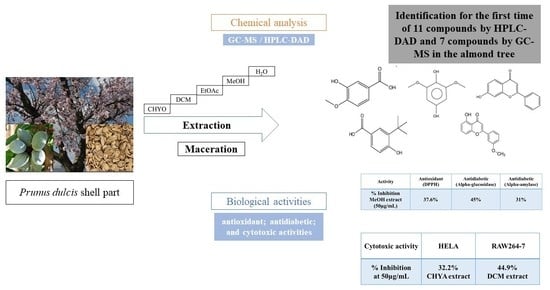
Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts
Abstract
1. Introduction
2. Results
2.1. Extraction Yield
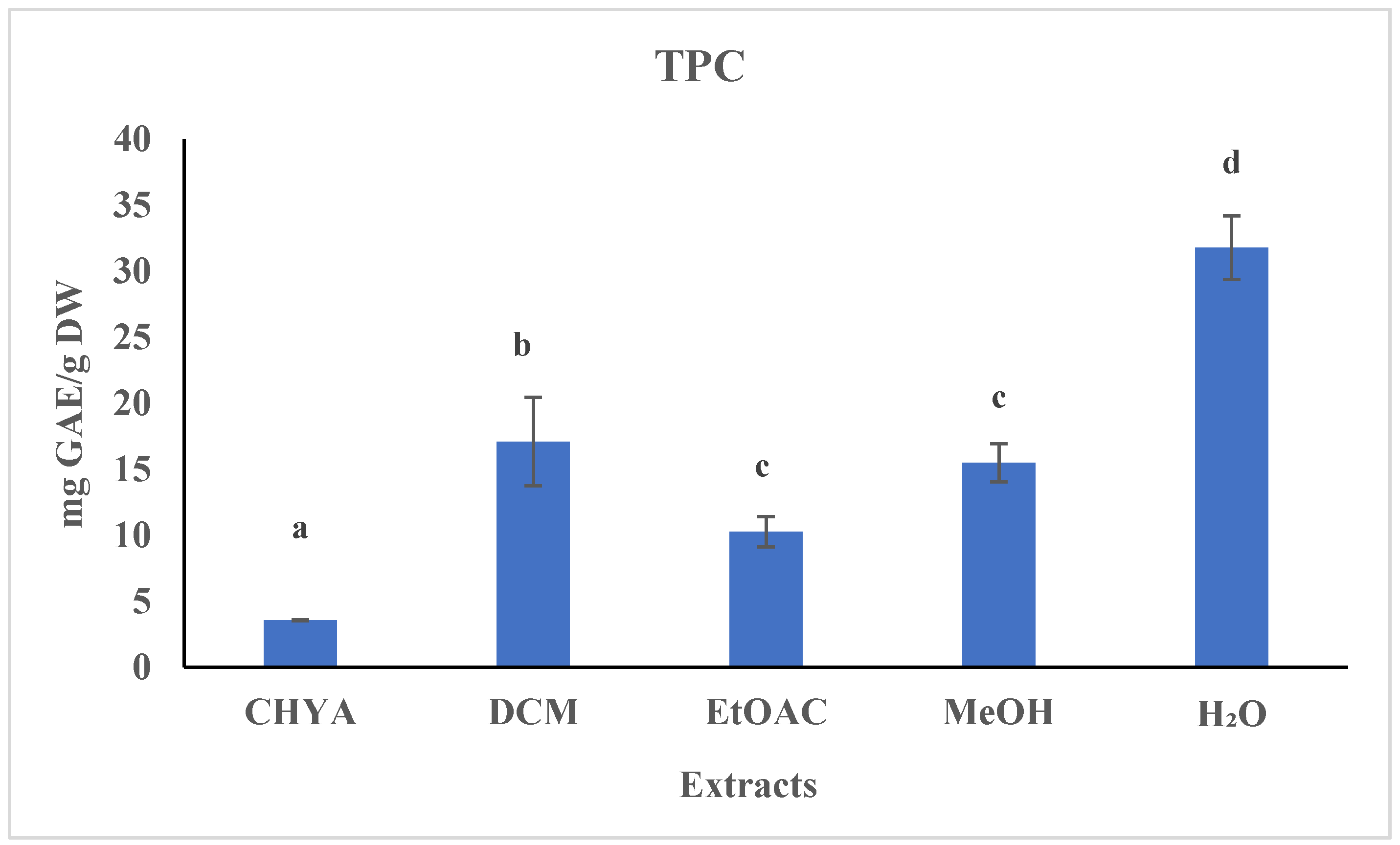
2.2. Determination of the Total Polyphenol Content (TPC)
2.3. Determination of the Antioxidant Activity (DPPH) of Shell Extracts
2.4. Chromatographic Analysis
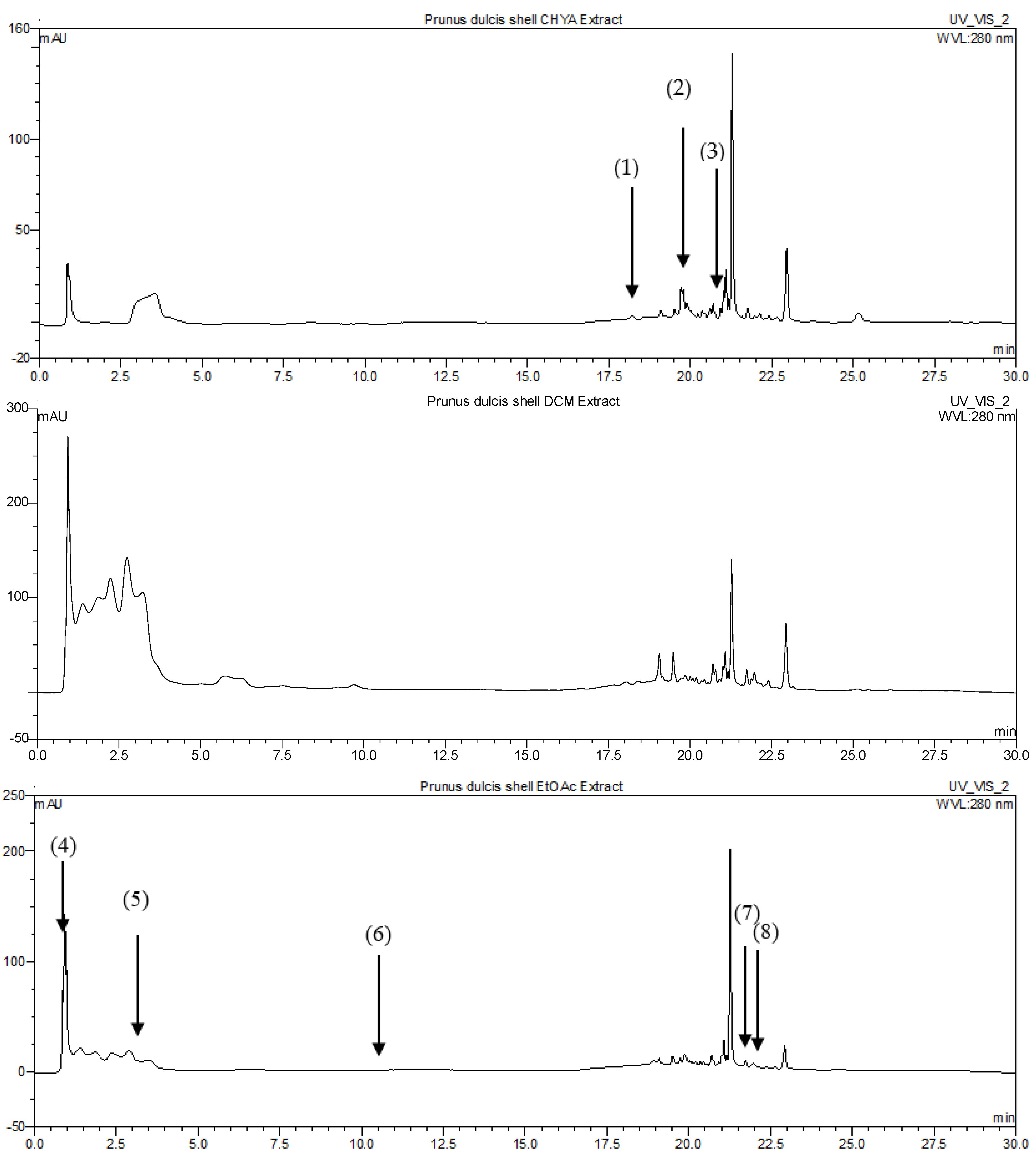
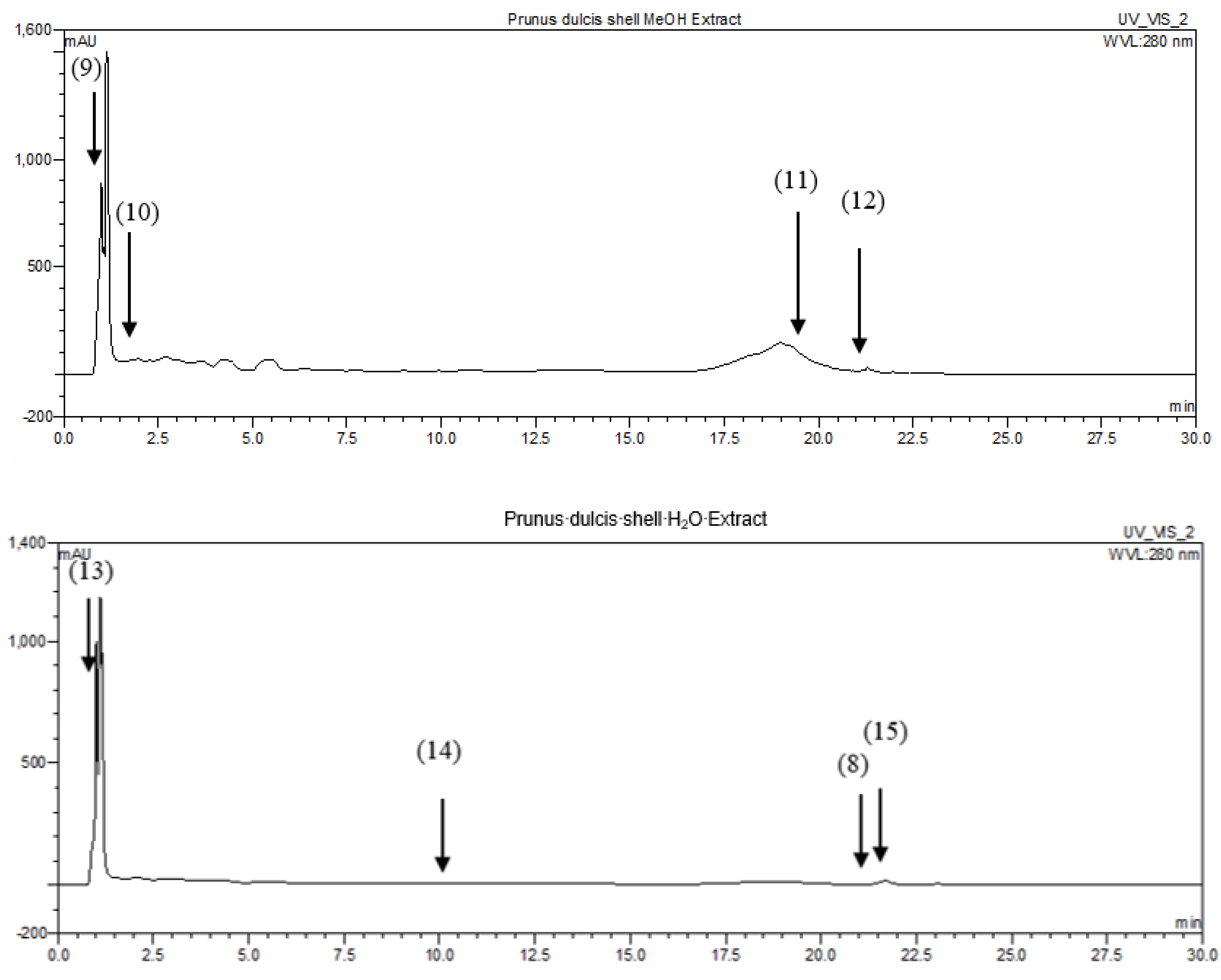
2.4.1. Identification of P. dulcis Shell Extracts Compounds by HPLC-DAD
2.4.2. Identification of Volatile Compounds in P. dulcis Shell Extracts by GC-MS (before and after Derivatization)
2.5. Biological Activities
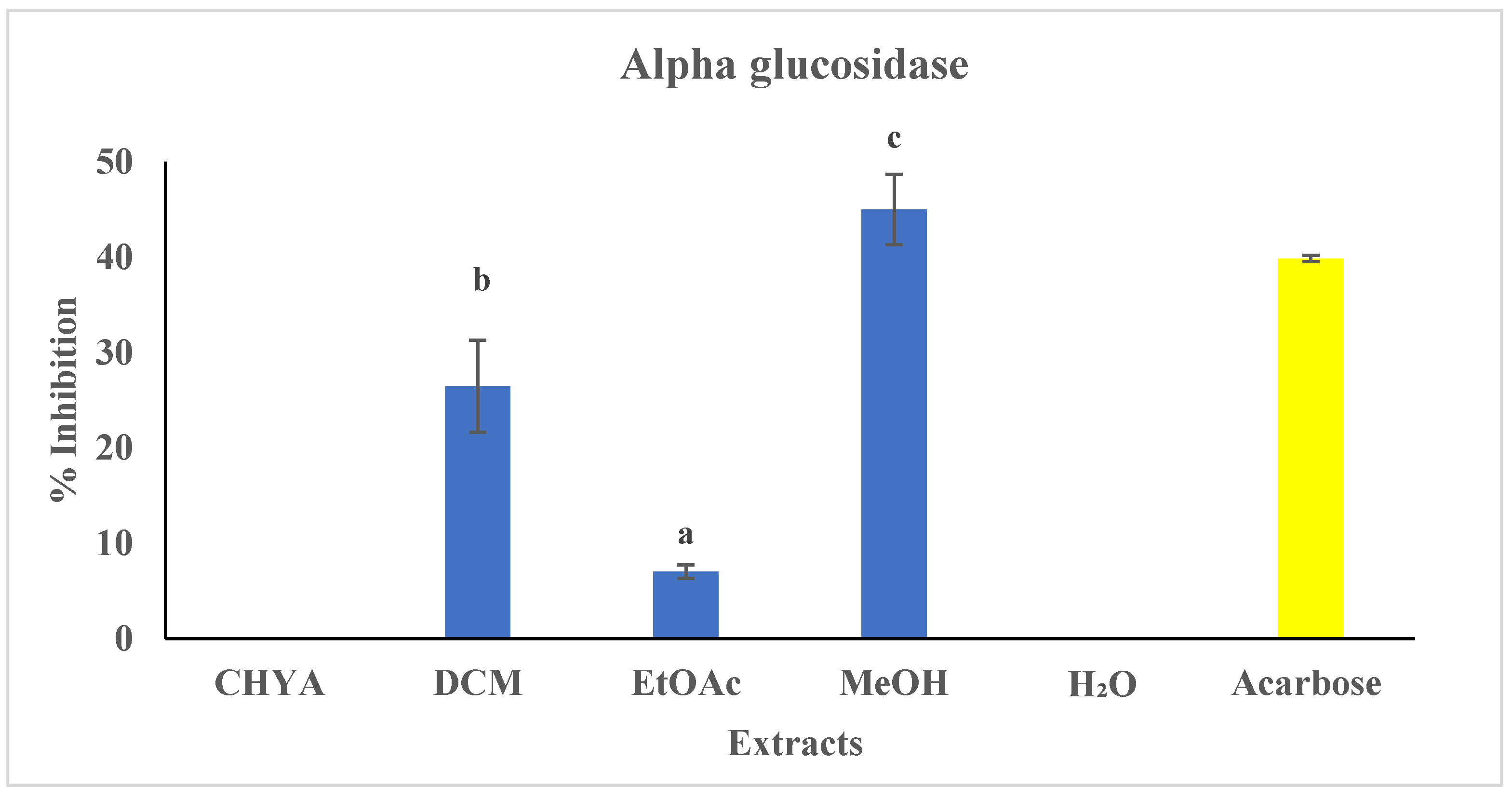
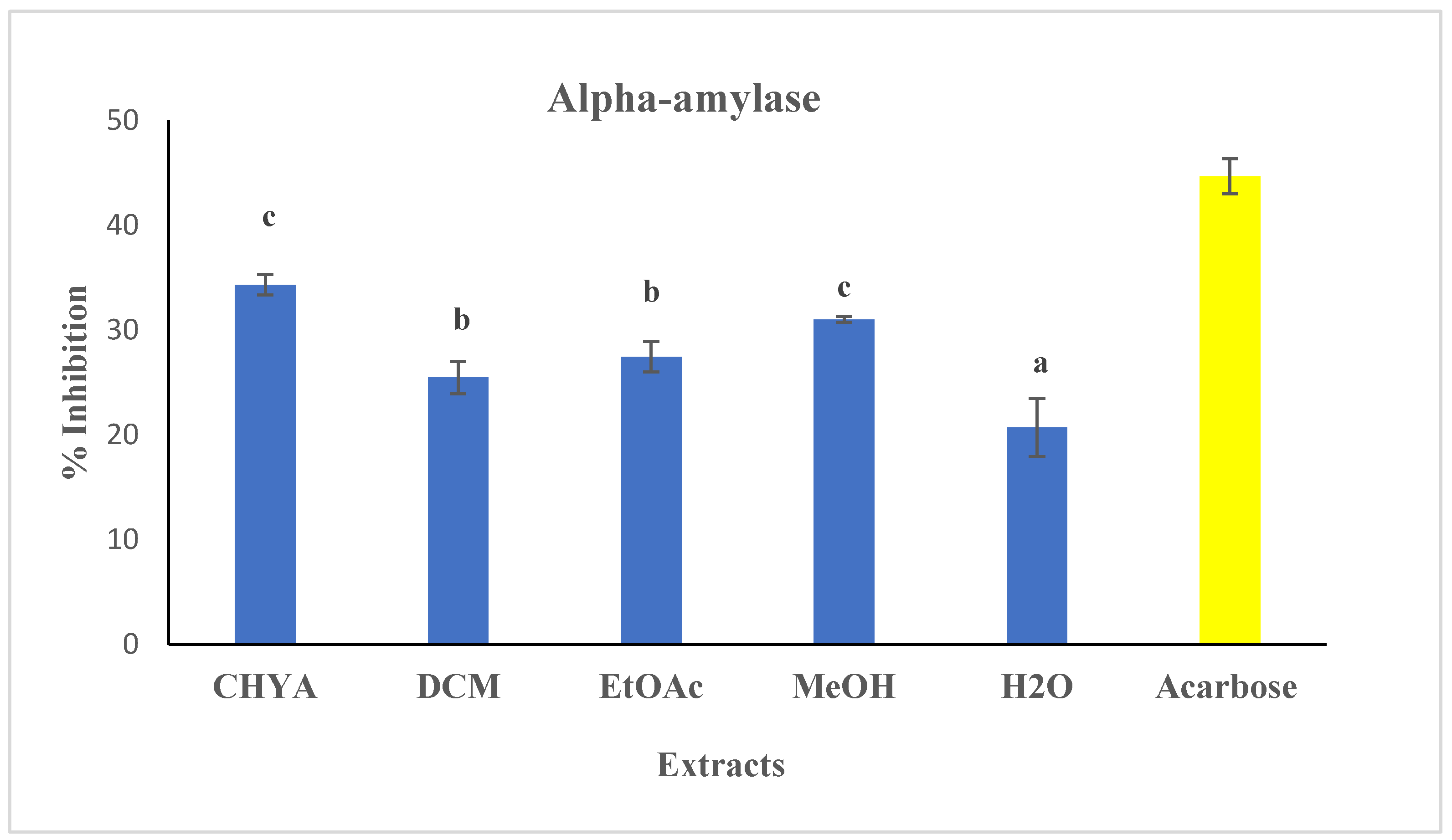
2.5.1. Antidiabetic Activity
- α-glucosidase activity:
- Alpha-amylase assay:
2.5.2. Cytotoxic Activity
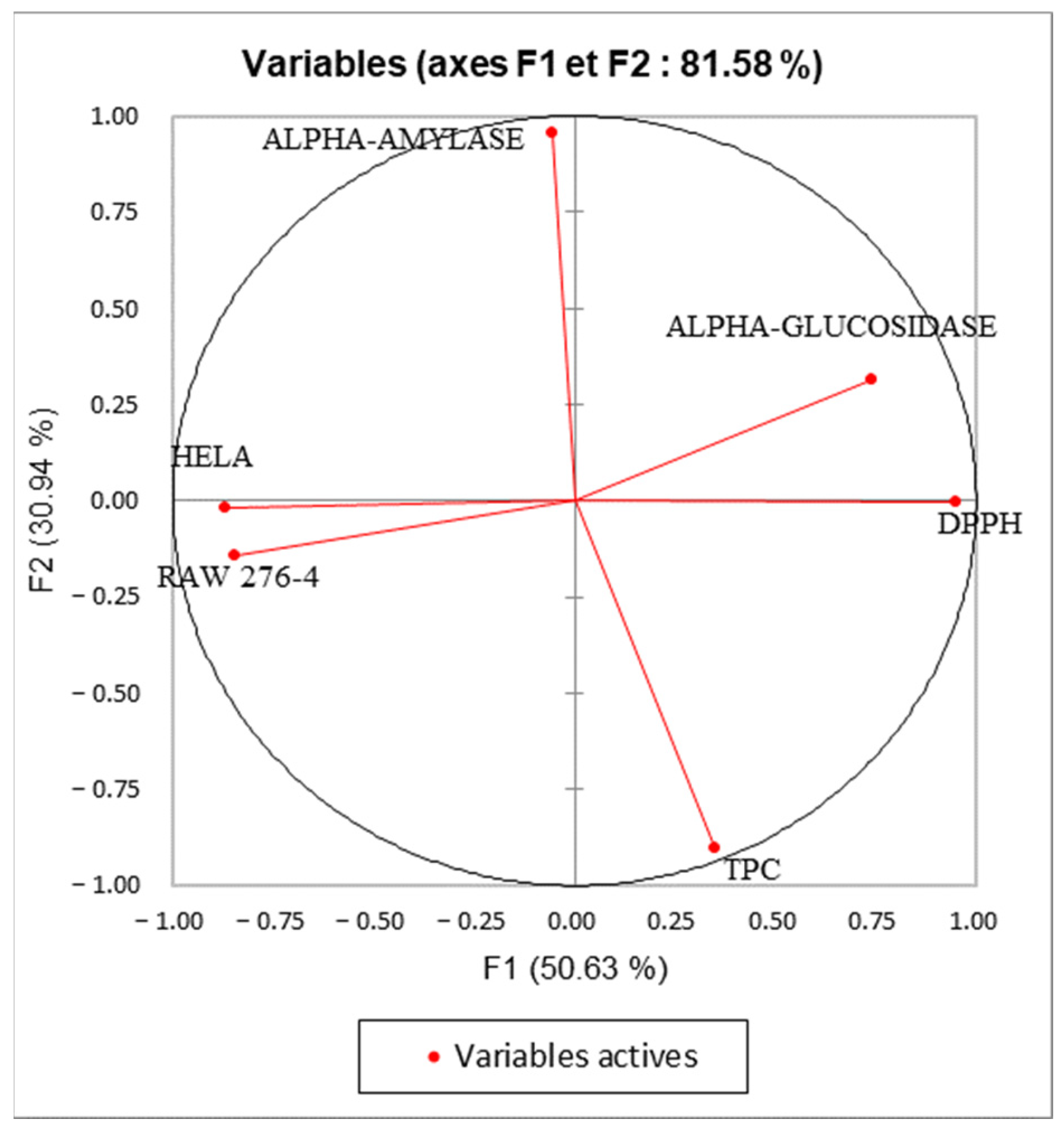
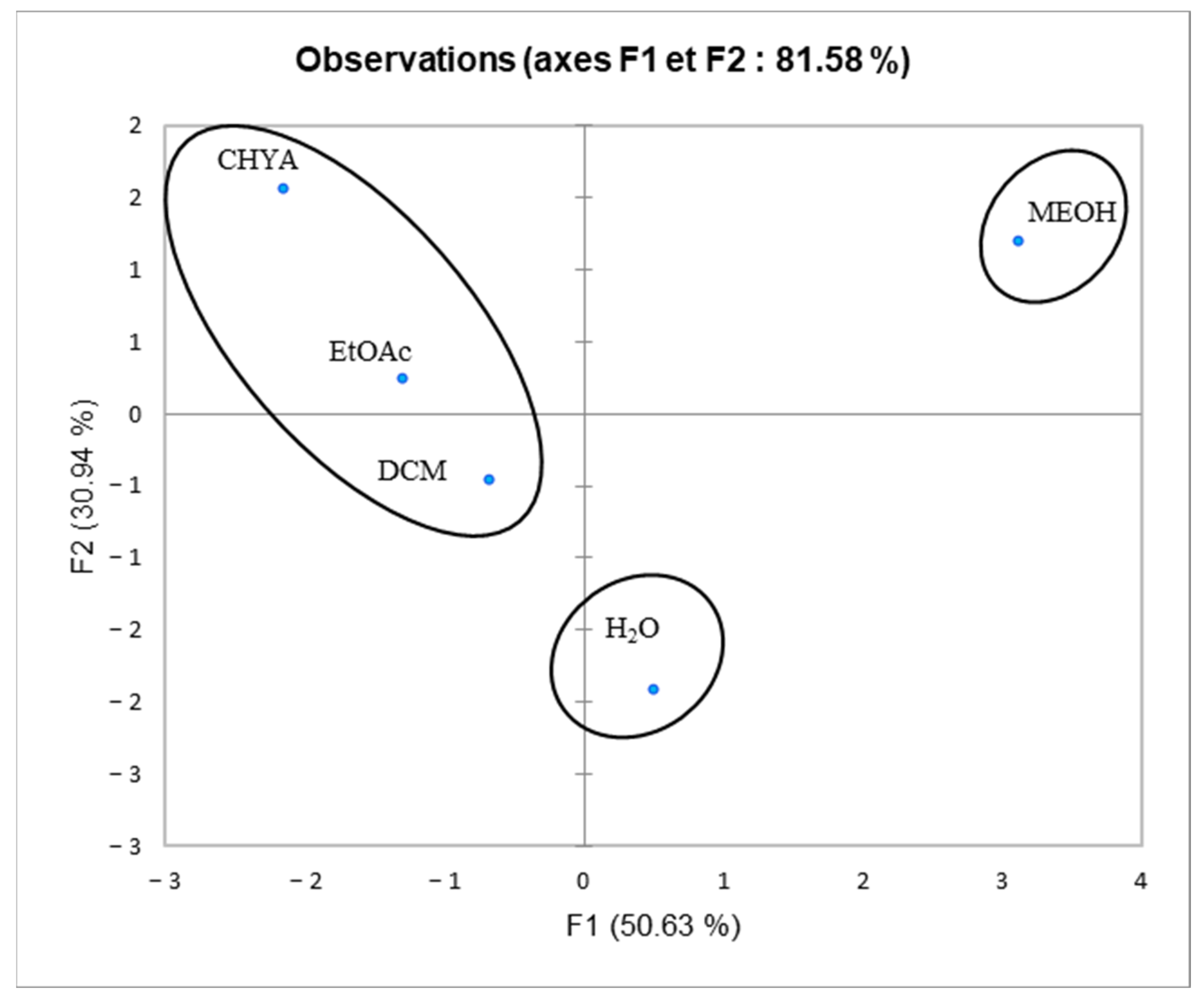
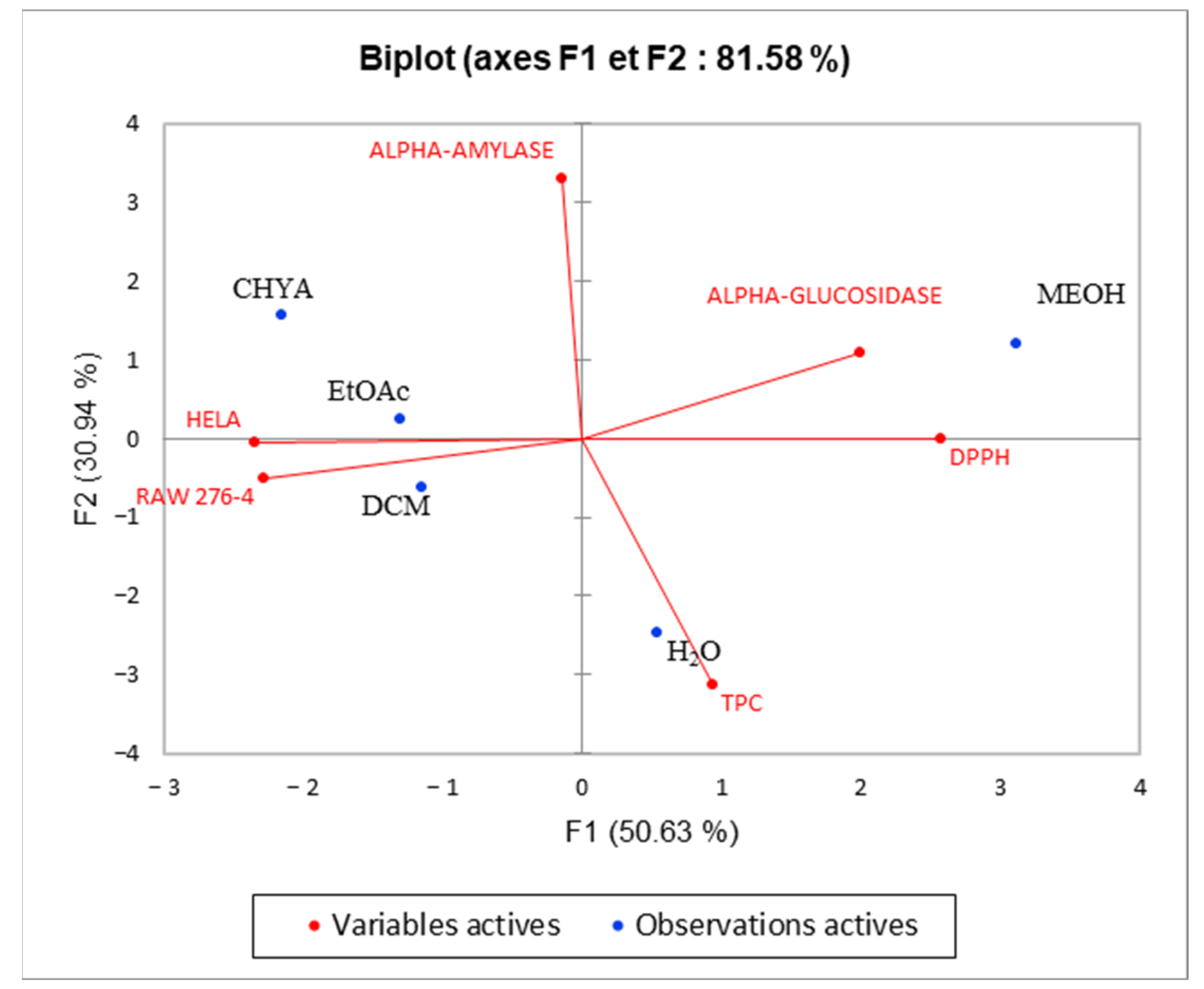
2.6. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
- Maceration:
3.3. Total Phenolic Content (TPC)
3.4. Determination of Radical Scavenging Activity (DPPH)
3.5. Biological Activity
3.5.1. Antidiabetic Activity
- Anti-α-Amylase Activity:
- Anti-α-glucosidase Activity:
3.5.2. Cytotoxic Activity
3.6. Chromatographic Analysis
3.6.1. High-Performance Liquid Chromatography Analysis (HPLC-DAD)
3.6.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
- Derivatization method:
- Compounds identification:
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qureshi, M.N.; Numonov, S.; Abudurexiti, A.; Aisa, H.A. Phytochemical Investigations and Evaluation of Antidiabetic Potential of Prunus dulcis Nuts. LWT Food Sci. Technol. 2016, 66, 311–317. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus amygdalus L.) and Its by-Products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from Almond (Prunus dulcis) Shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of Walnut, Almond, and Pine Nut Shells Regarding Chemical Composition and Extract Composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Sarwar, S.; Anwar, F.; Raziq, S.; Nadeem, M.; Zreen, Z.; Cecil, F. Antioxidant Characteristics of Different Solvent Extracts from Almond (Prunus dulcis L.) Shell. JMPR 2012, 6, 3311–3316. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2015, 2016, 5692852. [Google Scholar] [CrossRef]
- Moure, A.; Pazos, M.; Medina, I.; Domínguez, H.; Parajó, J.C. Antioxidant Activity of Extracts Produced by Solvent Extraction of Almond Shells Acid Hydrolysates. Food Chem. 2007, 101, 193–201. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef]
- Slimestad, R.; Vangdal, E.; Brede, C. Analysis of Phenolic Compounds in Six Norwegian Plum Cultivars (Prunus domestica L.). J. Agric. Food Chem. 2009, 57, 11370–11375. [Google Scholar] [CrossRef]
- He, D.; Shan, Y.; Wu, Y.; Liu, G.; Chen, B.; Yao, S. Simultaneous Determination of Flavanones, Hydroxycinnamic Acids and Alkaloids in Citrus Fruits by HPLC-DAD–ESI/MS. Food Chem. 2011, 127, 880–885. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Mozetič, B.; Simčič, M.; Trebše, P. Anthocyanins and Hydroxycinnamic Acids of Lambert Compact Cherries (Prunus avium L.) after Cold Storage and 1-Methylcyclopropene Treatment. Food Chem. 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Davarcı, F.; Paslı, A.A.; Demir, N.; Özçelik, B. Determination of Phenolic Compounds by Ultra High Liquid Chromatography-Tandem Mass Spectrometry: Applications in Nuts. LWT Food Sci. Technol. 2015, 64, 42–49. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Bouajila, J.; Cazaux, S.; Abderrabba, M. The Impact of Regional Locality on Chemical Composition, Anti-Oxidant and Biological Activities of Thymelaea hirsuta L. Extracts. Phytomedicine 2018, 41, 13–23. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Ao, W.-L.-J.; Wang, X.-L.; Bao, X.-H.; Wang, J.-H. Two New Flavonoid Glycosides from Artemisia frigida Willd. J. Asian Nat. Prod. Res. 2010, 12, 950–954. [Google Scholar] [CrossRef]
- Sancho, M.I.; Almandoz, M.C.; Blanco, S.E.; Castro, E.A. Spectroscopic Study of Solvent Effects on the Electronic Absorption Spectra of Flavone and 7-Hydroxyflavone in Neat and Binary Solvent Mixtures. Int. J. Mol. Sci. 2011, 12, 8895–8912. [Google Scholar] [CrossRef]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of Shikonin and Its Ester Derivatives from the Roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Ashrafian, S.; Farimani, M.M.; Sonboli, A.; Ashrafian, H.; Kabiri, M.; Rezadoost, H. Simultaneous Characterization of Nine Isolated Flavonoids in Iranian Dracocephalum Species and in Silico Study of Their Inhibitory Properties against MTH1 Enzyme. S. Afr. J. Bot. 2022, 146, 254–261. [Google Scholar] [CrossRef]
- Erdenetsogt, U.; Nadmid, S.; Paulus, C.; Chanagsuren, G.; Dolgor, E.; Gotov, C.; Dahse, H.-M.; Luzhetskyy, A.; Dagvadorj, E. Bioactive Flavonoids from Plant Extract of Pyrethrum pulchrum and Its Acute Toxicity. Nat. Prod. Res. 2021, 35, 5960–5963. [Google Scholar] [CrossRef] [PubMed]
- Sei-Ichi, K.; Toda, K.; Matsumoto, K.; Ishihara, C.; Nonobe, S.; Matsunaga, C.; Gomi, Y.K.; Senga, S.; Kawaguchi, K.; Yamamoto, A. Isolation and Characterization of a Novel Oligomeric Proanthocyanidin with Significant Anti-Cancer Activities from Grape Stems (Vitis vinifera). Sci. Rep. 2019, 9, 12046. [Google Scholar] [CrossRef] [PubMed]
- Bukvicki, D.R.; Tyagi, A.K.; Gottardi, D.G.; Veljic, M.M.; Jankovic, S.M.; Guerzoni, M.E.; Marin, P.D. Assessment of the Chemical Composition and In Vitro Antimicrobial Potential of Extracts of the Liverwort Scapania aspera. Nat. Prod. Commun. 2013, 8, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical Composition, Cytotoxicity and Molecular Profiling of Cordia africana Lam. on Human Breast Cancer Cell Line. Nat. Prod. Res. 2021, 35, 4133–4138. [Google Scholar] [CrossRef]
- Rath, D.; Panigrahi, S.K.; Kar, D.M.; Maharana, L. Identification of Bioactive Constituents from Different Fractions of Stems of Cuscuta reflexa Roxb. Using GC-MS. Nat. Prod. Res. 2018, 32, 1977–1981. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical Composition and Antifungal Properties of Essential Oils of Three Pistacia Species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Goyal, M. Qualitative and Quantitative Evaluation of Phytochemicals in Leaf Extract of Alstonia scholaris (L.) R. Br.By GC-MS Technique. Int. J. Pharm. Biol. Sci. IJPBST 2019, 9, 323–328. [Google Scholar]
- Guedes De Pinho, P.; Gonçalves, R.F.; Valentão, P.; Pereira, D.M.; Seabra, R.M.; Andrade, P.B.; Sottomayor, M. Volatile Composition of Catharanthus roseus (L.) G. Don Using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 674–685. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. BioMed Res. Int. 2022, 2022, e5938610. [Google Scholar] [CrossRef]
- Yetayih, M. Extraction and GC-MS Analysis of the Essential Oil from the Peel of Solanum incanum and Its Antibacterial Activity Studies. Asian J. Chem. 2020, 32, 2001–2006. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, I.T.; Nedelcheva, D.V.; Parushev, S.P.; Atanassov, A.I.; Hvarleva, T.D.; Djakova, G.J.; Bankova, V.S.; Popov, S.S. Preliminary Study on Biomarkers for the Fungal Resistance in Vitis vinifera Leaves. J. Plant Physiol. 2008, 165, 791–795. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis vinifera Cv. Pinot Noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Kumar, A. GC-MS Analysis of Phytochemical Constituents in Ethanolic Extract of Punica granatum Peel and Vitis vinifera Seeds. Int. J. Pharma Bio Sci. 2011, 2, 461–468. [Google Scholar]
- Orsavova, J.-M.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Sermakkani, M.; Thangapandian, V. GC-MS Analysis of Cassia italica Leaf Methanol Extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Fiehn, O.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Identification of Uncommon Plant Metabolites Based on Calculation of Elemental Compositions Using Gas Chromatography and Quadrupole Mass Spectrometry. Anal. Chem. 2000, 72, 3573–3580. [Google Scholar] [CrossRef]
- Eldjoudi, D.A.; Ruiz-Fernandez, C.; González-Rodriguez, M.; Atmane, S.A.; Cordero-Barreal, A.; Farrag, Y.; Pino, J.; Sineiro, J.; Lago, F.; Conde-Aranda, J.; et al. Analgesic and Antiinflammatory Effects of Nigella orientalis L. Seeds Fixed Oil: Pharmacological Potentials and Molecular Mechanisms. Phytother. Res. 2022, 36, 1372–1385. [Google Scholar] [CrossRef]
- Komakech, R.; Shim, K.-S.; Yim, N.-H.; Song, J.H.; Yang, S.; Choi, G.; Lee, J.; Kim, Y.; Omujal, F.; Okello, D.; et al. GC–MS and LC-TOF–MS Profiles, Toxicity, and Macrophage-Dependent in Vitro Anti-Osteoporosis Activity of Prunus africana (Hook f.) Kalkman Bark. Sci. Rep. 2022, 12, 7044. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayı, H.; Hanoglu, A.; Yigit Hanoglu, D.; Ozkum Yavuz, D.; Ozek, T.; Vatansever, S. Fatty Acid Composition and Anticancer Activity in Colon Carcinoma Cell Lines of Prunus dulcis Seed Oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative Terpenoids from Almond Hulls (Prunus dulcis): Identification and Structure—Activity Relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Bouajila, J.; Jouaidi, M.; Debouba, M. African Mustard (Brassica tournefortii) as Source of Nutrients and Nutraceuticals Properties. J. Food Sci. 2020, 85, 1856–1871. [Google Scholar] [CrossRef] [PubMed]


| Fractional Extraction | |||||
|---|---|---|---|---|---|
| CHYA | DCM | EtOAc | MeOH | H2O | |
| Maceration | 0.14 | 0.09 | 0.03 | 0.63 | 2.22 |
| Compounds | Rt (min) | Extracts | Ref. | ||||
|---|---|---|---|---|---|---|---|
| CHYA | DCM | EtOAc | MeOH | H2O | |||
| Catechin | 0.87 | 0.39 | [10] | ||||
| Rutin | 0.93 | 13.06 | [11] | ||||
| Synephrine | 1.09 | 1.62 | [12] | ||||
| Epicatechin | 1.9 | 4.42 | [13] | ||||
| trans-3-hydroxycinnamic acid | 3.5 | 0.08 | [14] | ||||
| trans-cinnamic acid | 10.8 | 0.02 | [15] | ||||
| 7,8-dihydroxy-2,2-dimethylchromane-6-carboxylic acid | 10.93 | 0.31 | [16] | ||||
| 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone | 19.51 | 0.31 | [17] | ||||
| 3-tert-butyl-4-hydroxybenzoic acid | 19.55 | 0.02 | [16] | ||||
| 7-hydroxyflavone | 19.71 | 0.59 | [18] | ||||
| Shikonin | 20.72 | 0.19 | [19] | ||||
| Isobutyl 4-hydroxybenzoate | 21.02 | 0.25 | [16] | ||||
| 3,3′-dimethoxyflavone | 21.59 | 0.23 | [20] | ||||
| 3,6,3′-trimethoxyflavone | 21.74 | 0.33 | 0.11 | [21] | |||
| 5-hydroxy-3′-methoxyflavone | 21.95 | 0.33 | [22] | ||||
| N | RI | Compounds | Area (107) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Extracts | ||||||||
| CHYA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1075 | 2,3-Dimethyldecane | 12.6 | [23] | ||||
| 2 | 1100 | Undecane | 0.0922 | [24] | ||||
| 3 | 1205 | Dodecane | 6.76 | [25] | ||||
| 4 | 1306 | Tridecane | 1.63 | [26] | ||||
| 5 | 1377 | 1,1′-Bicyclohexyl | 25,300 | 4.06 | [26] | |||
| 6 | 1535 | Benzaldehyde, 3-hydroxy-4-methoxy- | 370 | 4.99 | [25] | |||
| 7 | 1560 | 2,4-Di-tert-butylphenol | 6.24 | 3.55 | 7.868 | [27] | ||
| 8 | 1566 | Dodecanoic acid, 1-methylethyl ester | 3.69 | [28] | ||||
| N | RI | Compounds | Area (107) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Extracts | ||||||||
| CHYA | DCM | EtOAc | MeOH | H2O | ||||
| 1 | 1025 | Cyclohexanol | 15.4 | 4.02 | [29] | |||
| 2 | 1038 | Furfuryl alcohol | 3.13 | 14.3 | 39.1 | 23.4 | [29] | |
| 3 | 1075 | Lactic Acid | 45.9 | 58.2 | 86.5 | [30] | ||
| 4 | 1094 | Hexanoic acid | 15.1 | [30] | ||||
| 5 | 1094 | Glycolic acid | 121 | [30] | ||||
| 6 | 1162 | Hydracrylic acid | 13.8 | 32.9 | [31] | |||
| 7 | 1266 | Glycerol | 88.9 | 1130 | 621 | [26] | ||
| 8 | 1338 | Glyceric acid | 41.7 | 109 | [28] | |||
| 9 | 1351 | Butanedioic acid | 97.9 | 0.691 | [32] | |||
| 10 | 1384 | Nonanoic acid | 51.6 | [26] | ||||
| 11 | 1500 | Malic acid | 1760 | 1310 | [33] | |||
| 12 | 1591 | 2,4-Di-tert-butylphenol | 22.2 | [27] | ||||
| 13 | 1672 | Dodecanoic acid | 23.8 | [34] | ||||
| 14 | 1865 | Myristic acid | 12.8 | [34] | ||||
| 15 | 1959 | Pentadecanoic acid | 10.6 | [33] | ||||
| 16 | 2054 | Palmitic acid | 511 | 4.60 | 2.84 | [35] | ||
| 17 | 2074 | Myo-Inositol | 428 | 1150 | [36] | |||
| 18 | 2241 | 9-octadecenoic acid, (E)- | 278 | [37] | ||||
| 19 | 2248 | Stearic acid | 165 | 121 | 392 | [38] | ||
| F1 | F2 | |
|---|---|---|
| TPC | 0.121 | 0.818 |
| DPPH | 0.903 | 0.000 |
| ALPHA-GLUCOSIDASE | 0.547 | 0.100 |
| ALPHA-AMYLASE | 0.003 | 0.917 |
| RAW 276-4 | 0.711 | 0.021 |
| HELA | 0.753 | 0.000 |
| Variables | TPC | DPPH | ALPHA-GLUCOSIDASE | ALPHA-AMYLASE | RAW 276-4 | HELA |
|---|---|---|---|---|---|---|
| TPC | 1 | 0.388 | −0.018 | −0.828 | −0.150 | −0.209 |
| DPPH | 0.388 | 1 | 0.680 | 0.011 | −0.757 | −0.762 |
| ALPHA-GLUCOSIDASE | −0.018 | 0.680 | 1 | 0.185 | −0.490 | −0.535 |
| ALPHA-AMYLASE | −0.828 | 0.011 | 0.185 | 1 | −0.099 | 0.063 |
| RAW 276-4 | −0.150 | −0.757 | −0.490 | −0.099 | 1 | 0.679 |
| HELA | −0.209 | −0.762 | −0.535 | 0.063 | 0.679 | 1 |
| N° | Compound | RT | Calibration Curve | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| 1 | Catechin | 0.87 | y = 0.7845x − 2.2956 | 0.055 | 0.181 |
| 2 | Rutin | 0.93 | y = 0.1074x + 0.357 | 0.125 | 0.412 |
| 3 | Synephrine | 1.09 | y = 0.385x + 0.5679 | 0.003 | 0.010 |
| 4 | Epicatechin | 1.9 | y = 0.7845x − 2.2956 | 0.087 | 0.289 |
| 5 | trans-3-hydroxycinnamic acid | 3.5 | y = 5.134x − 0.7396 | 0.002 | 0.008 |
| 6 | trans-cinnamic acid | 10.8 | y = 5.134x − 0.7396 | 0.047 | 0.156 |
| 7 | 7,8-dihydroxy-2,2-dimethylchromane-6-carboxylic acid | 10.93 | y = 1.3215x + 2.8676 | 0.008 | 0.025 |
| 8 | 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone | 19.51 | y = 0.2384x + 1.3435 | 0.087 | 0.289 |
| 9 | 3-tert-butyl-4-hydroxybenzoic acid | 19.55 | y = 0.4259x − 0.082 | 0.009 | 0.029 |
| 10 | 7-hydroxyflavone | 19.71 | y = 1.3215x + 2.8676 | 0.226 | 0.746 |
| 11 | Shikonin | 20.72 | y = 0.1741x | 0.011 | 0.037 |
| 12 | Isobutyl 4-hydroxybenzoate | 21.02 | y = 0.4259x − 0.082 | 0.001 | 0.004 |
| 13 | 3,3′-dimethoxyflavone | 21.59 | y = 1.3215x + 2.8676 | 0.019 | 0.064 |
| 14 | 3,6,3′-trimethoxyflavone | 21.74 | y = 1.3215x + 2.8676 | 0.035 | 0.115 |
| 15 | 5-hydroxy-3′-methoxyflavone | 21.95 | y = 1.3215x + 2.8676 | 0.004 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Khadher, T.; Sassi-Aydi, S.; Aydi, S.; Mars, M.; Bouajila, J. Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts. Plants 2023, 12, 2733. https://doi.org/10.3390/plants12142733
Ben Khadher T, Sassi-Aydi S, Aydi S, Mars M, Bouajila J. Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts. Plants. 2023; 12(14):2733. https://doi.org/10.3390/plants12142733
Chicago/Turabian StyleBen Khadher, Talel, Sameh Sassi-Aydi, Samir Aydi, Mohamed Mars, and Jalloul Bouajila. 2023. "Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts" Plants 12, no. 14: 2733. https://doi.org/10.3390/plants12142733
APA StyleBen Khadher, T., Sassi-Aydi, S., Aydi, S., Mars, M., & Bouajila, J. (2023). Phytochemical Profiling and Biological Potential of Prunus dulcis Shell Extracts. Plants, 12(14), 2733. https://doi.org/10.3390/plants12142733