Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria
Abstract
1. Introduction
2. Results
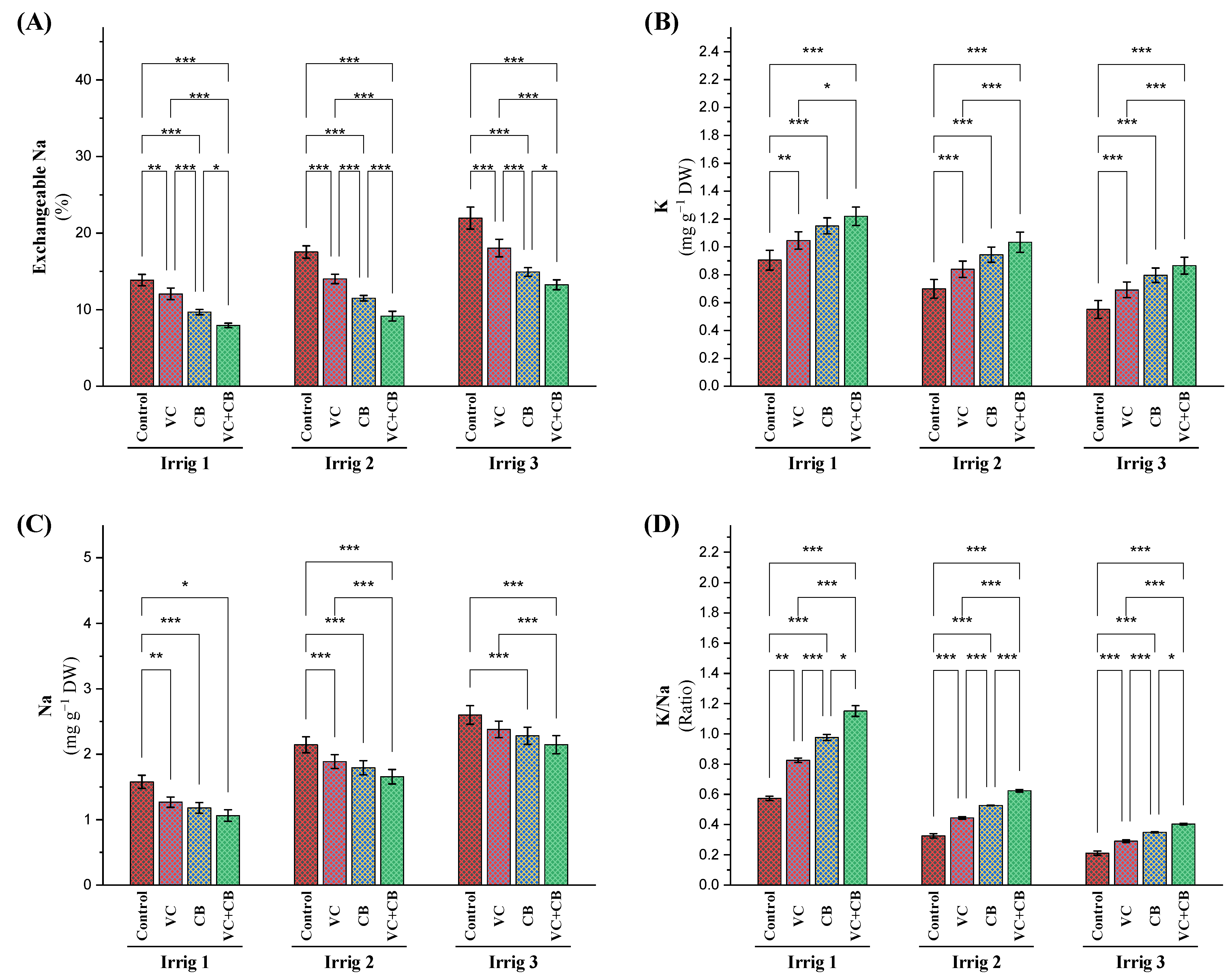
2.1. Exchangeable Sodium Percentage (ESP) in the Soil and Ratio of K/Na in Cotton Leaves
2.2. Physiological Traits
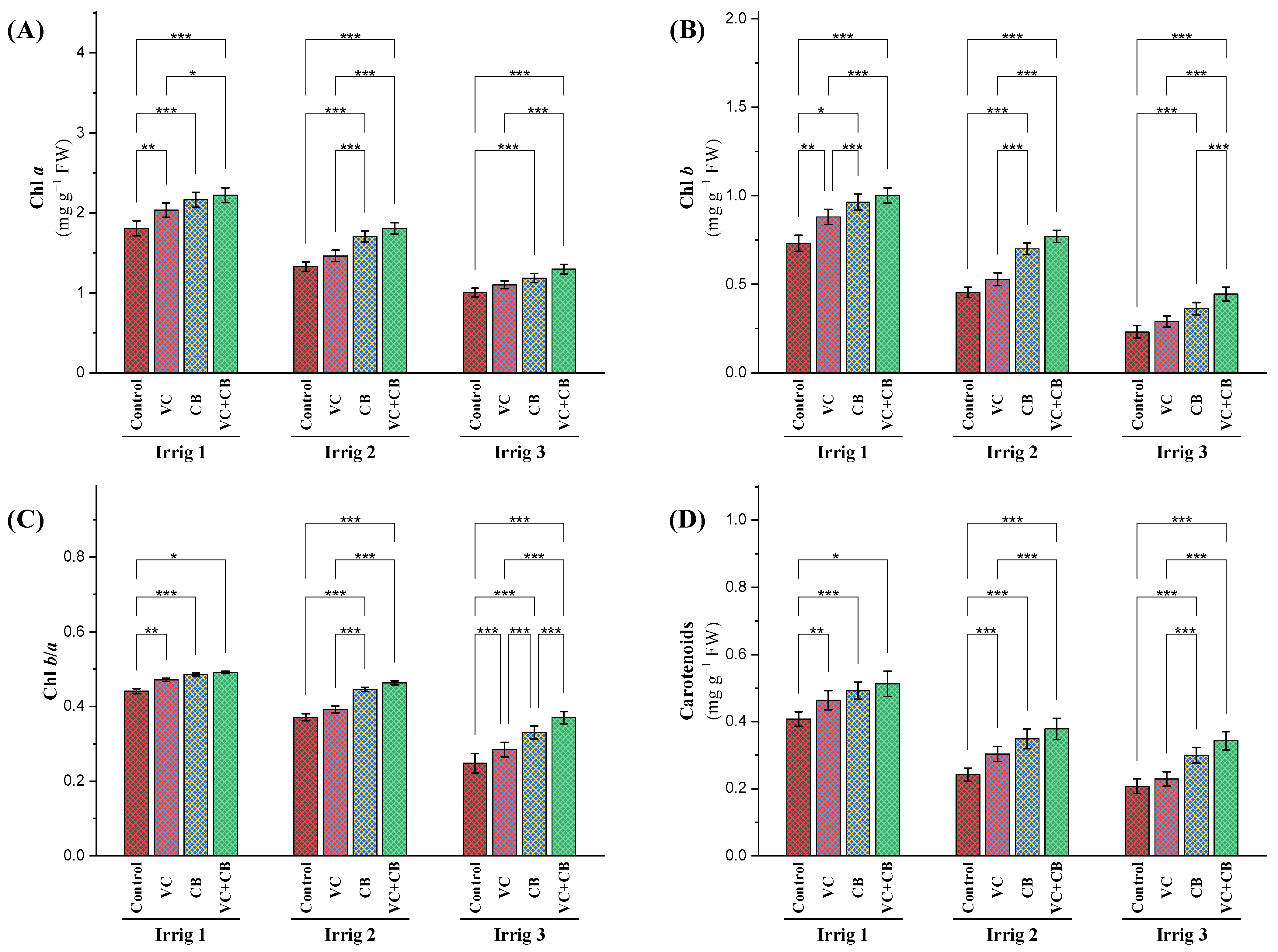
2.2.1. Photosynthetic Pigments
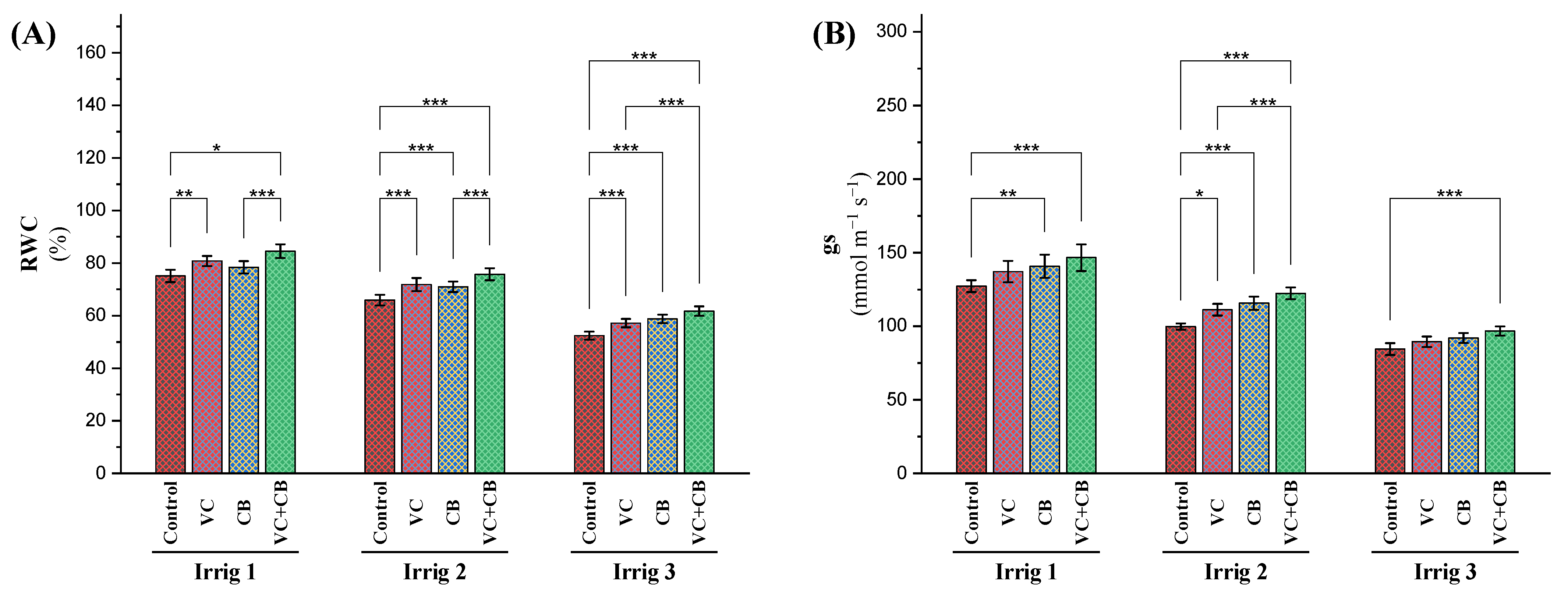
2.2.2. Water Relations
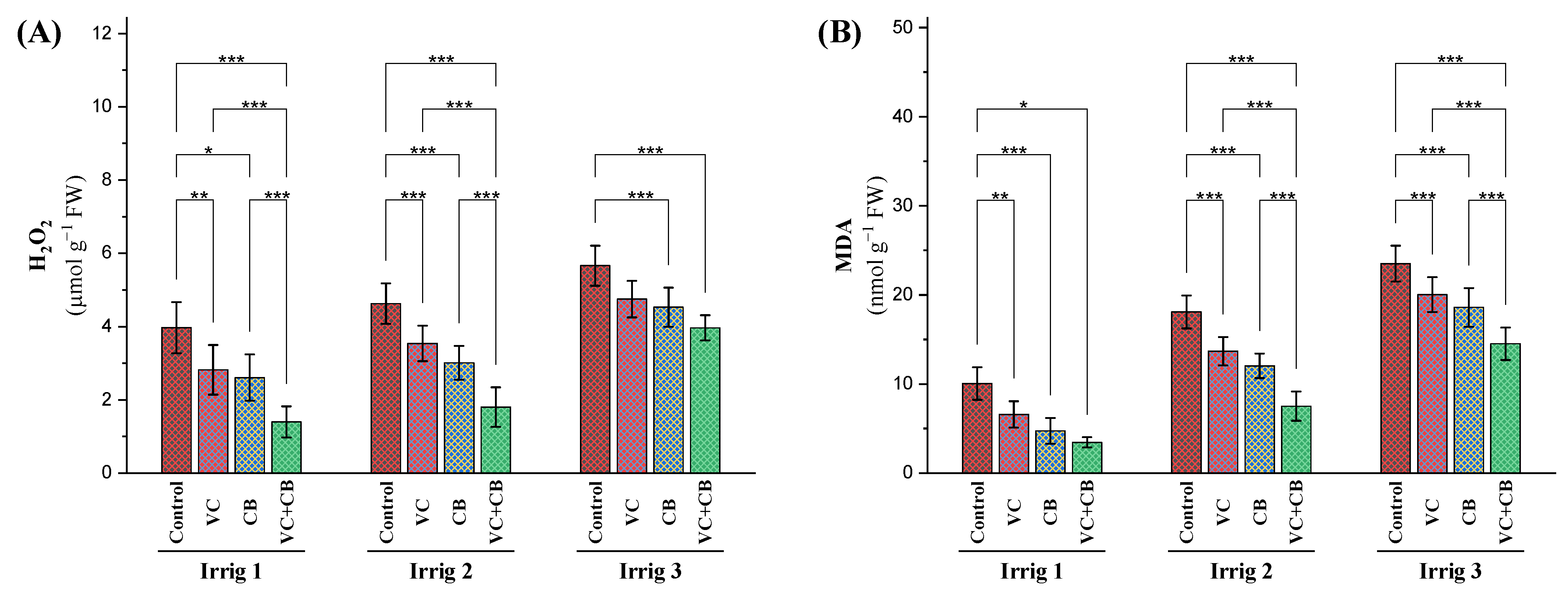
2.2.3. Oxidative Stress Indicators
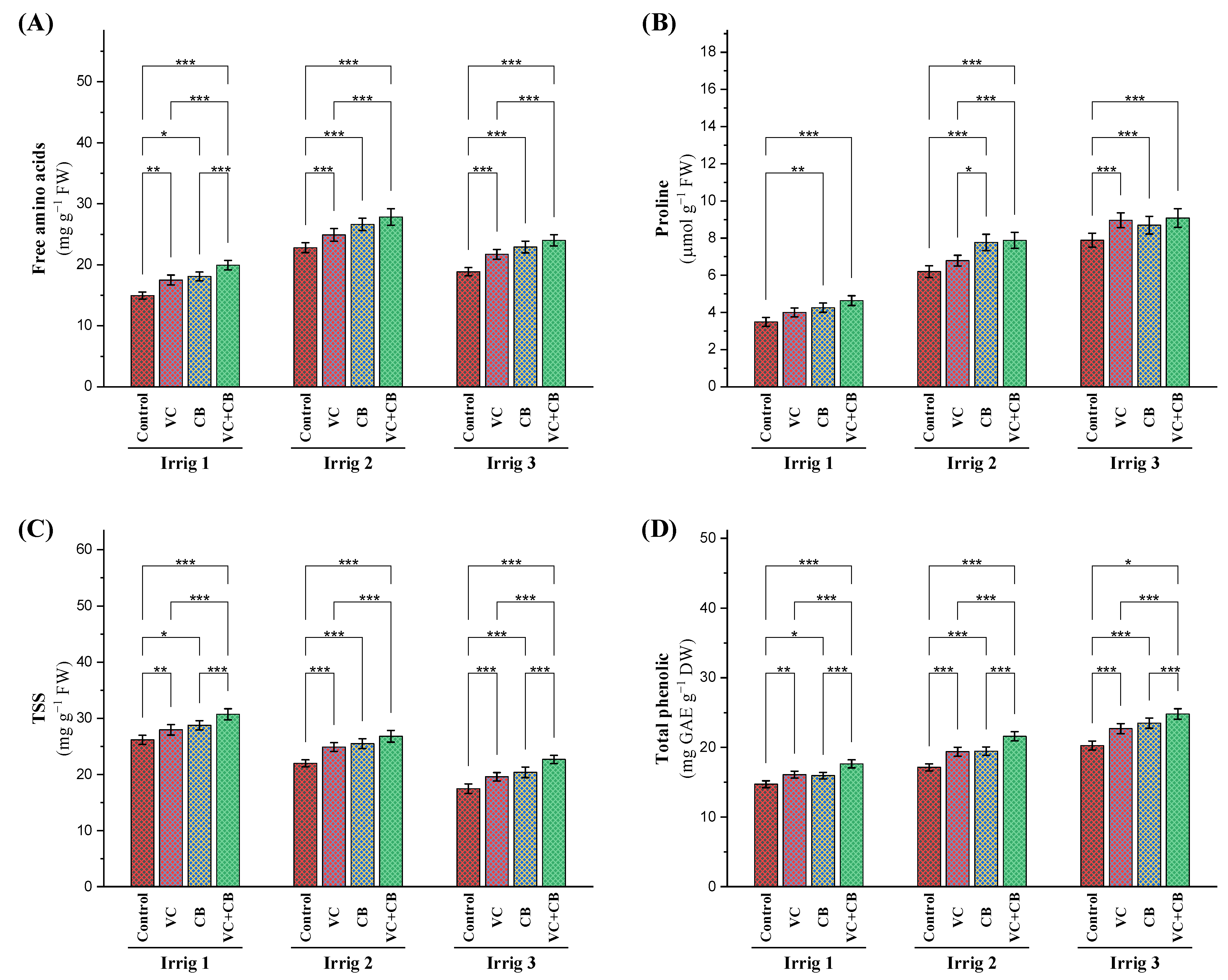
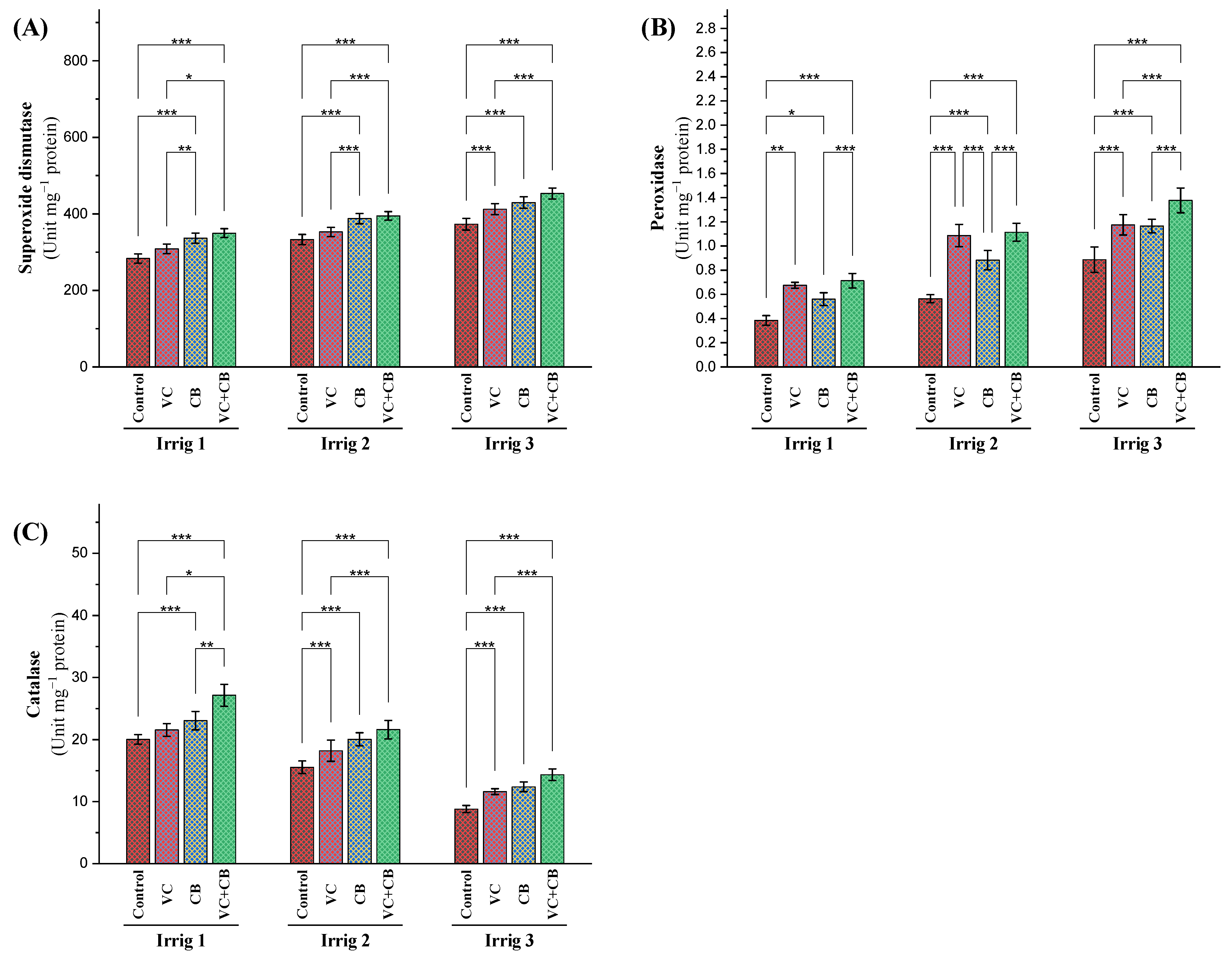
2.2.4. Antioxidant Defense System
Non-Enzymatic Antioxidants
Enzymatic Antioxidants
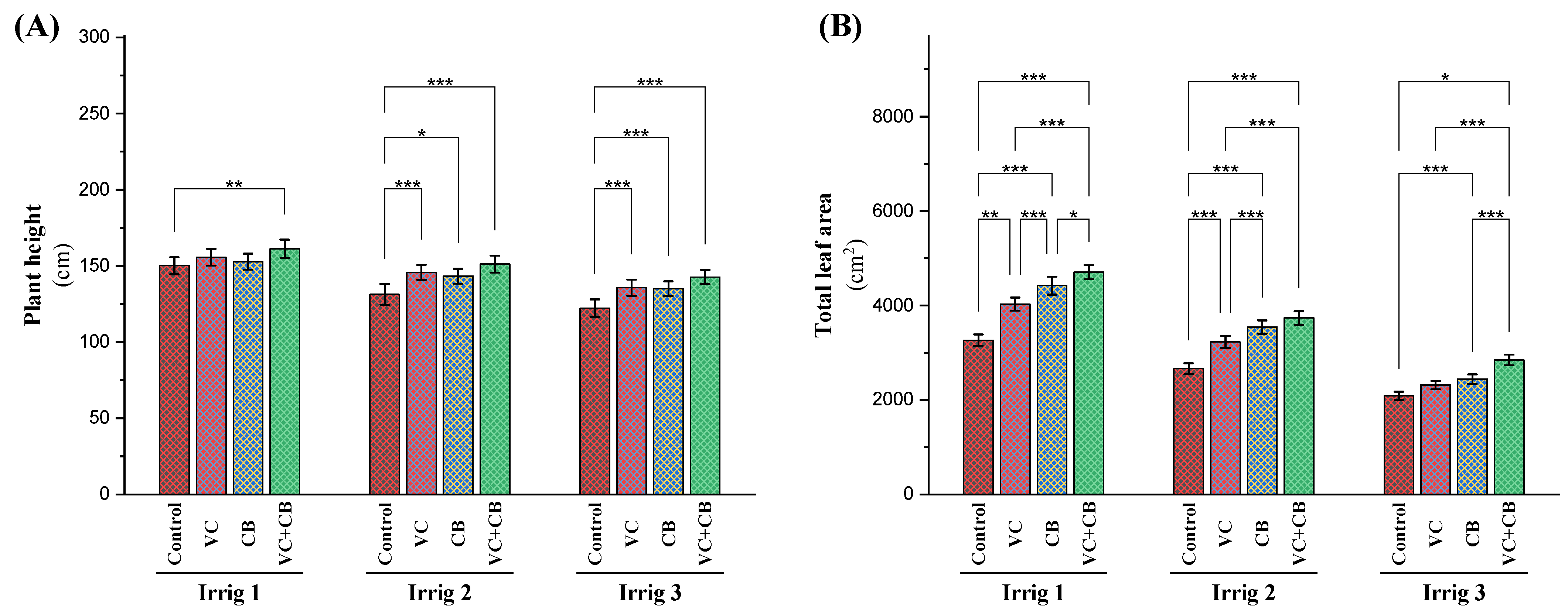
2.3. Vegetative Characteristics
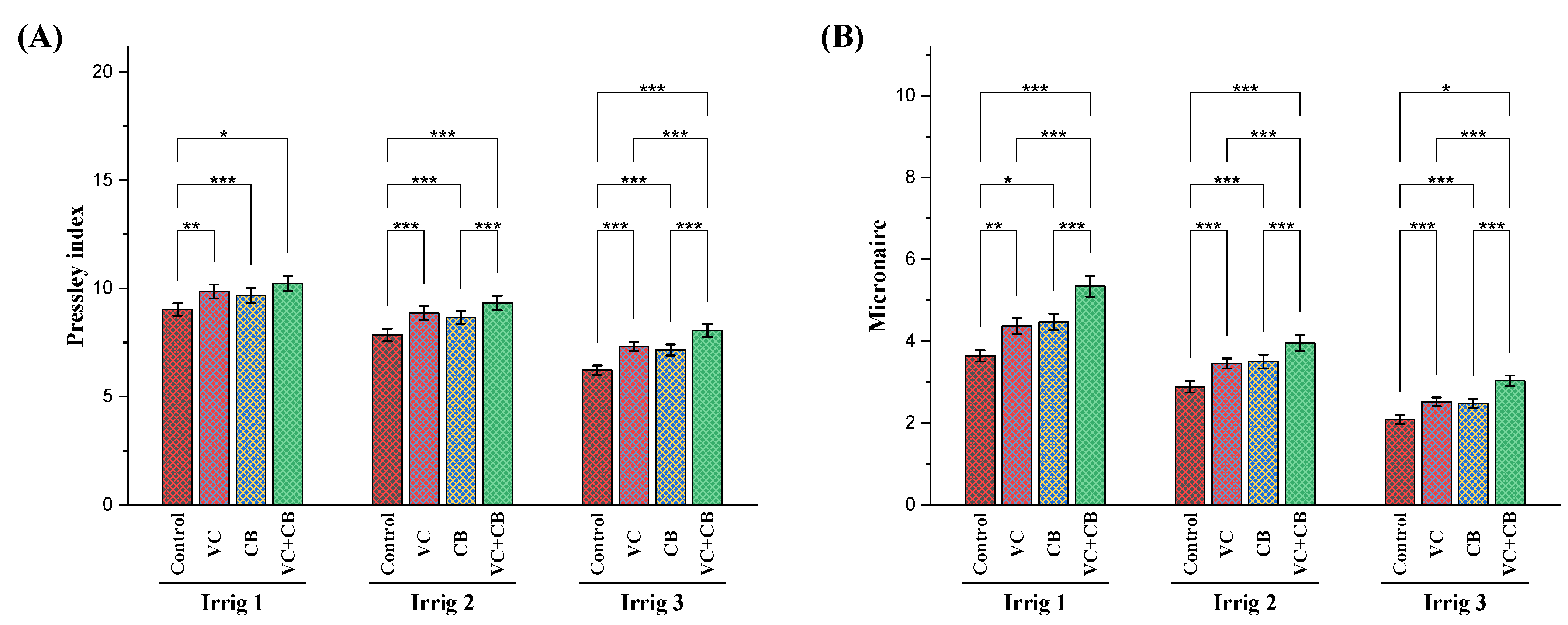
2.4. Yield Characteristics
3. Discussion
3.1. Effect of Soil and Foliar Treatments on Soil Exchangeable Sodium Percentage (ESP) under Osmotic Stress
3.2. Effect of Soil and Foliar Treatments on Physiological Attributes under Osmotic Stress
3.3. Effect of Soil and Foliar Treatments on Antioxidant Enzyme Activity and Oxidative Indicators under Osmotic Stress
3.4. Effect of Soil and Foliar Treatments on Osmolytes under Osmotic Stress
3.5. Effect of Soil and Foliar Treatments on Yield-Related Traits and Productivity under Osmotic Stress
4. Materials and Methods
4.1. Experimental Layout and Treatments
4.1.1. Vermicompost Characterization (VC)
4.1.2. Cyanobacteria Extract Characterization
4.2. Exchangeable Sodium Percentage (ESP) in Soil
4.3. Determination of Na+ and K+ in Cotton Leaves
4.4. Physiological Traits
4.4.1. Chlorophylls and Carotenoids
4.4.2. Water Relations
Relative Water Content (RWC)
Stomatal Conductance (gs; mmol H2O m−2 s−1)
4.4.3. Stress Indicators (Lipid Peroxidation and H2O2)
4.4.4. Antioxidant System
Non-Enzymatic Antioxidants
Enzymatic Antioxidants
4.5. Vegetative Growth Attributes
4.5.1. Plant Height
4.5.2. Total Leaf Area
4.6. Cotton Yield Attributes
4.6.1. Growth and Yield Parameters
4.6.2. Fiber Properties
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, G.; Grover, C.E.; Jareczek, J.; Yuan, D.; Dong, Y.; Miller, E.; Conover, J.L.; Wendel, J.F. Evolution and Diversity of the Cotton Genome. In Cotton Precision Breeding; Rahman, M.-U., Zafar, Y., Zhang, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 25–78. [Google Scholar]
- Shahzad, A.N.; Qureshi, M.K.; Ullah, S.; Latif, M.; Ahmad, S.; Bukhari, S.A.H. Exogenous trehalose improves cotton growth by modulating antioxidant defense under salinity-induced osmotic stress. Pak. J. Agric. Res. 2020, 33, 270–279. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Xu, L.; Kong, J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014, 73, 67–78. [Google Scholar] [CrossRef]
- Ali, S.O.; Ahmadikhah, A. The effects of drought stress on improved cotton varieties in Golesatn Province of Iran. Int. J. Plant Prod. 2009, 3, 1735–6814. [Google Scholar]
- Amin, A.; Nasim, W.; Mubeen, M.; Sarwar, S.; Urich, P.; Ahmad, A.; Wajid, A.; Khaliq, T.; Rasul, F.; Hammad, H.M.; et al. Regional climate assessment of precipitation and temperature in Southern Punjab (Pakistan) using SimCLIM climate model for different temporal scales. Theor. Appl. Climatol. 2018, 131, 121–131. [Google Scholar] [CrossRef]
- Saud, S.; Wang, L. Mechanism of cotton resistance to abiotic stress, and recent research advances in the osmoregulation related genes. Front. Plant Sci. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.; Läuchli, A.; Leidi, E.O. Plant Responses to Salinity. In Physiology of Cotton; Stewart, J.M., Oosterhuis, D.M., Heitholt, J.J., Mauney, J.R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 129–141. [Google Scholar]
- Hoque, M.N.; Imran, S.; Hannan, A.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Sarker, P.; Irin, I.J.; Brestic, M.; Rhaman, M.S. Organic amendments for mitigation of salinity stress in plants: A review. Life 2022, 12, 1632. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 1–36. [Google Scholar] [CrossRef]
- Ren, F.; Yang, G.; Li, W.; He, X.; Gao, Y.; Tian, L.; Li, F.; Wang, Z.; Liu, S. Yield-compatible salinity level for growing cotton (Gossypium hirsutum L.) under mulched drip irrigation using saline water. Agric. Water Manag. 2021, 250, 106859. [Google Scholar] [CrossRef]
- Alharbi, K.; Osman, H.S.; Rashwan, E.; Hafez, E.M.; Omara, A.E.-D. Stimulating the growth, anabolism, antioxidants, and yield of rice plants grown under salt stress by combined application of bacterial inoculants and nano-silicon. Plants 2022, 11, 3431. [Google Scholar] [CrossRef]
- Chen, W.; Jin, M.; Ferré, T.P.A.; Liu, Y.; Xian, Y.; Shan, T.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crop. Res. 2018, 215, 207–221. [Google Scholar] [CrossRef]
- Osman, H.S.; Rady, A.M.S.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M. Improving the antioxidants system, growth, and sugar beet quality subjected to long-term osmotic stress by phosphate solubilizing bacteria and compost tea. Int. J. Plant Prod. 2022, 16, 119–135. [Google Scholar] [CrossRef]
- Hejnák, V.; Tatar, Ö.; Atasoy, G.D.; Martinková, J.; Çelen, A.E.; Hnilička, F.; Skalický, M. Growth and photosynthesis of Upland and Pima cotton: Response to drought and heat stress. Plant Soil Environ. 2015, 61, 507–514. [Google Scholar] [CrossRef]
- Nazim, M.; Ali, M.; Shahzad, K.; Ahmad, F.; Nawaz, F.; Amin, M.; Anjum, S.; Nasif, O.; Ali Alharbi, S.; Fahad, S.; et al. Kaolin and Jasmonic acid improved cotton productivity under water stress conditions. Saudi J. Biol. Sci. 2021, 28, 6606–6614. [Google Scholar] [CrossRef] [PubMed]
- Ul-Allah, S.; Rehman, A.; Hussain, M.; Farooq, M. Fiber yield and quality in cotton under drought: Effects and management. Agric. Water Manag. 2021, 255, 106994. [Google Scholar] [CrossRef]
- Wang, R.; Ji, S.; Zhang, P.; Meng, Y.; Wang, Y.; Chen, B.; Zhou, Z. Drought effects on cotton yield and fiber quality on different fruiting branches. Crop Sci. 2016, 56, 1265–1276. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.E.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant 2020, 172, 587–602. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, E.K.; Ibrahim, D.A. Effects of vermicompost and water treatment residuals on soil physical properties and wheat yield. Int. Agrophys. 2015, 29, 157–164. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-des-Tureaux, T.; Rumpel, C.; Janeau, J.-L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Doan, T.T.; Ngo, P.T.; Rumpel, C.; Nguyen, B.V.; Jouquet, P. Interactions between compost, vermicompost and earthworms influence plant growth and yield: A one-year greenhouse experiment. Sci. Hortic. 2013, 160, 148–154. [Google Scholar] [CrossRef]
- Alamer, K.H.; Perveen, S.; Khaliq, A.; Zia Ul Haq, M.; Ibrahim, M.U.; Ijaz, B. Mitigation of salinity stress in maize seedlings by the application of vermicompost and sorghum water extracts. Plants 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Teli, B.; Mukherjee, A.; Bajpai, R.; Sarma, B.K. Secondary Metabolites from Cyanobacteria: A Potential Source for Plant Growth Promotion and Disease Management. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 239–252. [Google Scholar]
- Jhala, Y.K.; Panpatte, D.G.; Vyas, R.V. Cyanobacteria: Source of Organic Fertilizers for Plant Growth. In Microorganisms for Green Revolution: Volume 1: Microbes for Sustainable Crop Production; Panpatte, D.G., Jhala, Y.K., Vyas, R.V., Shelat, H.N., Eds.; Springer: Singapore, 2017; pp. 253–264. [Google Scholar]
- Mutale-joan, C.; Rachidi, F.; Mohamed, H.A.; Mernissi, N.E.; Aasfar, A.; Barakate, M.; Mohammed, D.; Sbabou, L.; Arroussi, H.E. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021, 33, 3779–3795. [Google Scholar] [CrossRef]
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Bayona-Morcillo, P.J.; Plaza, B.M.; Gómez-Serrano, C.; Rojas, E.; Jiménez-Becker, S. Effect of the foliar application of cyanobacterial hydrolysate (Arthrospira platensis) on the growth of Petunia x hybrida under salinity conditions. J. Appl. Phycol. 2020, 32, 4003–4011. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Shehawy, M.A.; Din, S.M.M.E.; Albalwe, F.M.; Albalawi, H.M.R.; Hussein, M.H. Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L. Green Process. Synth. 2022, 11, 648–658. [Google Scholar] [CrossRef]
- Omara, A.E.-D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.A.; Alharbi, K.; Abd El-Moneim, D.; Gowayed, S.M. Collaborative impact of compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef]
- Hafez, E.M.; Gowayed, S.M.; Nehela, Y.; Sakran, R.M.; Rady, A.M.S.; Awadalla, A.; Omara, A.E.-D.; Alowaiesh, B.F. Incorporated biochar-based soil amendment and exogenous glycine betaine foliar application ameliorate rice (Oryza sativa L.) tolerance and resilience to osmotic stress. Plants 2021, 10, 1930. [Google Scholar] [CrossRef]
- Goswami, L.; Nath, A.; Sutradhar, S.; Bhattacharya, S.S.; Kalamdhad, A.; Vellingiri, K.; Kim, K.-H. Application of drum compost and vermicompost to improve soil health, growth, and yield parameters for tomato and cabbage plants. J. Environ. Manag. 2017, 200, 243–252. [Google Scholar] [CrossRef]
- Munawar, W.; Hameed, A.; Khan, M.K.R. Differential morphophysiological and biochemical responses of cotton genotypes under various salinity stress levels during early growth stage. Front. Plant Sci. 2021, 12, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Cevheri, C.İ.; Sakin, E.; Ramazanoglu, E. Effects of different fertilizers on some soil enzymes activity and chlorophyll contents of two cotton (G. hirsutum L.) varieties grown in a saline and non-saline soil. J. Plant Nutr. 2022, 45, 95–106. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.R.; Rezende, M.O.O.; Tambone, F.; Adani, F. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Chen, X.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fertil. Soils 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.R.; Ahmadpour, R. Evaluation of vermicompost fertilizer application on growth, nutrient uptake and photosynthetic pigments of lentil (Lens culinaris Medik.) under moisture deficiency conditions. J. Plant Nutr. 2018, 41, 1276–1284. [Google Scholar] [CrossRef]
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Vermicompost leachate reduces temperature and water stress effects in tomato seedlings. HortScience 2014, 49, 1183–1187. [Google Scholar] [CrossRef]
- Tammam, A.A.; Shehata, M.R.A.M.; Pessarakli, M.; El-Aggan, W.H. Vermicompost and its role in alleviation of salt tress in plants—II. Impact of vermicompost on the physiological responses of salt-stressed plants. J. Plant Nutr. 2023, 46, 1458–1478. [Google Scholar] [CrossRef]
- Kumar, A.; Ramamoorthy, D.; Verma, D.K.; Kumar, A.; Kumar, N.; Kanak, K.R.; Marwein, B.M.; Mohan, K. Antioxidant and phytonutrient activities of Spirulina platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive impacts of beneficial microbes and Si-Zn nanocomposite on growth and productivity of soybean subjected to water deficit under salt-affected soil conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; El-Razek, U.A.A.; Elbagory, M.; Omara, A.E.-D.; Eid, M.A.; Gowayed, S.M. Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 2021, 10, 894. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Janda-Milczarek, K.; Szymczykowska, K.; Jakubczyk, K.; Kupnicka, P.; Skonieczna-Żydecka, K.; Pilarczyk, B.; Tomza-Marciniak, A.; Ligenza, A.; Stachowska, E.; Dalewski, B. Spirulina supplements as a source of mineral nutrients in the daily diet. Appl. Sci. 2023, 13, 1011. [Google Scholar] [CrossRef]
- Arahou, F.; Lijassi, I.; Wahby, A.; Rhazi, L.; Arahou, M.; Wahby, I. Spirulina-based biostimulants for sustainable agriculture: Yield improvement and market trends. BioEnergy Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Salim, B.B.M.; Hikal, M.S.; Osman, H.S. Ameliorating the deleterious effects of saline water on the antioxidants defense system and yield of eggplant using foliar application of zinc sulphate. Ann. Agric. Sci. 2019, 64, 244–251. [Google Scholar] [CrossRef]
- Salim, B.B.M.; Abou El-Yazied, A.; Salama, Y.A.M.; Raza, A.; Osman, H.S. Impact of silicon foliar application in enhancing antioxidants, growth, flowering and yield of squash plants under deficit irrigation condition. Ann. Agric. Sci. 2021, 66, 176–183. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Abdelrasheed, K.G.; Mazrou, Y.; Omara, A.E.-D.; Osman, H.S.; Nehela, Y.; Hafez, E.M.; Rady, A.M.S.; El-Moneim, D.A.; Alowaiesh, B.F.; Gowayed, S.M. Soil amendment using biochar and application of K-humate enhance the growth, productivity, and nutritional value of onion (Allium cepa L.) under deficit irrigation conditions. Plants 2021, 10, 2598. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.S.; Salim, B.B.M. Influence of exogenous application of some phytoprotectants on growth, yield and pod quality of snap bean under NaCl salinity. Ann. Agric. Sci. 2016, 61, 1–13. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Elashmouny, A.A.; Elsadany, A.Y. Differential response of cotton growth, yield and fiber quality to foliar application of Spirulina platensis and urea fertilizer. Asian J. Adv. Agric. Res. 2020, 12, 29–40. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer and biocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. Sci. Bio/Technol. 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Arshad, M.A.C.; Lowery, B.; Grossman, B. Physical Tests for Monitoring Soil Quality. SSSA Spec. Publ. 2015, 49, 123–141. [Google Scholar] [CrossRef]
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures; Springer: Dordrecht, The Netherlands, 2004; p. 179. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Rosen, H. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B.M. Enhancing antioxidants defense system of snap bean under NaCl salinity using foliar application of salicylic acid, spermidine and glycine betaine. Am.-Eurasian J. Agric. Environ. Sci. 2016, 16, 1200–1210. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analyt. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Vetter, J.L.; Steinberg, M.P.; Nelson, A.I. Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 1958, 6, 39–41. [Google Scholar] [CrossRef]
- Wallace, D.H.; Munger, H.M. Studies of the physiological basis for yield differences. I. Growth analysis of six dry bean varieties1. Crop Sci. 1965, 5, 343–348. [Google Scholar] [CrossRef]
- Standard on Textile Materials (D1448-59) and (D1445-67); A.S.T.M. American Society for Testing and Materials. The Society: Washington, DC, USA; Philladelphia, PA, USA, 1975.
| Irrigation | Treatments | No. of Fruiting Branches | Open Bolls No. Plant−1 | Boll Weight (g) | Lint % | Seeds Yield (kg ha−1) |
|---|---|---|---|---|---|---|
| 14 days | Control | 10.4 ± 0.5 e | 19.5 ± 0.6 e | 2.74 ± 0.10 e | 40.6 ± 1.3 bc | 3057 ± 126.7 e |
| Vermicompost | 12.5 ± 0.6 c | 22.4 ± 0.7 b | 2.94 ± 0.09 b | 41.7 ± 1.2 ab | 3459 ± 125.8 b | |
| Cyanobacteria | 13.0 ± 0.5 b | 21.3 ± 0.7 c | 2.86 ± 0.08 c | 41.2 ± 1.3 b | 3350 ± 112.7 c | |
| Combined | 14.3 ± 0.6 a | 23.8 ± 0.7 a | 3.03 ± 0.10 a | 42.9 ± 1.5 a | 3627 ± 138.5 a | |
| 21 days | Control | 8.5 ± 0.4 i | 13.8 ± 0.5 i | 2.55 ± 0.11 h | 36.5 ± 1.1 f | 2651 ± 97.3 i |
| Vermicompost | 9.9 ± 0.3 g | 17.9 ± 0.6 f | 2.67 ± 0.09 f | 39.7 ± 1.3 cd | 2922 ± 114.6 f | |
| Cyanobacteria | 10.2 ± 0.3 f | 16.6 ± 0.6 g | 2.65 ± 0.08 f | 39.1 ± 1.2 d | 2853 ± 104.5 g | |
| Combined | 10.8 ± 0.3 d | 20.0 ± 0.7 d | 2.77 ± 0.10 d | 40.7 ± 1.4 bc | 3118 ± 112.4 d | |
| 28 days | Control | 6.0 ± 0.3 l | 9.2 ± 0.3 l | 2.32 ± 0.09 j | 34.1 ± 1.8 g | 2319 ± 108.2 k |
| Vermicompost | 8.0 ± 0.4 k | 12.2 ± 0.5 j | 2.58 ± 0.10 gh | 37.7 ± 1.2 ef | 2577 ± 98.1 j | |
| Cyanobacteria | 8.2 ± 0.3 j | 11.6 ± 0.5 k | 2.51 ± 0.09 i | 37.0 ± 0.7 f | 2584 ± 100.6 j | |
| Combined | 8.8 ± 0.3 h | 15.0 ± 0.5 h | 2.6 ± 0.12 g | 38.6 ± 1.3 de | 2746 ± 103.7 h | |
| F-test | Irrigation | *** | *** | *** | *** | *** |
| Treatments | *** | *** | *** | *** | *** | |
| Irrigation X Treatments | *** | *** | ** | ** | *** |
| Year Month | 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Wind Speed (km Day−1) | RH ‡ (%) | Temperature (°C) | Wind Speed (km Day−1) | RH (%) | |||
| max ¥ | min † | max | min | |||||
| April | 27.21 | 11.98 | 119.25 | 57.45 | 26.12 | 12.54 | 110.32 | 55.41 |
| May | 29.7 | 13.6 | 105.0 | 59.8 | 27.9 | 15.4 | 91.0 | 62.7 |
| June | 34.6 | 19.0 | 105.1 | 65.5 | 34.5 | 15.4 | 101.0 | 63.4 |
| July | 34.9 | 21.9 | 97.1 | 64.5 | 33.0 | 21.0 | 101.1 | 64.1 |
| Aug. | 34.5 | 19.8 | 79.5 | 63.4 | 35.0 | 22.2 | 91.5 | 66.9 |
| Sept. | 33.5 | 19.5 | 83.3 | 68.5 | 34.4 | 19.8 | 82.2 | 67.4 |
| Average | 33.4 | 18.1 | 101.9 | 67.3 | 32.9 | 17.4 | 93.3 | 66.1 |
| Characteristics | 2020 | 2021 | |
|---|---|---|---|
| pH (1:2.5 soil/water suspension) | |||
| Soil depth (cm) | 0–20 | 8.22 ± 0.02 † | 8.28 ± 0.03 |
| 20–40 | 8.19 ± 0.02 | 8.21 ± 0.03 | |
| 40–60 | 8.16 ± 0.04 | 8.18 ± 0.02 | |
| Electrical conductivity (ECe, dS m−1) ¥ | |||
| Soil depth (cm) | 0–20 | 5.61 ± 0.01 | 5.66 ± 0.02 |
| 20–40 | 5.56 ± 0.02 | 5.59 ± 0.05 | |
| 40–60 | 5.36 ± 0.03 | 5.54 ± 0.04 | |
| ESP # (%) | |||
| Soil depth (cm) | 0–20 | 22.61 ± 0.42 | 21.50 ± 0.32 |
| 20–40 | 22.52 ± 0.02 | 21.36 ± 0.22 | |
| 40–60 | 22.46 ± 0.04 | 21.21 ± 0.35 | |
| Soil organic matter (g kg−1) | 11.2 ± 0.03 | 11.7 ± 0.05 | |
| Particle size distribution (%) | |||
| Sand | 27.22 ± 1.88 | 27.17 ± 1.98 | |
| Silt | 25.23 ± 2.02 | 25.55 ± 1.99 | |
| Clay | 47.55 ± 2.32 | 47.28 ± 2.03 | |
| Texture grade | clayey | clayey | |
| Soluble cations (meq L−1) ¥ | |||
| Ca++ | 7.54 ± 0.94 | 9.29 ± 0.87 | |
| Mg++ | 5.76 ± 1.11 | 6.23 ± 1.32 | |
| Na+ | 26.75 ± 2.06 | 22.63 ± 3.08 | |
| K+ | 0.33 ± 0.02 | 0.39 ± 0.02 | |
| Soluble anions (meq L−1) ¥ | |||
| CO3− − | nd ‡ | nd | |
| HCO3− | 4.61 ± 0.56 | 3.34 ± 0.68 | |
| Cl− | 24.56 ± 1.11 | 18.21 ± 1.15 | |
| SO4− − | 15.13 ± 3.03 | 11.15 ± 3.04 | |
| Available macronutrients (mg kg−1) | |||
| N | 9.70 ± 0.91 | 10.33 ± 1.71 | |
| P | 8.24 ± 1.33 | 8.94 ± 1.54 | |
| K | 344 ± 26.42 | 387 ± 24.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, K.; Hafez, E.M.; Omara, A.E.-D.; Osman, H.S. Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria. Plants 2023, 12, 1872. https://doi.org/10.3390/plants12091872
Alharbi K, Hafez EM, Omara AE-D, Osman HS. Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria. Plants. 2023; 12(9):1872. https://doi.org/10.3390/plants12091872
Chicago/Turabian StyleAlharbi, Khadiga, Emad M. Hafez, Alaa El-Dein Omara, and Hany S. Osman. 2023. "Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria" Plants 12, no. 9: 1872. https://doi.org/10.3390/plants12091872
APA StyleAlharbi, K., Hafez, E. M., Omara, A. E.-D., & Osman, H. S. (2023). Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria. Plants, 12(9), 1872. https://doi.org/10.3390/plants12091872







