.; Thamm, M. Distribution of serotonin (5-HT) and its receptors in the insect brain with a focus on the mushroom bodies: Lessons from Drosophila melanogaster and Apis mellifera. Arthropod Strut. Dev. 2011, 40, 381–394. [Google Scholar] [CrossRef]
- Wu, W.-H.; Cooper, R.L. Serotonin and synaptic transmission at invertebrate neuromuscular junctions. Exp. Neurobiol. 2012, 21, 101–112. [Google Scholar] [CrossRef]
- Woodring, J.; Hoffmann, K. The effects of octopamine, dopamine and serotonin on juvenile hormone synthesis, in vitro, in the cricket, Gryllus bimaculatus. J. Insect Physiol. 1994, 40, 797–802. [Google Scholar] [CrossRef]
- Ormerod, K.G.; Hadden, J.K.; Deady, L.D.; Mercier, A.J.; Krans, J.L. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J. Neurophysiol. 2013, 110, 1984–1996. [Google Scholar] [CrossRef]
- Charles, J.P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef]
- Araújo, C.R.M.; Santos, V.L.; Gonsalves, A.A. Acetilcolinesterase—AChE: Uma Enzima de Interesse Farmacológico. Rev. Virtual Quím. 2016, 8, 1818–1834. [Google Scholar] [CrossRef]
- Malaman, F.S.; Moraes, L.A.B.; West, C.; Ferreira, N.J.; Oliveira, A.L. Supercritical fluid extracts from the brazilian cherry (Eugenia uniflora L.): Relationship between the extracted compounds and the characteristic flavour intensity of the fruit. Food Chem. 2011, 124, 85–92. [Google Scholar] [CrossRef]
- Silva, R.T.; Assis, B.B.T.; Monção, É.C.; Fernandes, J.M.; Silva, M.E.S.; Grilo, M.M.S.; Coutinho, T.P.A.; Conceição, M.M. Análise microbiólogica e fisico-química de iogurte tipo grego adicionado de geleia de pitanga (Eugenia uniflora L.). Braz. J. Dev. 2020, 6, 24660–24677. [Google Scholar]
- Parra, J.R.P. Técnicas de Criação de Insetos Para Programas de Controle Biológico, 3rd ed.; FEALQ: Piracicaba, Brazil, 2001. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- He, L.M.; Wang, T.L.; Chen, Y.C.; Ge, S.S.; Wyckhuys, K.A.; Wu, K.M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 736–744. [Google Scholar]
- Chen, W.-H.; Itza, B.; Kafle, L.; Chang, T.-Y. Life Table Study of Fall Armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) on Three Host Plants under Laboratory Conditions. Insects 2023, 14, 329. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; Version 2020.01.12; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]
- Therneau, T.A. Package for Survival Analysis in R, R package version 3.2-7; 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 4 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993; p. 436. [Google Scholar]
- Hesterberg, T. It’s time to retire the “n >= 30” rule. In Proceedings of the Statistical Computing Section; American Statistical Association: Washington, DC, USA, 2008; pp. 1–9. [Google Scholar]
- Huang, Y.B.; Chi, H. The age-stage, two-sex life table with an offspring sex ratio 583 dependent on female age. J. Agric. Forest 2011, 60, 337–345. [Google Scholar]
- Yu, L.; Chen, Z.; Zheng, F.; Shi, A.; Guo, T.; Yeh, B.; Chi, H.; Xu, Y. Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: A case study of Chrysopa pallens (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akköprü, E.P.; Atlihan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Crowley, P.H. Resampling methods for data analysis in computation-intensive ecology and evolution. Annu. Rev. Ecol. Syst. 1992, 23, 405–447. [Google Scholar] [CrossRef]
- Hesterberg, T.; Monaghan, S.; Moore, D.S.; Clipson, A. Epstein R. Bootstrap methods and permutation tests. In The Practice of Business Statistics; Moore, D.S., McCabe, G.P., Duckworth, W.M., Sclove, S.L., Eds.; W. H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Smucker, M.D.; Allan, J.; Carterette, B. A comparison of statistical significance tests for information retrieval evaluation. In Proceedings of the Sixteenth ACM Conference on Information and Knowledge Management, Lisbon, Portugal, 6–10 November 2007; pp. 623–632. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]


| No. | Compound | RRI a | RRI b | Area (%) c ± SD | ||
|---|---|---|---|---|---|---|
| C. dinisii | E. uniflora | M. armillaris | ||||
| 1 | α-pinene | 932 | 932 | 19.90 ± 0.16 | nd | nd |
| 2 | Sabinene | 969 | 972 | 11.53 ± 0.15 | nd | nd |
| 3 | β-pinene | 974 | 976 | 13.71 ± 0.09 | nd | nd |
| 4 | myrcene | 991 | 988 | 1.72 ± 0.00 | nd | 1.27 ± 0.01 |
| 5 | α-phellandrene | 1005 | 1002 | nd | nd | 2.20 ± 0.00 |
| 6 | α-terpinene | 1016 | 1014 | nd | nd | 1.09 ± 0.00 |
| 7 | sylvestrene | 1027 | 1025 | 2.00 ± 0.00 | nd | nd |
| 8 | limonene | 1028 | 1024 | nd | nd | 2.99 ± 0.01 |
| 9 | 1,8-cineole | 1030 | 1026 | 2.54 ± 0.01 | nd | 21.81 ± 0.04 |
| 10 | (Z)-β-ocimene | 1036 | 1032 | 3.71 ± 0.00 | nd | nd |
| 11 | (E)-β-ocimene | 1045 | 1044 | 3.60 ± 0.00 | 1.14 ± 0.03 | nd |
| 12 | γ-terpinene | 1058 | 1054 | 1.27 ± 0.02 | nd | 2.97 ± 0.01 |
| 13 | terpine-4-ol | 1076 | 1077 | 1.31 ± 0.02 | nd | 2.98 ± 0.01 |
| 14 | terpinolene | 1090 | 1086 | nd | nd | 57.75 ± 0.17 |
| 15 | ni | 1101 | - | nd | nd | 1.03 ± 0.00 |
| 16 | α-terpineol | 1190 | 1173 | nd | nd | 2.62 ± 0.02 |
| 17 | β-elemene | 1390 | 1389 | nd | 6.00 ± 0.03 | nd |
| 18 | (E)-caryophyllene | 1416 | 1417 | 5.07 ± 0.11 | 2.53 ± 0.02 | nd |
| 19 | γ-elemene | 1432 | 1434 | nd | 2.07 ± 0.01 | nd |
| 20 | germacrene D | 1478 | 1480 | nd | 2.59 ± 0.04 | nd |
| 21 | bicyclogermacrene | 1495 | 1500 | 18.64 ± 0.57 | nd | nd |
| 22 | curzerene | 1497 | 1499 | nd | 41.22 ± 0.04 | nd |
| 23 | germacrene B | 1554 | 1559 | nd | 8.04 ± 0.07 | nd |
| 24 | (E)-nerolidol | 1564 | 1565 | 1.06 ± 0.04 | nd | nd |
| 25 | spathulenol | 1575 | 1577 | 2.09 ± 0.07 | 2.37 ± 0.02 | nd |
| 26 | caryophyllene oxide | 1581 | 1582 | 1.08 ± 0.07 | nd | nd |
| 27 | atractilone | 1658 | 1657 | nd | 1.17 ± 0.05 | nd |
| 28 | ni | 1690 | - | nd | 4.86 ± 0.12 | nd |
| 29 | germacrone | 1694 | 1693 | nd | 5.17 ± 0.05 | nd |
| 30 | ni | 1728 | - | nd | 4.50 ± 0.11 | nd |
| 31 | ni | 1743 | - | nd | 3.03 ± 0.06 | nd |
| Number of identified compounds | 15 | 13 | 10 | |||
| Total area (%) | 88.23 | 84.69 | 96.71 | |||
| Essential Oil | N | X2 | p | b | e | DL25 (µg of EO/Caterpillar) | DL50 (µg of EO/Caterpillar) | DL90 (µg of EO/Caterpillar) |
|---|---|---|---|---|---|---|---|---|
| C. dinisii | 100 | 4.22 | 0.234 | −1.79 | 5.62 | 3.04 ± 0.26 | 5.62 ± 0.34 | 19.16 ± 2.34 |
| E. uniflora | 100 | 7.15 | 0.0672 | −1.69 | 1.19 | 0.62 ± 0.06 | 1.19 ± 0.09 | 4.35 ± 0.54 |
| M. armillaris | 100 | 36.82 | 0 | −1.85 | 3.66 | 2.02 ± 0.20 | 3.66 ± 0.24 | 12.03 ± 1.32 |
| Parameter | Stages | Acetone | C. dinisii | E. uniflora | M. armillaris | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | N | Mean ± SE | N | Mean ± SE | ||
| Development time (days) | Egg | 100 | 3.00 ± 0.00a | 100 | 3.00 ± 0.00a | 100 | 3.00 ± 0.00a | 100 | 3.00 ± 0.00a |
| L1 | 100 | 2.00 ± 0.00a | 100 | 2.00 ± 0.00a | 100 | 2.00 ± 0.00a | 100 | 2.00 ± 0.00a | |
| L2 | 84 | 7.67 ± 0.31b | 37 | 6.86 ± 0.71b | 51 | 7.39 ± 0.38b | 100 | 3.51 ± 0.35a | |
| L3 | 84 | 5.11 ± 0.26a | 34 | 8.41 ± 0.59b | 48 | 4.33 ± 0.23c | 34 | 4.32 ± 0.28d | |
| L4 | 83 | 3.84 ± 0.15a | 34 | 3.74 ± 0.27ab | 48 | 4.12 ± 0.24b | 34 | 4.74 ± 0.43b | |
| L5 | 80 | 5.30 ± 0.11a | 30 | 5.63 ± 0.23a | 46 | 5.63 ± 0.15a | 34 | 5.79 ± 0.23a | |
| Pupa | 67 | 11.94 ± 0.27a | 22 | 12.14 ± 0.47a | 35 | 11.83 ± 0.37ab | 27 | 11.26 ± 0.20b | |
| Egg-Pupa | 67 | 38.40 ± 0.48b | 22 | 39.00 ± 0.00a | 35 | 37.63 ± 0.58b | 27 | 38.22 ± 0.67b | |
| Longevity (days) | Adult | 67 | 10.49 ± 0.52a | 22 | 11.27 ± 1.09a | 35 | 10.89 ± 0.66a | 27 | 12.89 ± 1.27a |
| Life cycle (days) * | Female | 35 | 49.57 ± 1.08a | 9 | 52.44 ± 2.46a | 15 | 48.40 ± 1.06a | 14 | 50.00 ± 1.80a |
| Male | 32 | 48.16 ± 0.55a | 13 | 53.85 ± 1.92b | 20 | 48.60 ± 0.88a | 13 | 52.31 ± 1.99b | |
| Egg-Adult | 67 | 48.90 ± 0.62a | 22 | 52.44 ± 2.46b | 35 | 48.51 ± 0.67ab | 27 | 51.11 ± 1.80b | |
| Demographic Parameter | Acetone | C. dinisii | E. uniflora | M. armillaris |
|---|---|---|---|---|
| Intrinsic growth rate (r) | 0.04 ± 0.01a | 0.01 ± 0.01b | 0.05 ± 0.02a | 0.04 ± 0.02a |
| Finite rate of growth (λ) | 1.04 ± 0.01a | 1.01 ± 0.01b | 1.04 ± 0.02a | 1.03 ± 0.02a |
| Average generation time (T) | 46.90 ± 0.06b | 56.31 ± 0.001a | 43.71 ± 0.096c | 44.63 ± 4.52bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.A.C.; Fernandes, L.A.; Figueiredo, K.G.; Corrêa, E.J.A.; Lima, L.H.F.; Alves, D.S.; Bertolucci, S.K.V.; Carvalho, G.A. Effects of Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda. Plants 2024, 13, 1801. https://doi.org/10.3390/plants13131801
Oliveira JAC, Fernandes LA, Figueiredo KG, Corrêa EJA, Lima LHF, Alves DS, Bertolucci SKV, Carvalho GA. Effects of Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda. Plants. 2024; 13(13):1801. https://doi.org/10.3390/plants13131801
Chicago/Turabian StyleOliveira, Júlia A. C., Letícia A. Fernandes, Karolina G. Figueiredo, Eduardo J. A. Corrêa, Leonardo H. F. Lima, Dejane S. Alves, Suzan K. V. Bertolucci, and Geraldo A. Carvalho. 2024. "Effects of Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda" Plants 13, no. 13: 1801. https://doi.org/10.3390/plants13131801
APA StyleOliveira, J. A. C., Fernandes, L. A., Figueiredo, K. G., Corrêa, E. J. A., Lima, L. H. F., Alves, D. S., Bertolucci, S. K. V., & Carvalho, G. A. (2024). Effects of Essential Oils on Biological Characteristics and Potential Molecular Targets in Spodoptera frugiperda. Plants, 13(13), 1801. https://doi.org/10.3390/plants13131801








