Abstract
Cereal plants under abiotic or biotic stressors to survive unfavourable conditions and continue growth and development, rapidly and precisely identify external stimuli and activate complex molecular, biochemical, and physiological responses. To elicit a response to the stress factors, interactions between reactive oxygen and nitrogen species, calcium ions, mitogen-activated protein kinases, calcium-dependent protein kinases, calcineurin B-like interacting protein kinase, phytohormones and transcription factors occur. The integration of all these elements enables the change of gene expression, and the release of the antioxidant defence and protein repair systems. There are still numerous gaps in knowledge on these subjects in the literature caused by the multitude of signalling cascade components, simultaneous activation of multiple pathways and the intersection of their individual elements in response to both single and multiple stresses. Here, signal transduction pathways in cereal plants under drought, salinity, heavy metal stress, pathogen, and pest attack, as well as the crosstalk between the reactions during double stress responses are discussed. This article is a summary of the latest discoveries on signal transduction pathways and it integrates the available information to better outline the whole research problem for future research challenges as well as for the creative breeding of stress-tolerant cultivars of cereals.
Keywords:
abiotic stress; biotic stress; cereal; crosstalk; drought; heavy metal; phytohormone; salinity; pathogen; pest 1. Introduction
The climatic conditions have changed many times during the history of Earth, but now, these alterations are strongly intensified by heavy industrial activity. As agriculture is a branch of the economy most dependent on climatic conditions, the progressing climate changes create a completely new situation for agricultural activity, especially for plant production [1]. The rate of temperature changes causes many extreme atmospheric phenomena that have not occurred or have appeared very rarely so far. Noticeable changes in the quantity and quality of rainfall (an increase in the number of storms followed by periods without rainfall) increase the risk of both flooding and drought. In addition, the increase in temperature may favour the overwintering of plant pathogens and pests, which have not so far posed a threat to native crops [2]. Moreover, in the era of global warming, the mobility of pollutants, including heavy metals in the environment increases [3].
Plant responses to environmental factors are extraordinarily complex. They can be observed at various levels of plant organisation, ranging from changes at the cell level, i.e., the changes in the activity of basic biochemical processes such as DNA replication, respiration, and photosynthesis to morphological and anatomical changes in plant organs [4,5,6,7]. However, mentioned biochemical changes are preceded by the activation of an efficient signalling system that endures environmental fluctuations [8]. The presented review highlights issues related to stress factor recognition/stress factor perception, induction, and transmission of the signal, and subsequent signalling responses at molecular and metabolic levels in cereals under the influence of various stress factors caused by global warming. This work shows the latest research results in the context of the defence mechanism induction of cereals to different abiotic stress and tolerance/existence under stressful environments.
It is well known that there are four main common signal transduction pathways in plants, which can interact with each other, i.e., reactive species signalling, calcium-dependent signalling, plant hormone signalling and signalling based on phosphorylation/dephosphorylation of proteins by kinase cascades [9]. Plant hormones are important regulators of the tailored responses to different stresses. The coordination of regulatory mechanisms among different hormones, or the interaction of hormone signalling with other molecules, such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and hydrogen sulphide (H2S), is flexible and changes over time [10,11,12].
Abiotic and biotic stressors can cause a state of excess excitation energy in plant cells which leads to universal consequences: disturbances in electron transport, increased reduction in plastoquinone and uncontrolled generation of ROS, mainly superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (HO−) and singlet oxygen (1O2). The reaction of ROS with nitric oxide leads also to the formation of RNS, and potentially the appearance of not only oxidative but also nitrosative stress [13]. As ROS and RNS can act as a double-edged sword, they also play an important role in redox signalling as secondary messengers or signalling molecules and take part in signal transmission to the nucleus through redox reactions using the mitogen-activated protein kinases (MAPK) pathway and control of the antioxidant system in the plant cell [10]. Emerging signals are also facilitated by a series of phosphorylation/dephosphorylation events. This protein modification can both activate or deactivate effective proteins, which adjust cell metabolism to current environmental conditions with low energy costs. Phosphorylation cascade can be calcium-dependent (calcium-dependent protein kinases; CDPKs, calcineurin B-like interacting protein kinase; CIPK) or calcium-independent, for example, MAPK.
All signalling pathways can lead to the activation of appropriate transcription factors (TFs), enabling the transcription of genes crucial for maintaining plant homeostasis under stress. Additionally, the gene expression may be regulated by the presence of microRNA (miRNAs)-key regulators of plant responses to abiotic and biotic stresses and plant development [14]. The end result is the translation of proteins whose role is to reduce stress (e.g., by sequestration of salt ions or inactivation of heavy metals) or to eliminate its negative consequences. The resulting metabolic adjustments are crucial for maintaining the balance between simultaneously occurring processes of growth and development and stress defence. In this review, we present, confront and discuss different recent views on signal transduction in cereal plants under various stresses.
2. Drought
During drought, disturbances in the water management of plants occur, leading, inter alia, to closing the stomata and limiting transpiration [15]. Overlapping structural and functional changes, including oxidative stress, inhibition of photosynthesis and alternations in the distribution of assimilation products lead to disruption of plant growth and development [5,16]. Under drought, assimilates move from leaves (donor organs) to the roots (acceptor organs), which are responsible for water and nutrient uptake, at the expense of the biomass of the aerial parts. Plants suffering from water deficit are usually smaller, light-coloured, lack turgor, and are more susceptible to disease and pest attacks. All of the above result in lesser nutrients supplementation, which reduces the yield of a crop [1].
Plants initially identify water scarcity conditions by the roots, which results in the initiation of several molecular signals that transfer from the roots to the shoots [17]. For this reason, the root is a key organ that determines the effectiveness of the plant’s response to the stress of water shortage. The root shows high plasticity of its features and its reaction to drought can vary. There can be observed root elongation [18] or shortening [19], but also its length may not change [20]. The root system of cereals varies, e.g., wheat (Triticum aestivum L.) produces coarser, moderately branched roots, which allows for more efficient water management, while rice (Oryza sativa L.) forms thin, more branched underground organs which can better penetrate soil [21].
A special role in signalling is attributed to chloroplasts and mitochondria that are considered sensors of changes occurring in the environment. Chloroplasts and mitochondria generate ROS and transmit retrograde signals to the nucleus [22]. The direct signals of drought are transduced in plants through ROS, such as singlet oxygen (1O2), superoxide anion radical (O2·−), hydroxyl radical (HO·) and hydrogen peroxide (H2O2) [23]. Signalling by ROS can take place through pathways based on susceptible proteins containing thiol groups which are subject to reversible oxidation [24]. Thiol reducing molecules, such as glutathione and specific isoforms of thiols reductases, thioredoxins (TRX) and glutaredoxins (GRX), were found in diverse nuclear subcompartments, further supporting the assumption that thiol-dependent systems are active in the nucleus [25]. Thioredoxins and glutaredoxins are not only responsible for the reduction of thiol groups of numerous metabolic enzymes and molecules belonging to ROS scavenging systems, but also regulate thiol-based post-transcriptional redox modifications of proteins [26]. In T. aestivum, TRX isoforms are accumulated in the nucleus upon oxidative stress. It is likely that the influence of ROS on the expression of nuclear genes may be based on the regulation of redox-sensitive TFs [27].
The MAPK cascade-mediated ROS removal is an important mechanism regulating drought stress tolerance [28]. The MAPK cascade leads to the activation of antioxidant enzymes such as superoxide dismutases (SOD), peroxidases (POX) and catalases (CAT) in many cereal plants [29,30]. A proteomic study of T. aestivum plants revealed the abundance of CAT and three isoforms of SOD (chloroplastic cytosolic Cu/Zn-SOD and mitochondrial Mn-SOD) in response to drought. These antioxidant enzymes were involved in the survival strategy of wheat by avoiding the excess generation of ROS [31]. Moreover, salicylic acid (SA), ethylene (ET), jasmonic acid (JA), cytokinins (CKs), gibberellins (GAs) and brassinosteroids (BRs) play a vital function in regulating various phenomena in cereal acclimatisation to drought stress [32]. For example, BR signalling regulates drought tolerance in wheat, which is partially achieved through brassinazole-resistant 2 (TaBZR2) TF. TaBZR2 increased the glutathione S-transferase-1 (TaGST1) expression and a decrease in ROS level was observed [33]. Furthermore, maize calcium/calmodulin-dependent protein kinase (ZmCCaMK) was involved in BR signalling and it was required for BR-induced antioxidant defence [34].
The key step in cereals’ response to drought is increased concentrations of abscisic acid (ABA) in the root, which may contribute to increased root hydraulic conductivity. By this mechanism, cereals adjust their cellular processes by triggering a network of long-distance signalling events that start with the perception of stress signals and lead through transduction of those signals to switch on acclimation cellular responses, such as changes in gene expression [35]. ABA regulates the expression of different stress-responsive genes involved in the accumulation of compatible osmolytes, synthesis of late embryogenesis abundant (LEA) proteins, dehydrins, chitinases, glucanases, as well as other protective proteins, such as the heat shock protein (HSP) [36]. The resulting osmotic adjustment helped to maintain higher leaf relative water content at low leaf water potential under drought. It enabled sustained growth while under reduced leaf water potential [37]. Recent evidence indicates that H2S is actively involved in the regulation of ethylene-induced stomatal closure and also interacts with H2O2 by impacting the activities of the inward K+ ion and anion channels [38]. Wheat can adapt to osmotic stress by H2S production and activation of the antioxidant system [12]. It was proven that H2S induced the ABA-triggered ascorbate–glutathione (AsA-GSH) cycle under osmotic stress. Obviously, H2S was involved in the ABA-related closing of stomata in response to various environmental stresses, however, the interaction between them is still unclear and requires further research [11].
In response to osmotic stress occurring often with drought, levels of growth-stimulating hormones: GAs, CKs, sometimes indole acetic acid (IAA) decrease, while there is an observed increase in the level of hormones that usually inhibit cell elongation growth or accelerate maturation and/or aging of tissues: ABA, ET, JA, methyl jasmonate (MeJA) and BRs [39]. Here, ABA acts as the hub of the hormonal crosstalk between several stress signalling cascades [40]. Osmotic stress-responsive gene expression is regulated by ABA-dependent and ABA-independent pathways [41]. In the ABA-dependent pathway, numerous types of TFs, such as MYeloBlastosis (MYB), a basic helix–loop–helix (bHLH), the basic region leucine zipper (bZIP), ethylene response factor (ERF) and homeodomain TF are involved [42]. It was proven that overexpression of JERF1 (ERF gene) significantly enhanced drought tolerance of transgenic rice [43]. According to Zhang et al. [43], the JERF1 activated the expression of stress-responsive genes and increased the synthesis of the osmolyte proline by regulating the expression of OsP5CS, encoding deltal-pyrroline-5-carboxylate synthetase (proline biosynthesis enzyme) [43]. JERF1 also triggered the expression of two rice genes encoding ABA biosynthesis enzymes, zeaxanthin epoxidase 2 (OsABA2) and xanthoxin dehydrogenase (Os03g0810800) [43].
Signal transduction of osmotic stress also depends on Ca2+, nitric oxide (NO), reactive sulphur species which induce MAPK [28], calcium-dependent protein kinase [44], and calcineurin B-like interacting protein kinase families of protein kinases [45], or phospholipid signalling [13]. The MAPK cascade plays an important role in the drought stress response mainly by responding to ABA and regulating ROS production. Numerous components of MAPK cascades were described as responding to water deficiency in cereals. For example in rice, the transcripts of OsMKK4, OsMKK1, OsMPK8, OsMPK7, OsMPK5 and OsMPK4 were accumulated under drought stress [46,47,48]. In wheat, the expression levels of TaMKKK16, TaMKK1 and TaMPK8 changed in response to drought stress [49]. Ma et al. [50] found that OsMKK10.2–OsMPK3 were responsible for conferring drought stress tolerance in rice via ABA signalling [50]. However, the exact relationship of the MAPK cascade with ABA has not yet been described [28]. In rice, MAPK5, MAPK7, MAPK8 and MAPK12 were induced by drought and MAPK4 was repressed under water shortage [51]. In rice, MAPK kinases regulated the activity of transcription factors such as OsWRKY30 and increased drought tolerance [52].
The CDPKs and CIPK are families of protein kinases. In rice, a Ca2+ dependent kinase such as OsCIPK12 increased the concentration of proline and soluble sugars, which may improve drought tolerance. Additionally, OsCDPK7 enhanced the expression of the gene whose product is the rab16A protein, potentially involved in drought tolerance [53]. In addition to the mentioned protein kinases, rice has a total of 74 heat shock proteins classified into four categories: sHSP, HSP70, HSP90 and HSP100 [54]. These HSPs are activated by ABA-dependent heat shock transcription factors (HSFs), but only some of them are activated by drought [55]. Furthermore, in wheat and barley, the expression of several dehydrins (Dhn) belonging to group two of LEA proteins was observed under drought [56]. Karami et al. [57] reported induction of several genes of Dhn, such as Dhn1, Dhn3, Dhn5, Dhn7, and Dhn9, in barley flag leaf under drought. Relative expression levels of Dhn3 and Dhn9 revealed positive correlations with chlorophyll a and b contents, osmotic adjustment, plant biomass and grain yield, and negative correlations with malondialdehyde (MDA), a marker of membrane oxidative lipids damage, and electrolyte leakage levels.
The majority of LEA proteins display a preponderance of hydrophilic and charged amino acid residues. On the basis of the literature, their function as antioxidants, membranes and protein stabilisers, and indirect participants as molecular shields in cell protection are considered [58]. HVA1 gene encoding group three of LEA protein from barley (Hordeum vulgare L.) was transformed into rice and the tolerance to water deficit of the transgenic rice was improved under the greenhouse conditions [59]. The overexpression of the HVA1 gene in the roots and leaves of wheat also tended to retain tolerance to drought stress. In wheat, in response to drought, the size of LEA proteins reached up to 200 kDa, therefore, these proteins were resistant to denaturation [60]. It was observed that overexpression of OsLEA6 and OsLEA3-1 led to enhanced drought tolerance of rice plants in the field [61].
As an important metabolic pathway, phosphatidylinositol metabolism generates signalling molecules that are essential for survival under drought [46]. Phospholipid molecules are involved in signalling processes leading to adjustments in root growth, pollen and vascular development, hormone effects and cell responses to environmental stimuli in plants [46]. Wang et al. [62] showed that the expression of maize ZmPLC1, encoding phospholipase C, was up-regulated under dehydration and it improved the drought tolerance of maize through the interaction with other signalling pathways in guard cells [62].
It is well known that the NO performs the signalling function in plant cells. Signal transduction by NO is mediated by cyclic guanosine monophosphate (cGMP) and activation of guanylate cyclase [63]. NO regulates the levels of cellular ROS content and toxicity through the activation of antioxidant enzymes [64]. Gan et al. [64] showed that NO (applied exogenously) increased drought resistance in barley. The application of NO not only increased the activity of antioxidant enzymes but also increased the content of proline. NO was also found to crosstalk with ABA, JA, SA and CKs to mitigate the adverse effect of drought stress [65]. There are also many studies showing the cooperation of H2S and NO in response to drought [11].
3. Salinity
Salinity is one of the most important abiotic stress that negatively influences plant growth and productivity, especially rice, wheat and barley, which are the main food crops worldwide [66,67]. Soil salinity caused 25–30% of the irrigated area worldwide to be commercially unproductive and it is estimated that progressive salinity, expanding at a rate of 10% per year, will lead to nearly 50% of agricultural land by 2050 being unproductive [68]. The high concentration of salts in the soil may cause a reduction in water and nutrient uptake due to salt accumulation in the root zone (physiological drought), therefore inducing ion and nutrient imbalance, and water stress in plants. Salinity, due to the presence of NaCl in the soil, is the most common, hence the most harmful effect of salinity is the accumulation of Na+ and Cl– [69]. Excess Na+ in the plant inhibits the uptake of essential micronutrients such as K+ and Ca2+ from the soil, a shortage of the second one is especially crucial because it participates in the maintenance of cell membrane integrity, as well as in the synthesis of new cell walls [70]. Thus, overaccumulation of Na+ leads to damage and enhanced permeability of membranes. Loss of membrane integrity can also lead to K+ leakage from cells, which can affect enzymatic reactions since many enzymes require K+ as a cofactor. Additionally, these enzymes are sensitive to high cytosolic Na+ content. The accumulation of Na+ also alters the activity of photosynthetic enzymes and it is harmful to other photosynthesis compounds, such as chlorophylls, and carotenoids [71]. What is more, it is assumed that disturbed ion homeostasis (excess of Na+ and shortage of Ca2+ and K+) might contribute to oxidative stress which is resulting in the overproduction of ROS and an inefficient ROS detoxification system. The following consequences are oxidative damage of various plant cellular components such as nucleic acids, proteins, sugars and lipids, and hence the inhibition of proper plant development and growth [72].
The response of plants to salinity occurs through the perception and transduction of a signal associated with the disruption of ion and osmotic homeostasis. It is considered that plant cells sense the increase in cytosol Na+ levels through a sensor or a receptor. Nonetheless, no specific sensor or receptor was identified in plants so far. Therefore, it is not known how an excess of Na+ is detected by plants, so it can be assumed that the perception of salt stress signal remains unrevealed [73]. However, the most common salt stress signalling pathways—the salt overly sensitive (SOS)—are well characterised in plants, including cereals. Additionally, the MAPK cascade, which transduces stress signals to a variety of transcription factors that further activate salt-responsive genes, plays an important role in salt stress signalling in plants [74].
SOS pathway genes encode proteins that are engaged in the active efflux of excess Na+ from the cytosol (Figure 1). SOS1 is a plasma membrane Na+/H+ antiporter, activated through phosphorylation catalysed by the SOS2–SOS3 kinase complex. SOS3 is a Ca2+ sensor, which belongs to the calcineurin B-like signal protein family [75]. It perceives the cytosolic Ca2+ signal, which is triggered by a salt-induced excess of Na+. ABA plays a key role in increasing Ca2+ content, which is released from intracellular storage compartments [76]. Then, SOS3 interacts with SOS2, which is a CIPK serine-threonine protein kinase. The SOS3/SOS2 kinase complex regulates the expression of SOS1 genes therefore it can stimulate SOS1 Na+/H+ antiporter activity [77]. In seedlings of three bread wheat genotypes, which were characterised as highly tolerant, moderately tolerant and sensitive to salinity stress, the expression of SOS1, SOS2 and SOS3 genes was observed at a significantly higher level in the salt-tolerant genotype. What is more, both constitutive and salt-induced expression of SOS1 was 2-fold higher in the leaf of this genotype. This was correlated with low Na+ levels in tissue and better leaf K+/Na+ ratio in leaves, which was probably a result of the facilitated exclusion of toxic Na+ into root apoplast [75]. Similarly in experiments by Jiang et al. [77], the expression of several genes belonging to the TaSOS1 gene family was up-regulated in response to salinity in the wheat-tolerant genotype after 1 day of salt treatment. What is more, overexpression of the wheat genes encoding TaSOS1 and TaSOS1-974 (with a deletion on the C-terminus) in tobacco resulted in improved Na+ efflux and K+ influx rates in the roots of the transgenic plant compared to wild-type (WT) tobacco upon salt stress. Among these three types of plants, the lowest content of MDA and electrolyte leakage was observed in TaSOS1-974 transgenic plants while the highest was observed in WT tobacco. This indicates that the overexpression of TaSOS1-974 might alleviate oxidative damage of the plasma membrane generated upon salinity [78]. Comparable results were obtained in Arabidopsis SOS1 mutant plants with the overexpression of durum wheat (Triticum durum Desf.) gene TdSOS1∆972 (with a deletion on the C-terminus). These plants showed greater water retention capacity and maintained a better K+/Na+ ratio in their shoots and roots, as well as their seeds, had a better germination rate upon salinity than in Arabidopsis SOS1 mutant transformed with empty binary vector or TdSOS1 (full-length) [79]. These results confirmed that in proteins belonging to the SOS1 family, the C-terminus function as an auto-inhibitory domain. Autoinhibition of SOS1 is released when the C-terminus domain is phosphorylated by activated SOS2 [80]. Additionally, in rice and barley, the involvement of SOS genes in response to salinity was observed. Fu et al. [81] showed that rice OsSOS3 was significantly up-regulated in roots under salt stress. Additionally, the expression of OsSOS2 and OsSOS1 was markedly up-regulated and a high transcript level of these genes was maintained. In turn, barley HvSOS3 was only slightly up-regulated in roots under stress. Other barley SOS genes, HvSOS1 and HvSOS3, showed slight changes in roots during salt treatment. All tested rice genes showed higher absolute expression than barley genes. However, rice was more sensitive to salt stress than barley. In rice, a higher excess of Na+ was observed in the shoots, which was harmful for physiological processes, e.g., protein degradation. On the other hand, in rice, the level of Na+ in the roots was lower than in barley, which might be the result of Na+ efflux through the SOS pathway. Despite this phenomenon, barley maintains normal metabolism. These results show the differences in salt tolerance between these two species [81].
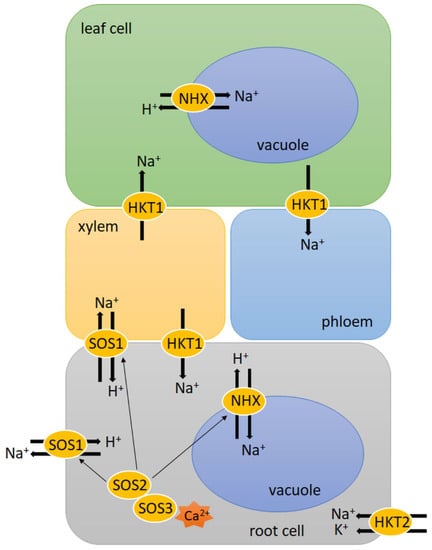
Figure 1.
Na+ transportation under salinity. Plants remove Na+ from the cytoplasm using plasma membrane Na+/H+ antiporter (SOS1), which is activated through phosphorylation, catalysed by the SOS2–SOS3 kinase complex, SOS3 is a Ca2+ sensor. Compartmentation of Na+ into vacuoles occurs by Na+/H+ antiporter (NHX), which is also activated by SOS2–SOS3 kinase complex. High-affinity K+ transport (HKT) proteins, are Na+ transporters (class 1) or Na+/K+ symporters (class 2). HKT1 proteins remove Na+ from xylem. HKT2 play role in Na+ uptake in the root. Details are described in Salinity paragraph.
Besides the exclusion of Na+ from the cytosol, compartmentation of Na+ into vacuoles by tonoplast Na+/H+ antiporter (NHX) is also another essential mechanism in salt stress response (Figure 1). The necessary proton gradient required for NHX activity is derived from vacuolar H+-pyrophosphatase and H+-ATPase. The SOS3/SOS2 kinase complex regulates both NHX and H+-ATPase activity under salt stress. In wheat, the expression of the NHX1 gene was markedly increased under saline conditions compared to the control [82]. Additionally, overexpression of the wheat TaNHX2 gene in eggplant (Solanum melongena L.) and sunflower (Helianthus annuus L.) increased salinity tolerance in comparison to WT plants. Both transgenic species showed improved growth as well as reduced ROS and MDA contents, which correlated with the high activity of antioxidant enzymes such as SOD and ascorbate peroxidase (APX) [83,84]. A comparison of salt stress response in barley and rice showed that the expression of one of the NHX genes was significantly higher in barley (HvNHX5) than in rice (OsNHX5) in the roots treated with salt. However, the expression of rice OsNHX1, OsNHX2 and OsNHX4 in shoots was higher than in barley HvNHXs. This may indicate that a higher concentration of Na+ in rice shoots is a result of the up-regulated expression of NHX genes [81]. Moreover, the overexpression of barley HvNHX2 in Arabidopsis showed that, under salt conditions, transgenic plants grew normally, while WT plants were not able to. Additionally, transgenic plants had a higher concentration of Na+ in the shoots and had longer roots than WT plants [85]. Similarly, the overexpression of OsNHX1 in transgenic rice showed increased salt tolerance in transgenic plants and delayed appearance of negative effects connected with damage or death [86]. These results suggest that the vacuolar Na+ compartmentalisation plays a beneficial role in improving cereals’ salt tolerance.
Another element involved in the response to salinity is the family of high-affinity K+ transport (HKT) proteins, which, contrary to their name, are Na+ transporters (class 1) or Na+/K+ symporters (class 2) (Figure 1). HKT1 proteins remove Na+ from xylem sap and sequestrate Na+ into xylem parenchyma cells. The function of this mechanism is to confine toxic Na+ to the roots, therefore, it prevents the accumulation of Na+ in shoots and leaves, protecting the photosynthetic tissues from damage [58,87]. By contrast, SOS1 plays a role in the protection of the root since it exports Na+ out of the root and facilitates its loading into the xylem. These two mechanisms function antagonistically, and it is not fully understood how they are activated and regulated to avoid Na+ loading and unloading. The role of HKT proteins differs between species in response to salinity. For most species, Na+ exclusion from the leaf blade is correlated with enhanced salinity tolerance and is due to HKT1. The comparison of two rice varieties with different sensitivity to salinity showed that, under salinity stress, the Na+ concentration in the leaf blades was much lower in Ouukan383 (salinity tolerant) than in Kanniho (salinity sensitive). It is the result of a high expression level of OsHKT1;4 in the leaf sheaths of in Ouukan383 cultivar, corresponding to higher Na+ accumulation in the leaf sheaths and lower Na+ accumulation in the leaf blades. What is more, under salinity conditions, the expression of the OsHKT1;5 gene was induced in the roots of Ouukan383 but was repressed in the roots of Kanniho. These findings indicate that the expression of OsHTS1s might be correlated with better tolerance to salt stress [88]. In addition, a mutation in OsHKT1;5 in rice showed that lack of OsHKT1;5 protein in roots leads to excess Na+ accumulation in leaves in response to salt stress [89]. On the other hand, the expression of ZmHKT1;5 in two maize genotypes (Zea mays L.), SC131 (more tolerant) and SC132 (less tolerant), was not significantly affected under salt stress. However, the expression of ZmHKT2 was highly induced in SC132 while its transcripts were absent in SC131. It can be concluded that differences in the salinity tolerance in these maize genotypes might be the result of weaker Na+ and K+ translocation to the shoots due to high expression of ZmHKT2 in the roots of SC132 since it is responsible for reduced leaf K+ concentration, enhanced Na+ uptake in the roots and later more translocation to the shoots [90].
Signalling through the MAPK cascade leads to cellular responses against various stresses. This pathway relies on successive phosphorylation reactions, thus maintaining proper cell phosphorus (P) content is crucial. During salt stress, Cl– may reduce plant P content due to ionic competition. Therefore, salinity may negatively affect the MAPK pathway. However, activation of the components of this signalling cascade does not always function as a positive regulator in the stress response. Hao et al. [91] showed that wheat TaMPK4, one of the members of MAPK, was a positive regulator in salt stress response. Sense- and antisense-expressing of TaMPK4 in tobacco strongly modified plant growth under salinity. TaMPK4-overexpressing plants were much larger and showed a larger dry mass, leaf number and leaf areas, while TaMPK4-knockout plants were much smaller and showed a lower dry mass, leaf number and leaf areas, compared to WT plants. What is more, under salinity, plants with overexpression of TaMPK4 had higher K+ and osmolyte contents and lower Na+ content than the WT plants, unlike TaMPK4-knockout plants [91]. Similarly, Arabidopsis plants with overexpression of ZmSIMK1, maize MAPK member, had increased tolerance to salt stress. Seeds of transgenic lines germinated better on medium containing NaCl, as well as at seedling stage, their growth was not inhibited, as was observed in WT plants [92]. On the other hand, the overexpression of wheat TMKP1, mitogen-activated protein kinase phosphatase (MKP), which is a negative regulator in the MAPK signalling pathways in Arabidopsis, resulted in improved tolerance to NaCl. Seeds of transgenic plants had a better germination rate and seedlings had lower content of MDA and ROS compared to WT. Improved resistance to salt stress in TMKP1-overexpressing plants was correlated with increased antioxidant enzyme activities, which resulted in less damage to cell components [93]. Additionally, Seong-Kon et al. [94] showed that rice OsMAPK33 could play a negative role in salt tolerance. The expression of OsMAPK33 was down-regulated until 8 h after the induction of salt stress, indicating that this is a negative regulator in response to salinity. Moreover, the overexpression of OsMAPK33 in rice enhanced sensitivity to salt stress. It was assumed that it was a consequence of disrupted ion homeostasis since transgenic plants had reduced expression of ion transporter genes, such as the K+/H+ antiporter [94].
It was also reported that H2S might be an important player in plants’ response to salinity. It was shown that exogenous application of H2S improved salt tolerance in some cereals such as rice [95], wheat [96] and barley [97]. The protective role of H2S was the result of maintaining ion homeostasis, as well as reducing oxidative stress, which was reflected in decreased ROS and MDA contents under salt stress. In addition, antioxidant enzyme activity was increased with H2S application. Exogenous H2S might also enhance photosynthetic capacity as well as improve primary and energy metabolism. As it was shown in rice under influence of exogenous H2S, proteins related to glycolysis, tricarboxylic acid cycle and ATP synthesis were up-regulated in salt-treated plants [95]. Moreover, exogenous H2S up-regulated transcript level of genes encoding proteins involved in the SOS pathway and the MAPK pathway, as was recently shown in wheat [96].
4. Heavy Metals
The impact of heavy metals (HMs) on plants depends not only on the concentration and type of xenobiotic elements but also on their availability to plants, which is related to such soil factors as pH, cation exchange capacity, organic matter content and adsorption by clays. HMs in high concentration affect membrane permeability, inhibit enzymes activity, inactivate photosystems and disturb mineral metabolism [98]. Furthermore, HMs cause secondary oxidative stress, which results in the oxidation of plant membranes, damage of nucleic acid, leading to mutations, oxidative modifications of proteins resulting in loss of their activity, disruption of pigment function, and finally, cell death [99]. The toxicity of a specific substance, including HMs, depends on a variety of factors, e.g., how much of the substance organisms are exposed to, how they are exposed and for how long. Understanding the mechanisms underlying plant resistance or tolerance of plants to abiotic and biotic stress factors is extremely important in the era of global warming, where the mobility of pollutants in the environment increases [3]. However, some HMs are necessary (in non-toxic concentrations) for the proper development and growth of cereals. This category includes, among others, copper (Cu), iron (Fe), cobalt (Co), zinc (Zn), molybdenum (Mo), manganese (Mn), boron (B) and nickel (Ni), the presence of which is required for the proper functioning of the plant. However, excessive concentrations of even these essential micronutrients can also stress the plants. There is also a group of particularly highly toxic HMs including Pb, Hg, As and Cd that are ranked as the first, second, third and sixth, respectively, in the list of the US Agency for Toxic Substances and Disease Registry (ATSDR) [100].
HMs negatively affect the plant cell on many levels. They can directly inhibit enzymes and cause an oxidative burst, leading to the overproduction of ROS and RNS, which changes the oxidative potential in the cell [101]. ROS and RNS not only damage proteins, which can lead to their degradation but also alter membrane permeability, which puts the integrity of the cell at risk. In addition, HMs can induce chloroplasts and mitochondria damage, which inhibits basic metabolic processes in the cell, such as photosynthesis and the respiration chain. What is more, HMs also influence the stomatal movements and subsequently affect the transpiration rate [102]. HM also caused damage to DNA and inhibition of transcription and translation, which hinders the synthesis of proteins that may be of fundamental importance in the survival of the cell. All these changes lead to the failure of cell division, which prevents the correct growth and development of crops [98]. However, it should be observed that each of the HMs can affect the plant in a slightly separate way. Cd causes a strong inhibition of cereal growth, browning of the roots and chlorotic changes in leaves. Cd particularly affects photosynthetic enzymes such as Fe (III) reductase. In turn, Hg blocks the flow of water in the plant by interacting with the water channels, thereby blocking them. The action of Pb focuses on changing the permeability of the cell membrane, disturbing the hormonal balance of the plant and inhibiting the activity of selected enzymes due to the interaction of Pb with their sulfhydryl groups. As has a similar effect on enzymes, as it also reacts with sulfhydryl-containing proteins, disrupting their function. As also binds to vicinal thiols present in dehydrogenases, which not only inhibits cellular respiration but also leads to overproduction of ROS [103].
Due to the different effects of individual HMs on cereals, the response of the plant to HM stress is multifaceted and is associated with the activation of several signalling pathways causing a change in the expression of the relevant TFs and/or genes: (a) calcium-dependent signalling; (b) signalling mediated by MAPK; (c) signalling via ROS; (d) hormone signalling [9]. Calcium signalling occurs through several sensors which include calmodulins (CaM), calmodulin-like proteins, calcineurin B-like proteins and CDPK. The activation of individual sensors depends on the concentration of Ca2+. It was observed that both the recurrent and long-term Cr (VI) stress in rice increased the activity of CDPK [104]. The signalling cascade based on MPAK caused the phosphorylation of selected transcription factors (ABA-responsive element; ABRE, dehydration-responsive element binding; DREB, bZIP, MYB, MYC, NAC and WRKY-containing a conserved WRKYGQK domain and a zinc finger-like motif) resulting in the altered expression of genes related to the HM stress response [9]. Induction of OsMAPK2 and myelin basic protein kinase was recorded in Cd-treated rice. In response to the increased production of ROS, cereals improve the activity of their antioxidant system by increasing both enzymatic (SOD, CAT, APX, dehydroascorbate reductase) and non-enzymatic (betaines, proline and ascorbate) activities, which allows them to avoid or reduce oxidative damage to the plant cell, however, some redox imbalance is necessary for the induction of a proper stress response [105]. ROS and kinase-related pathways may cross with each other. In rice, the activation of MPAK by excessive accumulation of ROS was reported as a result of secondary oxidative stress induced by HM stress. What is more, ROS also influence changes in the plant’s hormonal system, in particular, auxin (AUX), ET and JA and ABA signalling. Treatment of rice with JA was shown to increase the antioxidant response of rice to Cd [106]. Treatment of rice plants with As resulted in a change in ABA metabolism, which influenced the modulation of signal transduction and the plant defence stress response [107]. Besides those signal transduction pathways, miRNAs also play a crucial role in the response to HM stress. miRNAs are 20–24 nucleotide non-coding RNAs that regulate gene expression at the post-transcriptional level by targeting mRNA degradation or by translation repression [108]. Due to the different properties of individual HMs, their uptake pathways, as well as signal induction and transmission, differ from each other.
As (V), being the main form of As in the soil, is similar in structure to P ions and thus its uptake into the plant is possible via phosphate transporters. Under anaerobic conditions, As also reaches the cell via aquaporins (AQPs). AQPs include various family subclasses of proteins that can uptake As, including tonoplast intrinsic proteins, cell membrane intrinsic proteins, and nodulin-like proteins. In rice, As (III) ions can be taken up by silicon pathways and methylene forms of low silicon transporter proteins (Lsi1 and Lsi2), which have the ability to transport As (III) ions both from and into the cell [109]. Due to the similarity of As (V) to P ions, ATP synthesis in plant cells is disturbed. As (III) in turn reacts with thiol groups of proteins, including enzymes, leading to the disturbance of cell homeostasis. In rice, As caused the production of ROS and the activation of the MAPK-inducing phosphorylation cascade including MKK4, MPK3, MPK4 [110] and calcium-dependent signalling by CaM, CaM kinase and CaM-like protein [107,111]. Moreover, rice induces down-regulation of miR172 (miRNA) and up-regulation of miR393, miR397 and miR408. The last one (miR408) has a direct role in targeting Cu-containing proteins or SOD [112]. On the other hand, ROS down-regulated miR397 targeted laccase, which led to increased activity of the lignin biosynthesis pathway by the accumulation of laccase enzymes [113]. Additionally, one of the miRNAs, miR528, was crucial for As tolerance in rice [114].
Cd in the environment occurs in an ionic form (Cd2+) and is bound into chelates. Cd2+ is taken up into the plant by non-specific HM transporters, whose levels depend on transpiration. The most important uptake routes for both Cd forms include Zn-regulated transporters, Fe-regulated transporters, hyperpolarisation-activated Ca2+ channels, depolarisation-activated Ca2+ channels, voltage-insensitive cation channels, yellow-stripe 1-like proteins (YSL) and the natural resistance-associated macrophage protein (NRAMP). Transport to the xylem occurs via apoplastic ATP-binding cassette (ABC) transports and P1B ATPase and H+/Cd2+ antiports. During defence responses, cereals activate TFs such as DREB, APETALA2 (AP2) and bZIP [103]. Cd accumulation activated the MAPK pathway: MAPK2, MPK3, MPK6, MSRMK3, WJUMK in rice [110,115,116] and MPK3 [49] in maize. It also activated components of the hormonal pathway, mainly by auxins: MAPK3/6/7, YUCCA, PIN proteins, ARF (auxin response factors) and IAA [117]. Exposing rice to Cd stress led to the up-regulation of miR441, and down-regulation of 12 other miRNAs, including miR192, which targeted ABC transporters. Increased activity of ABC transporters enables Cd sequestration and stress alleviation [118]. Cd up-regulated the transcription factors belonging to MYB, AP2, DREB, WRKY and NAC at different time intervals in rice [119]. As for MYB, OsMYB45 was especially related to Cd toxicity, as its mutation increased H2O2 content in the leaves of mutant and decreased CAT activity compared to the WT plants [120], and OsARM1 (arsenite-responsive MYB1) regulated As-associated transporters genes OsLsi1, OsLsi2 and OsLsi6 [121].
In most plants, the occurrence of aluminium (Al) is limited to the roots, although the presence of Al-citrate in the xylem and Al-oxalate in the leaves of buckwheat was reported [122]. Al is excluded into the soil by organic acids aided secretion through transporters such as the Al-activated malate transporter (ALMT) family, ABC transporters family (STAR1 and STAR2), multidrug and toxic compound extrusion (MATE) family and aluminium transporter 1 (NRAMP/NRAT1) family [123]. In wheat, Al accumulation enabled pathways dependent on MAPK: 48 kDa MAPK, 42 kDa protein kinase [124], Ca: myosin, calpain, phospholipase C, phospholipase A2 [125], and ethylene: ALMT1, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), ACC oxidase (ACO) [126]. Similar to previously described HMs, Al also down-regulated most of the miRNAs in rice such as miR156, miR395, miR398, miR159 and only miR399, miR166, miR168 were up-regulated in response to Al [127]. This however is not true for all crops, as maize showed mostly miRNAs up-regulation with the exception of miR171 and miR396 [128]. MiR395 targets genes of ATP sulfurylase (APS) and SULTR2:1, which are crucial for GSH and phytochelatin (PCs) synthesis [129].
Another important HM is Hg. The bioavailable Hg compounds in the soil are Hg2+ and methylmercury. Hg with a hydrophilic character is easily trapped by the roots, transported to the shoots, and then released back into the atmosphere in gaseous form. Hg tends to accumulate in the roots and cannot be transferred to plant shoots. Transport of Hg in the plant is possible due to ABC transporters. They can pump Hg2+ conjugates to or from the vacuole of higher eukaryotes [130]. It was shown that an accumulation of Hg led to the activation of MAPK proteins in rice, especially MSRMK2, MSRMK3, WJUMK [115], and the ET pathway via OsACS2, OsACO1, OsACO2, OsACO5 and OsACO6, 5 MAPKKK, 1 MAPKK and 2 MAPK [131].
Pb in the form of a dipositive cation is passively aborted by root hairs. Its further transport is severely limited by its low solubility. Pb transport in the plant is accomplished by the apoplast of xylem tissues but is blocked in the Kasparian bands of the endoderm. It can then be sequestered via ABC transporters, P-type pumps, pleiotropic drug resistance (PDR1), inner membrane proteins of mitochondria, ATM1, leucine-rice repeat proteins (LRR), Ca2+ gated channels, cyclic nucleotide ion gated channels and K+ gated channels [132]. In rice, Pb activates 34 kDa, 40 kDa and 42 kDa MAPK, and a calcium-dependent pathway via CDPK-like kinase [133].
In order to limit the negative effects of HMs, the signal cascade causes adaptive changes in the plant cell, relying on detoxification to prevent the involvement of HMs in undesirable toxic reactions. Defence strategies include preventing or reducing the uptake by limiting the transport of metal ions to the apoplast by binding them to the cell wall or cell exudate, or by inhibiting long-distance transport [134]. To achieve that, activation of appropriate TFs and induction of the transcription of particular genes related to the HMs response is necessary. Some of the up-regulated genes are associated with the activation or amplification of selected signal transduction pathways. For example, As treatment of rice increased the expression of the ABA biosynthesis genes: OsNCED2 and OsNCED3 [135], while chromium treatment of rice increased the expression of four ET biosynthesis-related genes (ACS1, ACS2, ACO4 and ACO5) [136,137], two genes associated with MAP cascades (OsMPK3, OsCML31), three protein kinase-related genes (OsWAKL-Os, OsLRK10L-2, OsDUF26-If) and two TF-related genes (OsWRKY26, OsAP2/ERF-130) [137]. Another group of genes expressed by the action of HMs are genes encoding phosphatases. Phosphorylation/dephosphorylation is the most common post-translational modification, whose role is to activate and deactivate selected proteins, which results in the adaptation of the metabolism to the plants’ needs. In rice treated with chromium, increased expression of five families of genes encoding phosphatases (OsLMWP, OsDSP, OsPP2A, OsPTP and OsPP2C) was observed [137]. Due to the fact that one of the strategies for reducing the negative impact of HMs is their translocation, another group of genes up-regulated as a result of stress are those related to the transport of HMs. In rice, Cr strongly induced a number of genes involved in the vesicle trafficking pathway, including five OsExo70 genes (Os01g0763700, Os06g0255900, Os01g0905300, Os01g0905200 and Os11g0649900) and one Tom1 gene (Os05g0475300) [137]. In durum wheat, the exposure to Cd induced several vacuolar HM transporter genes, especially ZIF1, ZIF-like genes [138].
When HMs are present at elevated concentrations, cells activate a complex network of storage and detoxification strategies, such as chelating metal ions with phytochelatins (PC) and metallothioneins (MTs) in the cytosol, as well as transport and sequestration into the vacuole via vacuole transporters [139]. HMs activate the synthesis of phytochelatin synthase (PCS) and metallothionein, and then HM–PC and HM–MTs complexes of low molecular weight (LMW) are formed in the cytosol. LMW HM–PCs complexes are consistently transported across the tonoplast into the vacuole via the ATP binding cassette and the V-ATPase transporter (ABCC1/2). After compartmentalisation, the LMW complexes further integrate HMs and are generated by chloroplasts sulfide (S2−) to eventually form HM–PC complexes of high molecular weight (HMW). MTs regulate cellular redox homeostasis independently and by stimulating the antioxidant system and stabilising high cellular GSH concentrations. It was well documented that the biosynthesis of PCs can be regulated at the post-translational level by metals in many plant species. However, the overexpression of the phytochelatin synthase (PCS) gene in plants does not always result in enhanced tolerance to HM stress [140]. Moreover, MTs not only bind HM but also partake in the elevation of oxidative stress by acting as ROS scavengers, thus, integrating those two pathways [141]. MTs are tissue-specific. For example, the OsMT2c gene encoding for type 2 MT was expressed in the roots, leaf sheathes and leaves of rice, but was almost absent in seeds [142]. Moreover, to protect proteins against HM stress, HSP proteins are also synthesised, belonging to HSPs70, HSPs60, HSPs90, HSPs100 and HSPs classes. HSPs70 were induced in rice by As, Ag, Cu, Cd and Cr (HSP70, BiP), HSPs60s by Hg (cpn602), HSPs90 by Cu, As and Cd (HSP81-2, HSP82, HSP81-1), HSPs100 by As, Cu and Co (HSP101, ClpB-C), and HSPs by Cu, Cd, Fe, Al and Zn (HSP17.4, HSP23.9, HSP78.3) [140].
5. Biotic Stress
Plants are exposed to a wide variety of pathogens and pests, the life cycle of which and the impact on plants differ significantly. Therefore, it is difficult to identify one common signalling pathway associated with the biotic stress response. The plant–parasite relationship is quite specific and depends on both the defence mechanisms and the structure of the plant itself, as well as those of pathogen, therefore the signal transduction pathway is multifaceted and quite strongly individualised. Research on the subject is fairly limited, but in this review, we attempted to describe its known elements.
Plants have an innate immune system able to recognise evolutionarily conserved microbe/pathogen-associated molecular patterns or herbivore-associated molecular patterns [143,144]. The presence of transmembrane pattern recognition receptors and intracellular proteins of the nucleotide-binding domain and leucine-rich repeat superfamily enables the identification of pathogens/herbivores by plant cells which leads to induction of defence reactions including the synthesis of signalling molecules such as SA, ABA, JA, ET, H2O2 and NO [145]. The activation of those signalling patterns can cause alterations in gene expression, leading to specific defence responses. Both pathogens and insects can act locally and systematically [145].
Plants launch defence responses to shield themselves against pathogens and pests. Those responses are regulated by the infestation-induced production of hormones. SA, JA, ET and ABA are vital players in induced mechanisms against biotic stresses [146]. SA-dependent responses are usually efficacious against biotrophs, while JA-dependent responses are successful against necrotrophs and phytophagous insects [147]. Defence signalling of SA depends on the transcriptional co-factor called non-expresser of pathogenesis-related gene 1 (NPR1), ultimately leading to the activation of anti-microbial pathogenesis-related (PR) genes [148]. Following pathogen infection/insect infestation, molecules such as ABA, JA, SA, ET, H2O2 and NO are accumulated at different time points and convergence of signalling pathways can occur in a plant [149,150].
The biotrophic barley powdery mildew Blumeria graminis and the hemibiotrophic Bipolaris sorokiniana are economically significant pathogens of H. vulgare. To assess the barley defence responses to these pathogens, alternations in SA and genes of SA-dependent responses (PR1, PR2, PR3 and PR5) were studied, which revealed that the level of SA was significantly enhanced in infected barley plants (both resistant and susceptible) at 24 h post-inoculation compared to control plants. Furthermore, time-course experiments showed a clear contradiction in patterns of expression of SA-dependent genes upon barley inoculation with B. graminis and B. sorokiniana. These studies also showed that the expression of PR1 and PR2 genes was induced in resistant barley inoculated with B. sorokiniana contrary to B. graminis infestation, indicating different SA-dependent responses in barley plants infested with fungal pathogens with different lifestyles [2].
MYB transcription factors play a vital role in cereal plant defence including responses to fungal pathogens. Wei et al. [151] presented results on characterisation of the TaPIMP2 gene encoding a pathogen-activated MYB protein in T. aestivum. The expression of TaPIMP2 was altered to a different extent and speed upon inoculation with B. sorokiniana or Rhizoctonia cerealis. In addition, different expression patterns of TaPIMP2 were observed after T. aestivum plants were sprayed with ABA, 1-aminocyclopropane-1-carboxylic acid (ACC, precursor of ethylene) or SA. Silencing of TaPIMP2 decreased the resistance of B. sorokiniana-resistant wheat to B. sorokiniana infection but did not change the resistance of R. cerealis-resistant wheat to R. cerealis infection. On the other hand, the overexpression of TaPIMP2 remarkably increased resistance to B. sorokiniana rather than R. cerealis in transgenic wheat. Moreover, it was observed that TaPIMP2 is engaged in wheat resistance to B. sorokiniana due to stimulation of the expression of PR1a, PR2, PR5 and PR10.
After the plant is mechanically injured or infested with necrotrophic pathogens or insects, the accumulation of JA and its derivatives—oxylipins (called jasmonates)—occurs [152]. For example, infestation of maize with a lepidopteran pest, the beet armyworm caterpillars (Spodoptera exigua) induced synthesis of JA, MeJA and jasmonoyl-L-isoleucine in infested-maize leaves [153]. There are two separate branches of the JA signalling that have a negative influence on each other: the ERF branch and the MYC branch [154]. The ERF branch is induced upon infestation with necrotrophs and is controlled by the AP2/ERF-domain transcription factors such as ERF1 and octadecanoid-responsive AP2/ERF 59 (ORA59). Furthermore, the ERF branch is co-regulated by ET and triggers the expression of many ERF-branch genes including the marker gene encoding plant defensin 1.2 (PDF1.2) [155]. Dong et al. [156] identified and characterised B. sorokiniana-induced defence gene (TaPIEP1) from the ERF branch (B-3c subgroup) of wheat. The mRNA level of TaPIEP1 was induced upon both inoculations with B. sorokiniana and treatments with ET, JA, and ABA. Transgenic T. aestivum plants overexpressing TaPIEP1 showed enhanced resistance to B. sorokiniana. The increased resistance of transgenic wheat lines showed also increased transcript levels of defence-associated genes from the ET/JA pathways. Wheat is one of the main cereals crucial for food production worldwide, therefore its pathogens should be one of the main focuses in biotic stress studies. Besides B. sorokiniana, Puccinia striiformis, which also causes stripe rust, is an important wheat pathogen. In response to P. striiformis reactive oxygen species burst is observed. Early accumulation of ROS leads to an increase in chlorophyll a and b levels, as well as to activation of antioxidative enzymes. It contributes to plant resistance to this pathogen [157].
Jisha et al. [158] proposed a model for the role of the AP2/ERF transcription factor, OsEREBP1, during the response of rice plants to infection with the bacterium Xanthomonas oryzae pv. oryzae. The authors suggested that enhanced expression of OsEREBP1 can lead to accumulation of JA, which mediates activation of the helix–loop–helix transcription regulator RERJ1 and induces linalool synthase activity so that volatile monoterpene linalool molecules are accumulated resulting in improved tolerance to X. oryzae pv. oryzae infection.
The brown planthopper (Nilaparvata lugens) is a hemipteran pest infesting rice plants. This insect injures plants through feeding, and it also transmits rice grassy stunt virus and rice ragged stunt virus [159]. Xylanase inhibitors were described as players participating in plant defence. Zhan et al. [160] presented that infestation with imagines of N. lugens, wounding or MeJA treatment increased transcript and protein levels of OsXIP (an XIP-type rice xylanase inhibitor). By studying 5′ deletion in OsXIP promoter in rice mutant plants invaded by N. lugens, a 562 bp region was shown as crucial for the response to stress induced by pest feeding. Furthermore, a basic helix–loop–helix protein (OsbHLH59) and an AP2/ERF-transcription factor OsERF71 directly reacted with 562 bp sequence to induce the expression of OsXIP. The expression of genes OsbHLH59 and OsERF71 was also stimulated in rice roots and shoots by wounding and submerging in MeJA.
Fusarium head blight induced by Fusarium species such as F. graminearum is a globally important fungal disease of wheat. Transcriptional profiling of moderately resistant and susceptible to F. graminearum winter wheat cultivars have shown 2169 differentially expressed genes, induced by jasmonate and ethylene, e.g., encoding thionin, lipid-transfer protein, defensin and GDSL-like lipase. Moreover, defence-activated genes encoding jasmonate-dependent proteins were up-regulated in response to infection with F. graminearum, such as, for example, the subfamily of mannose-specific jacalin-like lectin-containing proteins [161].
During an infestation, pathogens and pests secrete effectors into host plant tissues. These effectors interact with plant defence systems, which may lead to effective colonisation and the spread of the infection [162]. Darino et al. [163] performed functional characterisation of the biotrophic fungus Ustilago maydis (causing smut disease on maize plants) effector jasmonate/ethylene signalling inducer 1 (Jsi1). Jsi1 reacts with members of the plant corepressor protein family Topless/Topless-related (TPL/TPR). It was shown that the increased expression of Jsi1 in maize led to activation of the ERF-branch pathway by an ET-responsive element-binding factor-associated amphiphilic repression (EAR) motif, which takes after EAR motifs from plant ERF transcription factors interacting with TPL/TPR proteins. Interestingly, phytopathogen effector candidates with EAR motifs were also found to be secreted by an ascomycete fungus Magnaporthe oryzae (affecting rice) and a Basidiomycota fungus Sporisorium reilianum (affecting maize and sorghum) [163].
In winter wheat field studies, it was shown that JA application induced resistance to cereal aphids (Metopolophium dirhodum, Sitobion avenae, Rhopalosiphum padi) and thrips (Limothrips denticornis and Thrips angusticeps). JA at first caused a significant decrease in the number of pests, which, even though it increased in time, remained lower on wheat treated with JA [164].
The MYC branch is induced upon mechanical injury or feeding by insects. This branch is controlled by basic helix–loop–helix leucine zipper transcription factors MYC2, MYC3 and MYC4, and it is also coordinated by ABA [165]. The MYC-branch activation results in the induction of JA-responsive gene expression including marker genes of the MYC-branch such as vegetative storage protein 1 and 2 (VSP1 and VSP2) [166].
The rice water weevil (Lissorhoptrus oryzophilus) is the most harmful coleopteran pest of O. sativa plants. It was proven that the treatment of rice seeds with jasmonates led to resistance against L. oryzophilus but rice growth and fitness were reduced. Jasmonates caused delayed emergence and heading, and after full development of plants, lower yield in comparison to plants grown from untreated seeds [167,168]. Therefore, it can be stated that plant fitness is decreased upon activation of JA-dependent defence responses, however, other hormones including ABA, SA, GAs, AUX and BRs are also substantial regulators of the immune–fitness balance caused by phytopathogens [169,170]. In addition, the decrease in plant growth elicited by JA is most probably regulated via signalling crosstalk with AUX, SA, BRs, GAs and CKs [171].
The crosstalk between hormonal pathways promotes the induction of efficient responses against pathogens and pests [172]. Many observations of the mutual interaction between the SA and JA pathways were made [173]. Pharmacological studies showed that the expression of PDF1.2 and VSP2 is sensitive to SA treatment. The opposed influence of SA on JA-depended responses was observed. It was shown that exogenous treatment with SA decreased the expression of the JA-responsive genes (PDF1.2 and VSP2) activated by MeJA, the necrotrophic fungi Alternaria brassicicola and Botrytis cinerea, and the western flower thrips (Frankliniella occidentalis) and P. rapae. However, infestation with the biotrophic oomycete Hyaloperonospora parasitica leading to SA-activation defence antagonised MeJA-dependent expression of PDF1.2 and VSP2 and infection with H. parasitica diminished P. rapae-activated expression of VSP2 [174]. Moreover, it was documented that this effect (induced by SA exogenous exposition) persists in the next plant generation [175]. The antagonism between SA and JA pathways can change resistance to biotic stressors. It was observed that activation of the SA signalling by exogenous exposition to SA or infestation with the hemibiotrophic bacterium Pseudomonas syringae, made the plants more susceptible to A. brassicicola [176,177]. Moreover, decreased SA responses in transgenic plants expressing a bacterial salicylate hydroxylase gene (nahG) and npr1 mutant plants were interdependent with attenuated feeding by the cabbage looper (Trichoplusia ni) caterpillars [178].
Similar antagonism is present in the ERF and the MYC branch. For example, it was proved that inducing the MYC2 branch in plants inhibits the ERF branch activated by P. rapae feeding, hence they are less alluring to the herbivore. Moreover, caterpillars of P. rapae preferred to feed on jin1 (MYC2 transcription factor) mutants and ORA59-overexpressing ones more than on WT plants, showing that the ERF and the MYC branch crosstalk changes host–insect herbivore interactions [154]. This antagonism between the ERF and the MYC branch can also change resistance to necrotrophic pathogens. The ERF branch was elevated in plants with MYC2-mutated jin1 and ABA biosynthesis mutant (aba2-1), leading to increased resistance to necrotrophs (B. cinerea, Plectosphaerella cucumerina, Fusarium oxysporum) [166,179,180,181,182].
Vast crosstalk between hormonal signalling pathways permits the plant under biotic stress for precise regulation of defence responses at various levels of plant organisation [183]. As elicitation of parasite-inducible responses is not without metabolic cost, trade-offs between immune defence and growth and development are clearly noticeable in plant organisms [146,184,185,186]. Hormonal crosstalk is sometimes discussed as an evolutionary cost-limiting strategy. Some researchers argue that this crosstalk may have evolved as a countermeasure to lessen energy costs by retardation of ineffective defence responses against specific invaders [187,188]. This hypothesis also seems to be confirmed by Vos et al. [189]. The authors analysed the effect of hormonal crosstalk on biotic stress resistance and host fitness upon multi-species infestation. Activation of SA- or JA/ABA-mediated responses by the biotrophs Hyaloperonospora arabidopsidis or P. rapae, respectively, decreased the level of induced JA/ET-response against the following infestation with B. cinerea. Notwithstanding, although there was increased susceptibility to this second invader, no long-term negative consequences were observed on host fitness when plants had been infected by multiple parasites. The authors concluded that host hormonal crosstalk during multi-parasite interactions gives the plants an opportunity to put their defence in order of importance while decreasing the energy fitness costs linked to activation of immune responses. This issue is extremely interesting and requires further research, especially in the context of crop plants including cereals.
6. Crosstalk Signalling between Abiotic and Biotic Stress
Current research on biotic and abiotic stress response pathways in plants suggests that there are significant similarities between them. The responses of cereals plants to biotic and abiotic stress are a complex web of interactions between secondary messengers, ROS, phytohormones, antioxidants, photosynthetic pigments, secondary metabolites, protein kinases, TFs, photosynthesis efficiency and chlorophyll a fluorescence parameters and ultrastructural adjustments [190,191,192]. Plants subjected to abiotic stress, e.g., high temperature, drought, salinity, are often more sensitive to subsequent attacks by pathogens [193]. There are reports about the decrease in the disease resistance of crops due to high humidity and high temperature [194]. Both types of stress factors cause the increase in such parameters as Ca2+, ROS, and pH levels in the apoplast. MAPK kinases are activated, which is a common response to both stresses [195]. For example, OsMPK5 kinase in rice is an ortholog of AtMPK3 in Arabidopsis and NtWIPK in tobacco, which are well known to be activated by both different pathogens and abiotic environmental stimuli [195]. ABA triggered a signal and it negatively imposed on the signalling of defence hormones, e.g., SA. ABA/SA interaction is two-sided, as activation of SA signalling by pathogens lowers ABA concentration [194]. On the other hand, positive interactions were observed for JA/ET signalling in response to double stress. ABA can act as a molecular switch between both responses and plays a dominant role in the response to stress [196]. It can take place through the ABA-inducible genes ERD15 and ATAF1, which may activate ABA-dependent biotic stress responses at the expense of abiotic responses [197]. A scheme for the interaction interface and overlapping signalling pathways of abiotic and biotic stress at the cellular level is presented in Figure 2.

Figure 2.
Scheme for the crosstalk signalling between abiotic and biotic stress. Both stress factors are first recognised by plant cells and then information is transduced through chemical signals such as Ca2+, reactive oxygen species (ROS), as well as mitogen-activated protein kinases (MAPK) cascades. Abscisic acid (ABA) is mostly involved in abiotic stress acclimation, while salicylic acid (SA) and jasmonate/ethylene (JA/ET) are responsible for the reaction to abiotic as well as biotic stresses. Finally, phytohormones up-regulate transcription factors (TFs), which then contribute to expression of genes related to stress response, e.g., late embryogenesis abundant proteins (LEA), heat shock proteins (HSP), phytochelatins (PC), metallothioneins (MT), defensis (DF).
CDPK families are also involved in crosstalk between biotic and abiotic stresses [198]. They are involved in various processes such as osmotic homeostasis, cell protection and root growth [44]. Some studies have reported that the CDPK genes not only behaved as positive regulators of abiotic or biotic stress signalling but also as negative regulators [44]. Overexpression of OsCDPK12 in rice led to positive regulation of salt tolerance and negative regulation of blast resistance [199].
Phytohormones regulate the activity of transcription factors such as WRKY, MYB, ERF, NAC and the HSF family, which respond to both biotic and abiotic stress and play a vital role in the plant’s response to simultaneously occurring stresses. WRKY30 and WRKY13 have a dualistic function in response to drought, salinity, cold and pathogen attack in rice [200]. Some WRKY such as OsWRKY76 antagonistically regulated the response of rice to blast disease and cold stress [201] but OsWRKY82 improved resistance against pathogens and tolerance against abiotic stress via the JA and ET pathways [202]. The rice OsWRKY45 is induced in response to ABA in various abiotic stress and also by infection with Pyricularia oryzae Cav. and Xanthomonas oryzae pv. oryzae. In a study by Qiu and Yu [203], it was shown that constitutive overexpression of the OsWRKY45 led to a significant increase in the expression of PR genes, resistance to bacterial pathogens, as well as tolerance to salt and drought stresses.
MYB transcription factors also may be common element of the response to various stresses. The MYB factor TaPIMP1 from wheat confers tolerance to drought and salt and pathogens stress when overexpressed in tobacco [204]. Another one of the candidates for common TF for multiple stresses response is JAmyb. JAmyb expression in response to salinity and osmotic stress was observed in rice seedlings. Microarray analysis showed that JAmyb overexpression stimulated the induction of several defence-related genes, some of which are predicted to be involved in osmosis regulation, ROS removal and ion homeostasis [205]. Additionally, transgenic rice plants overexpressing JAmyb exhibited improved resistance to blast [206]. A study by Yokotani et al. [205], showed that JAmyb expression was induced by H2O2 and paraquat. However, it is known that ROS overproduction is a common response to biotic and abiotic stress and could overlap with other stress responses. It is suggested that JAmyb might play a role in the crosstalk between JA and ROS-signal transduction pathways in dual stresses [205].
Another important TF is NAC. NAC are plant-specific TFs induced in various developmental stages and under abiotic and biotic stress [207]. The enhanced expression of the TaNAC4 gene in wheat was observed under the fungus, salinity, wounding and cold stress [208]. Expression of OsNAC6 in rice was induced by abiotic stresses, including cold, drought and high salinity, as well as by biotic stresses, such as wounding and blast disease [207]. OsNAC6, among others, increases the activity of peroxidase, which elevates oxidative stress.
It was shown that genes encoding cold-responsive/late embryogenesis abundant (COR/LEA) proteins, participate in improving cold resistance and protection of cells from dehydration and low-temperature [209]. It is known that the ABA participates in the regulation of COR gene (WRAB15 and WRAB18) expression in wheat. Studies by Talanova et al. [210] showed enhanced expression of WRAB15 and WRAB18 genes in wheat leaves caused by the Cd, hardening or their combination. This may indicate the participation of these genes in the protective and adaptive responses of plants to different stress factors [210].
As mentioned above, the effect of one stress can make plants more sensitive to the next stress. On the other hand, exposure of plants to one stress affects their response during the next stress leading to enhanced defence mechanisms to later stress. This phenomenon called “priming” results in a faster and stronger induction of basal defence mechanisms upon subsequent biotic stress factors [3]. “Metabolic memory” in higher plants requires less energy expenditure than defence directly induced by insect feeding or infection caused by pathogens.
A list of genes that may be crucial in signalling the response to biotic and abiotic stresses is given in Table 1 and Table 2.

Table 1.
The list of genes with a potential role under abiotic and biotic stress signalling pathways.

Table 2.
The list of mutants and transgenic plants with changed stress tolerance under abiotic and biotic stress.
7. Conclusions
Different stresses affect plants in various ways, therefore proper plant acclimation enabling plant survival is dependent on the crop’s ability to recognise the stress factor and its intensity, as well as on the ability to transmit the signal to the appropriate parts of both the cell and the plant in order to trigger an adequate response. While some plant defence mechanisms (such as ROS signalling) are not specific and occur under most stresses, others are strictly dependent on the specific stress factor (e.g., SOS). When cereals struggle to survive only with drought or with the presence of HMs, the situation is quite simple and well recognised in the literature. The problem appears when the same plant is affected by various stress factors at the same time or in short time intervals. In this case, the triggered defence mechanisms can be opposed to each other, which makes resistance to stress difficult. Therefore, learning about the signalling pathways and, more importantly, the interactions between them is crucial in plant cultivation, where multi-stress is common. It should be emphasised that these signal transduction pathways not only intersect with each other but are often opposed (ABA and SA), especially when both abiotic and biotic stress are present in the environment at the same time, which is of paramount importance for plant survival. By activating only selected response elements, and silencing others, it is possible to limit cereals’ energy expenditure on ineffective acclimatisation mechanisms. Reducing unnecessary energy consumption allows the plant to continue to develop and grow despite the presence of the stress factor, however, the same mechanism may lead to increase susceptibility to one stress when others occur. Therefore, in the near future, research should focus on signalling pathways crosstalk and multi-stress response.
Author Contributions
Conceptualisation, M.N.; writing—original draft preparation, M.N., J.F., M.G., M.L., B.P., I.M. and E.M.; writing—review and editing, M.N., J.F., M.G., M.L., A.R.-P., D.B.-M., J.G., I.M. and E.M.; supervision, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the Multidisciplinary Digital Publishing Institute (MDPI) publication discount voucher granted to Małgorzata Nykiel.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Al-Daoude, A.; Al-Shehadah, E.; Shoaib, A.; Jawhar, M.; Arabi, M.I.E. Salicylic acid pathway changes in barley plants challenged with either a biotrophic or a necrotrophic pathogen. Cereal Res. Commun. 2019, 47, 324–333. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Mai, V.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of Drought Damages by Exogenous Chitosan and Yeast Extract with Modulating the Photosynthetic Pigments, Antioxidant Defense System and Improving the Productivity of Garlic Plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Moghanm, F.S.; El-Banna, A.; El-Esawi, M.A.; Abdel-Daim, M.M.; Mosa, A.; Abdelaal, K.A.A. Genotoxic and Anatomical Deteriorations Associated with Potentially Toxic Elements Accumulation in Water Hyacinth Grown in Drainage Water Resources. Sustainability 2020, 12, 2147. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Wang, C.; Deng, Y.; Liu, Z.; Liao, W. Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12068. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Zhang, S.; Zhou, Y. Hydrogen sulfide is involved in the regulation of ascorbate-glutathione cycle by exogenous ABA in wheat seedling leaves under osmotic stress. Cereal Res. Commun. 2017, 45, 411–420. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Dolata, J.; Zielezinski, A.; Stepien, A.; Kruszka, K.; Bielewicz, D.; Pacak, A.; Jarmolowski, A.; Karlowski, W.; Szweykowska-Kulinska, Z. Quantitative Analysis of Plant Primary Transcripts. In RNA Abundance Analysis; Jin, H., Kaloshian, I., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2170, pp. 53–77. ISBN 978-1-07-160742-8. [Google Scholar]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. In Survival Strategies in Extreme Cold and Desiccation; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1081, pp. 215–232. ISBN 9789811312434. [Google Scholar]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; Xu, L. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gao, Z.; Wang, J.; Xu, W.; Huang, J. Morphological changes in roots of Bothriochloa ischaemum intercropped with Lespedeza davurica following phosphorus application and water stress. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2015, 149, 298–306. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials: Effects of drought on root traits. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Inagaki, M. Root development and water-uptake under water deficit stress in drought-adaptive wheat genotypes. Cereal Res. Commun. 2012, 40, 44–52. [Google Scholar] [CrossRef]
- Kadam, N.N.; Yin, X.; Bindraban, P.S.; Struik, P.C.; Jagadish, K.S.V. Does Morphological and Anatomical Plasticity during the Vegetative Stage Make Wheat More Tolerant of Water Deficit Stress Than Rice? Plant Physiol. 2015, 167, 1389–1401. [Google Scholar] [CrossRef]
- Kleine, T.; Leister, D. Retrograde signaling: Organelles go networking. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1313–1325. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Akter, S.; Jacques, S.; Huang, J.; Messens, J.; Van Breusegem, F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 2015, 66, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Trujillo-Hernandez, J.A.; Reichheld, J.-P. Thiol Based Redox Signaling in Plant Nucleus. Front. Plant Sci. 2018, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.-P. Thioredoxins and Glutaredoxins: Unifying Elements in Redox Biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome mining in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant Mitogen-Activated Protein Kinase Cascades in Environmental Stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Ford, K.L.; Cassin, A.; Bacic, A. Quantitative Proteomic Analysis of Wheat Cultivars with Differing Drought Stress Tolerance. Front. Plant Sci. 2011, 2, 44. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Gao, Y.; Guo, J.; Yu, T.-F.; Zheng, W.-J.; Liu, Y.-W.; Chen, J.; Xu, Z.-S.; Ma, Y.-Z. BES/BZR Transcription Factor TaBZR2 Positively Regulates Drought Responses by Activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guan, L.; Sun, Y.; Zhu, Y.; Liu, L.; Lu, R.; Jiang, M.; Tan, M.; Zhang, A. Calcium and ZmCCaMK are involved in brassinosteroid-induced antioxidant defense in maize leaves. Plant Cell Physiol. 2015, 56, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Z.; Ma, Q.; Sun, L.; Zhang, L.; Liu, Z.; Liu, D.; Hao, X.; Pei, Y. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 2017, 419, 141–152. [Google Scholar] [CrossRef]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.; Gao, X.; Tong, J.; Xiao, L.; Li, W.; Zhang, H. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene 2010, 457, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Li, D.; Zhang, H.; Huang, R. Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 2010, 232, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Mallikarjuna, M.G.; Rao, A.R.; Jain, P.A.; Dash, P.K.; Thirunavukkarasu, N. Comparative Analysis of CDPK Family in Maize, Arabidopsis, Rice, and Sorghum Revealed Potential Targets for Drought Tolerance Improvement. Front. Chem. 2017, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Wang, P.; Lu, Y.; Zhang, J.; Yang, X.; Cheng, T.; Shi, J.; Chen, J. CIPK11: A calcineurin B-like protein-interacting protein kinase from Nitraria tangutorum, confers tolerance to salt and drought in Arabidopsis. BMC Plant Biol. 2021, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.-W.; Chen, X.; Mei, Y. Function and regulation of phospholipid signalling in plants. Biochem. J. 2009, 421, 145–156. [Google Scholar] [CrossRef]
- Yoo, S.J.; Kim, S.H.; Kim, M.J.; Ryu, C.M.; Kim, Y.C.; Cho, B.H.; Yang, K.Y. Involvement of the OsMKK4-OsMPK1 Cascade and its Downstream Transcription Factor OsWRKY53 in the Wounding Response in Rice. Plant Pathol. J. 2014, 30, 168–177. [Google Scholar] [CrossRef]
- Kumar, K.; Rao, K.P.; Sharma, P.; Sinha, A.K. Differential regulation of rice mitogen activated protein kinase kinase (MKK) by abiotic stress. Plant Physiol. Biochem. 2008, 46, 891–897. [Google Scholar] [CrossRef]
- Wang, M.; Yue, H.; Feng, K.; Deng, P.; Song, W.; Nie, X. Genome-wide identification, phylogeny and expressional profiles of mitogen activated protein kinase kinase kinase (MAPKKK) gene family in bread wheat (Triticum aestivum L.). BMC Genom. 2016, 17, 668. [Google Scholar] [CrossRef]
- Ma, H.; Chen, J.; Zhang, Z.; Ma, L.; Yang, Z.; Zhang, Q.; Li, X.; Xiao, J.; Wang, S. MAPK kinase 10.2 promotes disease resistance and drought tolerance by activating different MAPKs in rice. Plant J. 2017, 92, 557–570. [Google Scholar] [CrossRef]
- Hadiarto, T.; Tran, L.-S.P. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011, 30, 297–310. [Google Scholar] [CrossRef]
- Shen, H.; Liu, C.; Zhang, Y.; Meng, X.; Zhou, X.; Chu, C.; Wang, X. OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 2012, 80, 241–253. [Google Scholar] [CrossRef]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca 2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Ran, J.; Zou, J.; Zhou, X.; Liu, A.; Zhang, X.; Peng, Y.; Tang, N.; Luo, G.; Chen, X. Heat shock factor OsHsfB2b negatively regulates drought and salt tolerance in rice. Plant Cell Rep. 2013, 32, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.; Khurana, N.; Agarwal, P.; Khurana, P. Heat shock factors in rice (Oryza sativa L.): Genome-wide expression analysis during reproductive development and abiotic stress. Mol. Genet. Genom. 2011, 286, 171. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and barley dehydrins under cold, drought, and salinity–what can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef]
- Karami, A.; Shahbazi, M.; Niknam, V.; Shobbar, Z.S.; Tafreshi, R.S.; Abedini, R.; Mabood, H.E. Expression analysis of dehydrin multigene family across tolerant and susceptible barley (Hordeum vulgare L.) genotypes in response to terminal drought stress. Acta Physiol. Plant 2013, 35, 2289–2297. [Google Scholar] [CrossRef]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant. 2021, 171, 546–558. [Google Scholar] [CrossRef]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.; Wu, R. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA Proteins During Water Stress: Not Just for Plants Anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef]
- Rodríguez-Valentín, R.; Campos, F.; Battaglia, M.; Solórzano, R.M.; Rosales, M.A.; Covarrubias, A.A. Group 6 Late Embryogenesis Abundant (LEA) Proteins in Monocotyledonous Plants: Genomic Organization and Transcript Accumulation Patterns in Response to Stress in Oryza sativa. Plant Mol. Biol. Rep. 2014, 32, 198–208. [Google Scholar] [CrossRef]
- Wang, C.-R.; Yang, A.-F.; Yue, G.-D.; Gao, Q.; Yin, H.-Y.; Zhang, J.-R. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta 2008, 227, 1127–1140. [Google Scholar] [CrossRef]
- Gehring, C.; Turek, I.S. Cyclic Nucleotide Monophosphates and Their Cyclases in Plant Signaling. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Hulless Barley. Plant Prod. Sci. 2015, 18, 52–56. [Google Scholar] [CrossRef]
- Lau, S.-E.; Hamdan, M.F.; Pua, T.-L.; Saidi, N.B.; Tan, B.C. Plant Nitric Oxide Signaling under Drought Stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Cheong, B.E.; Natera, S.; Roessner, U. Morphological and metabolic responses to salt stress of rice (Oryza sativa L.) cultivars which differ in salinity tolerance. Plant Physiol. Biochem. 2019, 144, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Alnusairi, G.S.H.; Mazrou, Y.S.A.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; ElNahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Abiala, M.A.; Abdelrahman, M.; Burritt, D.J.; Tran, L.P. Salt stress tolerance mechanisms and potential applications of legumes for sustainable reclamation of salt-degraded soils. Land Degrad. Dev. 2018, 29, 3812–3822. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.; Zhong, C.; Zhu, L.; Cao, X.; Yu, S.; Allen Bohr, J.; Hu, J.; Jin, Q. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Hossen, M.Z.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Riaz, M.; Arif, M.S.; Ashraf, M.A.; Mahmood, R.; Yasmeen, T.; Shakoor, M.B.; Shahzad, S.M.; Ali, M.; Saleem, I.; Arif, M.; et al. A Comprehensive Review on Rice Responses and Tolerance to Salt Stress. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–158. ISBN 978-0-12-814332-2. [Google Scholar]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants: Salt stress signaling. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Choudhary, P.; Pramitha, L.; Rana, S.; Verma, S.; Aggarwal, P.R.; Muthamilarasan, M. Hormonal crosstalk in regulating salinity stress tolerance in graminaceous crops. Physiol. Plant. 2021, 173, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Sathee, L.; Sairam, R.K.; Chinnusamy, V.; Jha, S.K. Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol. Plant 2015, 37, 169. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pan, R.; Buitrago, S.; Wu, C.; Abou-Elwafa, S.F.; Xu, Y.; Zhang, W. Conservation and divergence of the TaSOS1 gene family in salt stress response in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2021, 27, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lai, Z.; Yin, X.; Yu, S.; Xu, Y.; Wang, X.; Cong, X.; Luo, Y.; Xu, H.; Jiang, X. Hyperactive mutant of a wheat plasma membrane Na+/H+ antiporter improves the growth and salt tolerance of transgenic tobacco. Plant Sci. 2016, 253, 176–186. [Google Scholar] [CrossRef]
- Feki, K.; Quintero, F.J.; Khoudi, H.; Leidi, E.O.; Masmoudi, K.; Pardo, J.M.; Brini, F. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Rep. 2014, 33, 277–288. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ryu, C.-H.; Beilstein, M.A.; Schumaker, K.S. Distinct Roles for SOS1 in the Convergent Evolution of Salt Tolerance in Eutrema salsugineum and Schrenkiella parvula. Mol. Biol. Evol. 2014, 31, 2094–2107. [Google Scholar] [CrossRef]
- Fu, L.; Shen, Q.; Kuang, L.; Yu, J.; Wu, D.; Zhang, G. Metabolite profiling and gene expression of Na/K transporter analyses reveal mechanisms of the difference in salt tolerance between barley and rice. Plant Physiol. Biochem. 2018, 130, 248–257. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Aquib, A.; Anand, R. Analysis of physiological traits and expression of NHX and SOS3 genes in bread wheat (Triticum aestivum L.) under salinity stress. J. Pharmacogn. Phytochem 2020, 9, 362–366. [Google Scholar] [CrossRef]
- Yarra, R.; Kirti, P.B. Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct. Integr. Genom. 2019, 19, 541–554. [Google Scholar] [CrossRef]
- Mushke, R.; Yarra, R.; Kirti, P.B. Improved salinity tolerance and growth performance in transgenic sunflower plants via ectopic expression of a wheat antiporter gene (TaNHX2). Mol. Biol. Rep. 2019, 46, 5941–5953. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Shiran, B.; Belyaev, D.V. Overexpression of HvNHX2, a Vacuolar Na+/H+ Antiporter Gene from Barley, Improves Salt Tolerance in “Arabidopsis thaliana”. Aust. J. Crop Sci. 2011, 5, 428–432. [Google Scholar]
- Chen, H.; An, R.; Tang, J.-H.; Cui, X.-H.; Hao, F.-S.; Chen, J.; Wang, X.-C. Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol. Breed. 2007, 19, 215–225. [Google Scholar] [CrossRef]
- Ali, A.; Maggio, A.; Bressan, R.; Yun, D.-J. Role and Functional Differences of HKT1-Type Transporters in Plants under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef]
- Wangsawang, T.; Chuamnakthong, S.; Kohnishi, E.; Sripichitt, P.; Sreewongchai, T.; Ueda, A. A salinity-tolerant japonica cultivar has Na+ exclusion mechanism at leaf sheaths through the function of a Na+ transporter OsHKT1;4 under salinity stress. J. Agron. Crop Sci. 2018, 204, 274–284. [Google Scholar] [CrossRef]
- Kobayashi, N.I.; Yamaji, N.; Yamamoto, H.; Okubo, K.; Ueno, H.; Costa, A.; Tanoi, K.; Matsumura, H.; Fujii-Kashino, M.; Horiuchi, T.; et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017, 91, 657–670. [Google Scholar] [CrossRef]
- Rizk, M.S.; Mekawy, A.M.M.; Assaha, D.V.M.; Chuamnakthong, S.; Shalaby, N.E.; Ueda, A. Regulation of Na+ and K+ Transport and Oxidative Stress Mitigation Reveal Differential Salt Tolerance of Two Egyptian Maize (Zea mays L.) Hybrids at the Seedling Stage. J. Plant Growth Regul. 2021, 40, 1629–1639. [Google Scholar] [CrossRef]
- Hao, L.; Wen, Y.; Zhao, Y.; Lu, W.; Xiao, K. Wheat mitogen-activated protein kinase gene TaMPK4 improves plant tolerance to multiple stresses through modifying root growth, ROS metabolism, and nutrient acquisitions. Plant Cell Rep. 2015, 34, 2081–2097. [Google Scholar] [CrossRef]
- Gu, L.; Liu, Y.; Zong, X.; Liu, L.; Li, D.-P.; Li, D.-Q. Overexpression of maize mitogen-activated protein kinase gene, ZmSIMK1 in Arabidopsis increases tolerance to salt stress. Mol. Biol. Rep. 2010, 37, 4067–4073. [Google Scholar] [CrossRef]
- Zaidi, I.; Ebel, C.; Belgaroui, N.; Ghorbel, M.; Amara, I.; Hanin, M. The wheat MAP kinase phosphatase 1 alleviates salt stress and increases antioxidant activities in Arabidopsis. J. Plant Physiol. 2016, 193, 12–21. [Google Scholar] [CrossRef]
- Lee, S.-K.; Kim, B.-G.; Kwon, T.-R.; Jeong, M.-J.; Park, S.-R.; Lee, J.-W.; Byun, M.-O.; Kwon, H.-B.; Matthews, B.F.; Hong, C.-B.; et al. Overexpression of the mitogen-activated protein kinase gene OsMAPK33 enhances sensitivity to salt stress in rice (Oryza sativa L.). J. Biosci. 2011, 36, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-Y.; Liu, J.-Y.; Li, H.; Hu, W.-J.; Shen, Z.-J.; Qiao, F.; Zhu, C.-Q.; Chen, J.; Liu, X.; Zheng, H.-L. Proteomic analysis reveals the protective role of exogenous hydrogen sulfide against salt stress in rice seedlings. Nitric Oxide 2021, 111, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ma, D.; Huang, X.; Hou, J.; Wang, C.; Xie, Y.; Wang, Y.; Qin, H.; Guo, T. Exogenous hydrogen sulfide alleviates salt stress by improving antioxidant defenses and the salt overly sensitive pathway in wheat seedlings. Acta Physiol. Plant 2019, 41, 123. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.-H.; Wu, F.-H.; He, E.-M.; Liu, X.; Shangguan, Z.-P.; Zheng, H.-L. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.F.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, S.M. Remediation of heavy metal contaminated ecosystem: An overview on technology advancement. Int. J. Environ. Sci. Technol. 2015, 12, 353–366. [Google Scholar] [CrossRef]
- Mahmood, T.; Gupta, K.J.; Kaiser, W.M. Cadmium stress stimulates nitric oxide production by wheat roots. Pak. J. Bot. 2009, 41, 1285–1290. [Google Scholar]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead Toxicity in Cereals: Mechanistic Insight Into Toxicity, Mode of Action, and Management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- Singh, I.; Shah, K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 2014, 108, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, D.; Trivedi, P.K.; Misra, P.; Tiwari, M.; Shri, M.; Shukla, D.; Kumar, S.; Rai, A.; Pandey, A.; Nigam, D.; et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 2009, 74, 688–702. [Google Scholar] [CrossRef]
- Raghuram, B.; Sheikh, A.H.; Sinha, A.K. Regulation of MAP kinase signaling cascade by microRNAs in Oryza sativa. Plant Signal. Behav. 2014, 9, e972130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The Journey of Arsenic from Soil to Grain in Rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Rao, K.P.; Vani, G.; Kumar, K.; Wankhede, D.P.; Misra, M.; Gupta, M.; Sinha, A.K. Arsenic stress activates MAP kinase in rice roots and leaves. Arch. Biochem. Biophys. 2011, 506, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Peng, J.-S.; Zhong, C.; Yi, H.-Y.; Ow, D.W.; Gong, J.-M. Fission Yeast HMT1 Lowers Seed Cadmium through Phytochelatin-Dependent Vacuolar Sequestration in Arabidopsis. Plant Physiol. 2012, 158, 1779–1788. [Google Scholar] [CrossRef]
- Ma, C.; Burd, S.; Lers, A. miR408 is involved in abiotic stress responses in Arabidopsis. Plant J. 2015, 84, 169–187. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, M.; Lakhwani, D.; Tripathi, R.D.; Trivedi, P.K. Differential expression of microRNAs by arsenate and arsenite stress in natural accessions of rice. Metallomics 2015, 7, 174–187. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Wang, D.; Su, W.; Liu, L.; Wang, M.; Li, J. EBS7 is a plant-specific component of a highly conserved endoplasmic reticulum-associated degradation system in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 12205–12210. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Iwahashi, H.; Rakwal, R. Rice MAPKs. Biochem. Biophys. Res. Commun. 2003, 302, 171–180. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Chien, P.-S.; Huang, H.-J. Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J. Exp. Bot. 2006, 58, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Y.; Wang, K.; Zhang, S.Y.; Ren, J.; Liu, T.; Wang, X. Crosstalk between ABA, auxin, MAPK signaling, and the cell cycle in cadmium-stressed rice seedlings. Acta Physiol. Plant 2014, 36, 1879–1892. [Google Scholar] [CrossRef]
- Tang, M.; Mao, D.; Xu, L.; Li, D.; Song, S.; Chen, C. Integrated analysis of miRNA and mRNA expression profiles in response to Cd exposure in rice seedlings. BMC Genom. 2014, 15, 835. [Google Scholar] [CrossRef]
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2009, 325, 97–108. [Google Scholar] [CrossRef]
- Hu, S.; Yu, Y.; Chen, Q.; Mu, G.; Shen, Z.; Zheng, L. OsMYB45 plays an important role in rice resistance to cadmium stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Chen, M.-X.; Yu, L.-J.; Xie, L.-J.; Yuan, L.-B.; Qi, H.; Xiao, M.; Guo, W.; Chen, Z.; Yi, K.; et al. OsARM1, an R2R3 MYB Transcription Factor, Is Involved in Regulation of the Response to Arsenic Stress in Rice. Front. Plant Sci. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Vitorello, V.A.; Capaldi, F.R.; Stefanuto, V.A. Recent advances in aluminum toxicity and resistance in higher plants. Braz. J. Plant Physiol. 2005, 17, 129–143. [Google Scholar] [CrossRef]
- Hodson, M.J.; Evans, D.E. Aluminium–silicon interactions in higher plants: An update. J. Exp. Bot. 2020, 71, 6719–6729. [Google Scholar] [CrossRef]
- Osawa, H.; Matsumoto, H. Possible Involvement of Protein Phosphorylation in Aluminum-Responsive Malate Efflux from Wheat Root Apex. Plant Physiol. 2001, 126, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Kochian, L.V. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett. 1997, 400, 51–57. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, X.; Ramesh, S.; Gilliham, M.; Tyerman, S.D.; Zhang, W.-H. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J. Exp. Bot. 2014, 65, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Arenhart, R.A.; Margis-Pinheiro, M.; Margis, R. Aluminum triggers broad changes in microRNA expression in rice roots. Genet. Mol. Res. 2011, 10, 2817–2832. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, M.; Xu, X.; Li, X.; Li, C.; Ding, Z. System analysis of microRNAs in the development and aluminium stress responses of the maize root system. Plant Biotechnol. J. 2014, 12, 1108–1121. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chi, W.-C.; Trinh, N.N.; Huang, L.-Y.; Chen, Y.-C.; Cheng, K.-T.; Huang, T.-L.; Lin, C.-Y.; Huang, H.-J. Transcriptome Profiling and Physiological Studies Reveal a Major Role for Aromatic Amino Acids in Mercury Stress Tolerance in Rice Seedlings. PLoS ONE 2014, 9, e95163. [Google Scholar] [CrossRef]
- Wojas, S.; Ruszczyńska, A.; Bulska, E.; Wojciechowski, M.; Antosiewicz, D.M. Ca2+-dependent plant response to Pb2+ is regulated by LCT1. Environ. Pollut. 2007, 147, 584–592. [Google Scholar] [CrossRef]
- Huang, T.-L.; Huang, H.-J. ROS and CDPK-like kinase-mediated activation of MAP kinase in rice roots exposed to lead. Chemosphere 2008, 71, 1377–1385. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-L.; Nguyen, Q.T.T.; Fu, S.-F.; Lin, C.-Y.; Chen, Y.-C.; Huang, H.-J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.-N.; Huang, T.-L.; Chi, W.-C.; Fu, S.-F.; Chen, C.-C.; Huang, H.-J. Chromium stress response effect on signal transduction and expression of signaling genes in rice: Chromium stress response effect on signal transduction. Physiol. Plant. 2014, 150, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Sabella, E.; Vergine, M.; Genga, A.; Siciliano, M.; Nutricati, E.; Rampino, P.; De Pascali, M.; Luvisi, A.; Miceli, A.; et al. Activation of a gene network in durum wheat roots exposed to cadmium. BMC Plant Biol. 2018, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chu, C. Towards Understanding Plant Response to Heavy Metal Stress. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-394-1. [Google Scholar]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Ansarypour, Z.; Shahpiri, A. Heterologous expression of a rice metallothionein isoform (OsMTI-1b) in Saccharomyces cerevisiae enhances cadmium, hydrogen peroxide and ethanol tolerance. Braz. J. Microbiol. 2017, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.; Zhu, L.; Li, R.; Feng, Y.; Zhang, L.; Yang, Y.; Liu, X.; Zhang, H. Involvement of miR528 in the Regulation of Arsenite Tolerance in Rice (Oryza sativa L.). J. Agric. Food Chem. 2015, 63, 8849–8861. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Basu, S.; Varsani, S.; Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. MPMI 2018, 31, 13–21. [Google Scholar] [CrossRef]
- Woźniak, A.; Formela, M.; Bilman, P.; Grześkiewicz, K.; Bednarski, W.; Marczak, Ł.; Narożna, D.; Dancewicz, K.; Mai, V.; Borowiak-Sobkowiak, B.; et al. The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation. Int. J. Mol. Sci. 2017, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Naidoo, S.; van den Berg, N. The NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) and Related Family: Mechanistic Insights in Plant Disease Resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Formela-Luboińska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jeleń, H.; Bocianowski, J.; Kęsy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shan, T.; Hong, Y.; Xu, H.; Liu, X.; Zhang, Z. TaPIMP2, a pathogen-induced MYB protein in wheat, contributes to host resistance to common root rot caused by Bipolaris sorokiniana. Sci. Rep. 2017, 7, 1754. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Al-Zahrani, W.; Bafeel, S.O.; El-Zohri, M. Jasmonates mediate plant defense responses to Spodoptera exigua herbivory in tomato and maize foliage. Plant Signal. Behav. 2020, 15, 1746898. [Google Scholar] [CrossRef]
- Verhage, A. Rewiring of the jasmonate signaling pathway in Arabidopsis during insect herbivory. Front. Plant Sci. 2011, 2, 47. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Liu, X.; Lu, Y.; Du, L.; Xu, H.; Liu, H.; Xin, Z.; Zhang, Z. Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct. Integr. Genom. 2010, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Esmail, S.M.; Omara, R.I.; Abdelaal, K.A.A.; Hafez, Y.M. Histological and biochemical aspects of compatible and incompatible wheat- Puccinia striiformis interactions. Physiol. Mol. Plant Pathol. 2019, 106, 120–128. [Google Scholar] [CrossRef]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF Type Transcription Factor OsEREBP1 Confers Biotic and Abiotic Stress Tolerance in Rice. PLoS ONE 2015, 10, e0127831. [Google Scholar] [CrossRef] [PubMed]
- Otuka, A. Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Front. Microbiol. 2013, 4, 309. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Sun, X.; Rong, G.; Hou, C.; Huang, Y.; Jiang, D.; Weng, X. Identification of two transcription factors activating the expression of OsXIP in rice defence response. BMC Biotechnol. 2017, 17, 26. [Google Scholar] [CrossRef]
- Gottwald, S.; Samans, B.; Lück, S.; Friedt, W. Jasmonate and ethylene dependent defence gene expression and suppression of fungal virulence factors: Two essential mechanisms of Fusarium head blight resistance in wheat? BMC Genom. 2012, 13, 369. [Google Scholar] [CrossRef]
- Labudda, M.; Różańska, E.; Prabucka, B.; Muszyńska, E.; Marecka, D.; Kozak, M.; Dababat, A.A.; Sobczak, M. Activity profiling of barley vacuolar processing enzymes provides new insights into the plant and cyst nematode interaction. Mol. Plant Pathol. 2020, 21, 38–52. [Google Scholar] [CrossRef]
- Darino, M.; Chia, K.; Marques, J.; Aleksza, D.; Soto-Jiménez, L.M.; Saado, I.; Uhse, S.; Borg, M.; Betz, R.; Bindics, J.; et al. Ustilago maydis effector Jsi1 interacts with Topless corepressor, hijacking plant jasmonate/ethylene signaling. New Phytol. 2021, 229, 3393–3407. [Google Scholar] [CrossRef]
- El-Wakeil, N.E.; Volkmar, C.; Sallam, A.A. Jasmonic acid induces resistance to economically important insect pests in winter wheat: Jasmonic acid induces resistance to insect pests in wheat. Pest. Manag. Sci. 2010, 66, 549–554. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Saénchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 Encodes a MYC Transcription Factor Essential to Discriminate between Different Jasmonate-Regulated Defense Responses in Arabidopsis[W]. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Kraus, E.C.; Stout, M.J. Seed treatment using methyl jasmonate induces resistance to rice water weevil but reduces plant growth in rice. PLoS ONE 2019, 14, e0222800. [Google Scholar] [CrossRef] [PubMed]
- Bhavanam, S.; Stout, M. Seed Treatment With Jasmonic Acid and Methyl Jasmonate Induces Resistance to Insects but Reduces Plant Growth and Yield in Rice, Oryza sativa. Front. Plant Sci. 2021, 12, 691768. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Durán, R.; Zipfel, C. Trade-off between growth and immunity: Role of brassinosteroids. Trends Plant Sci. 2015, 20, 12–19. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Koornneef, A.; Leon-Reyes, A.; Ritsema, T.; Verhage, A.; Den Otter, F.C.; Van Loon, L.C.; Pieterse, C.M.J. Kinetics of Salicylate-Mediated Suppression of Jasmonate Signaling Reveal a Role for Redox Modulation. Plant Physiol. 2008, 147, 1358–1368. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-Generation Systemic Acquired Resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [PubMed]
- Leon-Reyes, A.; Spoel, S.H.; De Lange, E.S.; Abe, H.; Kobayashi, M.; Tsuda, S.; Millenaar, F.F.; Welschen, R.A.M.; Ritsema, T.; Pieterse, C.M.J. Ethylene Modulates the Role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in Cross Talk between Salicylate and Jasmonate Signaling. Plant Physiol. 2009, 149, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jander, G.; Racki, L.R.; Kim, P.D.; Pierce, N.E.; Ausubel, F.M. Signals Involved in Arabidopsis Resistance to Trichoplusia ni Caterpillars Induced by Virulent and Avirulent Strains of the Phytopathogen Pseudomonas syringae. Plant Physiol. 2002, 129, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic Interaction between Abscisic Acid and Jasmonate-Ethylene Signaling Pathways Modulates Defense Gene Expression and Disease Resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Nickstadt, A.; Thomma, B.P.H.J.; Feussner, I.; Kangasjarvi, J.; Zeier, J.; Loeffler, C.; Scheel, D.; Berger, S. The jasmonate-insensitive mutant jin1 shows increased resistance to biotrophic as well as necrotrophic pathogens. Mol. Plant Pathol. 2004, 5, 425–434. [Google Scholar] [CrossRef]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.-J.; Schmelz, E.A.; Solano, R. ABA Is an Essential Signal for Plant Resistance to Pathogens Affecting JA Biosynthesis and the Activation of Defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef]
- Sánchez-Vallet, A.; López, G.; Ramos, B.; Delgado-Cerezo, M.; Riviere, M.-P.; Llorente, F.; Fernández, P.V.; Miedes, E.; Estevez, J.M.; Grant, M.; et al. Disruption of Abscisic Acid Signaling Constitutively Activates Arabidopsis Resistance to the Necrotrophic Fungus Plectosphaerella cucumerina. Plant Physiol. 2012, 160, 2109–2124. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Heil, M. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Heil, M. Costs and trade-offs associated with induced resistance. Physiol. Mol. Plant Pathol. 2007, 71, 3–17. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Dicke, M. Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci. 2007, 12, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Vos, I.A.; Moritz, L.; Pieterse, C.M.J.; Van Wees, S.C.M. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front. Plant Sci. 2015, 6, 639. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef]
- Labudda, M.; Tokarz, K.; Tokarz, B.; Muszyńska, E.; Gietler, M.; Górecka, M.; Różańska, E.; Rybarczyk-Płońska, A.; Fidler, J.; Prabucka, B.; et al. Reactive oxygen species metabolism and photosynthetic performance in leaves of Hordeum vulgare plants co-infested with Heterodera filipjevi and Aceria tosichella. Plant Cell Rep. 2020, 39, 1719–1741. [Google Scholar] [CrossRef]
- Labudda, M.; Muszyńska, E.; Gietler, M.; Różańska, E.; Rybarczyk-Płońska, A.; Fidler, J.; Prabucka, B.; Dababat, A.A. Efficient antioxidant defence systems of spring barley in response to stress induced jointly by the cyst nematode parasitism and cadmium exposure. Plant Soil 2020, 456, 189–206. [Google Scholar] [CrossRef]
- Sharma, R.; De Vleesschauwer, D.; Sharma, M.K.; Ronald, P.C. Recent Advances in Dissecting Stress-Regulatory Crosstalk in Rice. Plant 2013, 6, 250–260. [Google Scholar] [CrossRef]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef]
- Rohila, J.S.; Yang, Y. Rice Mitogen-activated Protein Kinase Gene Family and Its Role in Biotic and Abiotic Stress Response. J. Integr. Plant Biol. 2007, 49, 751–759. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses: ABA in drought and pathogen responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Hagedorn, P.H.; de Torres-Zabala, M.; Grant, M.R.; Rung, J.H.; Collinge, D.B.; Lyngkjaer, M.F. Transcriptional regulation by an NAC (NAM-ATAF1,2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J. 2008, 56, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, X.; Leseberg, C.H.; Jia, J.; Mao, L. Biotic and abiotic stress responses through calcium-dependent protein kinase (CDPK) signaling in wheat ( Triticum aestivum L.). Plant Signal. Behav. 2008, 3, 654–656. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kobayashi, M.; Aoki, N.; Miyao, A.; Mitsuhara, I.; Ichikawa, H.; Komatsu, S.; Hirochika, H.; Kikuchi, S.; et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance: OsCPK12 modulates abiotic and biotic stress responses. Plant J. 2012, 69, 26–36. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, H.; Li, X.; Xiao, J.; Xu, C.; Wang, S. Rice WRKY13 Regulates Cross Talk between Abiotic and Biotic Stress Signaling Pathways by Selective Binding to Different cis-Elements. Plant Physiol. 2013, 163, 1868–1882. [Google Scholar] [CrossRef]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef]
- Peng, X.; Tang, X.; Zhou, P.; Hu, Y.; Deng, X.; He, Y.; Wang, H. Isolation and Expression Patterns of Rice WRKY82 Transcription Factor Gene Responsive to Both Biotic and Abiotic Stresses. Agric. Sci. China 2011, 10, 893–901. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Dong, N.; Liu, X.; Zhang, H.; Zhang, Z. Expression of a wheat MYB gene in transgenic tobacco enhances resistance to Ralstonia solanacearum, and to drought and salt stresses. Funct. Integr. Genom. 2011, 11, 431–443. [Google Scholar] [CrossRef]
- Yokotani, N.; Ichikawa, T.; Kondou, Y.; Iwabuchi, M.; Matsui, M.; Hirochika, H.; Oda, K. Role of the rice transcription factor JAmyb in abiotic stress response. J. Plant Res. 2013, 126, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate Signaling Enhances RNA Silencing and Antiviral Defense in Rice. Cell Host Microbe 2020, 28, 89–103.e8. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Tran, L.-S.P.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice: Rice OsNAC6 functions in stress responses. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhang, G.; Liu, X.-Y.; Deng, L.; Cai, G.-L.; Zhang, Y.; Wang, X.-J.; Zhao, J.; Huang, L.-L.; Kang, Z.-S. Characterization of a novel wheat NAC transcription factor gene involved in defense response against stripe rust pathogen infection and abiotic stresses. Mol. Biol. Rep. 2010, 37, 3703–3712. [Google Scholar] [CrossRef]
- Winfield, M.O.; Lu, C.; Wilson, I.D.; Coghill, J.A.; Edwards, K.J. Plant responses to cold: Transcriptome analysis of wheat: Plant responses to cold. Plant Biotechnol. J. 2010, 8, 749–771. [Google Scholar] [CrossRef]
- Talanova, V.V.; Titov, A.F.; Repkina, N.S.; Topchieva, L.V. Cold-responsive COR/LEA genes participate in the response of wheat plants to heavy metals stress. Dokl. Biol. Sci. 2013, 448, 28–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).