Contribution of Arbuscular Mycorrhizal and Endophytic Fungi to Drought Tolerance in Araucaria araucana Seedlings
Abstract
1. Introduction
2. Results
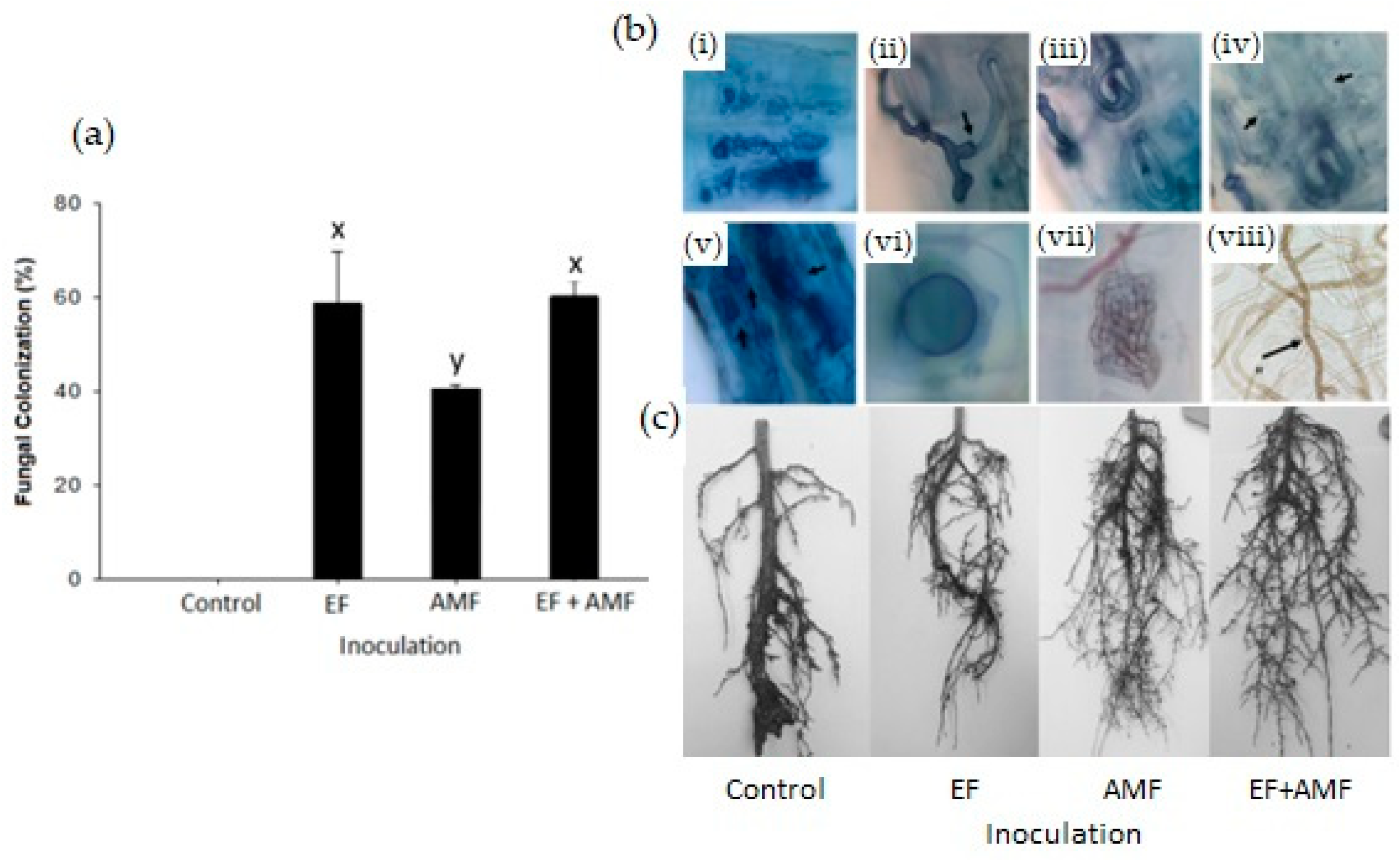
2.1. Fungal Root Colonization
2.2. Morphological Traits and Mortality Rate
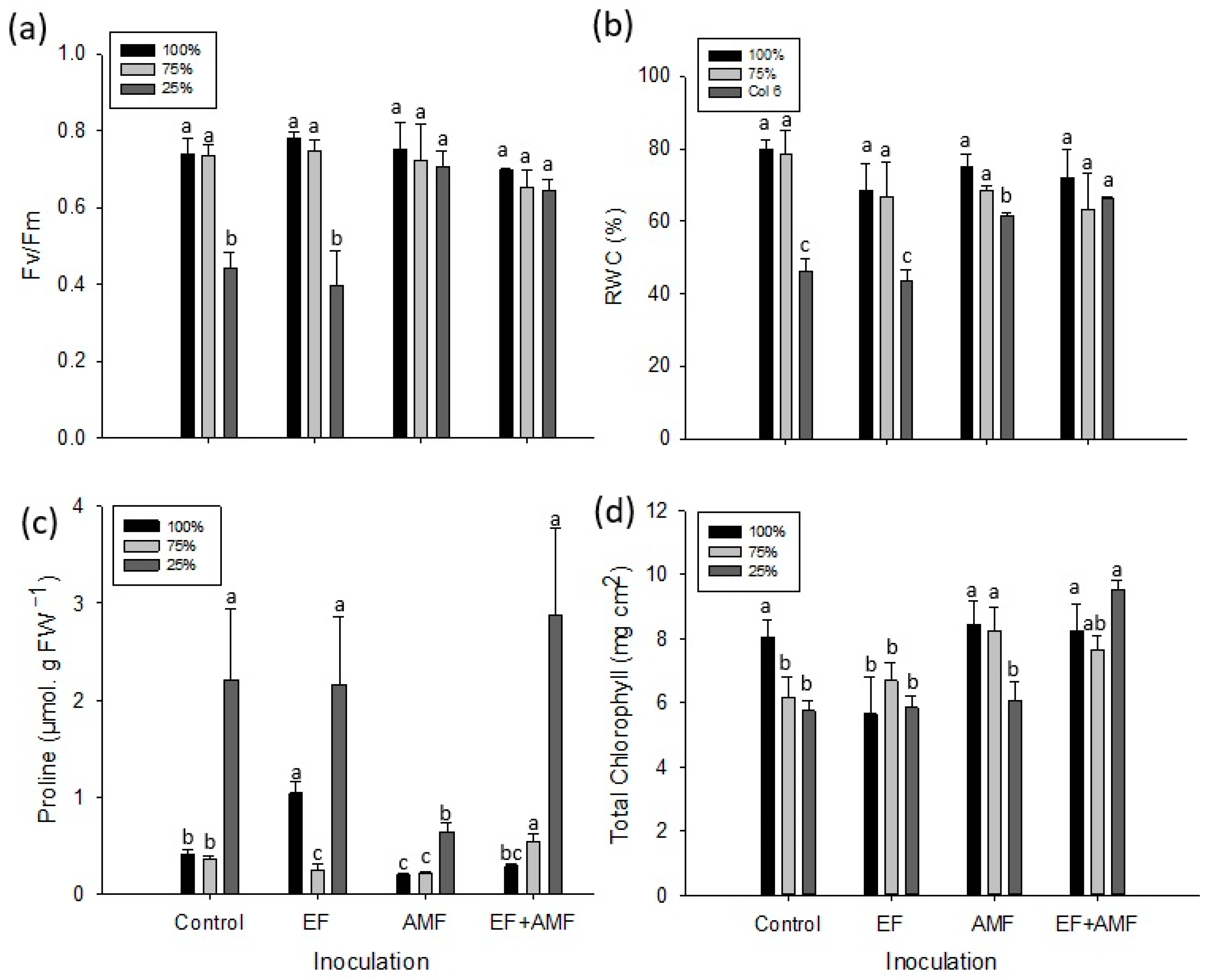
2.3. Maximum Quantum Yield of Photosystem II (Fv/Fm)
2.4. Relative Water Content (RWC)
2.5. Leaf Proline Determination
2.6. Concentration of Photosynthetic Pigments
2.7. CO2 Assimilation
2.8. Multivariate Associations
3. Discussion
4. Materials and Methods
4.1. Soil Samples and Plant Material
4.2. AMF Spore Isolation and Identification
4.3. Cultivation of Trap Plants
4.4. EF Isolation
4.5. Inoculation of A. araucana Seedlings and Different Water Regimes
4.6. Fungal Root Colonization
4.7. Maximum Quantum Efficiency of Photosystem II (Fv/Fm)
4.8. Relative Water Content (RWC)
4.9. Concentration of Photosynthetic Pigments
4.10. Proline Content
4.11. CO2 Assimilation
4.12. Morphological Traits and Mortality Rate
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrmann, T.M. Indigenous knowledge and management of Araucaria araucana forest in the Chilean Andes: Implications for native forest conservation. Biodivers. Conserv. 2006, 15, 647–662. [Google Scholar] [CrossRef]
- Aagesen, D.L. On the northern fringe of the South American temperate forest. The history and conservation of the monkeypuzzle Tree. Environ. Hist. 2006, 3, 64–85. [Google Scholar] [CrossRef]
- Valderrama, L.; Contreras-Reyes, J.; Carrasco, R. Ecological Impact of Forest Fires and Subsequent Restoration in Chile. Resources 2018, 7, 26. [Google Scholar] [CrossRef]
- Corporación Nacional Forestal (CONAF). Determinación del Daño Sanitario de Araucaria araucana y Medidas de Acción. Comisión de Agricultura del Senado. 2017. Available online: http://www.lib.udec.cl/wp-content/uploads/2018/05/Vargasetal2017.pdf (accessed on 15 May 2018).
- IPCC. Summary for Policymakers. In Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Boisier, J.P.; Rondanelli, R.; Garreaud, R.D.; Muñoz, F. Anthropogenic and natural contributions to the Southeast Pacific precipitation decline and recent mega drought in central Chile. Geophys. Res. Lett. 2016, 43, 413–421. [Google Scholar] [CrossRef]
- Amoroso, M.; Daniels, L.D.; Larson, B.C. Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: The use of tree-rings as an indicator of forest decline. For. Ecol. Manag. 2012, 265, 62–70. [Google Scholar] [CrossRef]
- Rodríguez-Catón, M.; Villalba, R.; Morales, M.; Srur, A. Influence of droughts on Nothofagus pumilio forest decline across northern Patagonia, Argentina. Ecosphere 2016, 7, e01390. [Google Scholar] [CrossRef]
- Papú, S.; Berli, F.; Piccoli, P.; Patón, D.; Rodriguez, D.O.; Roig, F.A. Physiological, biochemical, and anatomical responses of Araucaria araucana seedlings to controlled water restriction. Plant Physiol. 2021, 165, 47–56. [Google Scholar] [CrossRef]
- Reininger, V.; Sieber, T.N. Mycorrhiza Reduces Adverse Effects of Dark Septate Endophytes (DSE) on Growth of Conifers. PLoS ONE 2012, 7, e42865. [Google Scholar] [CrossRef]
- Wezowicz, K.; Rozpadek, P.; Turnau, K. Interactions of arbuscular mycorrhizal and endophytic fungi improve seedling survival and growth in post-mining waste. Mycorrhiza 2017, 27, 499–511. [Google Scholar] [CrossRef]
- Rivera, B.K.; Sáez, P.L.; Cavieres, L.A.; Capó-Bauçà, S.; Iñiguez, C.; Sanfuentes von Stowasser, E.; Fuentes, F.; Ramírez, C.F.; Vallejos, V.; Galmés, J. Anatomical and biochemical evolutionary ancient traits of Araucaria araucana (Molina) K. Koch and their effects on carbon assimilation. Tree Physiol. 2022, 42, 1957–1974. [Google Scholar] [CrossRef]
- Wu, H.; Zou, Y.; Rahman, M. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef] [PubMed]
- Diehl, P.; Fontenla, S.B. Arbuscular mycorrhizal infection in two morphological root types of Araucaria araucana (Molina) K. Koch. Rev. Argent Microbiol. 2010, 42, 133–137. [Google Scholar] [PubMed]
- Chávez, D.; Machuca, A.; Fuentes-Ramirez, A.; Fernández, N.; Cornejo, P. Shifts in soil traits and arbuscular mycorrhizal symbiosis represent the conservation status of Araucaria araucana forests and the effects after fire events. For. Ecol. Manag. 2020, 458, 117806. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef]
- Zimmer, H.C.; Brodribb, T.J.; Delzon, S.; Baker, P.J. Drought avoidance and vulnerability in the Australian Araucariaceae. Tree Physiol. 2016, 36, 218–228. [Google Scholar] [CrossRef]
- Alarcón, J.; Márquez, S.; Teunisse, G.; Mendoza, C.; Meneses, C.; Baldini, A.; Parra, P.; Zamora, P.; Boehmwald, F.; Castro-Nallar, E. Sequences of Endophytic Fungal and Bacterial Communities from Araucaria araucana [(Molina) K. Koch, 1869] in the Coastal and Andes Mountain Ranges, Chile. Microbiol. Resour. Announc. 2020, 9, e00544-20. [Google Scholar] [CrossRef]
- Stewart, S.; Griffiths, H. Effect of two species of arbuscularmycorrhizal fungi on growth, assimilation and leaf water relations in maize (Zea mays). Asp. Appl. Biol. 2001, 63, 73–76. [Google Scholar]
- Mumo, M.D.; Mugendi, N.E.; Mang’erere, N.M.; Maingi, J. Arbuscular mycorrhizal fungi and Bradyrhizobium co- inoculation enhances nitrogen fixation and growth of green grams (Vigna radiata L.) under water stress. J. Plant Nutr. 2020, 43, 1036–1047. [Google Scholar] [CrossRef]
- Khan, A.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Farsi, Z.; Al-Mamari, A.; Wagas, M.; Asaf, S.; Elyassi, A.; Mabood, F.; Shin, J. Endophytic Fungi from Frankincense Tree Improves Host Growth and Produces Extracellular Enzymes and Indole Acetic Acid. PLoS ONE 2016, 11, e0158207. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The Role of Phytohormones in Alleviating Salt Stress in Crop Plants. Aust. J. Crop. Sci. 2011, 5, 726–734. [Google Scholar]
- Ahlholm, J.U.; Helander, M.; Lehtimäki, S.; Wäli, P.; Saikkonen, K. Vertically transmitted fungal endophytes: Different responses of host-parasite systems to environmental conditions. Oikos 2002, 99, 173–183. [Google Scholar] [CrossRef]
- Hardoim, P.; Overbeek, L.; Berg, G.; Pirttilä, A.; Compant, S. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Carballar-Hernadez, S.; Hernandez-Cuevas, L.; Montaño, N.; Ferrera-Cerrato, R.; Alarcón, A. Species composition of native arbuscular mycorrhizal fungal consortial influences growth and nutrition of Poblano pepper plant (Capsicum annum L.). Appl. Soil Ecol. 2018, 130, 50–80. [Google Scholar] [CrossRef]
- Soufiani, M.; Aissam, S.; Boutay, H.; Ferradous, A.; Douira, A.; Meddich, A.; El Modafor, C. Effectiveness of indigenous arbuscular mycorrhizal consortium on the growth and mineral nutrition of Argaria spirosa (L.) skeel. Plant Biosyst. 2022, 156, 1365–1372. [Google Scholar] [CrossRef]
- Sharma, S.; Kashyap, S.; Vasudevan, R. In vitro rhizogenesis of Morus alba by mycorrhizal extracts under saline stress. Eur. J. Hortic. Sci. 2005, 70, 79–84. [Google Scholar]
- Adeleke, B.S.; Ayilara, M.S.; Akinola, S.A.; Babalola, O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control 2022, 32, 46. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Martinez del Río, C.M.; Suding, K.N. Rapid temporal changes in root colonization by arbuscular mycorrhizal fungi and fine root endophytes, not dark septate endophytes, track plant activity and environment in an alpine ecosystem. Mycorrhiza 2018, 28, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Q. Water Ecophysiology Effects of Arbuscular Mycorrhizal Fungi on Citrus grandis L. Osbeck cv. Shatianyou in Changshou. Master’s Thesis, Southwest Agriculture University, Changshou, China, 2004. [Google Scholar]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. Comptes Rendus Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 25–55. [Google Scholar]
- Salam, E.A.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2017, 25, 1772–1780. [Google Scholar] [CrossRef]
- Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought Stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—A meta-analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Li, Q.-S.; Xie, Y.-C.; Rahman, M.M.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf Antioxidant Defense System of Lane Late Navel Orange. J. Fungi 2022, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hort. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019, 167, 103821. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Ridout, M.; Houbraken, J.; Newcombe, G. Xerotolerance of Penicillium and Phialocephala fungi, dominant taxa of fine lateral roots of woody plants in the intermountain Pacific Northwest, USA. Rhizosphere 2017, 4, 94–103. [Google Scholar] [CrossRef]
- Toju, H.; Sato, H. Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Front. Microbiol. 2018, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.M.; Juan, R.; Herrera, M.A. An ecophysiographic approach for Araucaria araucana regeneration management. Cienc. e Investig. Agrar. 2012, 39, 159–176. [Google Scholar] [CrossRef]
- Bernier, P.; Lamhamedi, M.; Simpsom, D. Shoot: Root is of limited use in evaluating the quality of container conifer stock. Tree Plant. Notes 1995, 46, 102–106. [Google Scholar]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of arbuscular mycorrhizal fungi inoculation on growth and physiology performance of olive trees under regulated deficit irrigation and partial rootzone drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Valdebenito, A.; Nahuelcura, J.; Santander, C.; Cornejo, P.; Contreras, B.; Gómez-Alonso, S.; Ruiz, A. Physiological and Metabolic Effects of the Inoculation of Arbuscular Mycorrhizal Fungi in Solanum tuberosum Crops under Water Stress. Plants 2022, 11, 2539. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.D.A.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, 14823. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Ouahmane, L.; Hafidi, M.; Thioulouse, J.; Ducousso, M.; Kisa, M.; Prin, Y.; Galiana, A.; Boumezzough, A.; Duponnois, R. Improvement of Cupressus atlantica Gaussen growth by inoculation with native arbuscular mycorrhizal fungi. J. Appl. Microbiol. 2007, 103, 683–690. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, C.L.; Chen, Y.; Chen, Z.; Jiang, Q.B.; Wu, C.; Pinyopusarerk, K. Improving drought tolerance of Casuarina equisetifolia seedlings by arbuscular mycorrhizas under glasshouse conditions. New For. 2010, 40, 261–271. [Google Scholar] [CrossRef]
- Liu, C.Y.; Srivastava, A.K.; Wu, Q.S. Mycorrhizal fungi regulate root responses and leaf physiological activities in trifoliate orange. Not. Bot. Horti Agrobot. 2017, 45, 17–21. [Google Scholar] [CrossRef][Green Version]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R. Arbuscular Mycorrhizal Fungi and the Tolerance of Plants to Drought and Salinity. In Symbiotic Endophytes. Soil Biology Volume 37; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 1, pp. 271–288. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental factors influence plant vascular system and water regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Hamim, H. Underlying drought stress effect on plant: Inhibition of photosynthesis. Hayati 2004, 11, 164–169. [Google Scholar]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular Mycorrhizal Symbiosis Alleviates Salt Stress in Black Locust through Improved Photosynthesis, Water Status, and KC/NaC Homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Álvarez, S.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Root system response to drought and salinity: Root distribution and water transport. In A. Morte & A. Root Engineering; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 40, pp. 325–352. [Google Scholar]
- Kia, S.H.; Jurkechova, M.; Glynou, K.; Piepenbring, M.; Macia-Vicente, J.G. The effects of fungal root endophytes on plant growth are stable along gradients of abiotic habitat conditions. FEMS Microbiol. Ecol. 2018, 94, fix162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Zhang, J.C.; Huang, Y.Q. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Elharchli, E.H.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum. L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Fan, Q.J.; Liu, J.H. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol. Plant 2011, 33, 1533–1542. [Google Scholar] [CrossRef]
- Abbaspoura, H.; Saeidi-Sarb, S.; Afsharia, H.; Abdel-Wahhabc, M.A. Tolerance of Mycorrhiza infected Pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Saddique, M.A.B.; Ali, Z.; Khan, A.S. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Mrnka, L.; Tokarova, H.; Vosatka, M.; Matejka, P. Interaction of soil filamentous fungi affects needle composition and nutrition of Norway spruce seedlings. Trees 2009, 23, 887–897. [Google Scholar] [CrossRef]
- Larimer, A.L.; Bever, J.D.; Clay, K. The interactive effects of plant microbial symbionts: A review and meta-analysis. Symbiosis 2010, 51, 139–148. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mader, P.; Boiler, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef]
- Sieverding, E. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; GTZ: Eschborn, Germany, 1991; 371p. [Google Scholar]
- Blaszkowski, J.; Glomeromycota, W. Szafer Institute of Botany; Polish Academy of Sciences: Kraków, Poland, 2012; 304p. [Google Scholar]
- Oehl, F.; Silva, G.A.; Goto, B.T.; Sieverding, E. Glomeromycetes: Three new genera and glomoid species reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Liderman, R.; Davis, E. Varied response of manigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci. Hortic. 2004, 99, 67–88. [Google Scholar] [CrossRef]
- Vaz, A.B.M.; Fontenla, S.; Rocha, F.S.; Brandão, L.R.; Vieira, M.L.; De Garcia, V.; Góes-Neto, A.; Rosa, C.A. Fungal endophyte β-diversity associated with Myrtaceae species in an Andean Patagonian forest (Argentina) and an Atlantic forest (Brazil). Fungal Ecol. 2014, 8, 28–36. [Google Scholar] [CrossRef]
- Goes-Neto, A.; Loguercio-leite, C.; Guerrero, R. DNA extraction from frozen feeld-collecten and dehydrated herbarium fungal basidiomata. Performance of SDS and CTAB-based methods. Biotemas 2005, 18, 19–32. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.S., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Narisawa, K. The dark septate endophytic fungus Phialicephala fortinii is a potenvial decomposer of soil organic compounds and a promoter af Asparagus officianalis growth. Fungal Ecol. 2017, 28, 1–10. [Google Scholar]
- Tarroum, M.; Ben Romdhane, W.; Ali, A.A.M.; Al-Qurainy, F.; Al-Doss, A.; Fki, L.; Hassairi, A. Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities. Plants 2021, 10, 784. [Google Scholar] [CrossRef]
- Quin, D.; Wang, L.; Han, M.; Wang, J.; Song, H.; Yan, X.; Duan, X.; Dong, J. Effect of an endophytic fungus Umbelopsis dimorpha on the secondary metabolites of host-plant Kadsura agustifolia. Front. Microbiol. 2018, 9, 2845. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Menendez, V.; Martin, J.; Siles, J.A.; Reyes Gonzalez-Tejero, M.; Reyes, F.; Platas, G.; Tormo, J.R.; Genilloud, O. Biodiversity and chemotaxonomy of Preussia isolates from the Iberian Peninsula. Mycol. Prog. 2017, 16, 713–728. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–160. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- McGoingle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which give an objetive measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Galeano, E.; Vasconcelos, T.S.; Novais de Oliveira, P.; Carrer, H. Physiological and molecular responses to drought stress in teak (Tectona grandis L.f.). PLoS ONE 2019, 14, e0221571. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Aroca, R.; Irigoyen, J.; Sanchez-dia, M. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci. 2001, 161, 719–726. [Google Scholar] [CrossRef]
- Lichtenthaler, K. ChlorolShylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]




| Sources of Variation | Experimental Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Height (cm) | DHN (mm) | Shoot (g) | Root (g) | S/R | Fv/Fm | RWC (%) | Proline (µmol g FW−1) | T-Chl (mg cm−2) | |
| Inoculation | 13.7 *** | 3.70 * | 29.4 *** | 8.83 *** | 1.41 ns | 5.69 ** | 4.54 * | 9.40 *** | 23.4 *** |
| Irrigation | 35.8 *** | 25.1 *** | 23.4 *** | 14.4 *** | 0.94 ns | 50.0 *** | 35.9 *** | 63.4 *** | 4.78 * |
| Inoc. X Irrig. | 3.08 * | 0.60 ns | 3.41 * | 4.31 ** | 4.94 ** | 11.6 *** | 5.86 *** | 5.67 *** | 10.3 *** |
| Irrigation Levels | Control | EF | AMF | EF + AMF |
|---|---|---|---|---|
| 100 | 0 | 0 | 0 | 0 |
| 75 | 0 | 0 | 0 | 0 |
| 25 | 75 | 87.5 | 50 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, D.; Rivas, G.; Machuca, Á.; Santos, C.; Deramond, C.; Aroca, R.; Cornejo, P. Contribution of Arbuscular Mycorrhizal and Endophytic Fungi to Drought Tolerance in Araucaria araucana Seedlings. Plants 2023, 12, 2116. https://doi.org/10.3390/plants12112116
Chávez D, Rivas G, Machuca Á, Santos C, Deramond C, Aroca R, Cornejo P. Contribution of Arbuscular Mycorrhizal and Endophytic Fungi to Drought Tolerance in Araucaria araucana Seedlings. Plants. 2023; 12(11):2116. https://doi.org/10.3390/plants12112116
Chicago/Turabian StyleChávez, Daniel, Gustavo Rivas, Ángela Machuca, Cledir Santos, Christian Deramond, Ricardo Aroca, and Pablo Cornejo. 2023. "Contribution of Arbuscular Mycorrhizal and Endophytic Fungi to Drought Tolerance in Araucaria araucana Seedlings" Plants 12, no. 11: 2116. https://doi.org/10.3390/plants12112116
APA StyleChávez, D., Rivas, G., Machuca, Á., Santos, C., Deramond, C., Aroca, R., & Cornejo, P. (2023). Contribution of Arbuscular Mycorrhizal and Endophytic Fungi to Drought Tolerance in Araucaria araucana Seedlings. Plants, 12(11), 2116. https://doi.org/10.3390/plants12112116








