A Review on Remediation of Iron Ore Mine Tailings via Organic Amendments Coupled with Phytoremediation
Abstract
1. Introduction
2. Physicochemical and Biological Characterisation and Strategies to Improve Iron Ore Mine Tailings
2.1. Physicochemical Characterisation of Iron Ore Mine Tailings
2.2. Biological Characterisation of Iron Ore Mine Tailings
2.3. Strategies to Improve Physicochemical and Biological Characteristics of Iron Ore Tailings
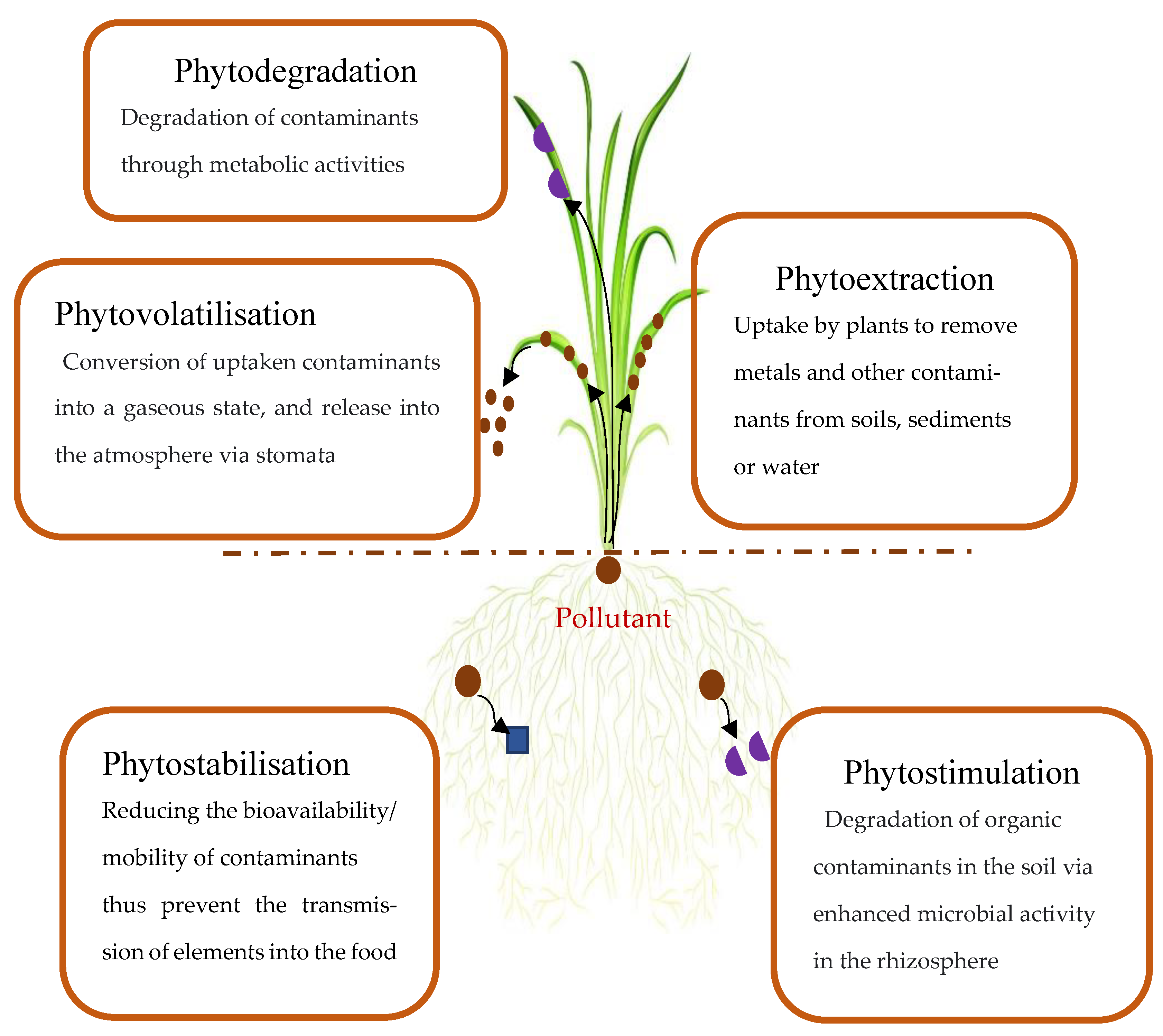
3. Phytoremediation
3.1. Phytoextraction
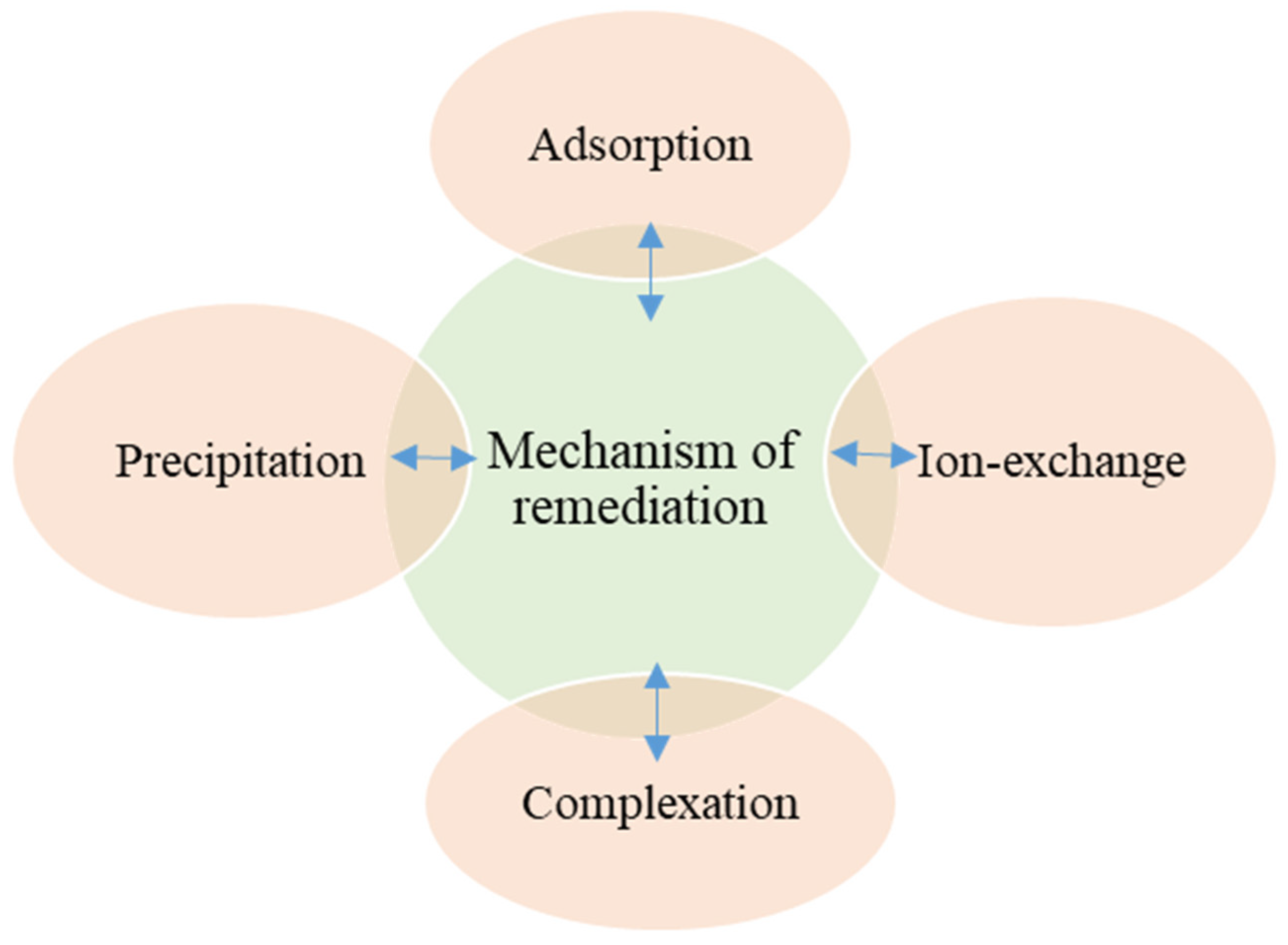
3.2. Phytostabilisation or Phytoimmobilisation
4. Phytoremediation with Amendments
4.1. Phytoremediation with Biochar Amendment
4.2. Phytoremediation with Compost Amendment
| Compost Feedstock | References |
|---|---|
| Dairy manure | [110] |
| Poultry manure and horse bedding | [111] |
| Pig manure | [112] |
| Rice straw | [113] |
| Sewage sludge, swine manure, sawdust, mushroom residue | [114] |
| Sewage sludge, barley straw, wood chips | [115] |
| Sewage sludge, kitchen waste and corn stalks | [116] |
| Municipal solid waste | [117] |
| Vegetable and fruit waste | [112] |
| Pine bark | [118] |
4.3. Phytoremediation with Topsoil Amendment
4.4. Phytoremediation with Straw Amendment
5. Plants in Poaceae Family Phytoremediating Heavy-Metal-Contaminated Mine Tailings
| Grass | Remediating Metal | Accumulation in Shoots (mg kg−1) | Reference |
|---|---|---|---|
| Vetiver grass (Vetiveria zizanioides) | Pb2+, | 300 | [142] |
| Signal grass (Brachiaria decumbens)) | Pb2+ | 70 | [143] |
| Italian ryegrass (Lolium multiflorium) | Pb2+, Cd2+ | 350, 800 | [144] |
| Perennial ryegrass (Lolium perenne) | Cu2+, Zn2+ | 15, 180 | [145] |
| Bermuda grass (Cynodon dactylon (L.) | Pb2+ | 400–1200 | [141] |
| Esparto grass (Lygeum spartum (L.) | Zn2+, | >4100 | [146] |
| Giant reed grass (Arundo donax) | As2+, Cd2+, Pb2+ | 23,25,26 | [147] |
| Amur silver grass (Miscanthus sacchariflorus) | Zn2+, Cr2+ | 320,100 | [139] |
| Rhodes grass (Chloris gayana) | Mn2+, Fe2+ | 164, 830 | [148] |
| Buffel grass (Cenchrus ciliaris) | Ni2+, Cu2+, Zn2+ | 100, 13,10, | [149] |
| Common reed (Phragmites australis) | As3+ | [150] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Owen, J.R.; Kemp, D.; Lèbre, É.; Svobodova, K.; Pérez Murillo, G. Catastrophic tailings dam failures and disaster risk disclosure. Int. J. Disaster Risk Reduct. 2020, 42, 101361. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Babel, S.; Chauhan, R.S.; Ali, N.; Yadav, V. Preparation of phosphate mine tailings and low grade rock phosphate enriched bio-fertilizer. J. Sci. Ind. Res. 2016, 75, 120–123. [Google Scholar]
- Thejas, H.K.; Hossiney, N. Alkali-activated bricks made with mining waste iron ore tailings. Case Stud. Constr. Mater. 2022, 16, e00973. [Google Scholar] [CrossRef]
- Ghazaryan, K.A.; Movsesyan, H.S.; Minkina, T.M.; Sushkova, S.N.; Rajput, V.D. The identification of phytoextraction potential of Melilotus officinalis and Amaranthus retroflexus growing on copper- and molybdenum-polluted soils. Environ. Geochem. Health 2021, 43, 1327–1335. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Ahmed, M.J.; Dhal, N.K. Effects of iron ore tailings on growth and physiological activities of Tagetes patula L. J. Soils Sediments 2013, 14, 721–730. [Google Scholar] [CrossRef]
- Yang, D.; Zeng, D.H.; Zhang, J.; Li, L.J.; Mao, R. Chemical and microbial properties in contaminated soils around a magnesite mine in northeast China. Land Degrad. Dev. 2012, 23, 256–262. [Google Scholar] [CrossRef]
- Sanchez-Lopez, A.S.; Carrillo-Gonzalez, R.; Gonzalez-Chavez Mdel, C.; Rosas-Saito, G.H.; Vangronsveld, J. Phytobarriers: Plants capture particles containing potentially toxic elements originating from mine tailings in semiarid regions. Environ. Pollut. 2015, 205, 33–42. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M.; Fantke, P. Ecological and human health risks associated with abandoned gold mine tailings contaminated soil. PLoS ONE 2017, 12, e0172517. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.; Liu, R.; Wang, L.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Nyenda, T.; Gwenzi, W.; Jacobs, S.M. Changes in physicochemical properties on a chronosequence of gold mine tailings. Geoderma 2021, 395, 115037. [Google Scholar] [CrossRef]
- Li, X.; Park, J.H.; Edraki, M.; Baumgartl, T. Understanding the salinity issue of coal mine spoils in the context of salt cycle. Environ. Geochem. Health 2014, 36, 453–465. [Google Scholar] [CrossRef]
- Choi, Y.D.; Wali, M.K. The role of Panicum virgatum (switch grass) in the revegetation of iron-mine tailings in northern new york. Restor. Ecol. 1995, 3, 123–132. [Google Scholar] [CrossRef]
- Cele, E.N.; Maboeta, M. A greenhouse trial to investigate the ameliorative properties of biosolids and plants on physicochemical conditions of iron ore tailings: Implications for an iron ore mine site remediation. J. Environ. Manag. 2016, 165, 167–174. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Jambhulkar, H.P. Phytoremediation of coal mine spoil dump through integrated biotechnological approach. Bioresour. Technol. 2008, 99, 4732–4741. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Cui, Z. Evaluation and analysis of soil migration and distribution characteristics of heavy metals in iron tailings. J. Clean. Prod. 2018, 172, 475–480. [Google Scholar] [CrossRef]
- Jung, M.C. Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a korean Cu-W mine. Sensors 2008, 8, 2413–2423. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O.; Naeth, M.A. Physicochemical properties, heavy metals, and metal-tolerant bacteria profiles of abandoned gold mine tailings in Krugersdorp, South Africa. Can. J. Soil Sci. 2020, 100, 217–233. [Google Scholar] [CrossRef]
- Cui, X.; Geng, Y.; Li, T.; Zhao, R.; Li, X.; Cui, Z. Field application and effect evaluation of different iron tailings soil utilization technologies. Resour. Conserv. Recycl. 2021, 173, 105746. [Google Scholar] [CrossRef]
- James, J.J.; Tiller, R.L.; Richards, J.H. Multiple resources limit plant growth and function in a saline-alkaline desert community. J. Ecol. 2005, 93, 113–126. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Alhwaimel, S.A.; Kuang, B.; Waine, T. Multiple on-line soil sensors and data fusion approach for delineation of water holding capacity zones for site specific irrigation. Soil Tillage Res. 2014, 143, 95–105. [Google Scholar] [CrossRef]
- Esteves, G.F.; de Souza, K.R.D.; Bressanin, L.A.; Andrade, P.C.C.; Veroneze Junior, V.; Dos Reis, P.E.; da Silva, A.B.; Mantovani, J.R.; Magalhaes, P.C.; Pasqual, M.; et al. Vermicompost improves maize, millet and sorghum growth in iron mine tailings. J. Environ. Manag. 2020, 264, 110468. [Google Scholar] [CrossRef]
- Renault, S.; Sailerova, E.; Fedikow, M.A.F. Phytoremediation of mine tailings and bio-ore production: Progress report on seed germination, survival and metal uptake of seedlings planted at central manitoba (AU) minesite. In Manitoba Industry, Trade and Mines; Manitoba Geological Survey: Winnipeg, MB, Canada, 2002; pp. 255–265. [Google Scholar]
- Han, X.; Wang, Y.; Zhang, N.; Meng, J.; Li, Y.; Liang, J. Facile synthesis of mesoporous silica derived from iron ore tailings for efficient adsorption of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126391. [Google Scholar] [CrossRef]
- Gagnon, V.; Rodrigue-Morin, M.; Tardif, A.; Beaudin, J.; Greer, C.W.; Shipley, B.; Bellenger, J.P.; Roy, S. Differences in elemental composition of tailings, soils, and plant tissues following five decades of native plant colonization on a gold mine site in Northwestern Quebec. Chemosphere 2020, 250, 126243. [Google Scholar] [CrossRef]
- Lange, C.A.; Kotte, K.; Smit, M.; van Deventer, P.W.; van Rensburg, L. Effects of different soil ameliorants on karee trees (Searsia lancea) growing on mine tailings dump soil-part I: Pot trials. Int. J. Phytoremediation 2012, 14, 908–924. [Google Scholar] [CrossRef]
- Friedlova, M. The influence of heavy metals on soil biological and chemical properties. Soil Water Res. 2010, 5, 21–27. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef]
- Castaldi, S.; Rutigliano, F.A.; de Santo Amalia, V. Suitability of soil microbial paramters as indicators of heavy metal pollution. Water Air Soil Pollut. 2004, 158, 21–35. [Google Scholar]
- Tordoff, G.M.; Baker, A.J.M.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Touceda-Gonzalez, M.; Alvarez-Lopez, V.; Prieto-Fernandez, A.; Rodriguez-Garrido, B.; Trasar-Cepeda, C.; Mench, M.; Puschenreiter, M.; Quintela-Sabaris, C.; Macias-Garcia, F.; Kidd, P.S. Aided phytostabilisation reduces metal toxicity, improves soil fertility and enhances microbial activity in Cu-rich mine tailings. J. Environ. Manag. 2017, 186, 301–313. [Google Scholar] [CrossRef]
- Konopka, A.; Zakharova, T.; Bischoff, M.; Oliver, L.; Nakatsu, C.; Turco, R.F. Microbial biomass and activity in lead-contaminated Soil. Appl. Environ. Microbiol. 1999, 65, 2256–2259. [Google Scholar] [CrossRef]
- Gardner, W.C.; Broersma, K.; Naeth, A.; Chanasyk, D.; Jobson, A. Influence of biosolids and fertilizer amendments on physical, chemical and microbiological properties of copper mine tailings. Can. J. Soil Sci. 2010, 90, 571–583. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef] [PubMed]
- Benidire, L.; Pereira, S.; Aboudrar, W.; Hafidi, M.; Castro, P.; Boularbah, A. Remediation of metal-contaminated mine tailings by the application of organic and mineral amendments. J. Soils Sediments 2021, 22, 482–495. [Google Scholar] [CrossRef]
- Zhan, J.; Sun, Q. Diversity of free-living nitrogen-fixing microorganisms in the rhizosphere and non-rhizosphere of pioneer plants growing on wastelands of copper mine tailings. Microbiol. Res. 2012, 167, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.R.; Lima, V.F.; Ferrer, S.A. The use of respiration indices in the composting process: A review. Waste Manag. Res. 2006, 24, 37–47. [Google Scholar] [CrossRef]
- Feketeova, Z.; Hulejova Sladkovicova, V.; Mangova, B.; Simkovic, I. Biological activity of the metal-rich post-flotation tailings at an abandoned mine tailings pond (four decades after experimental afforestation). Environ. Sci. Pollut. Res. 2015, 22, 12174–12181. [Google Scholar] [CrossRef]
- Pérez-de-Mora, A.; Burgos, P.; Madejón, E.; Cabrera, F.; Jaeckel, P.; Schloter, M. Microbial community structure and function in a soil contaminated by heavy metals: Effects of plant growth and different amendments. Soil Biol. Biochem. 2006, 38, 327–341. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Lacalle, R.G.; Becerril, J.M.; Garbisu, C. Biological methods of polluted soil remediation for an effective economically-optimal recovery of soil health and ecosystem services. J. Environ. Sci. Public Health 2020, 4, 112–133. [Google Scholar] [CrossRef]
- Chen, F.; Yao, Q.; Tian, J. Review of ecological restoration technology for mine tailings in china. Eng. Rev. 2016, 36, 115–121. [Google Scholar]
- Khan, J.M.; Jones, D.L. Chemical and organic immobilization treatments for reducing phytoavailability of heavy metals in copper-mine tailings. J. Plant Nutr. Soil Sci. 2008, 171, 908–916. [Google Scholar] [CrossRef]
- McGowen, S.L.; Basta, N.T.; Brown, G.O. Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J. Environ. Qual. 2001, 30, 493–500. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Salt, D.E. Fortified foods and phytoremediation. two sides of the same coin. Plant Physiol. 2001, 125, 164–167. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Garcia-Figueroa, A.C.; Amabilis-Sosa, L.E.; Molina-Freaner, F.E.; Pat-Espadas, A.M. Stabilization of potentially toxic elements contained in mine waste: A microbiological approach for the environmental management of mine tailings. J. Environ. Manag. 2020, 270, 110873. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Xu, D.M.; Zhan, C.L.; Liu, H.X.; Lin, H.Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef] [PubMed]
- Kotrba, P.; Najmanova, J.; Macek, T.; Ruml, T.; Mackova, M. Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol. Adv. 2009, 27, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.; Perez-Esteban, J.; Escolastico, C.; Masaguer, A.; Moliner, A. Phytoremediation of Cu and Zn by vetiver grass in mine soils amended with humic acids. Environ. Sci. Pollut. Res. 2016, 23, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Shahid, M.; Pinelli, E.; Dumat, C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J. Hazard. Mater. 2012, 219–220, 1–12. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, Z.; Li, X.; Guan, Z.; Cai, Y.; Liao, X. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci. Total Environ. 2019, 670, 950–960. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2013, 11, 843–872. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–900. [Google Scholar] [CrossRef]
- Seth, C.S. A Review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot. Rev. 2011, 78, 32–62. [Google Scholar] [CrossRef]
- Van Nevel, L.; Mertens, J.; Oorts, K.; Verheyen, K. Phytoextraction of metals from soils: How far from practice? Environ. Pollut. 2007, 150, 34–40. [Google Scholar] [CrossRef]
- Robinson, B.; Fernández, J.; Madejon, P.; Marañon, T.; Murillo, J.; Green, S.; Clothier, B. Phytoextraction: An assessment of biogeochemical and economic viability. Plant Soil 2003, 249, 117–125. [Google Scholar] [CrossRef]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Shabani, N.; Sayadi, M.H. Evaluation of heavy metals accumulation by two emergent macrophytes from the polluted soil: An experimental study. Environmentalist 2012, 32, 91–98. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Zacchini, M.; Pietrini, F.; Scarascia Mugnozza, G.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2008, 197, 23–34. [Google Scholar] [CrossRef]
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the transfer of trace metals to spontaneous plants on abandoned pyrrhotite mine: Potential application for phytostabilisation of phosphate wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Netw. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Ippolito, J.A.; Ducey, T.F.; Johnson, M.G.; Spokas, K.A. Phytostabilization of Zn and Cd in mine soil Using corn in combination with biochars and manure-based compost. Environments 2019, 6, 69. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, B.; Theng, B.K.G.; Wu, P.; Liu, F.; Wang, S.; Lee, X.; Chen, M.; Li, L.; Zhang, X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Laird, D.A. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Ok, Y.S.; Bhatnagar, A.; Hou, D.; Bhaskar, T.; Masek, O. Advances in algal biochar: Production, characterization and applications. Bioresour. Technol. 2020, 317, 123982. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.J.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. J. Soil Sci. Soc. Am. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef]
- Elad, Y.; Cytryn, E.; Harel, Y.M.; Lew, B.; Graber, E.R. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2011, 50, 335–349. [Google Scholar]
- Zimmerman, A.R.; Gao, B.; Ahn, M. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Moreno-Barriga, F.; Díaz, V.; Acosta, J.A.; Muñoz, M.Á.; Faz, Á.; Zornoza, R. Organic matter dynamics, soil aggregation and microbial biomass and activity in technosols created with metalliferous mine residues, biochar and marble waste. Geoderma 2017, 301, 19–29. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akter, F.; Solaiman, Z.M.; Strezov, V. Biochar: An emerging panacea for remediation of soil contaminants from mining, industry and sewage wastes. Pedosphere 2015, 25, 654–665. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Kelly, C.N.; Peltz, C.D.; Stanton, M.; Rutherford, D.W.; Rostad, C.E. Biochar application to hardrock mine tailings: Soil quality, microbial activity, and toxic element sorption. Appl. Geochem. 2014, 43, 35–48. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- El-Gamal, E.H.; Saleh, M.; Elsokkary, I.; Rashad, M.; El-Latif, M.M.A. Comparison between properties of biochar produced by traditional and controlled pyrolysis. Alex. Sci. Exch. J. 2017, 38, 412–425. [Google Scholar]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. Int. 2018, 25, 11875–11883. [Google Scholar] [CrossRef]
- Tan, G.; Wu, Y.; Liu, Y.; Xiao, D. Removal of Pb(II) ions from aqueous solution by manganese oxide coated rice straw biochar A low-cost and highly effective sorbent. J. Taiwan Inst. Chem. Eng. 2018, 84, 85–92. [Google Scholar] [CrossRef]
- Yan, S.; Yu, W.; Yang, T.; Li, Q.; Guo, J. The adsorption of corn stalk biochar for Pb and Cd: Preparation, characterization, and batch adsorption study. Separations 2022, 9, 22. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Quainoo, A.K.; Abubakari, A.H. Simultaneous adsorption of toxic metals in binary systems using peanut and sheanut shells biochars. Heliyon 2022, 8, e10558. [Google Scholar] [CrossRef]
- Islam, M.S.; Gao, R.L.; Gao, J.Y.; Song, Z.T.; Ali, U.; Hu, H.Q. Cadmium, lead, and zinc immobilization in soil using rice husk biochar in the presence of citric acid. Int. J. Environ. Sci. Technol. 2021, 19, 567–580. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Agnew, J.M.; Leonard, J.J. The Physical Properties of Compost. Compost. Sci. Util. 2003, 11, 238–264. [Google Scholar] [CrossRef]
- Perez, R.; Tapia, Y.; Antilen, M.; Casanova, M.; Vidal, C.; Santander, C.; Aponte, H.; Cornejo, P. Interactive effect of compost application and inoculation with the fungus Claroideoglomus claroideum in Oenothera picensis plants growing in mine tailings. Ecotoxicol. Environ. Saf. 2021, 208, 111495. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils--to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.J.; Berton, R.S.; Coscione, A.R.; Saes, L.A. Biosolids application on banana production: Soil chemical properties and plant nutrition. Appl. Environ. Soil Sci. 2011, 2011, 238185. [Google Scholar] [CrossRef]
- Chiu, K.K.; Ye, Z.H.; Wong, M.H. Growth of Vetiveria zizanioides and Phragmities australis on Pb/Zn and Cu mine tailings amended with manure compost and sewage sludge: A greenhouse study. Bioresour. Technol. 2006, 97, 158–170. [Google Scholar] [CrossRef]
- Gil-Loaiza, J.; White, S.A.; Root, R.A.; Solis-Dominguez, F.A.; Hammond, C.M.; Chorover, J.; Maier, R.M. Phytostabilization of mine tailings using compost-assisted direct planting: Translating greenhouse results to the field. Sci. Total Environ. 2016, 565, 451–461. [Google Scholar] [CrossRef]
- González, Á.; García-Gonzalo, P.; Gil-Díaz, M.M.; Alonso, J.; Lobo, M.C. Compost-assisted phytoremediation of As-polluted soil. J. Soils Sediments 2019, 19, 2971–2983. [Google Scholar] [CrossRef]
- Alege, F.P.; Tao, H.; Miito, G.J.; DeVetter, L.W.; Ndegwa, P.M. Influence of moisture content on recovery and durability of dairy manure compost pellets. Bioresour. Technol. Rep. 2022, 18, 101076. [Google Scholar] [CrossRef]
- Rizzo, P.F.; Young, B.J.; Pin Viso, N.; Carbajal, J.; Martinez, L.E.; Riera, N.I.; Bres, P.A.; Beily, M.E.; Barbaro, L.; Farber, M.; et al. Integral approach for the evaluation of poultry manure, compost, and digestate: Amendment characterization, mineralization, and effects on soil and intensive crops. Waste Manag. 2022, 139, 124–135. [Google Scholar] [CrossRef]
- Shi, W.; Dong, Q.; Saleem, M.; Wu, X.; Wang, N.; Ding, S.; Huang, J.; Wang, X.; Zhou, B.; Gao, Z. Microbial-based detonation and processing of vegetable waste for high quality compost production at low temperatures. J. Clean. Prod. 2022, 369, 133276. [Google Scholar] [CrossRef]
- Wu, D.; Qu, F.; Li, D.; Zhao, Y.; Li, X.; Niu, S.; Zhao, M.; Qi, H.; Wei, Z.; Song, C. Effect of Fenton pretreatment and bacterial inoculation on cellulose-degrading genes and fungal communities during rice straw composting. Sci. Total Environ. 2022, 806, 151376. [Google Scholar] [CrossRef]
- Bao, H.; Chen, Z.; Wen, Q.; Wu, Y.; Fu, Q. Effect of calcium peroxide dosage on organic matter degradation, humification during sewage sludge composting and application as amendment for Cu (II)-polluted soils. J. Hazard. Mater. 2022, 439, 129592. [Google Scholar] [CrossRef]
- Leśniańska, A.; Janowska, B.; Sidełko, R. Immobilization of Zn and Cu in conditions of reduced C/N ratio during sewage sludge composting process. Energies 2022, 15, 4507. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Zhan, J.; Guo, X. Variation and factors on heavy metal speciation during co-composting of rural sewage sludge and typical rural organic solid waste. J. Environ. Manag. 2022, 306, 114418. [Google Scholar] [CrossRef]
- Kabasiita, J.K.; Opolot, E.; Malinga, G.M. Quality and fertility assessments of municipal solid waste compost produced from cleaner development mechanism compost projects: A case study from Uganda. Agriculture 2022, 12, 582. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.; Al-Degs, Y.; Paradelo, R. Competitive removal of textile dyes from solution by pine bark-compost in batch and fixed bed column experiments. Environ. Technol. Innov. 2022, 27, 102421. [Google Scholar] [CrossRef]
- Sydnor, M.E.W.; Redente, E.F. Reclamation of high-elevation, acidic mine waste with organic amendments and topsoil. J. Environ. Qual. 2002, 31, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rojas, M.; Erickson, T.E.; Martini, D.C.; Dixon, K.W.; Merritt, D.J. Climate and soil factors influencing seedling recruitment of plant species used for dryland restoration. Soil 2016, 2, 287–298. [Google Scholar] [CrossRef]
- Kneller, T.; Harris, R.J.; Bateman, A.; Munoz-Rojas, M. Native-plant amendments and topsoil addition enhance soil function in post-mining arid grasslands. Sci. Total Environ. 2018, 621, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Merino-Martín, L.; Commander, L.; Mao, Z.; Stevens, J.C.; Miller, B.P.; Golos, P.J.; Mayence, C.E.; Dixon, K. Overcoming topsoil deficits in restoration of semiarid lands: Designing hydrologically favourable soil covers for seedling emergence. Ecol. Eng. 2017, 105, 102–117. [Google Scholar] [CrossRef]
- Limón, Á.; Peco, B. Germination and emergence of annual species and burial depth: Implications for restoration ecology. Acta Oecologica 2016, 71, 8–13. [Google Scholar] [CrossRef]
- Machado, N.A.; Leite, M.G.; Figueiredo, M.A.; Kozovits, A.R. Growing Eremanthus erythropappus in crushed laterite: A promising alternative to topsoil for bauxite-mine revegetation. J. Environ. Manag. 2013, 129, 149–156. [Google Scholar] [CrossRef]
- Brevik, E.C.; Lazari, A.G. Rates of pedogenesis in reclaimed lands as compared to rates of natural pedogenesis. Soil Horiz. 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Ma, Y.; Dickinson, N.M.; Wong, M.H. Beneficial effects of earthworms and arbuscular mycorrhizal fungi on establishment of leguminous trees on Pb/Zn mine tailings. Soil Biol. Biochem. 2006, 38, 1403–1412. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; Zhou, S.; Sun, H.; Zhao, Y.; Zheng, X.; Li, S.; Zhang, J.; Lv, W. Long-term effects of straw return and straw-derived biochar amendment on bacterial communities in soil aggregates. Sci. Rep. 2020, 10, 7891. [Google Scholar] [CrossRef]
- Shi, T.; Liu, Y.; Zhang, L.; Hao, L.; Gao, Z. Burning in agricultural landscapes: An emerging natural and human issue in China. Landsc. Ecol. 2014, 29, 1785–1798. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Lu, C. Crop yield and soil organic matter after long-term straw return to soil in China. Nutr. Cycl. Agroecosystems 2015, 102, 371–381. [Google Scholar] [CrossRef]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Chen, X.; He, H.Z.; Chen, G.K.; Li, H.S. Effects of biochar and crop straws on the bioavailability of cadmium in contaminated soil. Sci. Rep. 2020, 10, 9528. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, T.; Zhang, X.; Yu, H.; Zheng, Z.; Wang, Y.; Hao, X.; Pu, Y. Changes in pH, dissolved organic matter and Cd species in the rhizosphere soils of Cd phytostabilizer Athyrium wardii (Hook.) Makino involved in Cd tolerance and accumulation. Environ. Sci. Pollut. Res. 2014, 21, 4605–4613. [Google Scholar] [CrossRef]
- Zhao, H.; Shar, A.G.; Li, S.; Chen, Y.; Shi, J.; Zhang, X.; Tian, X. Effect of straw return mode on soil aggregation and aggregate carbon content in an annual maize-wheat double cropping system. Soil Tillage Res. 2018, 175, 178–186. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lu, L.; Lin, X. Effects of organic amendment on soil aggregation and microbial community composition during drying-rewetting alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhu, J.; Fu, Q.; Hu, H.; Huang, Q.; Violante, A. Sorption of Cu by organic matter from the decomposition of rice straw. J. Soils Sediments 2016, 16, 2203–2210. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Jockwer, F. Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ. Pollut. 1999, 104, 145–155. [Google Scholar] [CrossRef]
- Li, C.; Xiao, B.; Wang, Q.H.; Yao, S.H.; Wu, J.Y. Phytoremediation of Zn- and Cr-contaminated soil using two promising energy grasses. Water Air Soil Pollut. 2014, 225, 2027. [Google Scholar] [CrossRef]
- Mugica-Alvarez, V.; Cortés-Jiménez, V.; Vaca-Mier, M.; Domínguez-Soria, V. Phytoremediation of mine tailings using Lolium Multiflorum. Int. J. Environ. Sci. Dev. 2015, 6, 246–251. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Biotechnol. 2007, 7, 47–59. [Google Scholar] [CrossRef]
- Otunola, B.O.; Aghoghovwia, M.P.; Thwala, M.; Ololade, O.O. Heavy metal phytoremediation potential of Vetiver grass and Indian mustard update on enhancements and research opportunities. Water Air Soil Pollut. 2022, 233, 154. [Google Scholar] [CrossRef]
- Andreazza, R.; Bortolon, L.; Pieniz, S.; Camargo, F.A.O.; Bortolon, E.S.O. Copper Phytoextraction and Phytostabilization by Brachiaria decumbens Stapf. in Vineyard Soils and a Copper Mining Waste. Open J. Soil Sci. 2013, 3, 273–282. [Google Scholar] [CrossRef]
- WeiXie, L.; Yang, R.; Liu, B.; Lei, N.; Peng, S.; Li, J.; Tong, J.; Deng, R.; Li, J. Effects of Pb-, Cd-resistant bacterium Pantoea sp. on growth, heavy metal uptake and bacterial communities in oligotrophic growth substrates of Lolium multiflorum Lam. Environ. Sci. Pollut. Res. 2022, 29, 50742–50754. [Google Scholar] [CrossRef]
- Sarathchandra, S.S.; Rengel, Z.; Solaiman, Z.M. Remediation of heavy metal-contaminated iron ore tailings by applying compost and growing perennial ryegrass (Lolium perenne L.). Chemosphere 2022, 288, 132573. [Google Scholar] [CrossRef]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Growth of Lygeum spartum in acid mine tailings: Response of plants developed from seedlings, rhizomes and at field conditions. Environ. Pollut. 2007, 145, 700–707. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, X.; Miao, X.; Guo, Z.; Wang, F. Effect of amendments on growth and metal uptake of giant reed (Arundo donax L.) grown on soil contaminated by arsenic, cadmium and lead. Trans. Nonferrous Met. Soc. China 2012, 22, 1462–1469. [Google Scholar] [CrossRef]
- Itanna, F.; Coulman, B. Phyto-extraction of copper, iron, manganese, and zinc from environmentally contaminated sites in Ethiopia, with three grass species. Commun. Soil Sci. Plant Anal. 2011, 34, 111–124. [Google Scholar] [CrossRef]
- Alfaifi, T. Evaluation and assessment of metal(loids) adsorptions by Cenchrus ciliaris L. in a cement contaminated area. BioResources 2022, 17, 4360–4377. [Google Scholar] [CrossRef]
- Alvarez-Robles, M.J.; Bernal, M.P.; De Brasi-Velasco, S.; Sevilla, F.; Clemente, R. Response of Phragmites australis to increasing As(V) concentrations: Accumulation and speciation of As, and plant oxidative stress. Chemosphere 2022, 302, 134937. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.M.; Copani, V.; Patanè, C.; Cosentino, S.L. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crops Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Lee, J.; Jeffries, T.W. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis. Biomass Bioenergy 2012, 39, 296–305. [Google Scholar] [CrossRef]
- Keeling, S.M.; Werren, G. Phytoremediation: The uptake of metals and metalloids by rhodes grass grown on metal-contaminated soil. Remediat. J. 2005, 15, 53–61. [Google Scholar] [CrossRef]
- Kirk, J.L.; Klironomos, J.N.; Lee, H.; Trevors, J.T. The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ. Pollut. 2005, 133, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Bartucca, M.L.; Del Buono, D.; Cesco, S. Italian ryegrass for the phytoremediation of solutions polluted with terbuthylazine. Chemosphere 2015, 119, 31–36. [Google Scholar] [CrossRef]
- Gu, C.; Bai, Y.; Tao, T.; Chen, G.; Shan, Y. Effect of sewage sludge amendment on heavy metal uptake and yield of ryegrass seedling in a mudflat soil. J. Environ. Qual. 2013, 42, 421–428. [Google Scholar] [CrossRef]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Sinha, S.; Mishra, R.K.; Sinam, G.; Mallick, S.; Gupta, A.K. Comparative evaluation of metal phytoremediation potential of trees, grasses, and flowering plants from tannery-wastewater-contaminated soil in relation with physicochemical properties. Soil Sediment Contam. Int. J. 2013, 22, 958–983. [Google Scholar] [CrossRef]

| Technique | Cost (AUD$/ton) | Factors to Be Considered | |
|---|---|---|---|
| [4] | [49] | ||
| Physical remediation | 140–720 | - | Transport/excavation/monitoring |
| Chemical remediation | 140–720 | 90–420 | Recycling of pollutants |
| Phytoremediation | 7–60 | - | Long-term monitoring |
| Feedstock | Pyrolysis Temperature | Type of Metal Pollutant | Removal Rate (%) | Reference |
|---|---|---|---|---|
| Rice straw | 500 °C | Cd2+ | 98% | [96] |
| Rice straw | 420 °C | Pb2+ | 95% | [97] |
| Corn straw | 400–600 °C | Pb2+, Cd2+ | 60%, 80% | [98] |
| Peanut shells and shea nut shells | 700 °C | Pb2+, Cd2+, Hg2+ | 100% | [99] |
| Rice husk | 500 °C | Cd2+, Pb2+, Zn2+ | 25%, 18%, 17% | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarathchandra, S.S.; Rengel, Z.; Solaiman, Z.M. A Review on Remediation of Iron Ore Mine Tailings via Organic Amendments Coupled with Phytoremediation. Plants 2023, 12, 1871. https://doi.org/10.3390/plants12091871
Sarathchandra SS, Rengel Z, Solaiman ZM. A Review on Remediation of Iron Ore Mine Tailings via Organic Amendments Coupled with Phytoremediation. Plants. 2023; 12(9):1871. https://doi.org/10.3390/plants12091871
Chicago/Turabian StyleSarathchandra, Sajeevee S., Zed Rengel, and Zakaria M. Solaiman. 2023. "A Review on Remediation of Iron Ore Mine Tailings via Organic Amendments Coupled with Phytoremediation" Plants 12, no. 9: 1871. https://doi.org/10.3390/plants12091871
APA StyleSarathchandra, S. S., Rengel, Z., & Solaiman, Z. M. (2023). A Review on Remediation of Iron Ore Mine Tailings via Organic Amendments Coupled with Phytoremediation. Plants, 12(9), 1871. https://doi.org/10.3390/plants12091871











