Chemical Compositions and Fumigation Effects of Essential Oils Derived from Cardamom, Elettaria cardamomum (L.) Maton, and Galangal, Alpinia galanga (L.) Willd, against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae)
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
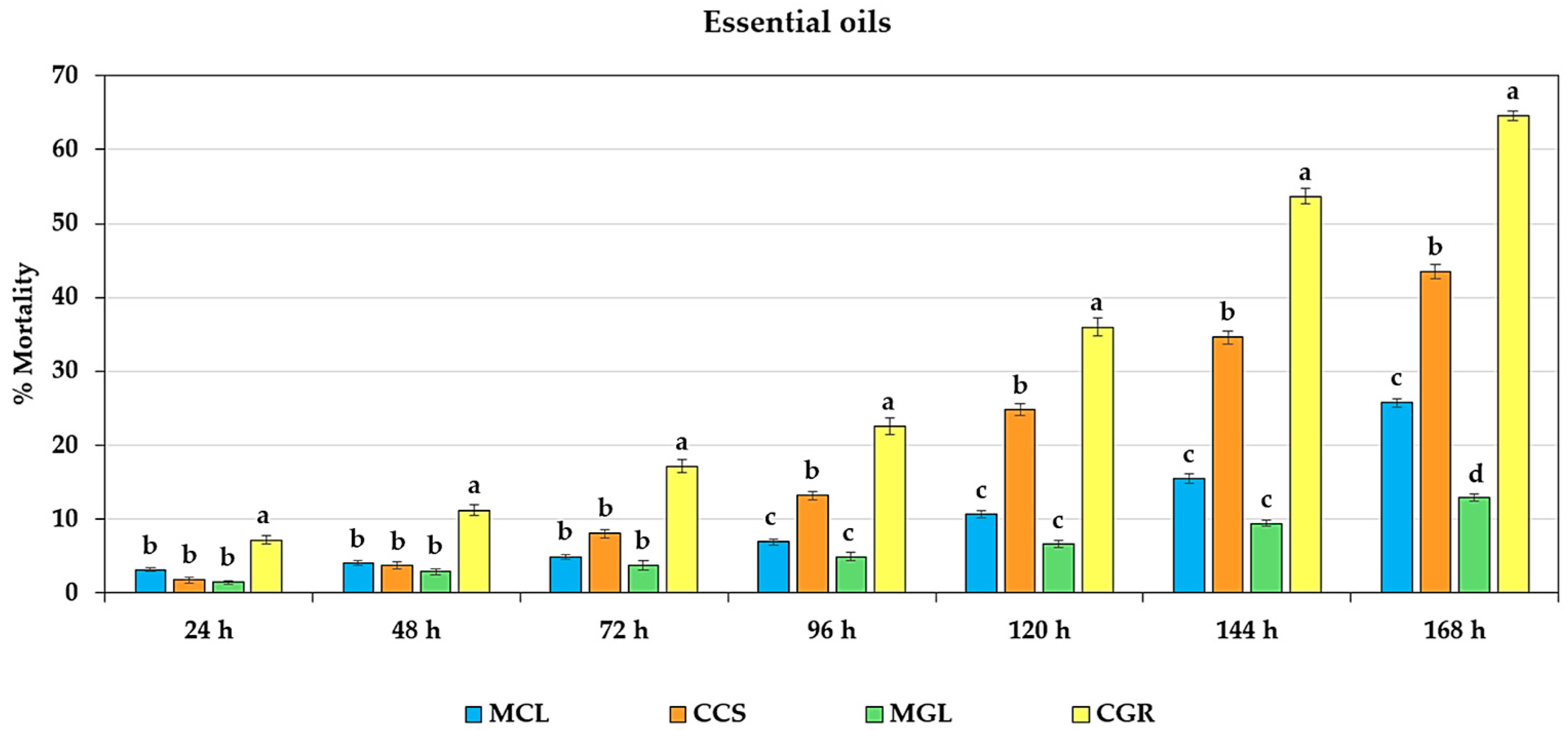
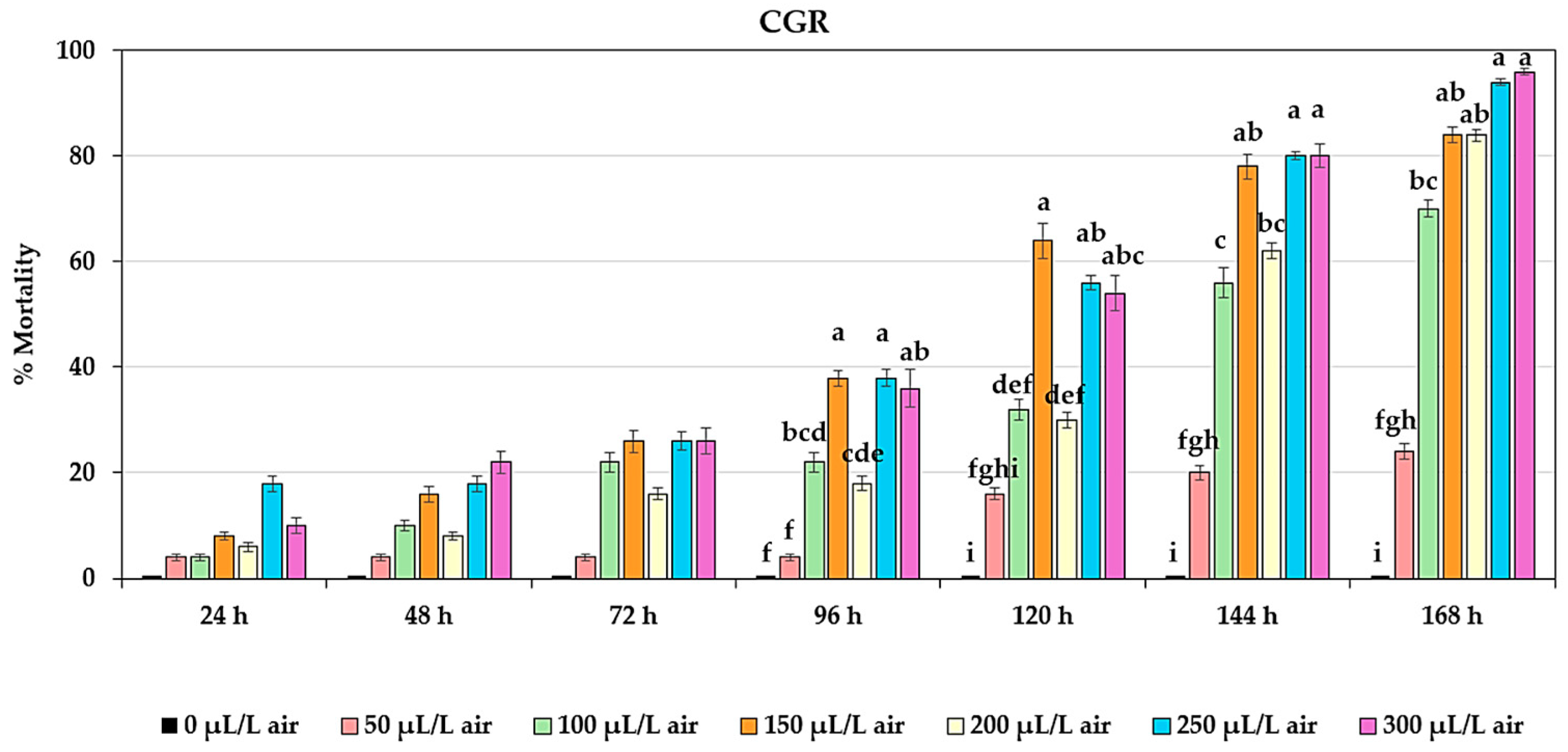
2.2. Effect of Fumigation on Adult Mortality
3. Materials and Methods
3.1. Insect Rearing
3.2. Preparation of Essential Oils
3.3. Chemical Compositon Analysis
3.4. Effects of Fumigation Bioassay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wijayaratne, L.K.W.; Arthur, F.H.; Whyard, S. Methoprene and control of stored-product insects. J. Stored. Prod. Res. 2016, 76, 161–169. [Google Scholar] [CrossRef]
- Pugazhvendan, S.R.; Elumalai, K.; Ross, P.R.; Soundarajan, M. Repellent activity of chosen plant species against Tribolium castaneum. World J. Zool. 2009, 4, 188–190. [Google Scholar]
- Bezabih, G.; Satheesh, N.; Fanta, S.W.; Wale, M.; Atlabachew, M. Reducing postharvest loss of stored grains using plant-based biopesticides: A review of past research efforts. Adv. Agric. 2022, 2022, 6946916. [Google Scholar] [CrossRef]
- Campbell, J.F.; Athanassiou, C.G.; Hagstrum, D.W.; Zhu, K.Y. Tribolium castaneum: A model insect for fundamental and applied research. Annu. Rev. Entomol. 2022, 67, 347–365. [Google Scholar] [CrossRef]
- Zhang, S.F.; Chen, H.J.; Xue, G.H. Atlas of Beetles Associated with Stored Products; China Agricultural Science and Technology Press: Beijing, China, 2008; p. 161. [Google Scholar]
- Phankaen, Y.; Manaprasertsak, A.; Pluempanupat, W.; Koul, O.; Kainoh, Y.; Bullangpoti, V. Toxicity and repellent action of Coffea arabica against Tribolium castaneum (Herbst) adults under laboratory conditions. J. Stored Prod. Res. 2017, 71, 112–118. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Dai, J.; Zhu, L. Chemical composition and biological activity against Tribolium castaneum (Coleoptera: Tenebrionidae) of Artemisia brachyloba essential oil. Ind. Crops Prod. 2019, 128, 29–37. [Google Scholar] [CrossRef]
- Rajendran, S.; Srianjini, V. Plant products as fumigants for stored product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Anusree, R.P.; Pathrose, B.; Chellappan, M. Malathion resistance in red flour beetle (Tribolium castaneum) (Herbst) (Coleoptera: Tenebrionidae) from FCI godowns of Kerala, India. J. Trop. Agric. 2019, 57, 201–205. [Google Scholar]
- Nayak, M.K.; Jagadeesan, R.; Singarayan, V.T.; Nath, N.S.; Pavic, H.; Dembowski, B.; Daglish, G.J.; Schlipalius, D.I.; Ebert, P.R. First report of strong phosphine resistance in stored grain insects in a far northern tropical region of Australia, combining conventional and genetic diagnostics. J. Stored Prod. Res. 2021, 92, 101813. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, Q.Y.; Li, D.W.; Zhang, Z.M.; You, C.X. Antagonistic storage potential of Tagetes minuta, Eupatorium fortunei and Ocimum basilicum oils with volatile secondary metabolites against Tribolium castaneum and Lasioderma serricorne. Ind. Crops Prod. 2022, 187, 115502. [Google Scholar] [CrossRef]
- Sharaby, A.; EL-Nujiban, A. Biological activity of essential oil of sage plan leaves Salvia offecinalis L. against the black cutworm Agrotis ipsilon (Hubn.). Int. J. Sci. Res. 2015, 4, 737–741. [Google Scholar]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Auti, S.T.; Kulkarni, Y.A. Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front. Neurol. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Rehman, N.U.; Ansari, M.N.; Palla, A.H. Effects of essential oils of Elettaria cardamom grown in India and Guatemala on gram-negative bacteria and gastrointestinal disorders. Molecules 2021, 26, 2546. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Tomas, M.; Toydemir, G.; Kamiloglu, S.; Capanoglu, E. Introduction to nutraceuticals, medicinal foods, and herbs. In Aromatic Herbs in Food; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–34. [Google Scholar]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]–A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef] [PubMed]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 2020, 61, 102089. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Vellaikumar, S.; Murugan, M.; Dhanya, M.K.; Ariharasutharsan, G.; Aiswarya, S.; Akilan, M.; Warkentin, T.D.; Karthikeyan, A. Essential oil profile diversity in cardamom accessions from Southern India. Front. Sustain. Food Syst. Sec. Nutr. Sustain. Diets 2021, 5, 2021. [Google Scholar] [CrossRef]
- Jha, V.; Shaikh, D.; Bhargava, A.; Marick, A.; Khan, F.; Dhamapurkar, V.; Jhangiani, A.; Narvekar, S.; Shinde, R.; Nair, M. Characterization of physio-chemical properties and evaluation of bioactive potentials of essential oils from Elettaria cardamomum. J. Plant Biol. Crop Res. 2022, 5, 1068. [Google Scholar]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Stashenko, E.E.; Olivero-Verbel, J. Repellent and fumigant actions of the essential oils from Elettaria cardamomum (L.) Maton, Salvia officinalis (L.) Linnaeus, and Lippia origanoides (V.) Kunth against Tribolium castaneum and Ulomoides dermestoides. J. Essent. Oil Bear. Plants 2019, 22, 18–30. [Google Scholar] [CrossRef]
- Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Hosseinpour, M.H. Fumigant toxicity and oviposition deterrency of the essential oil from cardamom, Elettaria cardamomum, against three stored product insects. J. Insect Sci. 2011, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Managi, H.; Yoshikawa, M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-a and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2003, 13, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Latha, C.; Shriram, V.D.; Jahagirdar, S.S.; Dhakephalkar, P.K.; Rojatkar, S.R. Antiplasmid activity of 1′- acetoxychavicol acetate from Alpinia galanga against multidrug resistant bacteria. J. Ethnopharmacol. 2009, 123, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Sukhirun, N.; Pluempanupat, W.; Bullangpoti, V.; Koul, O. Bioefficacy of Alpinia galanga (Zingiberaceae) rhizome extracts, (E)-p-acetoxycinnamyl alcohol, and (E)-p-coumaryl alcohol ethyl ether against Bactrocera dorsalis (Diptera: Tephritidae) and the impact on detoxification enzyme activities. J. Econ. Entomol. 2011, 104, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Yadav, J.; Kaushik, P.; Sacher, D.; Rani, R. Current pharmacological and phytochemical studies of the plant Alpinia galanga. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Sawangjaroen, N.; Subhadhirasakul, S.; Phongpaichit, S.; Siripanth, C.; Jamjaroen, K.; Sawangjaroen, K. The in vitro anti-giardial activity of extracts from plants that are used for self-medication by AIDS patients in southern Thailand. Parasitol. Res. 2005, 95, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Soussi, M.; Noumi, E.; Dehmani, A.; Flamini, G.; Aouni, M.; Al-sieni, M.; Al-sieni, A. Chemical composition and antimicrobial activities of Elettaria cardamomum L. (Maton) essential oil: A high activity against a wide range of food borne and medically important bacteria and fungi. J. Chem. Biol. Phys. Sci. 2015, 6, 248–259. [Google Scholar]
- Asghar, A.; Butt, M.S.; Shahid, M.; Huang, Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol. 2017, 54, 2306–2315. [Google Scholar]
- Noshad, M.; Behbahani, B.A. Identification of chemical compounds, antioxidant activity, and antimicrobial effect of Elettaria cardamomum essential oil on a number of pathogenic microorganisms in vitro. Qom Univ. Med. Sci. J. 2019, 13, 57–69. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Ben Said, M.; Shater, A.F.; Al-Sabi, M.N.S. Acaricidal, larvacidal, and repellent activity of Elettaria cardamomum essential oil against Hyalomma anatolicum ticks infesting Saudi Arabian cattle. Plants 2022, 11, 1221. [Google Scholar] [CrossRef]
- Pirmohammadi, M.; Abai, M.R.; Shayeghi, M.; Vatandoost, H.; Rahimi, S.; Pirmohammadi, M. Influence of agro-climatic conditions on chemical compositions and repellency effect of Mentha longifolia plant against malaria vector, Anopheles stephensi. Toxin Rev. 2023, 42, 115–121. [Google Scholar] [CrossRef]
- Oommen, M.; Reivax, X.; Vadiraj, B.A.; Kumar, P.M.S.; Remashree, A.B. Quality appraisal of small cardamom (Elettaria cardamomum, Maton) Sourced from A, B and C Zones of CHR in Idukki district of Kerala, India. J. Essent. Oil Bear. Plants 2018, 21, 1315–1326. [Google Scholar] [CrossRef]
- Menon, A.N. Chemical composition of the volatile oils of Alpinia galanga plant parts from Kerala. J. Essent. Oil Bear. Plants 2006, 9, 277–282. [Google Scholar] [CrossRef]
- Raina, V.K.; Srivastava, S.K.; Syamasunder, K.V. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flav. Fragr. J. 2002, 17, 358–360. [Google Scholar] [CrossRef]
- Akhtar, P.; Ali, M.; Mir, S.R.; Sharma, M.P. Volatile constituents of rhizomes of Alpinia galanga (Linn.) Wild. J. Essent. Oil Bear. Plants 2004, 7, 243–246. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, S.; Ray, A.; Sahoo, A.; Nayak, S.; Kar, B. Thermal desorption modulation based detection of volatile constituents of Alpinia galanga by two dimensional gas chromatography and time of flight mass spectrometry. Nat. Prod. Res. 2019, 35, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Abeywickrama, K.; Adhikari, A.A.C.K.; Paranagama, P.; Gamage, C.S.P. The efficacy of essential oil of Alpinia calcarata (Rosc.) and its major constituent, 1.8-cineole, as protectants of cowpea against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Can. J. Plant Sci. 2006, 86, 821–827. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Li, Z.H.; Wang, C.F.; Wei, J.Y.; Li, X.L.; Wang, P.J.; Zhou, Z.F.; Du, S.S.; Huang, D.Y.; et al. Composition of the essential oil from Alpinia galanga rhizomes and its bioactivity on Lasioderma serricorne. Bull. Insectology 2014, 67, 247–254. [Google Scholar]
- Chalchat, J.; Özcan, M.M. Comparative essential oil composition of flowers, leaves and stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008, 110, 501–503. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Lee, S.B.; Lee, H.S.; Kim, G.H. Insecticidal and acaricidal activity of carvacrol and β-thujaplicine derived from Thujopsis dolabrata var. hondai Sawdust. J. Chem. Eco. 1998, 24, 81–90. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say) (Coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Sener, O.; Arslan, M.; Demirel, N.; Uremis, I. Insecticidal effects of some essential oils against the confused flour beetle (Tribolium confusum du Val) (Col.: Tenebrinoidea) in stored wheat. Asian J. Chem. 2009, 21, 3995–4000. [Google Scholar]
- Lee, B.H.; Choi, W.S.; Lee, S.E.; Park, B.S. Fumigant toxicity of essential oil and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-Armani, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Erler, F. Fumigant activity of six monoterpenoids from aromatic plants in Turkey against two stored product pests confused flour beetle, Tribolium confusum, and Mediterranean flour moth, Ephestia kuehniella. J. Plant Dis. Prot. 2005, 112, 602–611. [Google Scholar] [CrossRef]
- Reis, S.; Mantello, A.; Macedo, J.; Gelfuso, E.; da Silva, C.; Fachin, A.; Cardoso, A.; Beleboni, R. Typical monoterpenes as insecticides and repellents against stored grain pests. Molecules 2016, 21, 258. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Gupta, S.; Chauhan, N.; Bhushan, S.; Arora, R.; Arora, S.; Kaur, S. Insecticidal, food utilisation and biochemical effect of essential oils extracted from seeds of Brassica juncea (Czern.) against Spodoptera litura (Lepidoptera: Noctuidae) (Fabricius). Arthropod 2017, 6, 93–106. [Google Scholar]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. 2001, C130, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Aziza, S.; AL-Dosary, M. An electric air flow olfactometer and the olfactory Response of Rhynchophorous ferrugineus weevil to some volatile compounds. J. Agric. Ecol. Res. Int. 2014, 1, 40–50. [Google Scholar]
- Liu, Z.L.; Jiang, G.H.; Zhou, L.; Liu, Q.Z. Analysis of the essential oil of Dipsacus japonicus flowering aerial parts and its insecticidal activity against Sitophilus zeamais and Tribolium castaneum. Z. Naturforschung. C J. Biosci. 2013, 68, 13–18. [Google Scholar] [CrossRef]
- Emekci, M.; Navarro, S.; Donahaye, E.; Rindner, M.; Azrieli, A. Respiration of Rhyzopertha dominica (F.) at reduced oxygen concentration. J. Stored Prod. Res. 2004, 40, 27–38. [Google Scholar] [CrossRef]
- Araújo, M.O.; Pérez-Castillo, Y.; Oliveira, L.H.G.; Nunes, F.C.; de Sousa, D.P. Larvicidal activity of cinnamic acid derivatives: Investigating alternative products for Aedes aegypti L. Control Mol. 2021, 26, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qian, L.; Litao, S.; Jizhi, Y.; Huabao, C.; Surong, J.; Chunxian, J.; Wang, H. Fumigant, contact, and repellent activities of essential oils against the darkling beetle, Alphitobius diaperinus. J. Stored Prod. Res. 2014, 14, 2–11. [Google Scholar] [CrossRef]
- Lu, J.H.; Su, X.H.; Zhong, J.J. Fumigant activity of Elsholtzia stauntonii extract against Lasioderma serricorne. S. Afr. J. Sci. 2012, 108, 7–8. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Safaralizadeh, M.; Pourmirza, A.; Gheibi, S. Toxicity of essential oil of Agastache Foeniculum (Pursh) Kuntze to Oryzaephilus surinamensis L. and Lasioderma Serricorne F. J. Plant Prot. Res. 2010, 50, 215–219. [Google Scholar] [CrossRef]
- Nour, A.H.; Elhussein, S.A.; Nour, A.O.; Nour, A.H.; Yusoff, M.M. A study of the essential oils of four Sudanese accessions of basil (Ocimum basilicum L.) against Anopheles mosquito larvae. Am. J. Appl. Sci. 2009, 6, 1359–1363. [Google Scholar] [CrossRef]
- Dekker, T.; Ignell, R.; Ghebru, M.; Glinwood, R.; Hopkins, R. Identification of mosquito repellent odours from Ocimum forskolei. Parasit. Vectors 2011, 4, 183. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.R.; Choi, D.R.; Anh, Y.J. Toxicity of cassia and cinnamon oil compounds and cinnamaldehyde-related compounds to Sitophilus oryzae (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Sunnerheim, K.; Nordqvist, A.; Nordlander, G.; Borg-Karlson, A.K.; Unelius, C.R.; Bohman, B.; Nordenhem, H.; Hellqvist, C.; Karlen, A. Quantitative structure-activity relationships of pine weevil antifeedants, a multivariate approach. J. Agric. Food Chem. 2007, 55, 9365–9372. [Google Scholar] [CrossRef]
- Kim, N.J.; Byun, S.G.; Cho, J.E.; Chung, K.; Ahn, Y.J. Larvicidal activity of Kaempferia galanga rhizome phenylpropanoids towards three mosquito species. Pest Manag. Sci. 2008, 64, 857–862. [Google Scholar] [PubMed]
- Fujiwara, G.M.; Annies, V.; de Oliveira, C.F.; Lara, R.A.; Gabriel, M.M.; Betim, F.C.M.; Nadal, J.M.; Farago, P.V.; Dias, J.F.G.; Miguel, O.G.; et al. Evaluation of larvicidal activity and ecotoxicity of linalool, methyl cinnamate and methyl cinnamate/linalool in combination against Aedes aegypti. Ecotoxicolo. Environ. Saf. 2017, 139, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.H.; Kini, R.M. Bioactivities of safrole and isosafrole on Sitophilus zeamais (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 1999, 92, 676–683. [Google Scholar] [CrossRef]
- Franco, O.L.; Melo, F.R.; Mendes, P.A.; Peas, N.S.; Yokoyama, M.; Coutinho, M.V.; Bloch, C., Jr.; Grossi-de-Sa, M.F. Characterization of two Acanthoscelides obtectus alpha-amylases and their inactivation by wheat inhibitors. J. Agric. Food Chem. 2005, 53, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar]
- Durden, K.; Sellars, S.; Pszczolkowski, M. Preventing fruit infestation by codling moth neonates with Artemisia extracts. Pestycydy/Pesticides 2009, 1, 51–56. [Google Scholar]
- Satongrod, B.; Wanna, R.; Khaengkhan, P.; Chumpawadee, S. Fumigant toxicity and bioactivity of Wedelia trilobata essential oil against cowpea weevil (Callosobruchus maculatus). Int. J. Agri. Technol. 2021, 17, 1591–1604. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corp: Carol Stream, IL, USA, 2001. [Google Scholar]
- Wongsawas, M.; Bunphan, D.; Wanna, R.; Bozdoğan, H. Toxicity of essential oil from Plectranthus amboinicus (Lamiales: Lamiaceae) against Sitophilus zeamais (Coleoptera: Curculionidae). J. Entomol. Sci. 2024, 59, 323–331. [Google Scholar] [CrossRef]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]



| No. | Compound | RI | RT | % Peak Area |
|---|---|---|---|---|
| 1 | eucalyptol | 1030 | 8.071 | 25.2 |
| 2 | trans-p-mentha-1(7),8-dien-2-ol | 1170 | 13.841 | 3.2 |
| 3 | trans-verbenol | 1172 | 15.560 | 1.4 |
| 4 | α-guaiene | 1380 | 26.043 | 4.1 |
| 5 | α-cubebene | 1400 | 26.381 | 3.1 |
| 6 | trans-calamenene | 1570 | 27.587 | 13.4 |
| 7 | caryophyllene oxide | 1574 | 29.656 | 1.4 |
| 8 | isospathulenol | 1575 | 30.307 | 13.1 |
| 9 | ledol | 1580 | 30.747 | 4.1 |
| 10 | junenol | 1598 | 30.945 | 2.7 |
| 11 | isoaromadendrene epoxide | 1621 | 31.950 | 1.9 |
| 12 | epicubenol | 1650 | 32.058 | 1.6 |
| 13 | ylangenal | 1674 | 31.783 | 5.0 |
| Total | 80.3 |
| No. | Compound | RI | RT | % Peak Area |
|---|---|---|---|---|
| 1 | α-pinene | 931 | 6.012 | 4.7 |
| 2 | eucalyptol | 1030 | 8.026 | 31.2 |
| 3 | linalool | 1095 | 10.291 | 2.5 |
| 4 | terpineol | 1189 | 14.046 | 1.9 |
| 5 | linalyl acetate | 1267 | 16.388 | 4.7 |
| 6 | α-terpinyl acetate | 1345 | 20.907 | 46.1 |
| 7 | nerolidol | 1560 | 28.971 | 1.1 |
| Total | 92.2 |
| No. | Compound | RI | RT | % Peak Area |
|---|---|---|---|---|
| 1 | caryophyllene | 1419 | 24.405 | 28.7 |
| 2 | aciphyllene | 1425 | 27.159 | 18.3 |
| 3 | α-farnesene | 1450 | 27.415 | 7.0 |
| 4 | α-bisabolene | 1490 | 27.822 | 10.7 |
| 5 | β-Sesquiphellandrene | 1525 | 28.145 | 1.9 |
| 6 | caryophyllene oxide | 1582 | 30.217 | 4.2 |
| 7 | isoaromadendrene epoxide | 1621 | 32.814 | 1.4 |
| 8 | farnesyl butanoate | 1960 | 39.247 | 6.1 |
| 9 | n-hexadecanoic acid | 2100 | 44.776 | 2.3 |
| Total | 80.4 |
| No. | Compound | RI | RT | % Peak Area |
|---|---|---|---|---|
| 1 | α-pinene | 931 | 6.200 | 6.0 |
| 2 | α-phellandrene | 1007 | 6.973 | 1.5 |
| 3 | eucalyptol | 1030 | 7.924 | 7.7 |
| 4 | terpineol | 1189 | 14.044 | 1.1 |
| 5 | safrole | 1209 | 22.488 | 19.8 |
| 6 | p-vinylphenylisothiocyanate | 1330 | 22.658 | 11.5 |
| 7 | methyl cis-cinnamate | 1410 | 23.274 | 47.3 |
| Total | 94.8 |
| No. | Compound | % Peak Area | |||
|---|---|---|---|---|---|
| MCL | CCS | MGL | CGR | ||
| 1 | eucalyptol | 25.2 | 31.2 | - | 7.7 |
| 2 | α-terpinyl acetate | - | 46.1 | - | - |
| 3 | methyl cis-cinnamate | - | - | - | 47.3 |
| 4 | caryophyllene | - | - | 28.7 | - |
| 5 | safrole | - | - | - | 19.8 |
| 6 | aciphyllene | - | - | 18.3 | - |
| 7 | trans-calamenene | 13.4 | - | - | - |
| 8 | isospathulenol | 13.1 | - | - | - |
| 9 | p-vinylphenylisothiocyanate | - | - | - | 11.5 |
| 10 | α-bisanolene | - | - | 10.7 | - |
| 11 | caryophyllene oxide | 1.4 | - | 4.2 | - |
| 12 | terpineol | - | 1.9 | - | 1.1 |
| 13 | linalool | - | 2.5 | - | - |
| 14 | α-pinene | - | 4.7 | - | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanna, R.; Khaengkhan, P.; Bozdoğan, H. Chemical Compositions and Fumigation Effects of Essential Oils Derived from Cardamom, Elettaria cardamomum (L.) Maton, and Galangal, Alpinia galanga (L.) Willd, against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Plants 2024, 13, 1845. https://doi.org/10.3390/plants13131845
Wanna R, Khaengkhan P, Bozdoğan H. Chemical Compositions and Fumigation Effects of Essential Oils Derived from Cardamom, Elettaria cardamomum (L.) Maton, and Galangal, Alpinia galanga (L.) Willd, against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Plants. 2024; 13(13):1845. https://doi.org/10.3390/plants13131845
Chicago/Turabian StyleWanna, Ruchuon, Parinda Khaengkhan, and Hakan Bozdoğan. 2024. "Chemical Compositions and Fumigation Effects of Essential Oils Derived from Cardamom, Elettaria cardamomum (L.) Maton, and Galangal, Alpinia galanga (L.) Willd, against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae)" Plants 13, no. 13: 1845. https://doi.org/10.3390/plants13131845
APA StyleWanna, R., Khaengkhan, P., & Bozdoğan, H. (2024). Chemical Compositions and Fumigation Effects of Essential Oils Derived from Cardamom, Elettaria cardamomum (L.) Maton, and Galangal, Alpinia galanga (L.) Willd, against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Plants, 13(13), 1845. https://doi.org/10.3390/plants13131845









