Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa)
Abstract
1. Introduction
2. Results and Discussion
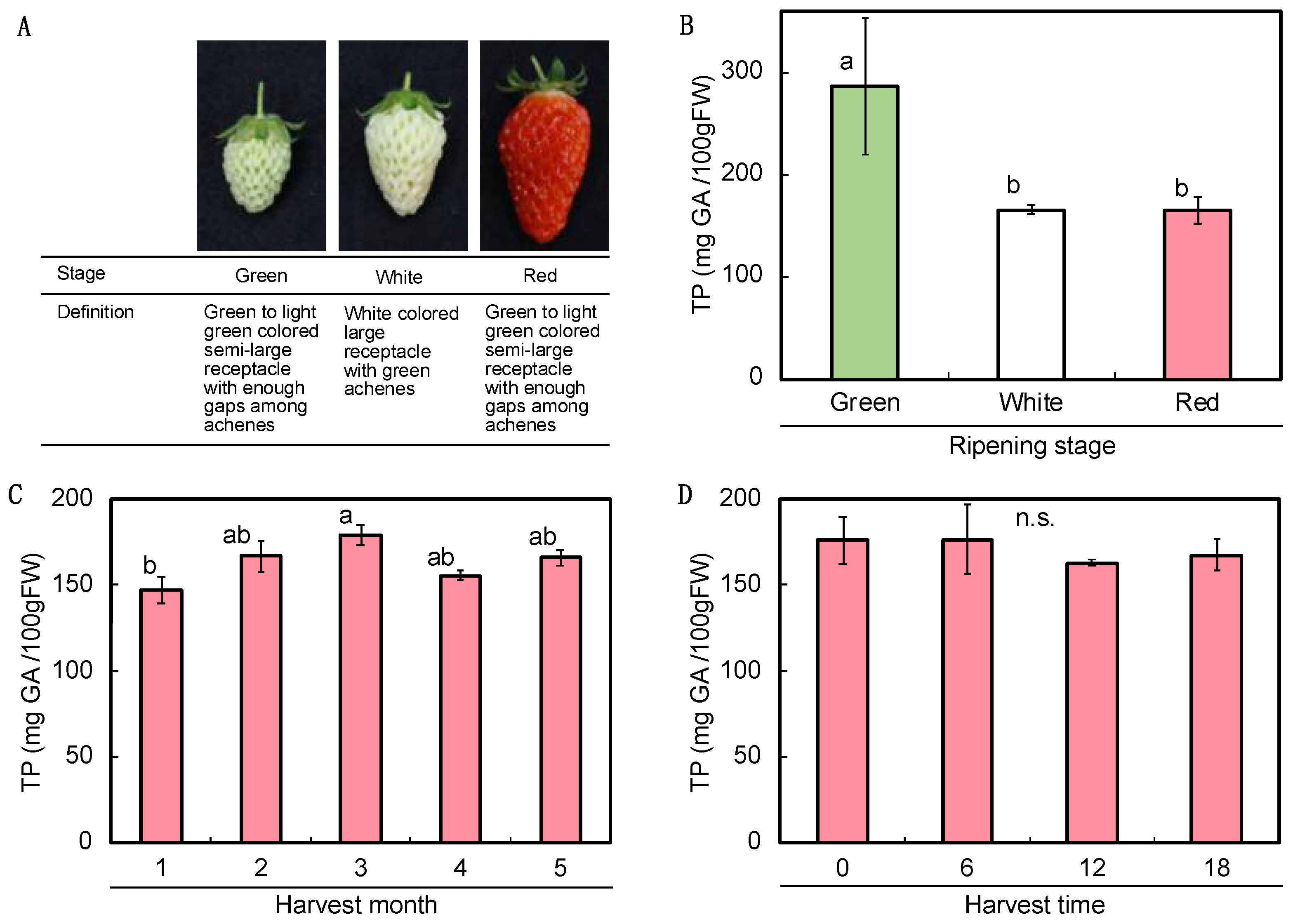
2.1. A Pre-Survey of the Basic Conditions during the Growth and Development of the Fruits
2.2. Variation in Polyphenol Content under Different Pre- and Post-Harvest Conditions
2.3. Transcriptome Analysis to Verify the Conditions of Polyphenol Richness
2.4. Improvement of Cold Storage Conditions
2.4.1. Quality Preservation by Modifying Storage Methods
2.4.2. Polyphenol Contents and Antioxidant Activity in the Improved Cold Condition
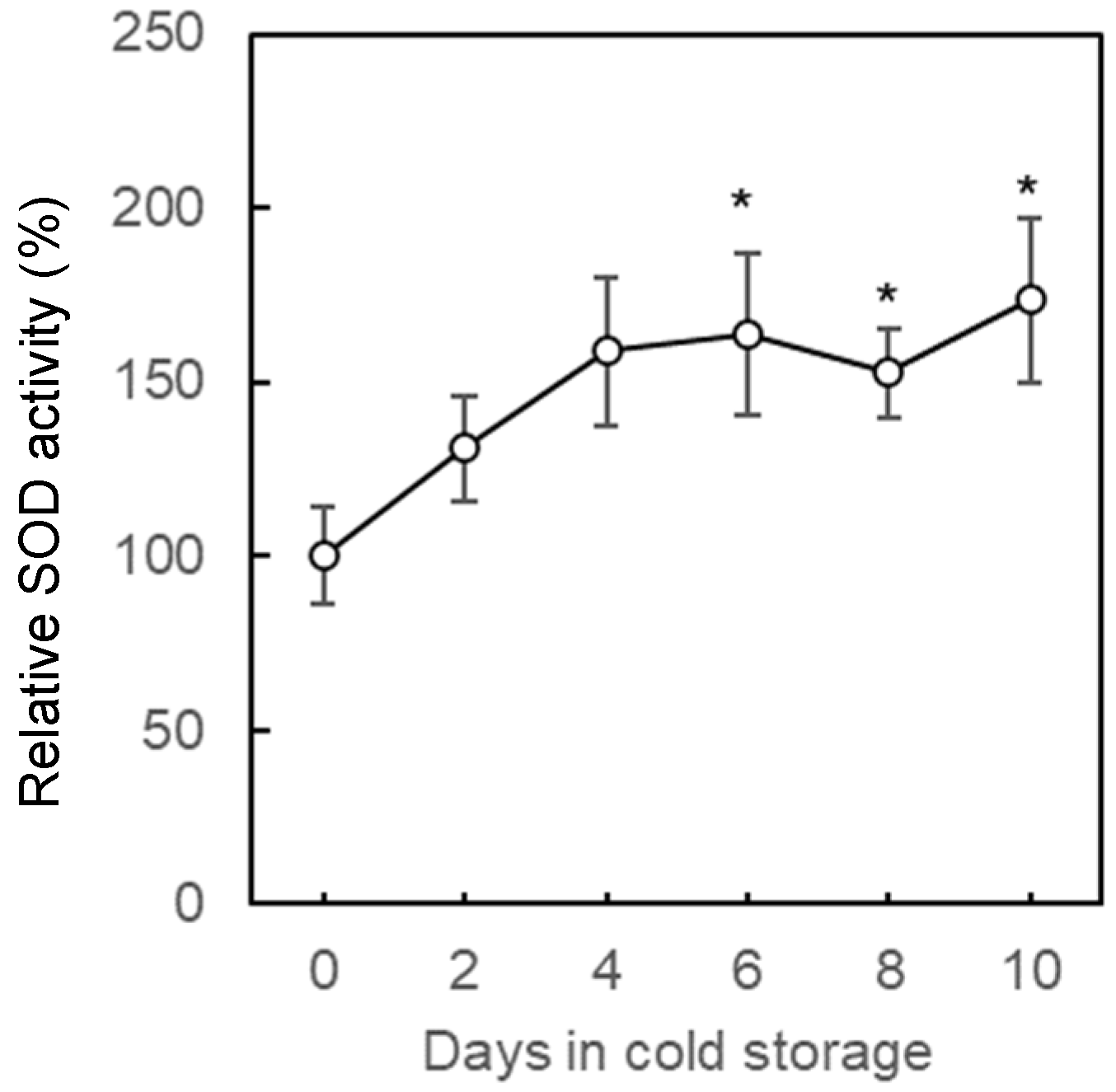
2.4.3. Stress Response to Low Temperature Based on Indicator Substances
3. Materials and Methods
3.1. Plant Material, Culture, and Treatment in a Pre-Survey of the Basic Conditions
3.2. Screening of Effective Treatment in Pre- and Post-Harvest
3.3. Improve Cold Storage Methods (MAP) and Detailed Analysis
3.4. Quantification of Total Polyphenol
3.5. RNA Extraction
3.6. RNA-Seq Analysis
3.7. Determination of Antioxidant Capacity
3.8. Measurement of Antioxidant Enzyme Activity
3.9. Quantification of Abscisic Acid (ABA)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Weber, N.; Veberic, R.; Stampar, F.; Munda, A.; Koron, D. Alteration of the content of primary and secondary metabolites in strawberry fruit by Colletotrichum nymphaeae infection. J. Agric Food Chem. 2013, 61, 5987–5995. [Google Scholar] [CrossRef] [PubMed]
- Cardeñosa, V.; Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Cuevas, F.; Pradas, I.; Moreno-Rojas, J.M. Effects of salinity and nitrogen supply on the quality and health-related compounds of strawberry fruits (Fragaria × ananassa cv. Primoris). J. Sci. Food Agric. 2015, 95, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moidu, H.; Charles, M.T.; Dubé, C.; Khanizadeh, S. Differential regulation of superoxide dismutase activity in selected strawberry lines exposed to Mycosphaerella fragariae. J. Plant Stud. 2015, 4, 30–41. [Google Scholar] [CrossRef]
- Rugienius, R.; Bendokas, V.; Siksnianas, T.; Stanys, V.; Sasnauskas, A.; Kazanaviciute, V. Characteristics of Fragaria vesca yield parameters and anthocyanin accumulation under water deficit stress. Plants 2021, 10, 557. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Zhang, D.; Dong, Y.; Meng, Q.; Zhu, S.; Zhang, L. Strigolactone maintains strawberry quality by regulating phenylpropanoid, NO, and H2S metabolism during storage. Postharvest Biol. Technol. 2021, 178, 111546. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Uno, Y.; Nitta, Y.; Ishibashi, M.; Noguchi, Y.; Kikuzaki, H. Inhibition of recombinant human histidine decarboxylase activity in different strawberry cultivars. Acta Physiol. Plant. 2017, 39, 134. [Google Scholar] [CrossRef]
- Urün, I.; Attar, S.H.; Sönmez, D.A.; Gündeşli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of polyphenol, sugar, organic acid, volatile compounds, and antioxidant capacity of commercially grown strawberry cultivars in Turkey. Plants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.; Orsucci, S.; Moser, M.; Brilli, M.; Giongo, L.; Si-Ammour, A. Gene expression and metabolite accumulation during strawberry (Fragaria × ananassa) fruit development and ripening. Planta 2018, 248, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma, C.; Hammerbacher, A.; Heil, M.; Gershenzon, J.; Méndez-Alonzo, R.; Oyama, K. Specific polyphenols and tannins are associated with defense against insect herbivores in the tropical oak Quercus oleoides. J. Chem. Ecol 2014, 40, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Sato-Furukawa, M.; Sone, K.; Oki, T. Effect of harvest time on changes in hydrophilic oxygen radical absorbance capacity of fruits from different strawberry cultivars (Fragaria × ananassa Duch.). Nippon. Shokuhin Kagaku Kogaku Kaishi 2020, 67, 109–114. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Palmieri, L.; Masuero, D.; Martinatti, P.; Baratto, G.; Martens, S.; Vrhovsek, U. Genotype-by-environment effect on bioactive compounds in strawberry (Fragaria × ananassa Duch.). J. Sci. Food Agric. 2017, 97, 4180–4189. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yoshikawa, H.; Uno, Y. Expression profiling of strawberry allergen Fra a during fruit ripening controlled by exogenous auxin. Int. J. Mol. Sci. 2017, 18, 1186. [Google Scholar] [CrossRef]
- Okochi, S.; Ishibashi, M.; Yoshikawa, H.; Uno, Y. Response of the major allergen Fra a 1.01 in strawberry to cold. Hortic. J. 2020, 89, 182–190. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M. The commercial storage of fruits, vegetables, and florist and nursery stocks. In Agriculture Handbook; United States Department of Agriculture: Beltsville, MD, USA, 2016. [Google Scholar]
- Wang, H.-G.; Gemma, H.; Oogaki, C. Physiological characteristics and keeping qualities on the strawberry and kiwifruits during chilled storage. J. Jpn. Soc. Cold Preserv. Food 1988, 14, 8–14. [Google Scholar] [CrossRef]
- Yamagishi, A. Cold adaptation and molecular evolution of enzyme. Netsu Sokutei 2006, 33, 2–9. [Google Scholar]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Boo, H.O.; Heo, B.G.; Gorinstein, S.; Chon, S.U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kurata, R.; Kai, Y. Seasonal Variation in the yield and polyphenol content of sweet potato (Ipomoea batatas L.) Foliage. Hortic. J. 2019, 88, 270–275. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Jiang, Y.; He, H.; Liu, T.; Lv, M.; Ji, S. Influence of long-term cold storage on phenylpropanoid and soluble sugar metabolisms accompanied with peel browning of ‘Nanguo’ pears during subsequent shelf life. Sci. Hortic. 2020, 260, 108888. [Google Scholar] [CrossRef]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Pott, D.M.; de Abreu E Lima, F.; Soria, C.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S.; Vallarino, J.G. Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 2020, 321, 126747. [Google Scholar] [CrossRef] [PubMed]
- Sanz Cervera, S.; Olarte, C.; Echávarri, J.F.; Ayala, F. Influence of exposure to light on the sensorial quality of minimally processed cauliflower. J. Food Sci. 2007, 72, S012–S018. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Inamasu, K.; Yokoyama, A.; Nishida, Y.; Furuichi, Y. Nutrient composition and nutritional functions of Nigaichigo (Rubus microphyllus) Fruits. Nippon. Shokuhin Kagaku Kogaku Kaishi 2010, 57, 483–488. [Google Scholar] [CrossRef][Green Version]
- Rohloff, J.; Kopka, J.; Erban, A.; Winge, P.; Wilson, R.C.; Bones, A.M.; Davik, J.; Randall, S.K.; Alsheikh, M.K. Metabolite profiling reveals novel multi-level cold responses in the diploid model Fragaria vesca (woodland strawberry). Phytochemistry 2012, 77, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Koehler, G.; Rohloff, J.; Wilson, R.C.; Kopka, J.; Erban, A.; Winge, P.; Bones, A.M.; Davik, J.; Alsheikh, M.K.; Randall, S.K. Integrative “omic” analysis reveals distinctive cold responses in leaves and roots of strawberry, Fragaria × ananassa ‘Korona’. Front. Plant Sci. 2015, 6, 826. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends. Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Zhang, X.; Dong, C.; Ji, H.; Ke, R.; Ban, Z.; Hu, Y.; Lin, S.; Chen, C. Effects of ozone treatment on the antioxidant capacity of postharvest strawberry. RSC Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Liu, Y.; Yang, M.; Wang, L.; Liu, X.; Chen, Q.; Li, M.; Lin, Y.; Tang, H.; et al. MAPK5 and MAPK10 overexpression influences strawberry fruit ripening, antioxidant capacity and resistance to Botrytis cinerea. Planta 2021, 255, 19. [Google Scholar] [CrossRef]
- Ishibashi, M.; Okochi, S.; Sone, K.; Noguchi, Y.; Uno, Y. Seasonal variation of the major allergen Fra a 1 in Strawberry Fruit. Hortic. J. 2019, 88, 354–363. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Nabe, T.; Nitta, Y.; Uno, Y. Efficient isolation of high-quality total RNA from strawberry. Hortscience 2019, 54, 380–384. [Google Scholar] [CrossRef]
- Edger, P.P.; VanBuren, R.; Colle, M.; Poorten, T.J.; Wai, C.M.; Niederhuth, C.E.; Alger, E.I.; Ou, S.; Acharya, C.B.; Wang, J.; et al. Single-molecule sequencing and optical mapping yields an improved genome of woodland strawberry (Fragaria vesca) with chromosome-scale contiguity. Gigascience 2018, 7, gix124. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Koyama, R.; Yoshimoto, A.; Ishibashi, M.; Itoh, H.; Uno, Y. Enzymatic activities and gene transcript levels associated with the augmentation of antioxidant constituents during drought stress in lettuce. Horticulturae 2021, 7, 444. [Google Scholar] [CrossRef]




| Conditions | Total Polyphenol (mg GA/100 g FW) | |||
|---|---|---|---|---|
| Pre-harvest treatment | ||||
| Wounding | Control | 176.0 ± 9.6 | n.s. | |
| Vertical | 205.1 ± 8.7 | |||
| Phytohormone | Control | 166.0 ± 13.1 | n.s. | |
| NAA | 155.7 ± 9.5 | |||
| Post-harvest treatment | ||||
| Cold storage | Control (0 d) | 150.7 ± 7.5 | b | |
| 0 °C for 1 d | 170.2 ± 11.5 | b | ||
| 0 °C for 5 d | 178.7 ± 14.0 | b | ||
| 0 °C for 10 d | 198.3 ± 11.4 | a | ||
| Phytohormone | Control | 136.7 ± 9.0 | n.s. | |
| IAA | 155.9 ± 11.7 | |||
| Plasma | Control | 141.7 ± 5.0 | n.s. | |
| Air (−) | 157.2 ± 5.8 | |||
| Air (+) | 148.2 ± 13.3 | |||
| O2 (−) | 151.7 ± 18.1 | |||
| O2 (+) | 147.6 ± 10.1 | |||
| Pathway | Pathway | Category | DEGs * |
|---|---|---|---|
| ko00254 | Aflatoxin biosynthesis | Metabolism | 1 |
| ko00261 | Monobactam biosynthesis | Metabolism | 1 |
| ko00500 | Starch and sucrose metabolism | Metabolism | 7 |
| ko04113 | Meiosis—yeast | Cellular Processes | 8 |
| ko00940 | Phenylpropanoid biosynthesis | Metabolism | 10 |
| ko03030 | DNA replication | Genetic Information Processing | 8 |
| ko00982 | Drug metabolism—cytochrome P450 | Metabolism | 6 |
| ko00980 | Metabolism of xenobiotics by cytochrome P450 | Metabolism | 7 |
| ko00196 | Photosynthesis—antenna proteins | Metabolism | 2 |
| ko00480 | Glutathione metabolism | Metabolism | 7 |
| ko02025 | Biofilm formation—Pseudomonas aeruginosa | 1 | |
| ko04111 | Cell cycle—yeast | Cellular Processes | 8 |
| ko04110 | Cell cycle | Cellular Processes | 8 |
| ko00592 | Alpha-linolenic acid metabolism | Metabolism | 4 |
| ko00550 | Peptidoglycan biosynthesis | Metabolism | 1 |
| Days in Cold Storage | Relative Fresh Weight (%) | Brix (%) | Acidity (%) |
|---|---|---|---|
| Control (without MAP) | |||
| 0 | 100.0 ± 0.0 a | - | - |
| 5 | 94.2 ± 0.4 b | - | - |
| 10 | 87.3 ± 1.0 c | - | - |
| MAP | |||
| 0 | 100.0 ± 0.0 n.s. | 8.27± 0.36 n.s. | 0.57 ± 0.03 n.s. |
| 2 | 99.5 ± 0.2 | 8.43 ± 0.41 | 0.53 ± 0.02 |
| 4 | 99.4 ± 0.1 | 8.27 ± 0.48 | 0.52 ± 0.06 |
| 6 | 99.5 ± 0.1 | 8.83 ± 0.29 | 0.48 ± 0.02 |
| 8 | 99.3 ± 0.1 | 8.12 ± 0.18 | 0.49 ± 0.04 |
| 10 | 99.2 ± 0.2 | 7.90 ± 0.40 | 0.49 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, R.; Ishibashi, M.; Fukuda, I.; Okino, A.; Osawa, R.; Uno, Y. Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa). Plants 2022, 11, 2220. https://doi.org/10.3390/plants11172220
Koyama R, Ishibashi M, Fukuda I, Okino A, Osawa R, Uno Y. Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa). Plants. 2022; 11(17):2220. https://doi.org/10.3390/plants11172220
Chicago/Turabian StyleKoyama, Ryohei, Misaki Ishibashi, Itsuko Fukuda, Akitoshi Okino, Ro Osawa, and Yuichi Uno. 2022. "Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa)" Plants 11, no. 17: 2220. https://doi.org/10.3390/plants11172220
APA StyleKoyama, R., Ishibashi, M., Fukuda, I., Okino, A., Osawa, R., & Uno, Y. (2022). Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa). Plants, 11(17), 2220. https://doi.org/10.3390/plants11172220







