Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Adult V. coerulea Plants
2.3. V. coerulea Protocorm
2.4. Extraction of V. coerulea Adult Plant and Protocorm
2.5. Determination of Chemical Compositions of V. coerulea Plant and Protocorm Extracts Using High-Performance Liquid Chromatography (HPLC)
2.6. Determination of Biological Activities of V. coerulea Adult Plant and Protocorm Extracts
2.6.1. Antioxidant Activities
- 1.
- 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
- 2.
- Ferric-Reducing Antioxidant Power (FRAP) Assay
2.6.2. Anti-Skin Aging Activities
- 3.
- Matrix metalloproteinase-1 (MMP-1) inhibition
- 4.
- Elastase Inhibition
2.6.3. Anti-Tyrosinase Activities
2.6.4. Anti-Inflammatory Activities
2.7. Determination of Irritation Potency of V. coerulea Adult Plant and Protocorm Extracts by Hen’s Egg-Chorioallantoic Membrane (HET-CAM) Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Adult V. coerulea Plants and Protocorms
3.2. Chemical Compositions of V. coerulea Plant and Protocorm Extracts
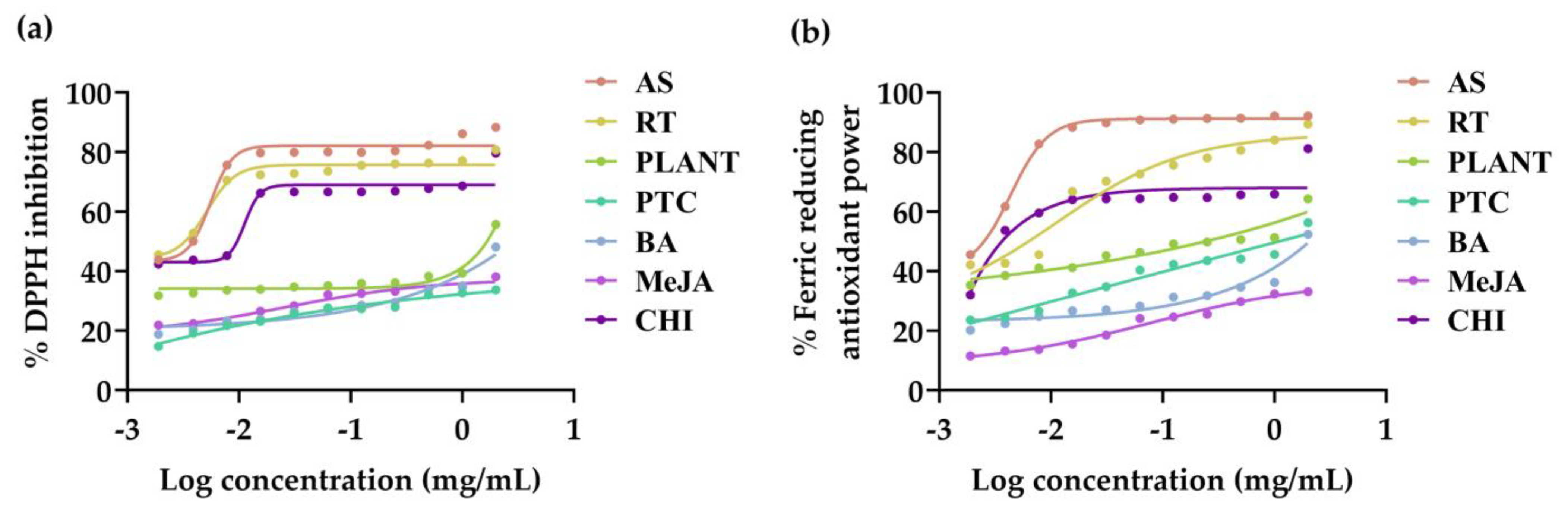
3.3. Antioxidant Activities of V. coerulea Plant and Protocorm Extracts
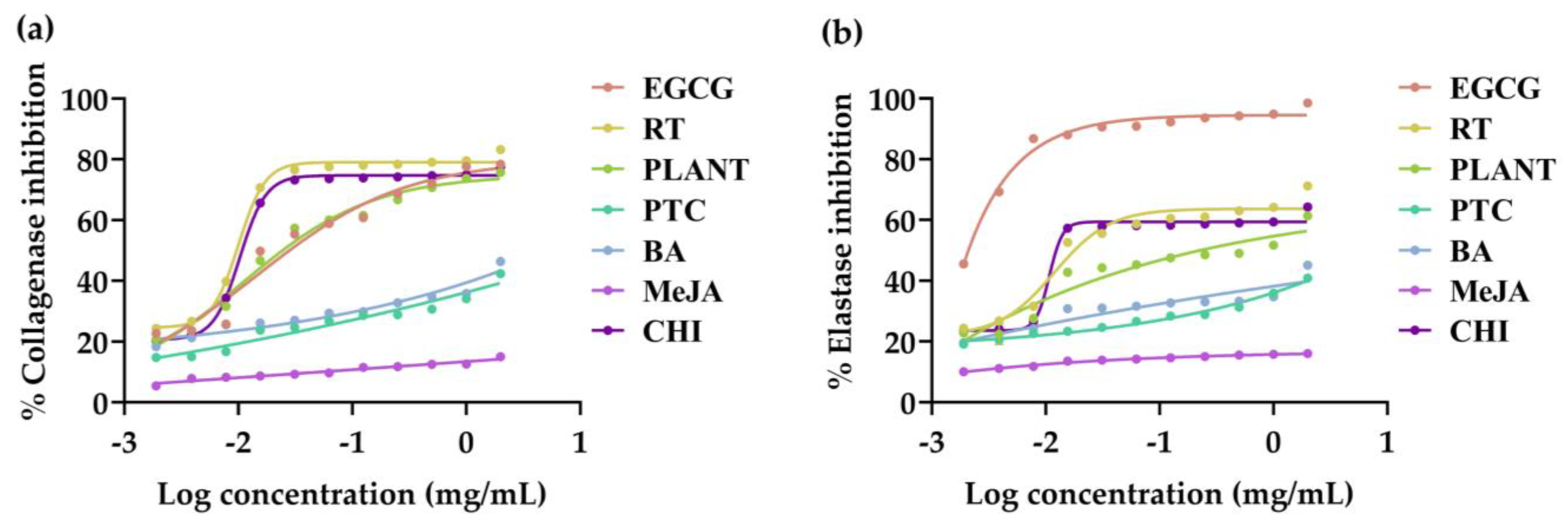
3.4. Anti-Skin Aging Activities of V. coerulea Plant and Protocorm Extracts
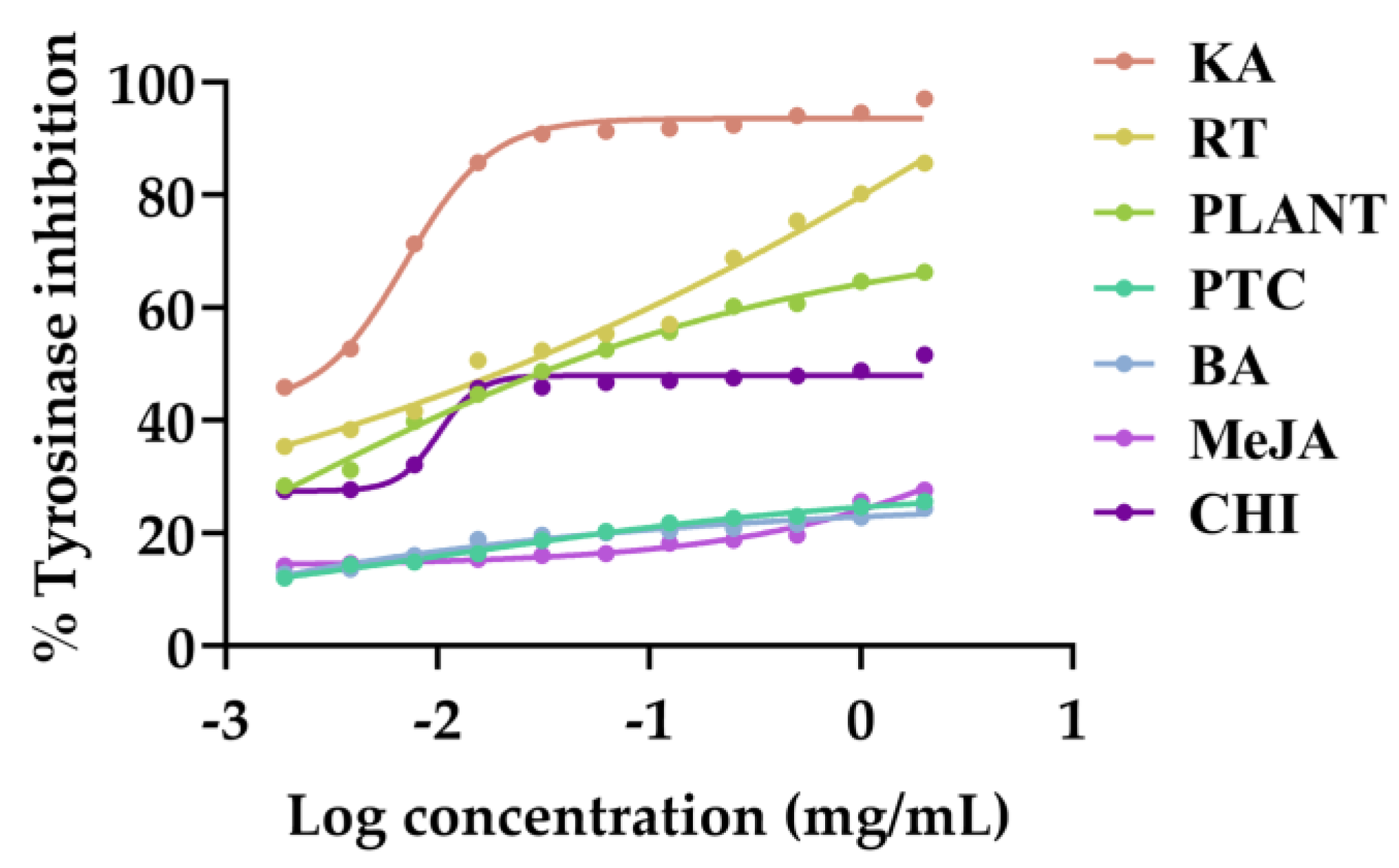
3.5. Anti-Tyrosinase Activities of V. coerulea Plant and Protocorm Extracts
3.6. Anti-Inflammatory Activities of V. coerulea Plant and Protocorm Extracts
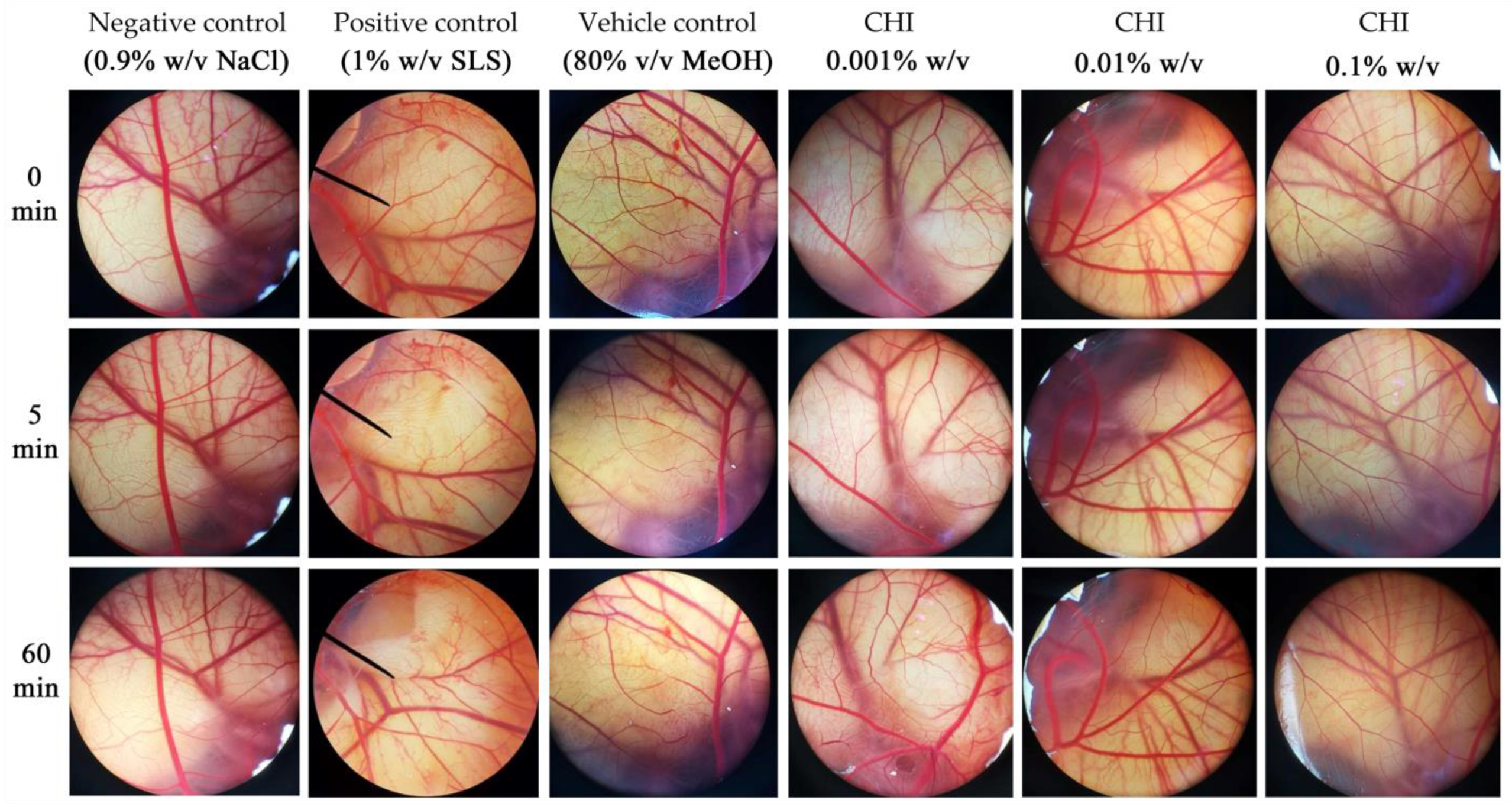
3.7. Irritation Potency of V. coerulea Protocorm Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taneja, S. Therapeutic Products from Botanical Sources: The Indian Scenario. Planta Med. 2009, 75, S-25. [Google Scholar] [CrossRef]
- Abass, S.; Parveen, R.; Irfan, M.; Jan, B.; Husain, S.A.; Ahmad, S. Synergy based Extracts of Medicinal Plants: Future Antimicrobials to Combat Multidrug Resistance. Curr. Pharm. Biotechnol. 2022, 23, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Belwal, T.; Tariq, M.; Atanasov, A.G.; Devkota, H.P. Genus Vanda: A review on traditional uses, bioactive chemical constituents and pharmacological activities. J. Ethnopharmacol. 2019, 229, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pathak, P. The budding potential of orchids in the cosmeceutical sector: Role of orchids in skincare and health. J. Orchid. Soc. India 2020, 34, 79–85. [Google Scholar]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant tissue culture: Current status and opportunities. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L., Eds.; IntechOpen Limited: London, UK, 2012; Volume 6, pp. 1–28. [Google Scholar] [CrossRef]
- Mampan, K.P.; Hill, J.; Saleem, M. Natural resources management and food security in the context of sustainable development. Sains Malays. 2011, 40, 1331–1340. [Google Scholar]
- Arroo, R.R.J.; Alfermann, A.W.; Medarde, M.; Petersen, M.; Pras, N.; Woolley, J.G. Plant cell factories as a source for anti-cancer lignans. Phytochem. Rev. 2002, 1, 27–35. [Google Scholar] [CrossRef]
- Hitmi, A.; Coudret, A.; Barthomeuf, C. The production of pyrethrins by plant cell and tissue cultures of Chrysanthemum cinerariaefolium and Tagetes species. CRC Crit. Rev. Plant Sci. 2000, 19, 69–89. [Google Scholar] [CrossRef]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Food Biotechnol. 2008, 111, 187–228. [Google Scholar]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.K.; Mehrotra, S.; Kukreja, A.K. Elicitor-induced cellular and molecular events are responsible for productivity enhancement in hairy root cultures: An insight study. Appl. Biochem. Biotechnol. 2011, 165, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Krishnamurthy, R. Elicitors in plant tissue culture. J. Pharmacogn. Phytochem. 2013, 2, 60–65. [Google Scholar] [CrossRef]
- Giri, L.; Dhyani, P.; Rawat, S.; Bhatt, I.D.; Nandi, S.K.; Rawal, R.S.; Pande, V. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Ind. Crops Prod. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Nag, S.; Kumaria, S. In vitro propagation of medicinally threatened orchid Vanda coerulea: An improved method for the production of phytochemicals, antioxidants and phenylalanine ammonia lyase activity. J. Pharmacogn. Phytochem. 2018, 7, 2973–2982. [Google Scholar]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. Ther. 2011, 5, 144–149. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Lee, B.N.; Hong, J.U.; Kim, S.M.; Jang, J.H.; Chang, H.S.; Hwang, Y.C. Anti-inflammatory and osteogenic effects of calcium silicate–based root canal sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Lönnroth, E.C.; Dahl, J.; Shahnavaz, H. Evaluating the potential occupational hazard of handling dental polymer products using the HET-CAM technique. Int. J. Occup. Saf. Ergon. 1999, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Cool, S.M.; Nurcombe, V. Substrate induction of osteogenesis from marrow-derived mesenchymal precursors. Stem. Cells Dev. 2005, 14, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, Z.; Chen, X.; Jin, P.; Wang, X.; Zheng, Y. 6-Benzylaminopurine delays senescence and enhances health-promoting compounds of harvested broccoli. J. Agric. Food Chem. 2012, 60, 234–240. [Google Scholar] [CrossRef]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Werner, T.; Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant. Biol. 2009, 12, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ. Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef]
- Ram, M.; Prasad, K.V.; Singh, S.K.; Hada, B.S.; Kumar, S. Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue Organ. Cult. 2013, 113, 459–467. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- Bhavanam, S.; Stout, M. Seed treatment with jasmonic acid and methyl jasmonate induces resistance to insects but reduces plant growth and yield in rice, Oryza sativa. Front. Plant Sci. 2021, 12, 691768. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Kim, K.H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and chitosan fragments responsible for plant elicitor and growth stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; MaheriSis, N.; Eshratkhah, B. Plants and secondary metabolites (Tannins): A Review. Int. J. Softw. Eng. 2011, 1, 47–53. [Google Scholar]
- Banerjee, J.; Chauhan, N.; Dey, B.K. Pharmacognostical, physiochemical and phytochemical evaluation of leaf, Stem and Root of Orchid Dendrobium ochreatum. J. Appl. Pharm. Res. 2018, 6, 16–25. [Google Scholar] [CrossRef]
- Arora, M.; Arora, K.; Kaur, R. Pharmacognostic, physicochemical, phytochemical, nutraceutical evaluation and in vitro antioxidant potency of Habenaria intermedia (D. Don) Szlach-A rare orchid. S. Afr. J. Bot. 2023, 152, 278–287. [Google Scholar] [CrossRef]
- Adams, E.; Miyazaki, T.; Moon, J.Y.; Sawada, Y.; Sato, M.; Toyooka, K.; Hirai, M.Y.; Shin, R. Syringic acid alleviates cesium-induced growth defect in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 9116. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.; Petersen, M. Rosmarinic acid and related metabolites. In Biotechnology of Natural Products; Springer: Cham, Switzerland, 2018; pp. 25–60. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yun, D.J.; Yamada, Y. Production of tropane alkaloids in genetically engineered root cultures. Phytochemistry 1993, 32, 713–718. [Google Scholar] [CrossRef]
- Sevón, N.; Hiltunen, R.; Oksman-Caldentey, K.M. Chitosan increases hyoscyamine content in hairy root cultures of Hyoscyamus muticus. Pharm. Pharmacol. Lett. 1992, 2, 96–99. [Google Scholar] [CrossRef]
- Conrath, U.; Domard, A.; Kauss, H. Chitosan-elicited synthesis of callose and coumarin derivatives in parsley cell suspension cultures. Plant Cell Rep. 1989, 8, 152–155. [Google Scholar] [CrossRef]
- Sauerwein, M.; Flores, H.M.; Yamazaki, T.; Shimomura, K. Lippia dulcis shoot cultures as a source of the sweet sesquiterpene hernandulcin. Plant Cell Rep. 1991, 9, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Merkli, A.; Christen, P.; Kapetanidis, I. Production of diosgenin by hairy root cultures of Trigonella foenum-graecum L. Plant Cell Rep. 1997, 16, 632–636. [Google Scholar] [CrossRef]
- Niazian, M.; Sabbatini, P. Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Planta 2021, 254, 111. [Google Scholar] [CrossRef] [PubMed]
- Abualhasan, M.N.; Mansour, J.; Jaradat, N.; Zaid, A.N.; Khadra, I. Formulation and development of a validated UV-spectrophotometric analytical method of rutin tablet. Int. Sch. Res. Not. 2017, 2017, 2624947. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tejada, M.M.; Durán, J.D.G.; Ontiveros-Ortega, A.; Espinosa-Jimenez, M.; Perea-Carpio, R.; Chibowski, E. Investigation of alumina/(+)-catechin system properties. Part I: A study of the system by FTIR-UV–Vis spectroscopy. Colloids Surf. B Biointerfaces 2002, 24, 297–308. [Google Scholar] [CrossRef]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT—Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Uthairatanakij, A.; Teixeira da Silva, J.A.; Obsuwan, K. Chitosan for improving orchid production and quality. Orchid. Sci. Biotechnol. 2007, 1, 1–5. [Google Scholar]
- Cho, H.S.; Lee, M.H.; Lee, J.W.; No, K.O.; Park, S.K.; Lee, H.S.; Hong, J.T. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultra-violet B irradiation. Photodermatol. Photoimmunol. Photomed. 2007, 23, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Choi, J.S.; Choi, Y.J.; Shin, S.Y.; Kang, S.W.; Han, S.J.; Kang, Y.H. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food. Chem. Toxicol. 2008, 46, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The Role of Elastases Secreted by Fibroblasts in Wrinkle For-mation: Implication Trough Selective Inhibition of Elastase Activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; Saber, W.I. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Fellah, B.H.; Delorme, B.; Sohier, J.; Magne, D.; Hardouin, P.; Layrolle, P. Macrophage and osteoblast responses to biphasic calcium phosphate microparticles. J. Biomed. Mater. Res. A 2010, 93, 1588–1595. [Google Scholar] [CrossRef]
- Lederle, W.; Depner, S.; Schnur, S.; Obermueller, E.; Catone, N.; Just, A.; Mueller, M.M. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int. J. Cancer 2011, 128, 2803–2814. [Google Scholar] [CrossRef]
- Bashir, M.; Sharma, M.; Werth, V. TNF-α production in the skin. Arch. Dermatol. Res. 2009, 301, 87–91. [Google Scholar] [CrossRef]
- Jirik, F.R.; Podor, T.J.; Hirano, T.; Kishimoto, T.; Loskutoff, D.J.; Carson, D.A.; Lotz, M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J. Immunol. 1989, 142, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczak, D.; Górska, D.; Czarnecka, E. Influence of amlodipine and atenolol on lipopolysaccharide (LPS)-induced serum concentrations of TNF-alpha, IL-1, IL-6 in spontaneously hypertensive rats (SHR). Pharmacol. Rep. 2006, 58, 711–719. [Google Scholar] [PubMed]
- Kundeková, B.; Máčajová, M.; Meta, M.; Čavarga, I.; Bilčík, B. Chorioallantoic membrane models of various avian species: Differences and applications. Biology 2021, 10, 301. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| IL-6 | Forward | GAGACTTCCATCCAGTTGCC |
| Reverse | TACTCCAGAAGACCAGAGG | |
| TNF-α | Forward | GGGACAGTGACCTGGACTGT |
| Reverse | GCAGAGGTTCAGTGATGTAG | |
| β-Actin | Forward | TGGATGGCTACGTACATGGCTGGG |
| Reverse | TTCTTTGCAGCTCCTTCGTTGCCG |
| Standard Compounds | Retention Time (min) | Chemical Compositions (mg/g Extract) | ||||
|---|---|---|---|---|---|---|
| PLANT | PTC | BA | MeJA | CHI | ||
| Catechin | 10.920 | 17.5 ± 0.0 a | 1.5 ± 0.0 c | 5.7 ± 0.0 b | 1.3 ± 0.0 c | 5.8 ± 0.0 b |
| Syringic acid | 13.671 | 0.1 ± 0.0 d | 1.4 ± 0.0 b | 0.9 ± 0.0 c | 1.7 ± 0.0 a | 1.3 ± 0.0 b |
| Rutin | 16.880 | 33.4 ± 0.0 a | 5.0 ± 0.0 c | 12.8 ± 0.0 b | 4.6 ± 0.0 d | 12.1 ± 0.0 b |
| Ellagic acid | 17.605 | 3.9 ± 0.0 a | 0.4 ± 0.0 c | 1.0 ± 0.0 b | 0.2 ± 0.0 d | 0.9 ± 0.0 b |
| Rosmarinic acid | 21.346 | 0.1 ± 0.0 a | 0.0 ± 0.0 b | 0.1 ± 0.0 a | 0.0 ± 0.0 b | 0.1 ± 0.0 a |
| Quercetin | 26.769 | 0.2 ± 0.0 b | 0.0 ± 0.0 c | 0.2 ± 0.0 b | 0.0 ± 0.0 c | 0.3 ± 0.0 a |
| Samples | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| DPPH Inhibition | Ferric Reducing Capacity | Collagenase Inhibition | Elastase Inhibition | Tyrosinase Inhibition | |
| AS | 3.5 ± 2.9 a | 2.4 ± 0.7 a | N/A | N/A | N/A |
| EGCG | N/A | N/A | 16.9 ± 3.2 b | 0.1 ± 0.0 a | N/A |
| KA | N/A | N/A | N/A | N/A | 2.8 ± 0.6 a |
| RT | 3.2 ± 2.7 a | 12.9 ± 2.0 b | 7.6 ± 3.4 a | 10.5 ± 1.9 b | 7.7 ± 3.1 b |
| PLANT | ND | 25.8 ± 4.7 c | 12.3 ± 2.3 b | 11.3 ± 7.9 b | 10.8 ± 3.5 b |
| PTC | ND | 27.3 ± 5.0 c | 27.3 ± 8.6 c | ND | ND |
| BA | ND | 60.3 ± 5.0 d | 24.5 ± 3.2 c | ND | ND |
| MeJA | ND | 73.8 ± 8.8 e | ND | ND | ND |
| CHI | 9.3 ± 2.7 b | 2.1 ± 0.1 a | 7.8 ± 3.5 a | 8.4 ± 3.3 b | 8.1 ± 2.8 b |
| Treatment | Irritation Score (IS) | Irritation Level |
|---|---|---|
| Negative control (0.9% w/v NaCl) | 0.0 ± 0.0 | No irritation |
| Positive control (1% w/v SLS) | 15.13 ± 0.3 | Severe irritation |
| Vehicle control (80% v/v MeOH) | 9.1 ± 0.6 | Severe irritation |
| CHI 0.001% w/v | 0.0 ± 0.0 | No irritation |
| CHI 0.01% w/v | 0.0 ± 0.0 | No irritation |
| CHI 0.1% w/v | 0.46 ± 0.01 | No irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amnuaykan, P.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan. Plants 2024, 13, 1770. https://doi.org/10.3390/plants13131770
Amnuaykan P, Juntrapirom S, Kanjanakawinkul W, Chaiyana W. Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan. Plants. 2024; 13(13):1770. https://doi.org/10.3390/plants13131770
Chicago/Turabian StyleAmnuaykan, Piyatida, Saranya Juntrapirom, Watchara Kanjanakawinkul, and Wantida Chaiyana. 2024. "Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan" Plants 13, no. 13: 1770. https://doi.org/10.3390/plants13131770
APA StyleAmnuaykan, P., Juntrapirom, S., Kanjanakawinkul, W., & Chaiyana, W. (2024). Enhanced Antioxidant, Anti-Aging, Anti-Tyrosinase, and Anti-Inflammatory Properties of Vanda coerulea Griff. Ex Lindl. Protocorm through Elicitations with Chitosan. Plants, 13(13), 1770. https://doi.org/10.3390/plants13131770







