Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques
Abstract
1. Introduction
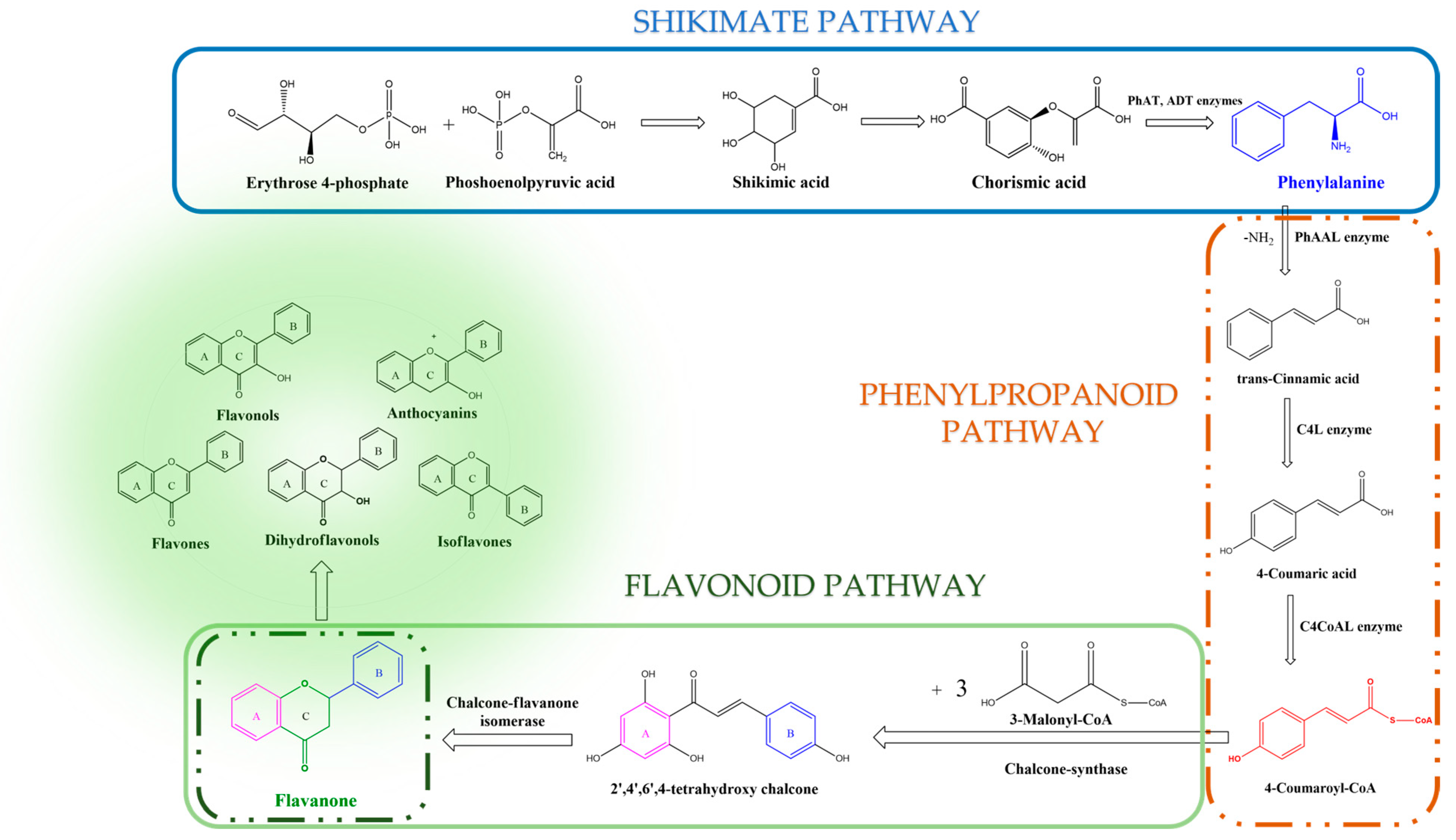
2. Biosynthesis of Flavonoids
3. Classification and Biological Activity
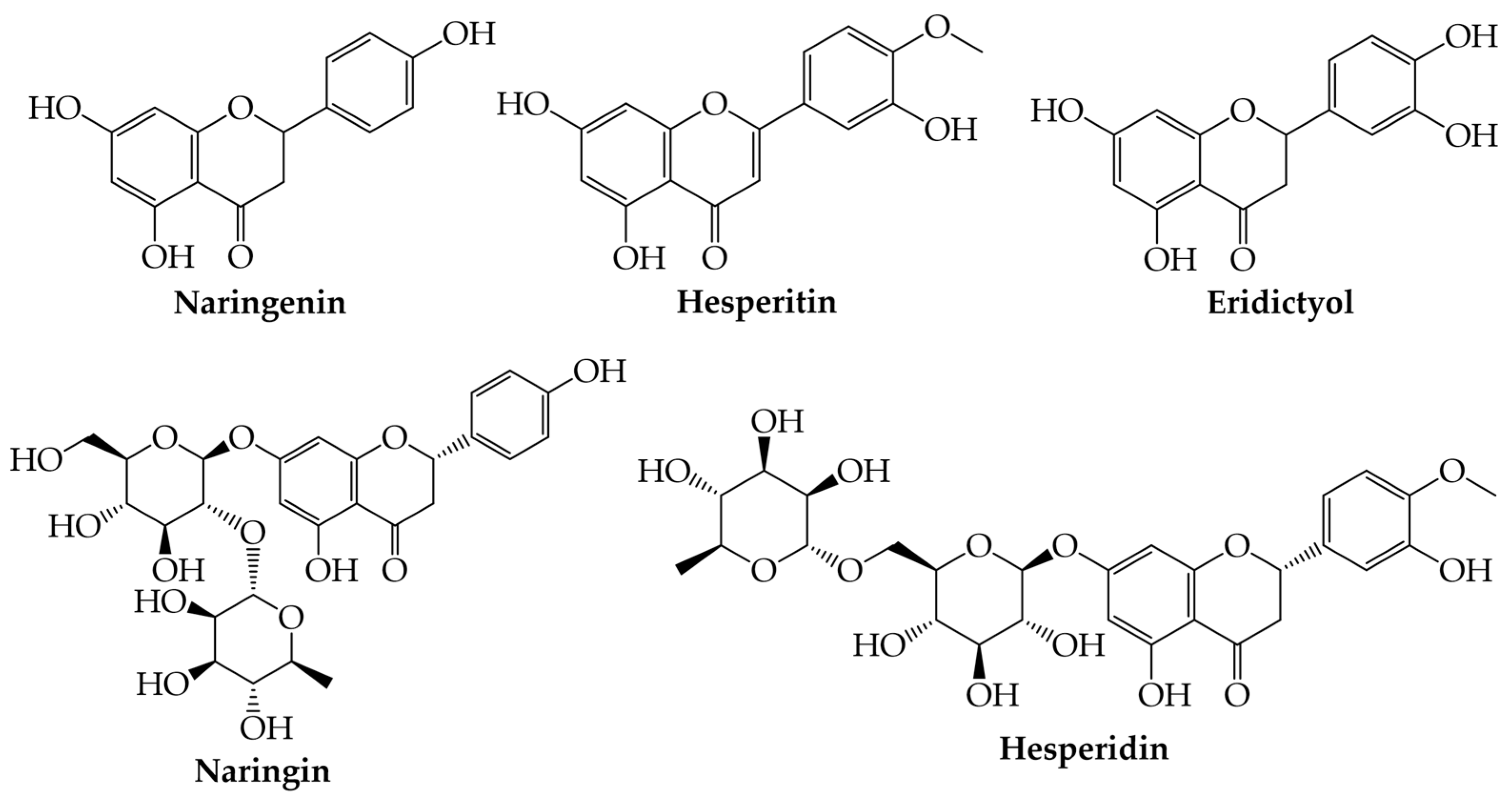
3.1. Flavanones
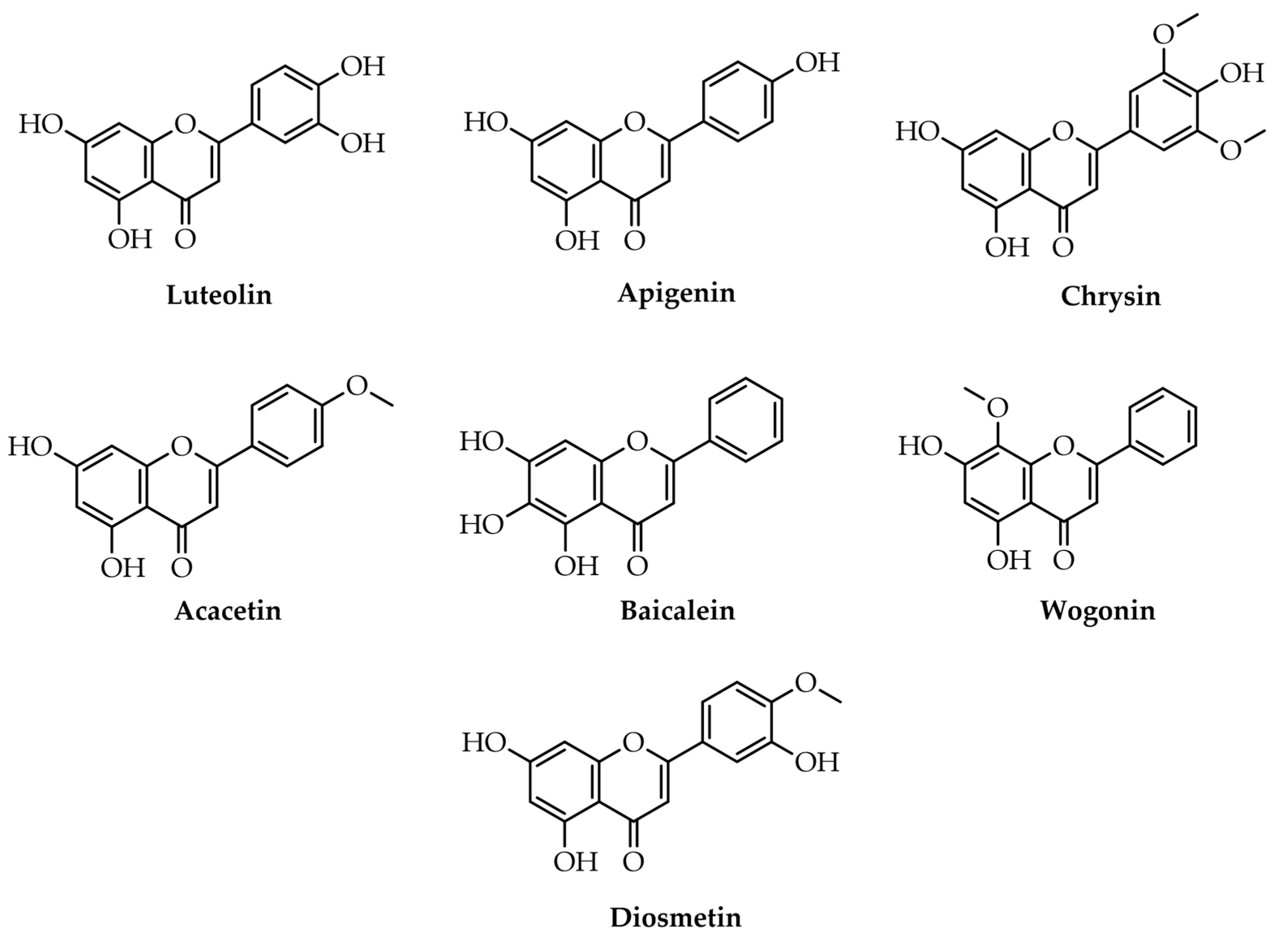
3.2. Flavones
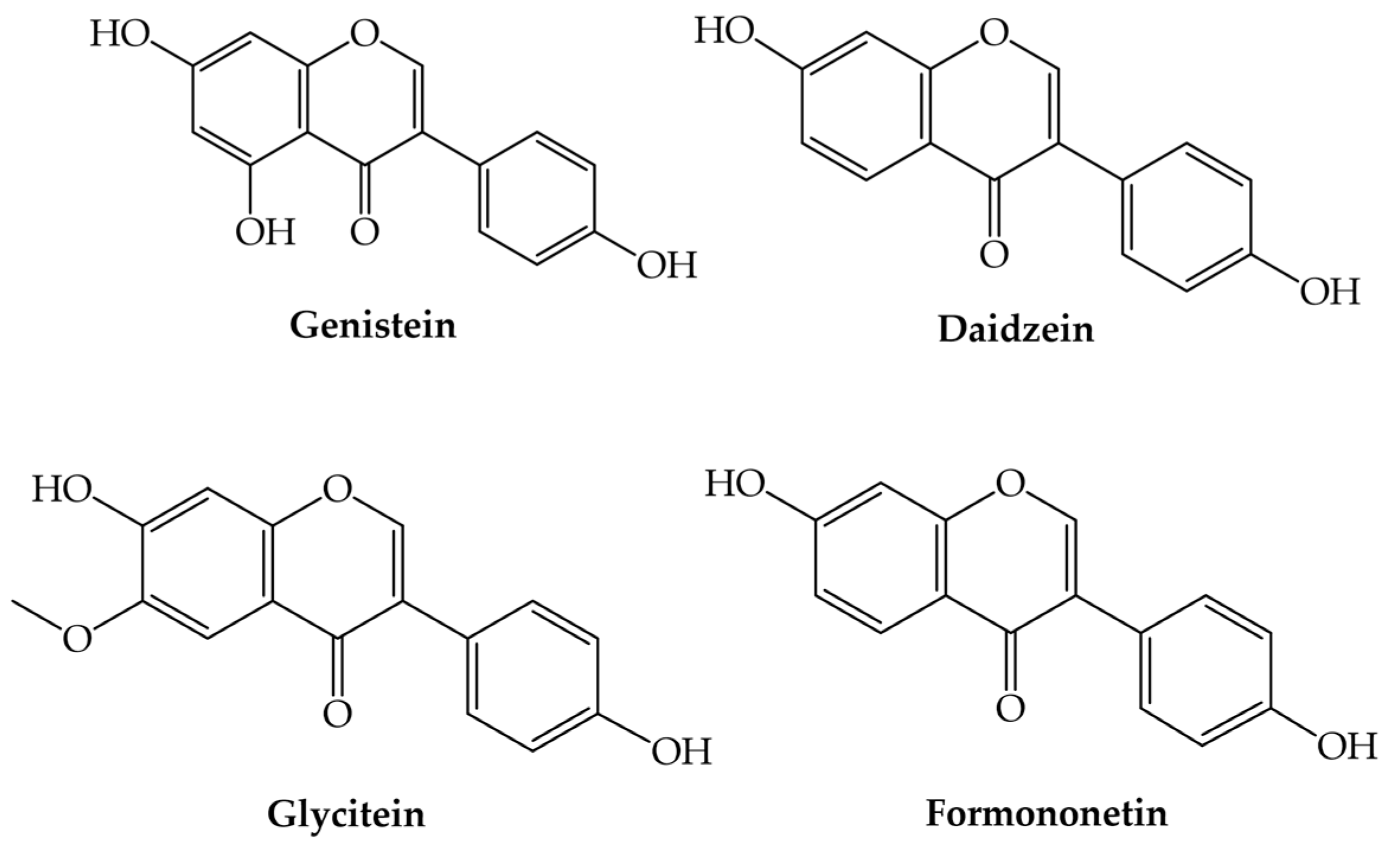
3.3. Isoflavones
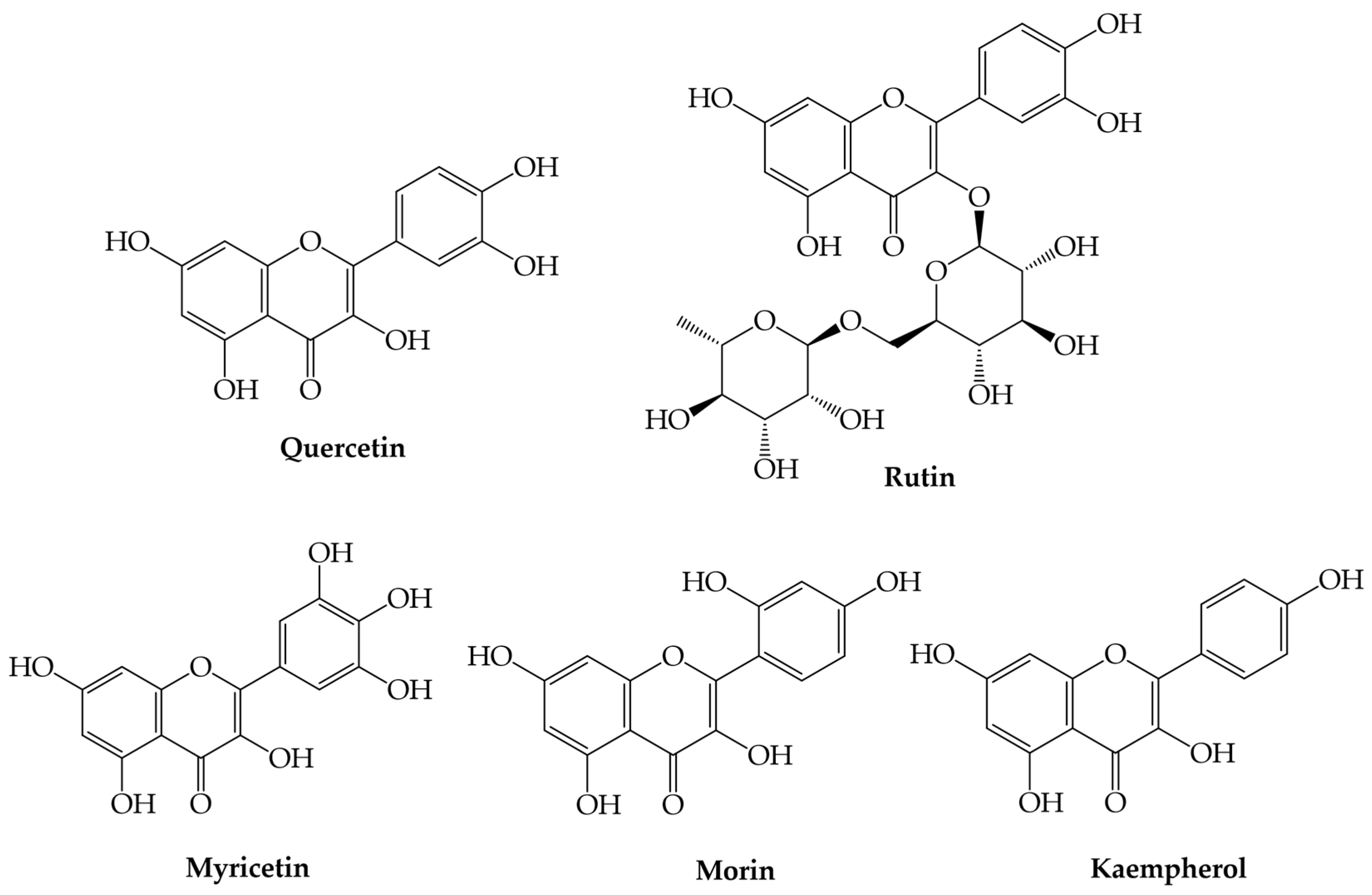
3.4. Flavonols
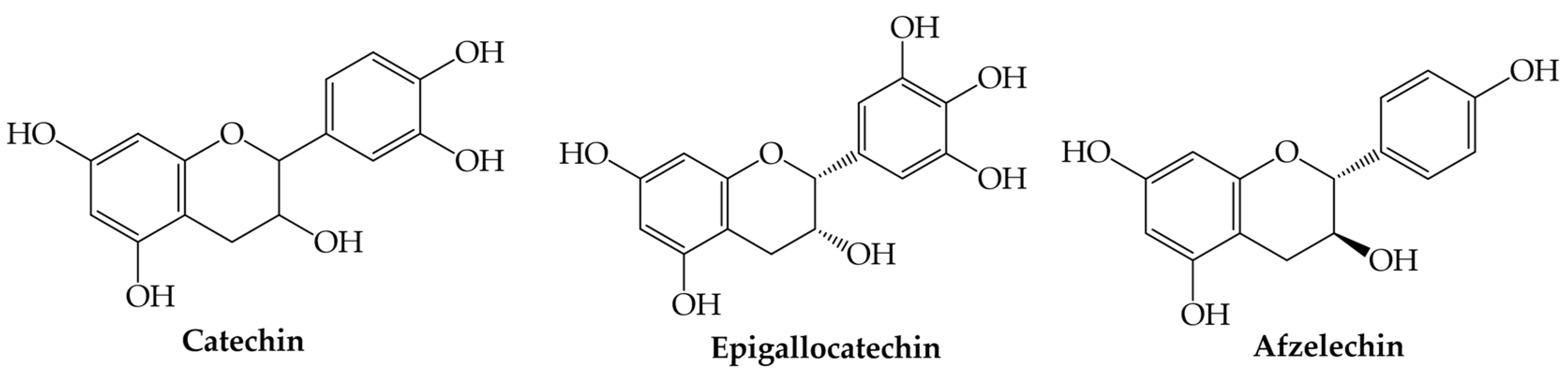
3.5. Flavanols
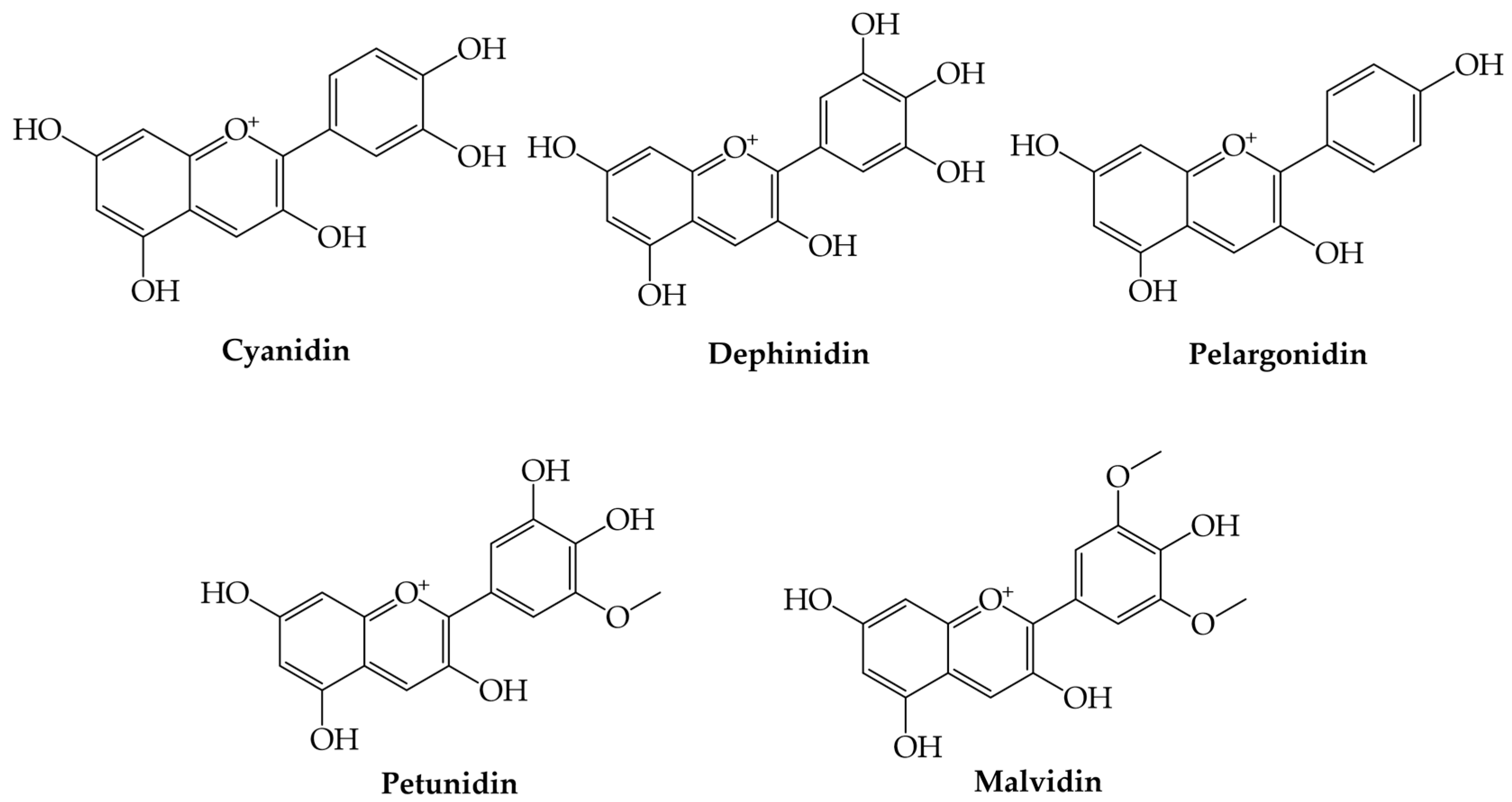
3.6. Anthocyanins
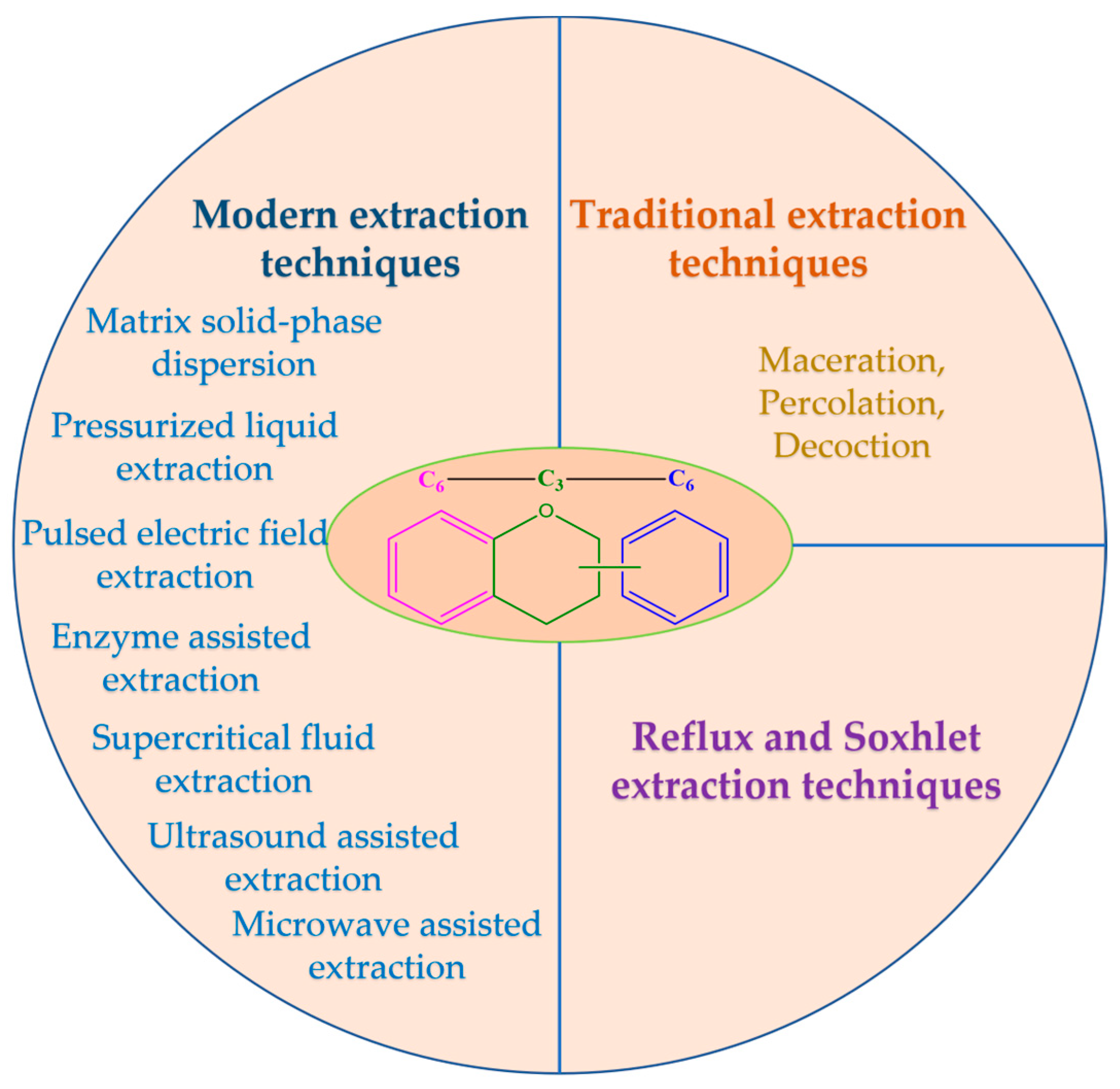
4. Actual Limitations of Extended Utilization of Flavonoids
5. Current Extraction Techniques
5.1. Traditional Extraction Techniques
5.2. Reflux and Soxhlet Extraction Techniques
5.3. Modern Extraction Techniques
5.3.1. Microwave-Assisted Extraction
5.3.2. Ultrasound-Assisted Extraction
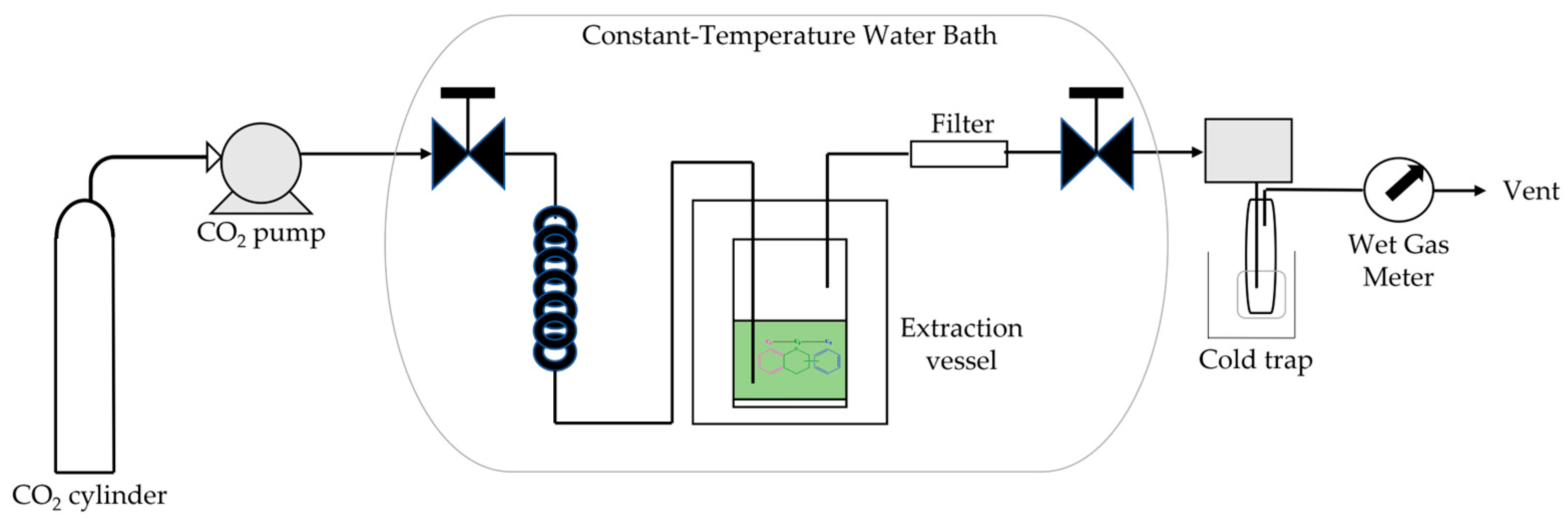
5.3.3. Supercritical Fluid Extraction
5.3.4. Matrix Solid-Phase Dispersion Extraction
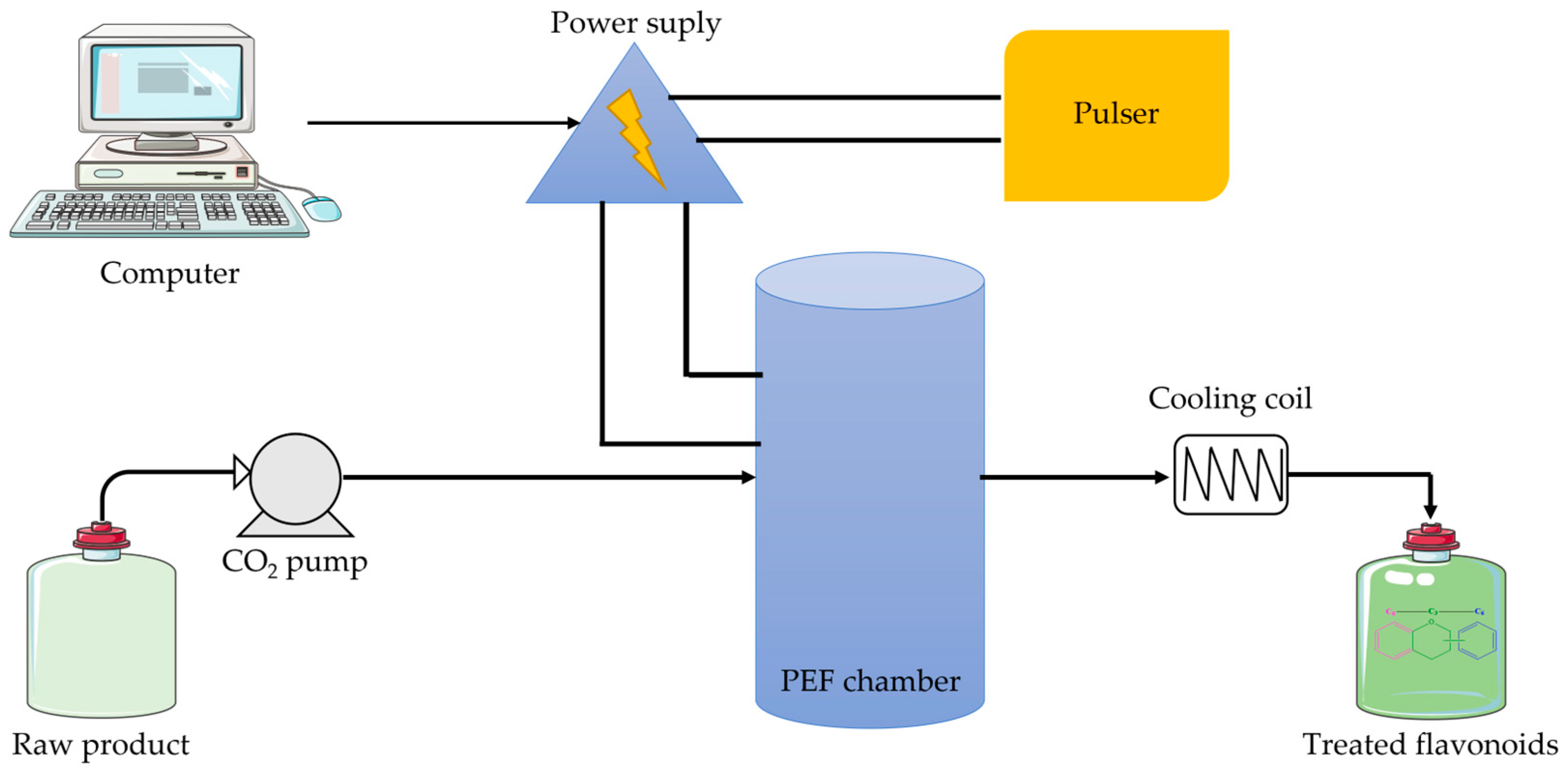
5.3.5. Pulsed Electric Field Extraction
5.3.6. Enzyme-Assisted Extraction
6. Outlook and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ADT | Arogenate-dehydratase |
| C4CoAL | 4-coumarate-coenzymeA-ligase |
| C4L | Cinnamate-4-hydroxylase |
| CoA | Coenzyme A |
| GAE | Gallic acid equivalent |
| IC50 | Half maximal inhibitory concentration |
| IFN | Interferon |
| LDL | Low-density lipoprotein |
| PhAAL | Phenylalanine-ammonia lipase |
| PhAT | Prephenate-amino-transferase |
| QE | Quercetin equivalents |
| TE | Trolox equivalent antioxidant capacity |
References
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Bioactive Compounds; Zepka, L.Q., Nascimento, T.C.D., Jacob-Lopes, E., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 6. [Google Scholar]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current Trends in Plant Metabolic Engineering and De Novo Microbial Production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Studies in Natural Products Chemistry; Rahman, A.-u., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 257–312. [Google Scholar]
- Brahmachari, G. Naturally Occurring Flavanones: An Overview. Nat. Prod. Commun. 2008, 3, 1934578X0800300820. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jiang, H.; Wu, Y.; Li, X.; Gao, L.; Wang, Y.; Jiang, J. Bacterial biosynthesis of flavonoids: Overview, current biotechnology applications, challenges, and prospects. J. Cell. Physiol. 2023. early view. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Chapter 7—Bioactive Phytocomponents and Their Analysis. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–328. [Google Scholar]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Wei, W.; Rao, F.; Liu, F.; Xue, Y.; Deng, C.; Wang, Z.; Zhu, J.; Yang, H.; Li, X.; Zhang, M.; et al. Involvement of Smad3 pathway in atrial fibrosis induced by elevated hydrostatic pressure. J. Cell. Physiol. 2018, 233, 4981–4989. [Google Scholar] [CrossRef]
- Chtourou, Y.; Fetoui, H.; Jemai, R.; Ben Slima, A.; Makni, M.; Gdoura, R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur. J. Pharmacol. 2015, 746, 96–105. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Patel, Y.M. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation. Breast Cancer Res. Treat. 2004, 85, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gumushan Aktas, H.; Akgun, T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed. Pharmacother. 2018, 106, 770–775. [Google Scholar] [CrossRef]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell. Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef]
- Song, H.M.; Park, G.H.; Eo, H.J.; Lee, J.W.; Kim, M.K.; Lee, J.R.; Lee, M.H.; Koo, J.S.; Jeong, J.B. Anti-Proliferative Effect of Naringenin through p38-Dependent Downregulation of Cyclin D1 in Human Colorectal Cancer Cells. Biomol. Ther. 2015, 23, 339–344. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Landes, B.; Ramful-Baboolall, D.; Bourdon, E.; Neergheen-Bhujun, V.; Wagner, K.-H.; Bahorun, T. Functional benefits of citrus fruits in the management of diabetes. Prev. Med. 2012, 54, S12–S16. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hassan, S.; Rafiq, M.; Li, H.; He, Y.; Cai, Y.; Kang, X.; Liu, Z.; Yan, T. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evid. Based Complement. Altern. Med. 2020, 2020, 6681352. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C.C. Eriocitrin: A review of pharmacological effects. Biomed Pharm. 2022, 154, 113563. [Google Scholar] [CrossRef]
- Li, W.; Du, Q.; Li, X.; Zheng, X.; Lv, F.; Xi, X.; Huang, G.; Yang, J.; Liu, S. Eriodictyol Inhibits Proliferation, Metastasis and Induces Apoptosis of Glioma Cells via PI3K/Akt/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Catarino, M.; Alves-Silva, J.; Pereira, O.; Cardoso, S. Antioxidant Capacities of Flavones and Benefits in Oxidative-Stress Related Diseases. Curr. Top. Med. Chem. 2014, 15, 105–119. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The Flavonoid Apigenin Protects Brain Neurovascular Coupling against Amyloid-β 25-35-Induced Toxicity in Mice. J. Alzheimer’s Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef]
- Pan, L.; Cho, K.-S.; Yi, I.; To, C.-H.; Chen, D.F.; Do, C.-W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxidative Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Phillips, D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef] [PubMed]
- Mahalanobish, S.; Saha, S.; Dutta, S.; Ghosh, S.; Sil, P.C. Chapter 3—Anti-inflammatory efficacy of some potentially bioactive natural products against rheumatoid arthritis. In Discovery and Development of Anti-Inflammatory Agents from Natural Products, Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–100. [Google Scholar]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Ma, J.; Li, X.-Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.-M.; Lu, G.-D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Sui, C.; Wu, Y.; Zhang, R.; Zhang, T.; Zhang, Y.; Xi, J.; Ding, Y.; Wen, J.; Hu, Y. Rutin Inhibits the Progression of Osteoarthritis Through CBS-Mediated RhoA/ROCK Signaling. DNA Cell Biol. 2022, 41, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ji, Y.; Yi, C.; Wang, X.; Liu, C.; Wang, C.; Lu, X.; Xu, X.; Wang, X. Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules 2022, 27, 4201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, R.; Yan, H.; Wang, F.; Huang, S.; Zhang, Y.; Zhong, M.; Zhang, W.; Wang, Z. Inhibition of vascular smooth muscle cells premature senescence with rutin attenuates and stabilizes diabetic atherosclerosis. J. Nutr. Biochem. 2018, 51, 91–98. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from Nature: A Phytochemistry and Biological Activity Review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Al-Dashti, Y.A.; Holt, R.R.; Stebbins, C.L.; Keen, C.L.; Hackman, R.M. Dietary Flavanols: A Review of Select Effects on Vascular Function, Blood Pressure, and Exercise Performance. J. Am. Coll. Nutr. 2018, 37, 553–567. [Google Scholar] [CrossRef]
- Martín, M.Á.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.Á.; Ramos, S. Impact of cocoa flavanols on human health. Food Chem. Toxicol. 2021, 151, 112121. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Rasool, M. Cyanidin attenuates IL-17A cytokine signaling mediated monocyte migration and differentiation into mature osteoclasts in rheumatoid arthritis. Cytokine 2021, 142, 155502. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cui, S.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Tang, X.; Chen, W. Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-κB Signaling Pathways. Antioxidants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Wu, A.; Zhu, Y.; Han, B.; Peng, J.; Deng, X.; Chen, W.; Du, J.; Ou, Y.; Peng, X.; Yu, X. Delphinidin induces cell cycle arrest and apoptosis in HER-2 positive breast cancer cell lines by regulating the NF-κB and MAPK signaling pathways. Oncol. Lett. 2021, 22, 832. [Google Scholar] [CrossRef]
- Kang, S.H.; Bak, D.-H.; Chung, B.Y.; Bai, H.-W.; Kang, B.S. Delphinidin enhances radio-therapeutic effects via autophagy induction and JNK/MAPK pathway activation in non-small cell lung cancer. Korean J. Physiol. Pharm. 2020, 24, 413–422. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A randomized placebo-controlled cross-over study on the effects of anthocyanins on inflammatory and metabolic responses to a high-fat meal in healthy subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Luo, Y.; Li, X.; Huang, G.; Chen, H.; Li, A.; Qin, S. The Role of Flavonoids in the Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Pharmacol. 2022, 13, 849513. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- Koushki, M.; Farrokhi Yekta, R.; Amiri-Dashatan, N. Critical review of therapeutic potential of silymarin in cancer: A bioactive polyphenolic flavonoid. J. Funct. Foods 2023, 104, 105502. [Google Scholar] [CrossRef]
- Khazeei Tabari, M.A.; Iranpanah, A.; Bahramsoltani, R.; Rahimi, R. Flavonoids as Promising Antiviral Agents against SARS-CoV-2 Infection: A Mechanistic Review. Molecules 2021, 26, 3900. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Shang, J.; Jiao, J.; Yan, M.; Wang, J.; Li, Q.; Shabuerjiang, L.; Lu, Y.; Song, Q.; Bi, L.; Huang, G.; et al. Chrysin protects against cerebral ischemia-reperfusion injury in hippocampus via restraining oxidative stress and transition elements. Biomed. Pharmacother. 2023, 161, 114534. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Nagaraja, S.; Hafsa, N.E.; Meravanige, G.; Asdaq, S.M.B.; Anwer, M.K. Polyphenol chrysin for management of skin disorders: Current status and future opportunities. J. King Saud Univ. Sci. 2022, 34, 102026. [Google Scholar] [CrossRef]
- Khalid, A.; Naseem, I. Antidiabetic and antiglycating potential of chrysin is enhanced after nano formulation: An in vitro approach. J. Mol. Struct. 2022, 1261, 132906. [Google Scholar] [CrossRef]
- Tew, W.Y.; Tan, C.S.; Yan, C.S.; Loh, H.W.; Wen, X.; Wei, X.; Yam, M.F. Evaluation of vasodilatory effect and antihypertensive effect of chrysin through in vitro and sub-chronic in vivo study. Biomed. Pharmacother. 2023, 157, 114020. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Ding, H.; Ding, H.; Mu, P.; Lu, X.; Xu, Z. Diosmetin inhibits subchondral bone loss and indirectly protects cartilage in a surgically-induced osteoarthritis mouse model. Chem. Biol. Interact. 2023, 370, 110311. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, Y.; Shang, J.; Huang, H.; Zhang, Y.; Ding, Y.; Liang, Y.; Xie, Z.; Chen, C. Effects of Genistein on Common Kidney Diseases. Nutrients 2022, 14, 3768. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, L. The protective activity of genistein against bone and cartilage diseases. Front. Pharmacol. 2022, 13, 1016981. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef]
- Xie, K.; Li, Y.; He, G.; Zhao, X.; Chen, D.; Yu, B.; Luo, Y.; Mao, X.; Huang, Z.; Yu, J.; et al. Daidzein supplementation improved fecundity in sows via modulation of ovarian oxidative stress and inflammation. J. Nutr. Biochem. 2022, 110, 109145. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grewal, S.; Sharma, N.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Mohan, S.; Bungau, S.G.; Bumbu, A. Unveiling the Pharmacological and Nanotechnological Facets of Daidzein: Present State-of-the-Art and Future Perspectives. Molecules 2023, 28, 1765. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-E.; Lien, J.-C.; Tsai, C.-W.; Wu, C.-R. Therapeutic Potential and Mechanisms of Novel Simple O-Substituted Isoflavones against Cerebral Ischemia Reperfusion. Int. J. Mol. Sci. 2022, 23, 10394. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Jin, W. Mechanism of Glycitein in the Treatment of Colon Cancer Based on Network Pharmacology and Molecular Docking. Lifestyle Genom. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Geng, L.-M.; Jiang, J.-G. The neuroprotective effects of formononetin: Signaling pathways and molecular targets. J. Funct. Foods 2022, 88, 104911. [Google Scholar] [CrossRef]
- Fu, H.; Li, M.; Huan, Y.; Wang, X.; Tao, M.; Jiang, T.; Xie, H.; Wujisiguleng; Gegentana; He, Y. Formononetin Inhibits Microglial Inflammatory Response and Contributes to Spinal Cord Injury Repair by Targeting the EGFR/MAPK Pathway. Immunol. Investig. 2023, 52, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J. Formononetin: A Pathway to Protect Neurons. Front. Integr. Neurosci. 2022, 16, 908378. [Google Scholar] [CrossRef]
- Balaga, V.K.R.; Pradhan, A.; Thapa, R.; Patel, N.; Mishra, R.; Singla, N. Morin: A Comprehensive Review on Its Versatile Biological Activity and Associated Therapeutic Potential in Treating Cancers. Pharmacol. Res. Mod. Chin. Med. 2023, 7, 100264. [Google Scholar] [CrossRef]
- Sati, P.; Dhyani, P.; Bhatt, I.D.; Pandey, A. Ginkgo biloba flavonoid glycosides in antimicrobial perspective with reference to extraction method. J. Tradit. Complement. Med. 2019, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.M.E.; Omar, F.A.; Emam, M.M.A.-A.; Farag, M.A. UPLC-MS/MS and GC-MS based metabolites profiling of Moringa oleifera seed with its anti- Helicobacter pylori and anti-inflammatory activities. Nat. Prod. Res. 2022, 36, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, Z.; Zhang, X.; Chen, M.; Zhang, X. Identification of MAP Kinase Kinase 3 as a protein target of myricetin in non-small cell lung cancer cells. Biomed. Pharmacother. 2023, 161, 114460. [Google Scholar] [CrossRef]
- Pan, H.; He, J.; Yang, Z.; Yao, X.; Zhang, H.; Li, R.; Xiao, Y.; Zhao, C.; Jiang, H.; Liu, Y.; et al. Myricetin possesses the potency against SARS-CoV-2 infection through blocking viral-entry facilitators and suppressing inflammation in rats and mice. Phytomedicine 2023, 116, 154858. [Google Scholar] [CrossRef]
- Rostami, A.; Baluchnejadmojarad, T.; Roghani, M. Hepatoprotective Effect of Myricetin following Lipopolysaccharide/DGalactosamine: Involvement of Autophagy and Sirtuin 1. Curr. Mol. Pharmacol. 2023, 16, 419–433. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, H.; Luo, C.; Hu, W.; Weng, T.-j.; Shuang, F. Pelargonidin ameliorates inflammatory response and cartilage degeneration in osteoarthritis via suppressing the NF-κB pathway. Arch. Biochem. Biophys. 2023, 743, 109668. [Google Scholar] [CrossRef]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, e02115. [Google Scholar] [CrossRef]
- Cai, X.; Yang, C.; Shao, L.; Zhu, H.; Wang, Y.; Huang, X.; Wang, S.; Hong, L. Targeting NOX 4 by petunidin improves anoxia/reoxygenation-induced myocardium injury. Eur. J. Pharmacol. 2020, 888, 173414. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Fan, H.; Cui, J.; Liu, F.; Zhang, W.; Yang, H.; He, N.; Dong, Z.; Dong, J. Malvidin protects against lipopolysaccharide-induced acute liver injury in mice via regulating Nrf2 and NLRP3 pathways and suppressing apoptosis and autophagy. Eur. J. Pharmacol. 2022, 933, 175252. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Machado, M.; Morais, R.; Calhau, C.; Pintado, M. Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites 2022, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Zarricueta, M.L.; Fagundes, F.L.; Pereira, Q.C.; Pantaleão, S.Q.; Santos, R.D. Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach. Pharmaceutics 2022, 14, 565. [Google Scholar] [CrossRef] [PubMed]
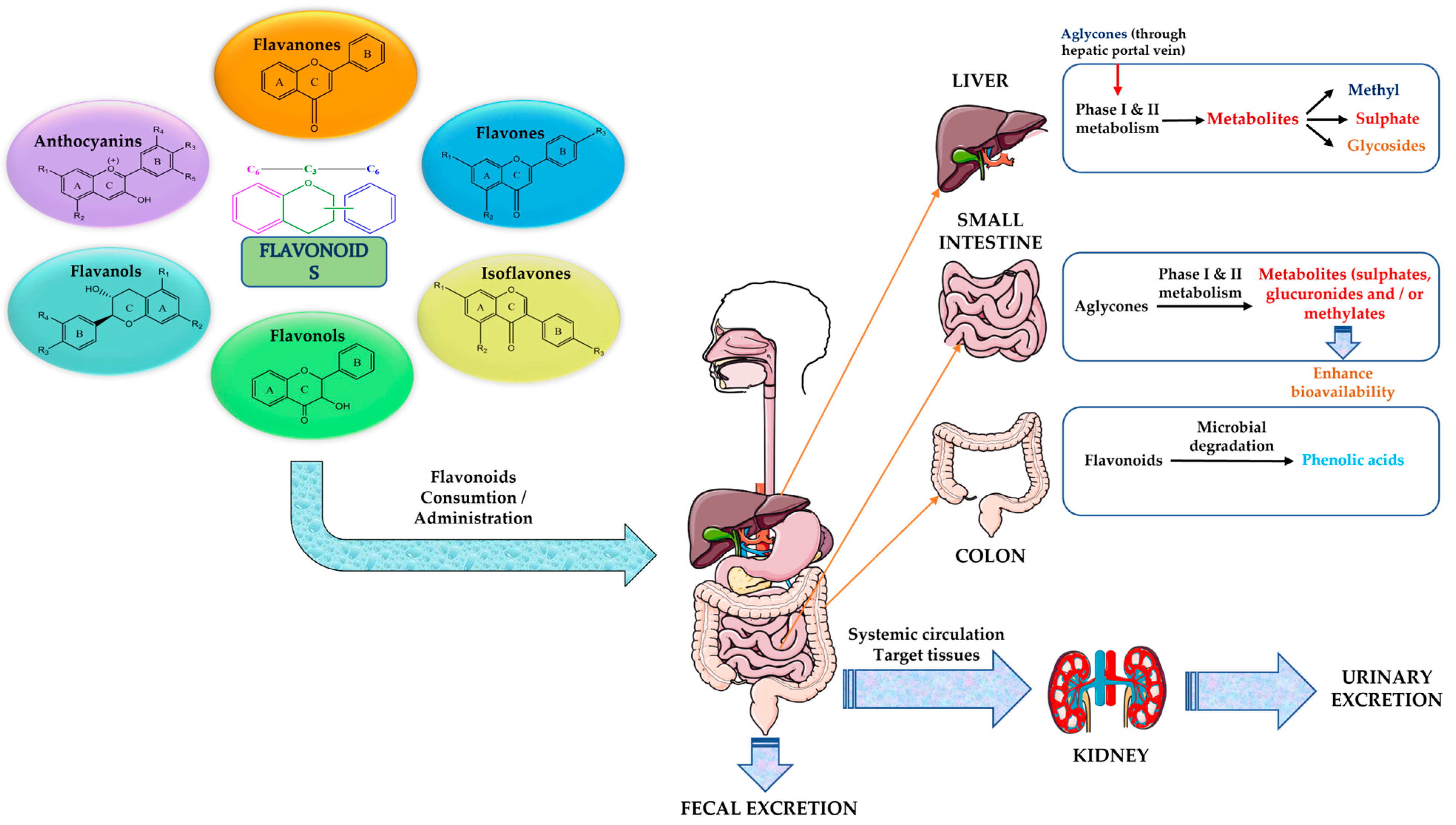
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids1. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol Mechanisms against Gastric Cancer and Their Interactions with Gut Microbiota: A Review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Chapter 6—Extraction and Other Downstream Procedures for Evaluation of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–236. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Chua, L.S.; Latiff, N.A.; Mohamad, M. Reflux extraction and cleanup process by column chromatography for high yield of andrographolide enriched extract. J. Appl. Res. Med. Aromat. Plants 2016, 3, 64–70. [Google Scholar] [CrossRef]
- Babich, O.; Ivanova, S.; Ulrikh, E.; Popov, A.; Larina, V.; Frolov, A.; Prosekov, A. Study of the Chemical Composition and Biologically Active Properties of Glycyrrhiza glabra Extracts. Life 2022, 12, 1772. [Google Scholar] [CrossRef]
- Nuzul, M.I.; Jong, V.Y.; Koo, L.F.; Chan, T.H.; Ang, C.H.; Idris, J.; Husen, R.; Wong, S.W. Effects of Extraction Methods on Phenolic Content in the Young Bamboo Culm Extracts of Bambusa beecheyana Munro. Molecules 2022, 27, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Meng, A.; Liu, P.; Chen, Y.; Yuan, A.; Dai, Y.; Ye, K.; Yang, Y.; Wang, Y.; Li, Z. Reflux Extraction Optimization and Antioxidant Activity of Phenolic Compounds from Pleioblastus amarus (Keng) Shell. Molecules 2022, 27, 362. [Google Scholar] [CrossRef] [PubMed]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Gao, Y.; Liu, P. Optimization of microwave-assisted extraction, antioxidant capacity, and characterization of total flavonoids from the leaves of Alpinia oxyphylla Miq. Prep. Biochem. Biotechnol. 2020, 50, 82–90. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Zhang, J.-J.; Li, Y.; Meng, X.; Li, H.-B. Microwave-Assisted Extraction of Phenolic Compounds from Melastoma sanguineum Fruit: Optimization and Identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef]
- Choommongkol, V.; Punturee, K.; Klumphu, P.; Rattanaburi, P.; Meepowpan, P.; Suttiarporn, P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules 2022, 27, 1397. [Google Scholar] [CrossRef]
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Separation Science and Technology; Snow, N.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 12, pp. 141–203. [Google Scholar]
- Pham, D.-C.; Nguyen, H.-C.; Nguyen, T.-H.L.; Ho, H.-L.; Trinh, T.-K.; Riyaphan, J.; Weng, C.-F. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Celastrus hindsii Leaves Using Response Surface Methodology and Evaluation of Their Antioxidant and Antitumor Activities. BioMed Res. Int. 2020, 2020, 3497107. [Google Scholar] [CrossRef]
- Mai, Y.-H.; Zhuang, Q.-G.; Li, Q.-H.; Du, K.; Wu, D.-T.; Li, H.-B.; Xia, Y.; Zhu, F.; Gan, R.-Y. Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ′Jinfeng′ Kiwifruit. Foods 2022, 11, 827. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-Serrano, D.J.; Christodoulou, M.C.; Orellana-Palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-Christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Abhari, K.; Mousavi Khaneghah, A. Chapter Two—Alternative extraction techniques to obtain, isolate and purify proteins and bioactive from aquaculture and by-products. In Advances in Food and Nutrition Research; Lorenzo, J.M., Barba, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 92, pp. 35–52. [Google Scholar]
- Buelvas-Puello, L.M.; Franco-Arnedo, G.; Martínez-Correa, H.A.; Ballesteros-Vivas, D.; Sánchez-Camargo, A.D.; Miranda-Lasprilla, D.; Narváez-Cuenca, C.-E.; Parada-Alfonso, F. Supercritical Fluid Extraction of Phenolic Compounds from Mango (Mangifera indica L.) Seed Kernels and Their Application as an Antioxidant in an Edible Oil. Molecules 2021, 26, 7516. [Google Scholar] [CrossRef] [PubMed]
- Végh, K.; Riethmüller, E.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, Á.; Tóth, A.; Béni, S.; Könczöl, Á.; Balogh, G.T.; et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Laganà, A. Recent advances and developments in matrix solid-phase dispersion. TrAC Trends Anal. Chem. 2015, 71, 186–193. [Google Scholar] [CrossRef]
- Barker, S.A. Matrix solid phase dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [Google Scholar] [CrossRef]
- Mansur, A.R.; Kim, K.J.; Kim, D.-B.; Yoo, M.; Jang, H.W.; Kim, D.-O.; Nam, T.G. Matrix solid-phase dispersion extraction method for HPLC determination of flavonoids from buckwheat sprouts. LWT 2020, 133, 110121. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds from Arthrospira Platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ko, M.-J.; Park, C.-H.; Chung, M.-S. Application of Pulsed Electric Field as a Pre-Treatment for Subcritical Water Extraction of Quercetin from Onion Skin. Foods 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 171–201. [Google Scholar]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef] [PubMed]
- Amulya, P.R.; ul Islam, R. Optimization of enzyme-assisted extraction of anthocyanins from eggplant (Solanum melongena L.) peel. Food Chem. X 2023, 18, 100643. [Google Scholar] [CrossRef] [PubMed]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef] [PubMed]
- Gil, K.A.; Jokić, S.; Cikoš, A.-M.; Banožić, M.; Jakovljević Kovač, M.; Fais, A.; Tuberoso, C.I. Comparison of Different Green Extraction Techniques Used for the Extraction of Targeted Flavonoids from Edible Feijoa (Acca sellowiana (O.Berg) Burret) Flowers. Plants 2023, 12, 1461. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tian, B.; Ge, Z.; Feng, J.; Wang, J.; Yang, K.; Sun, P.; Cai, M. Ultrasound and deep eutectic solvent as green extraction technology for recovery of phenolic compounds from Dendrobium officinale leaves. Process Biochem. 2023, 128, 1–11. [Google Scholar] [CrossRef]
- Park, N.; Cho, S.-D.; Chang, M.-S.; Kim, G.-H. Optimization of the ultrasound-assisted extraction of flavonoids and the antioxidant activity of Ruby S apple peel using the response surface method. Food Sci. Biotechnol. 2022, 31, 1667–1678. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhuo, Y.; Li, Y.; Meng, J.; Wang, Y.; Li, H. Ultrasound-Assisted Extraction of Anthocyanins from Malus ‘Royalty’ Fruits: Optimization, Separation, and Antitumor Activity. Molecules 2022, 27, 4299. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Optimization of Ultrasound-Assisted Extraction of Yields and Total Methoxyflavone Contents from Kaempferia parviflora Rhizomes. Molecules 2022, 27, 4162. [Google Scholar] [CrossRef]
- Arias, J.; Mejía, J.; Córdoba, Y.; Martínez, J.R.; Stashenko, E.; del Valle, J.M. Optimization of flavonoids extraction from Lippia graveolens and Lippia origanoides chemotypes with ethanol-modified supercritical CO2 after steam distillation. Ind. Crops Prod. 2020, 146, 112170. [Google Scholar] [CrossRef]
- Arias, J.; Muñoz, F.; Mejía, J.; Kumar, A.; Villa, A.L.; Martínez, J.R.; Stashenko, E.E. Simultaneous extraction with two phases (modified supercritical CO2 and CO2-expanded liquid) to enhance sustainable extraction/isolation of pinocembrin from Lippia origanoides (Verbenaceae). Adv. Sample Prep. 2023, 6, 100059. [Google Scholar] [CrossRef]
- Long, T.; Lv, X.; Xu, Y.; Yang, G.; Xu, L.-Y.; Li, S. Supercritical fluid CO2 extraction of three polymethoxyflavones from Citri reticulatae pericarpium and subsequent preparative separation by continuous high-speed counter-current chromatography. J. Chromatogr. B 2019, 1124, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-J.; Liu, W.; Zu, Y.-G.; Tong, M.-H.; Li, S.-M.; Yan, M.-M.; Efferth, T.; Luo, H. Enzyme assisted extraction of luteolin and apigenin from pigeonpea [Cajanuscajan (L.) Millsp.] leaves. Food Chem. 2008, 111, 508–512. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Enzyme-Assisted Extraction Enhancing the Phenolic Release from Cauliflower (Brassica oleracea L. var. botrytis) Outer Leaves. J. Agric. Food Chem. 2014, 62, 7468–7476. [Google Scholar] [CrossRef]
- Ma, X.-D.; Zhang, X.-G.; Guo, S.-J.; Ma, G.-Y.; Liu, W.-J.; Wang, N.; Feng, M.; Su, Y. Application of enzyme-assisted extraction of baicalin from Scutellaria baicalensis Georgi. Prep. Biochem. Biotechnol. 2021, 51, 241–251. [Google Scholar] [CrossRef]
- Bernal-Millán, M.D.; Carrasco-Portugal, M.D.; Heredia, J.B.; Bastidas-Bastidas, P.D.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Angulo-Escalante, M.Á. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants 2023, 12, 1692. [Google Scholar] [CrossRef]
- Li, F.; Xiao, L.; Lin, X.; Dai, J.; Hou, J.; Wang, L. Deep Eutectic Solvents-Based Ultrasound-Assisted Extraction of Antioxidants from Kudingcha (llex kudingcha C.J. Tseng): Process Optimization and Comparison with Other Methods. Foods 2023, 12, 1872. [Google Scholar] [CrossRef]
- Myo, H.; Yaowiwat, N.; Pongkorpsakol, P.; Aonbangkhen, C.; Khat-udomkiri, N. Butylene Glycol Used as a Sustainable Solvent for Extracting Bioactive Compounds from Camellia sinensis Flowers with Ultrasound-Assisted Extraction. ACS Omega 2023, 8, 4976–4987. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, S.-Q.; Li, L.-Y.; Gai, Q.-Y. Deep Eutectic Solvent-Based Microwave-Assisted Extraction for the Extraction of Seven Main Flavonoids from Ribes mandshuricum (Maxim.) Kom. Leaves. Separations 2023, 10, 191. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Deng, G.; Xu, K.; Xu, H.; Liu, J. Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology. Molecules 2022, 27, 4344. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, C.; Yang, J.; Ma, X.; Jiang, Y.; Qiu, S.; Wang, G. Ionic Liquid-Microwave-Based Extraction of Biflavonoids from Selaginella sinensis. Molecules 2019, 24, 2507. [Google Scholar] [CrossRef] [PubMed]
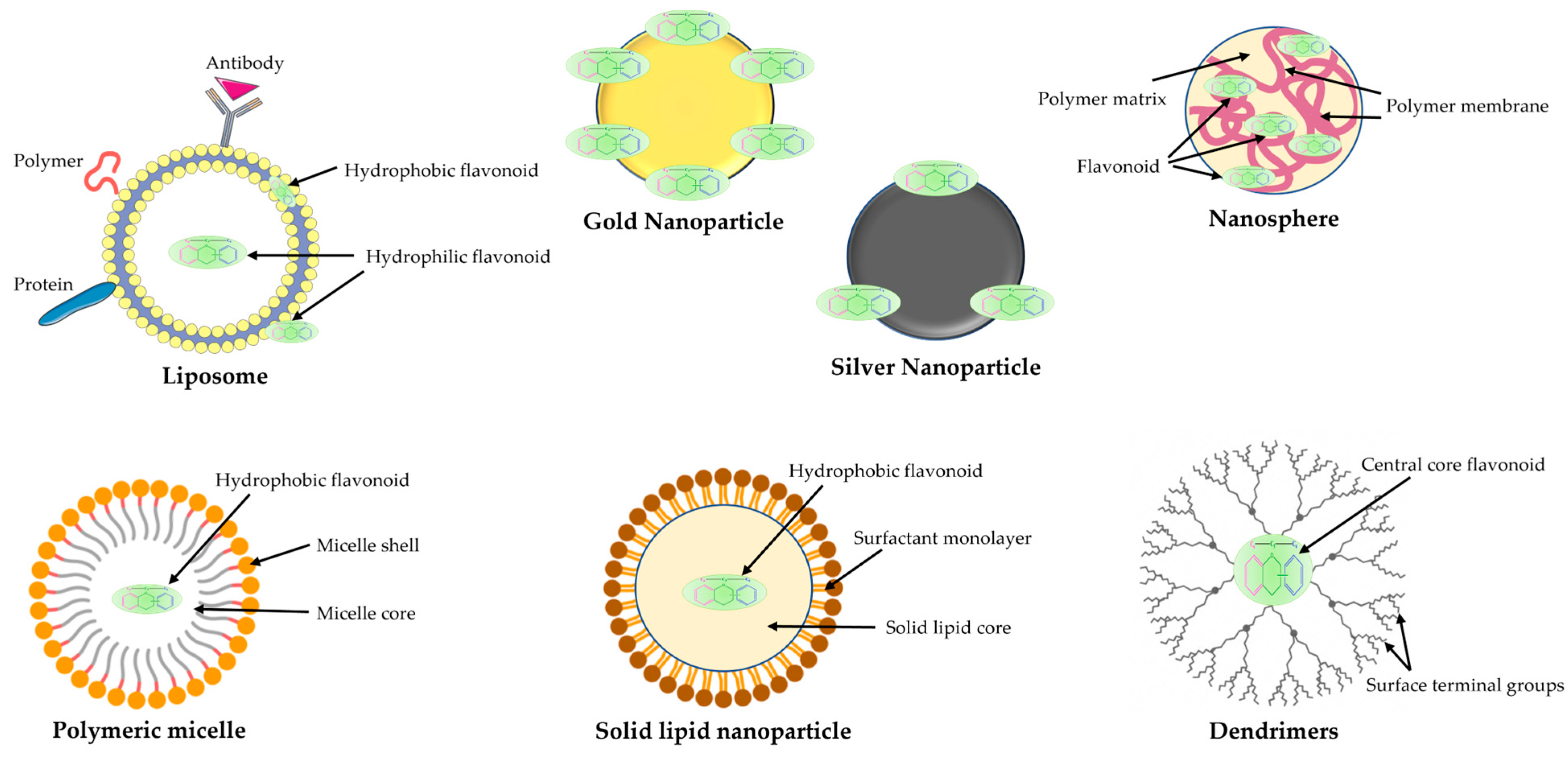
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Acharyya, A.; Sankar Chakraborti, A. Flavonoid-based polymeric nanoparticles: A promising approach for cancer and diabetes treatment. Eur. Polym. J. 2022, 177, 111455. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Y.; Chen, M.; Liu, G. Application of Nanomicelles in Enhancing Bioavailability and Biological Efficacy of Bioactive Nutrients. Polymers 2022, 14, 3278. [Google Scholar] [CrossRef]
- Sysak, S.; Czarczynska-Goslinska, B.; Szyk, P.; Koczorowski, T.; Mlynarczyk, D.T.; Szczolko, W.; Lesyk, R.; Goslinski, T. Metal Nanoparticle-Flavonoid Connections: Synthesis, Physicochemical and Biological Properties, as Well as Potential Applications in Medicine. Nanomaterials 2023, 13, 1531. [Google Scholar] [CrossRef]



| Classification | Flavonoid | Biological Activity | References |
|---|---|---|---|
Flavanones | Naringenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-chroman-4-one) | Antifibrinolytic, decreases pro-inflammatory cytokines in hepatocytes, antineoplastic activity (breast cancer, prostate cancer, lung cancer, and colon cancer), a promising treatment strategy against COVID-19, and therapeutic application in bone disorders and bone tissue engineered constructs | [11,12,13,14,15,16,17,18,19,20,62,63] |
| Hesperitin ((2S)-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydrochromen-4-one) | Anti-diabetic, antioxidant, anti-inflammatory, anticancer, antiviral, antibiofilm, antimicrobial, and cardiovascular protective activities | [21,22,23,24,25,64] | |
| Eriodictyol (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one) | Cardiotonic, skin protection, antitumoral, antioxidant, anti-inflammatory, immunomodulatory, and hepatoprotective | [26,27,28] | |
| Silymarin (3,5,7-trihydroxy-2-[3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one) | Antioxidant, anti-inflammatory, and anticancer | [65] | |
Flavones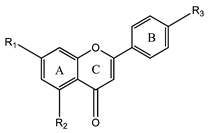 | Apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one) | Neuroprotective | [32] |
| Baicalein (5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one) | Neuroprotective, antiviral activity, possible treatment agent against SARS-Cov2 | [31,33,66] | |
| Wogonin (5,7-dihydroxy-8-methoxy-2-phenyl-4H-chromen-4-one) | Neuroprotective | [31,33] | |
| Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one) | Anti-inflammatory, antiallergy, and anticancer | [67] | |
| Chrysin (5,7-Dihydroxy-2-phenyl-4H-chromen-4-one) | Neuroprotective, anti-aging, skin protective, antioxidant, anti-inflammatory, anticancer, anti-diabetic, vasodilatory effect, and antihypertensive | [68,69,70,71] | |
| Acacetin (5,7-dihydroxy-2-(4-methoxyphenyl)-chromen-4-one) | Antiproliferative, neuroprotective, cardioprotective, anticancer, anti-inflammatory, anti-inflammatory diabetic, antimicrobial | [72] | |
| Diosmetin (5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-chromen-4-one) | Antioxidant, anti-inflammatory, anti-apoptotic, and anticancer properties | [73] | |
Isoflavones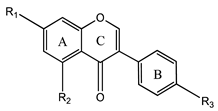 | Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)-chromen-4-one) | Fungistatic, antibacterial, antiviral, anti-inflammatory, kidney protective, antioxidant, prevents angiogenesis, exerts estrogenic and/or antiestrogenic effects, and promising therapeutic application in bone disorders and bone tissue engineered constructs | [34,38,39,63,74,75,76,77] |
| Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-chromen-4-one) | Fungistatic, antibacterial, antiviral, antioxidant, prevents angiogenesis, exerts estrogenic and/or antiestrogenic effects, and promising therapeutic application in bone disorders and bone tissue engineered constructs | [34,38,39,63,77,78,79] | |
| Glycitein (7-hydroxy-3-(4-hydroxyphenyl)-6-methoxychromen-4-one) | Fungistatic, antibacterial, antiviral, antioxidant, prevents angiogenesis, and exerts estrogenic and/or antiestrogenic effects | [34,38,39,80,81] | |
| Formononetin (7-hydroxy-3-(4-methoxyphenyl)-chromen-4-one) | Fungistatic, antibacterial, antiviral, antioxidant, anti-inflammatory, neuroprotective, prevents angiogenesis, and exerts estrogenic and/or antiestrogenic effects | [34,38,39,82,83,84] | |
Flavonols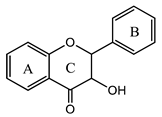 | Quercetin (3,3,4,5,7-pentahydroxyflavone) | Downregulates malondialdehyde level and scavenges several free radicals (e.g., hydrogen peroxide, superoxide, and hydroxyl radicals), increases cell IFN-γ expression, decreases interleukine-4-positive cell expression, induces extrinsic and intrinsic pathways of apoptosis and autophagy, antimicrobial activity, inhibits biofilm formation, inhibits nucleic acid synthesis, antiviral activity, and possible treatment against SARS-Cov2 | [41,42,43,44,45,66] |
| Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoside) | Anti-inflammatory, anti-hypolipemiant, anti-atherosclerotic, antiallergic, anti-inflammatory, and antiviral | [46,47,48,49] | |
| Morin (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one) | Antioxidant, anti-inflammatory, anticancer, anti-diabetic, anti-inflammatory, antihypertensive, and gastric protector effects | [85] | |
| Kaempherol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-chromen-4-one) | Antioxidant, antimicrobial, and anti-inflammatory | [86,87] | |
| Myricetin (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-chromen-4-one) | Antioxidant, anti-inflammatory, anti-diabetic, anti-epileptic, anti-Alzheimer, anti-apoptotic, antithrombotic, neuroprotective, potential therapeutic agent against COVID-19, and hepatoprotective effect | [88,89,90] | |
Flavanols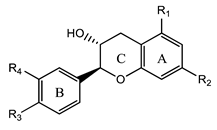 | Catechin (2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) | Anti-inflammatory, anticancer, antiviral, antimicrobial, and protective cardiovascular properties | [50,51,52,53] |
| Epigallocatechin (2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) | Anti-inflammatory, anticancer, antiviral, antifungal, antimicrobial, and protective cardiovascular properties | [50,51,52,53,66] | |
| Afzelechin (2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) | Anti-inflammatory, anticancer, antiviral, antimicrobial, and protective cardiovascular properties | [50,51,52,53] | |
Anthocyanins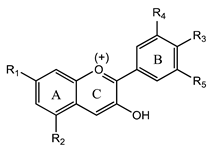 | Cyanidin (2-(3,4-dihydroxyphenyl)-chromenylium-3,5,7-triol) | Immunomodulator and anti-inflammatory | [57,58,61] |
| Delphinidin (2-(3,4,5-trihydroxyphenyl)-chromenylium-3,5,7-triol) | Anti-HER-2 effect, regulates the protein expression level of Bax and Bcl-2, inhibits the activation of NF-κB, induces G2/M phase arrest and apoptosis, pro-apoptotic, anti-proliferative effects, and anti-inflammatory | [59,60,61] | |
| Pelargonidin (2-(4-hydroxyphenyl)-chromenylium-3,5,7-triol) | Antioxidant and anti-inflammatory | [91,92,93] | |
| Petunidin (2-(3,4-dihydroxy-5-methoxyphenyl)-chromenylium-3,5,7-triol) | Antioxidant and anti-MIRI effects | [92,93] | |
| Malvidin (2-(4-hydroxy-3,5-dimethoxyphenyl)-chromenylium-3,5,7-triol) | Hypoglycemic, hypolipidemic, gastroprotective, hepatoprotective, antiadhesive, and antibiofilm effects | [94,95,96,97] |
| Extraction Technique | Target Flavonoids | References |
|---|---|---|
| Microwave-assisted extraction (MAE) | Flavonol, catechins | [113,131] |
| Ultrasound-assisted extraction (UAE) | Flavonols (isoquercitrin, quercetin) | [132,133,134] |
| Anthocyanins | [135] | |
| Flavones (methoxyflavones) | [136] | |
| Supercritical fluid extraction (SFE) | Flavanone (pinocembrin) | [137] |
| Flavonol (galangin) | [138] | |
| Flavones | [121,139] | |
| Pulsed electric field extraction (PEFE) | Flavanones | [140] |
| Enzyme-assisted extraction (EAE) | Anthocyanins | [129,130,141] |
| Flavones | [142,143,144] | |
| UAE + deep eutectic solvents | Flavanones, flavonols | [145,146] |
| UAE + butylene Glycol | Flavonols (catechins) | [147] |
| MAE + deep eutectic solvents | Flavones (trifolin, isoquercetin, kaempferol), flavonols (astragalin, quercetin, hyperoside) | [148] |
| UAE + EAE | Anthocyanins | [149] |
| MAE + ionic liquids | Flavones | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. https://doi.org/10.3390/plants12142732
Liga S, Paul C, Péter F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants. 2023; 12(14):2732. https://doi.org/10.3390/plants12142732
Chicago/Turabian StyleLiga, Sergio, Cristina Paul, and Francisc Péter. 2023. "Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques" Plants 12, no. 14: 2732. https://doi.org/10.3390/plants12142732
APA StyleLiga, S., Paul, C., & Péter, F. (2023). Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants, 12(14), 2732. https://doi.org/10.3390/plants12142732