Spraying Ethephon Effectively Increased Canopy Light Transmittance of Densely Planted Summer Maize, Thus Achieving Synergistic Improvement in Stalk Lodging Resistance and Grain Yield
Abstract
:1. Introduction
2. Results
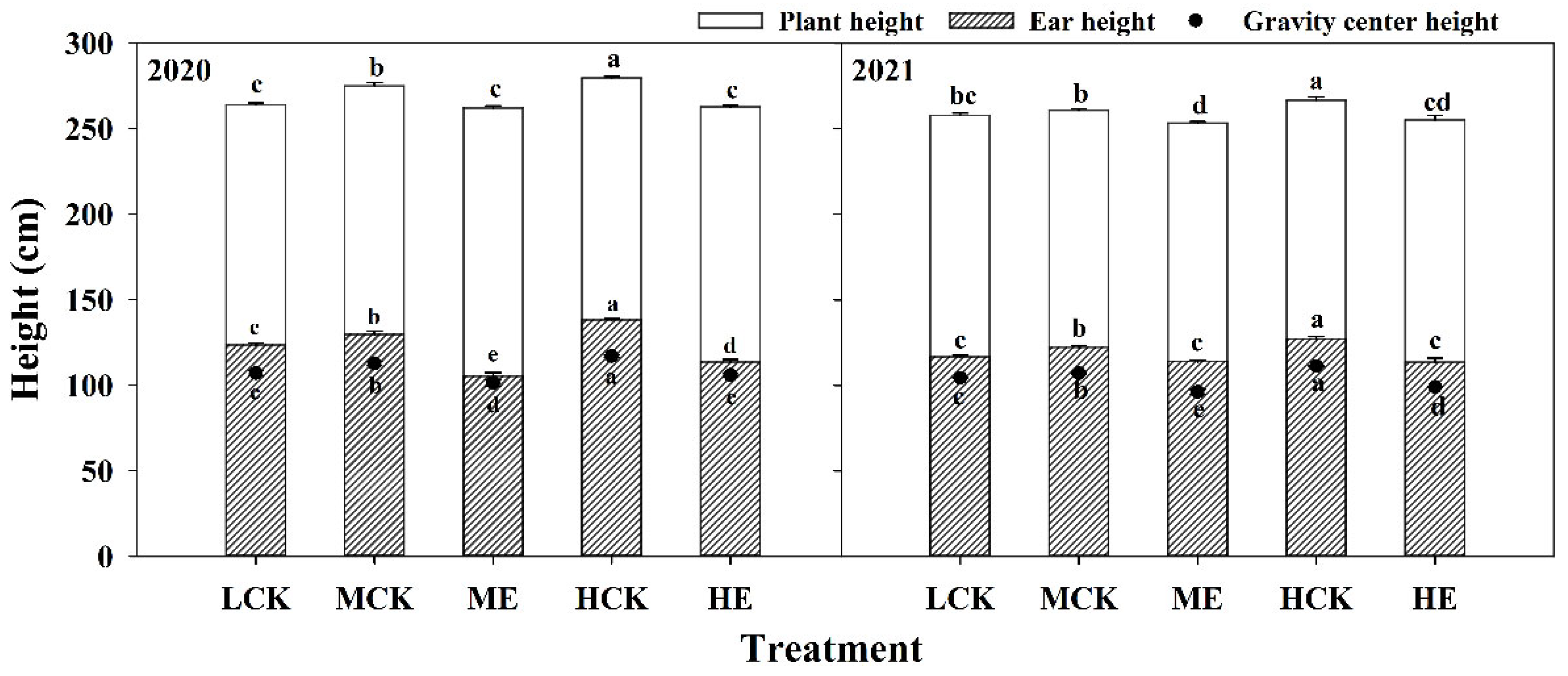
2.1. Effects of Spraying Ethephon on Plant Height, Ear Height and Gravity Center Height of Summer Maize
2.2. Effects of Spraying Ethephon on Leaf Area Index (LAI) and Soil and Plant Analysis Development (SPAD) of Summer Maize
2.3. Effects of Spraying Ethephon on Canopy Light Transmittance of Summer Maize
2.4. Effects of Spraying Ethephon on Stalk Traits of Summer Maize
2.4.1. Internode Length
2.4.2. Stem Diameter
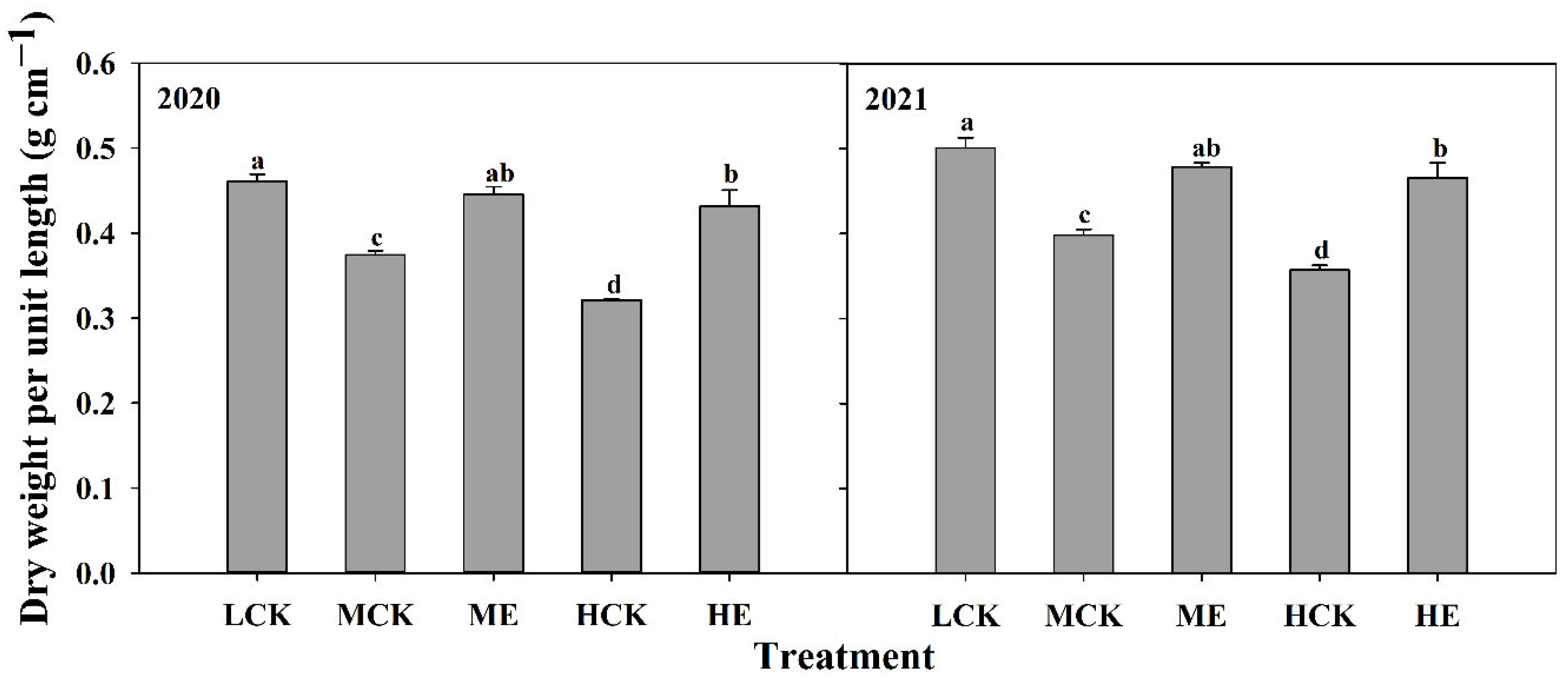
2.4.3. Internode Dry Weight per Unit Length
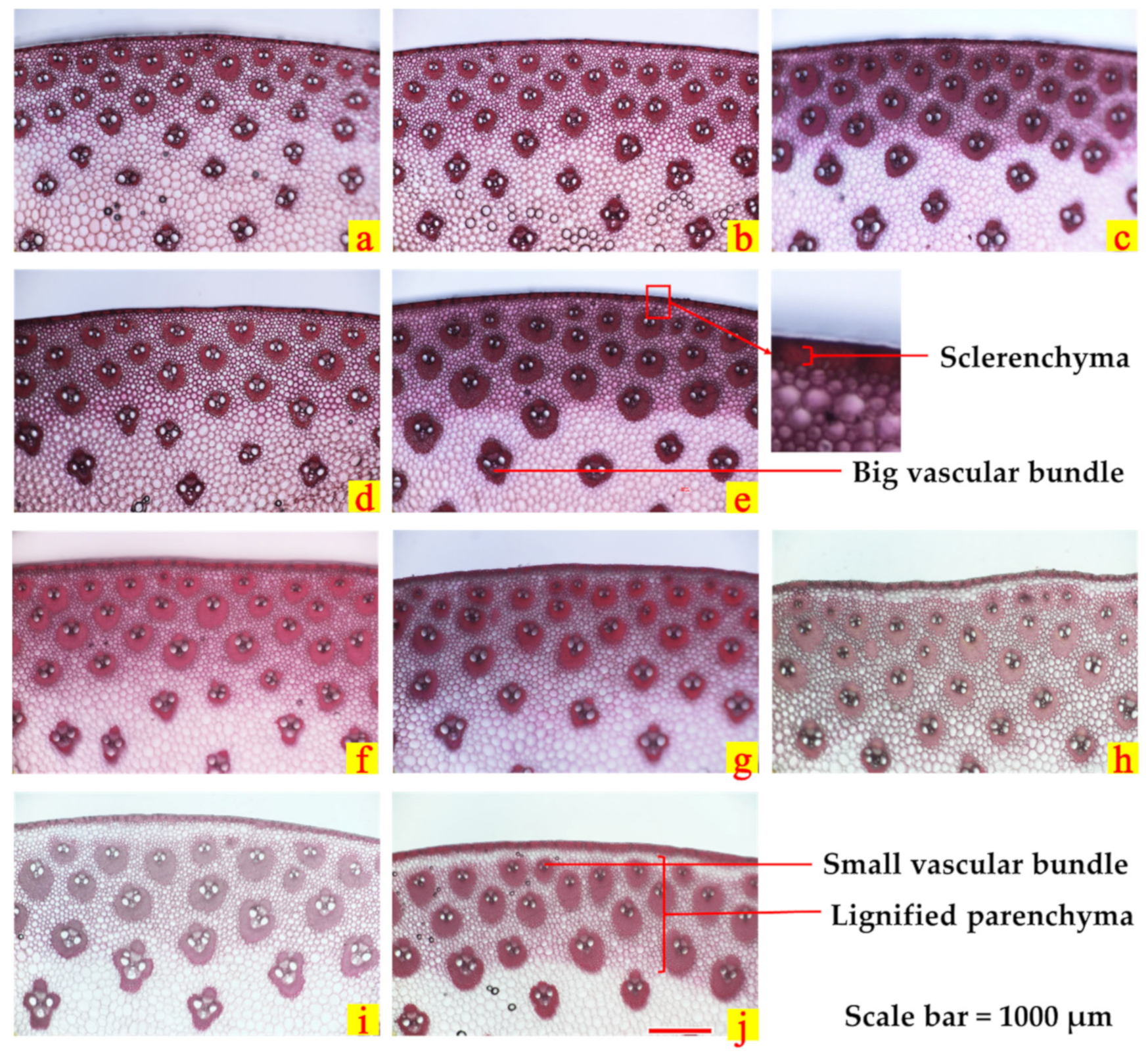
2.4.4. Stem Anatomical Structure
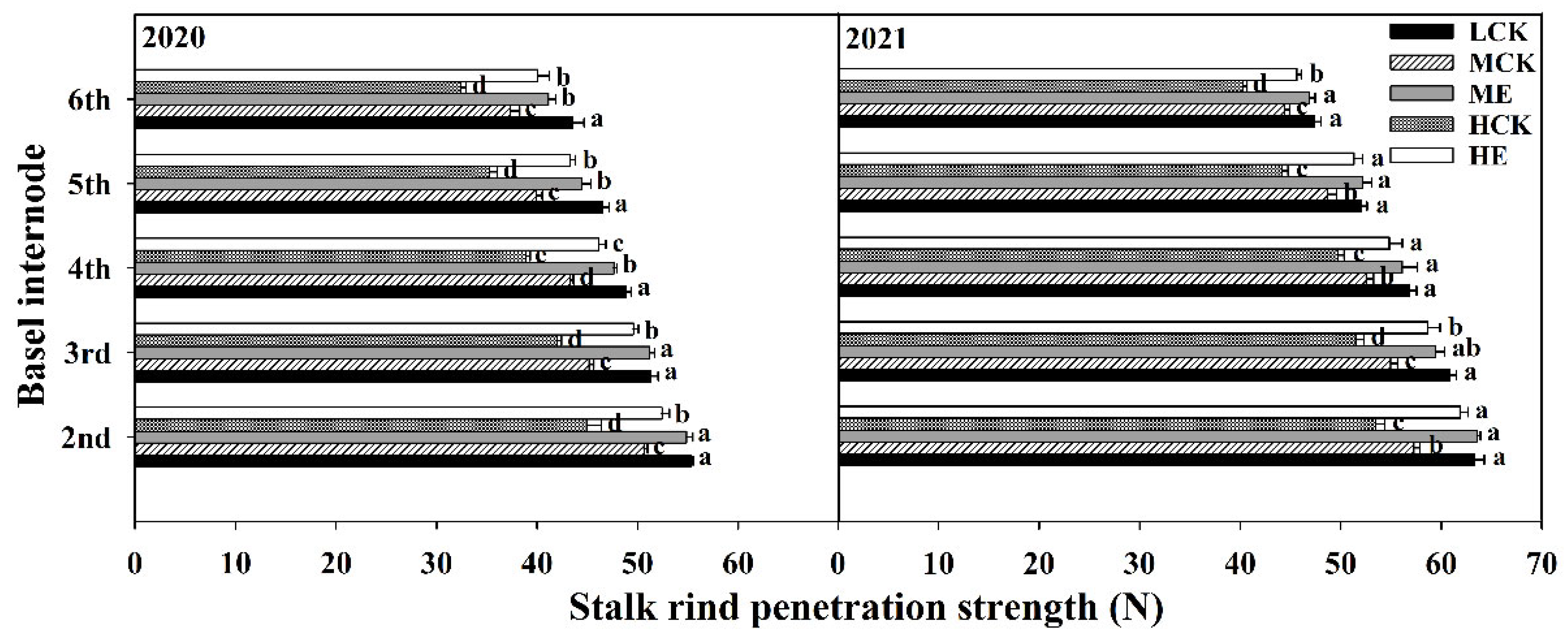
2.4.5. Stalk Rind Penetration Strength
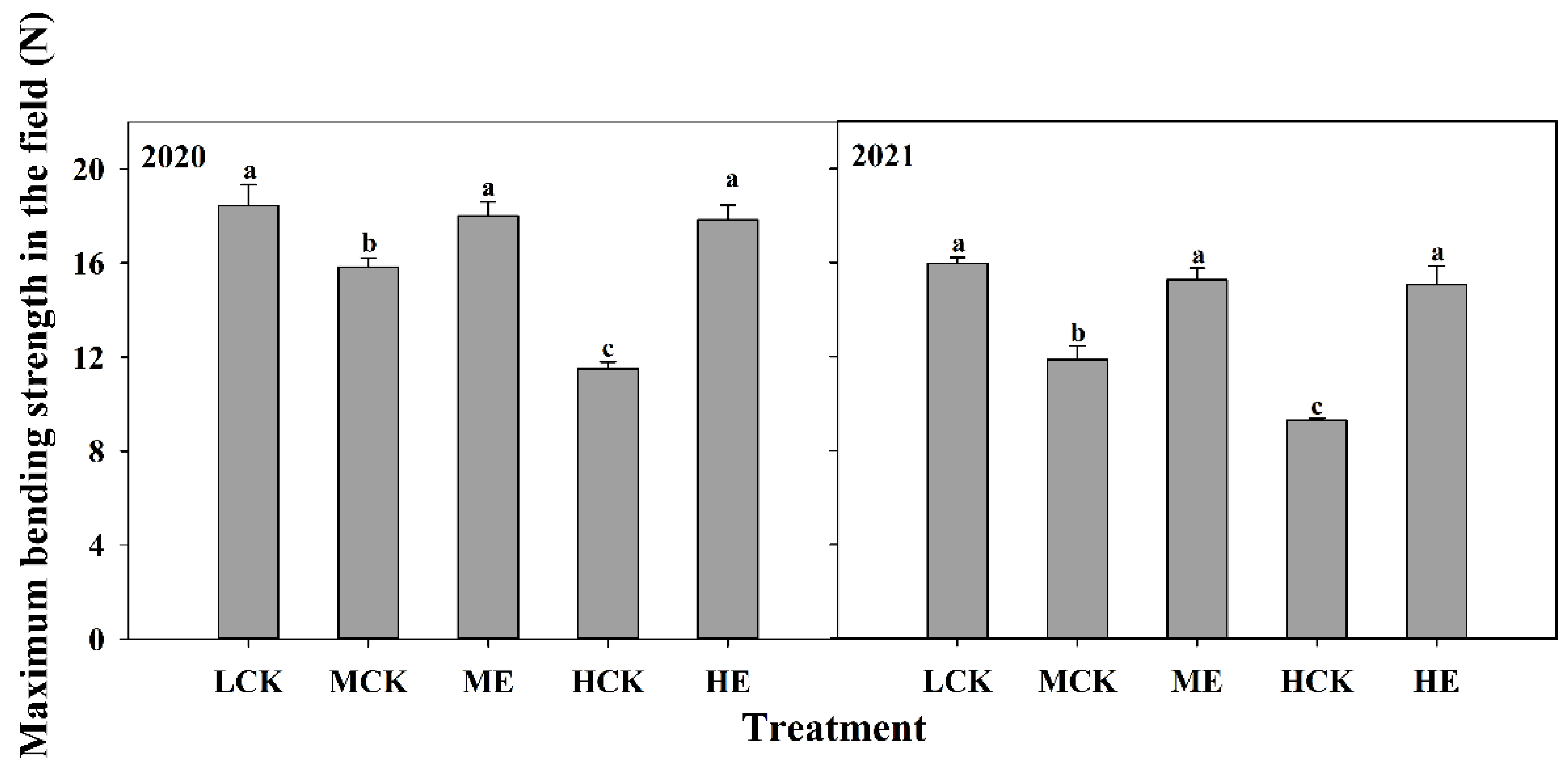
2.4.6. Maximum Bending Strength in the Field
2.5. Effects of Spraying Ethephon on Lodging Percentage of Summer Maize
2.6. Effects of Spraying Ethephon on Grain Yield of Summer Maize
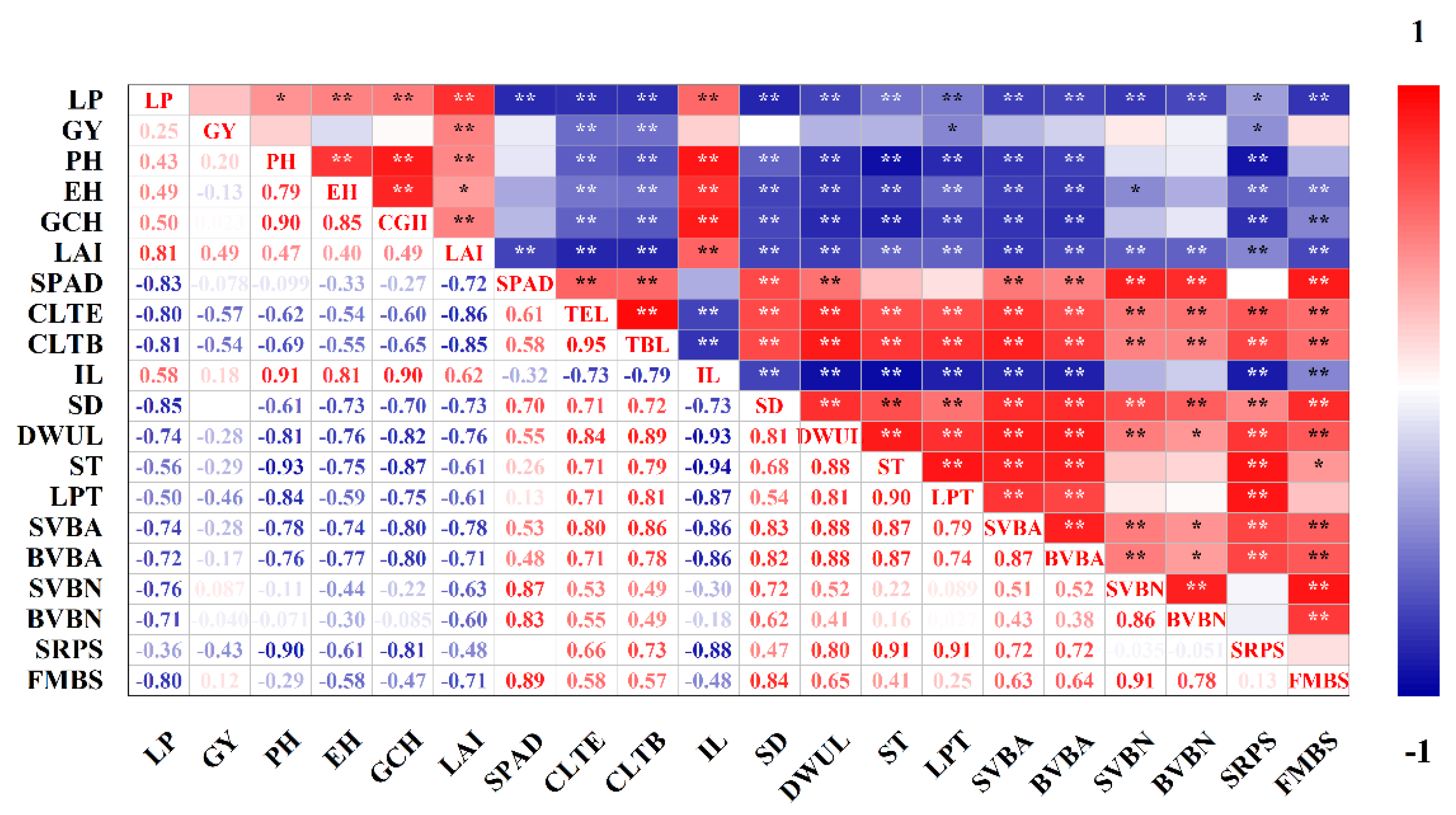
2.7. Correlation Analysis
3. Discussion
3.1. Increasing Planting Density Significantly Reduced Stalk Lodging Resistance of Summer Maize
3.2. The Regulation of Spraying Ethephon on Stalk Lodging Resistance and Grain Yield of Densely Planted Summer Maize
3.3. The Possibility of Synergistic Improvement in Stalk Lodging Resistance and Grain Yield of Densely Planted Summer Maize
4. Materials and Methods
4.1. Materials
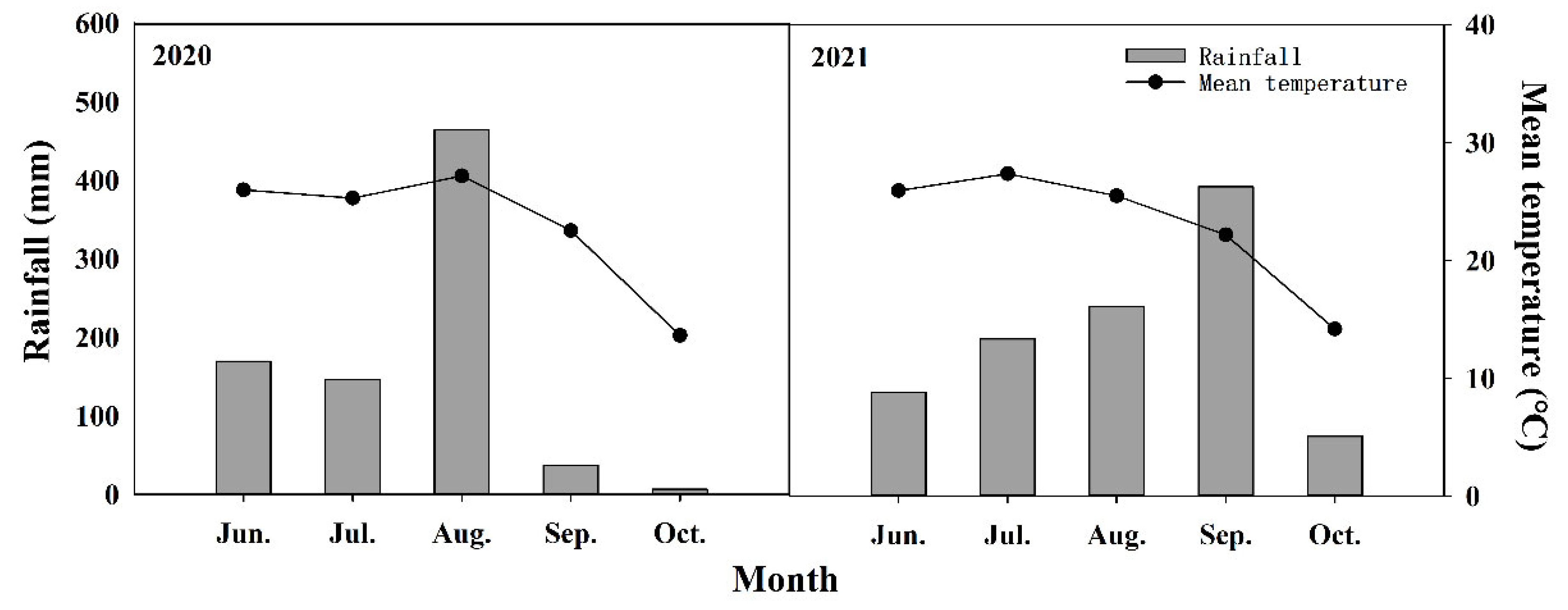
4.2. Experimental Design
4.3. Measurement Items and Methods
4.3.1. Plant Height, Ear Height and Gravity Center Height
4.3.2. LAI and SPAD
4.3.3. Crop Canopy
4.3.4. Internode Length and Stem Diameter
4.3.5. Internode Dry Weight per Unit Length
4.3.6. Stem Anatomical Structure
4.3.7. Stalk Rind Penetration Strength
4.3.8. Maximum Bending Strength in the Field
4.3.9. Lodging Percentage
4.3.10. Grain Yield
4.3.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, J.; Xie, R.Z.; Zhang, W.F.; Wang, K.R.; Hou, P.; Ming, B.; Gou, L.; Li, S.K. Research progress on reduced lodging of high-yield and -density maize. J. Integr. Agric. 2017, 16, 2717–2725. [Google Scholar]
- Cui, X.M.; Yang, Z.B.; Yang, W.R.; Zhang, G.G.; Jiang, S.Z.; Liu, L.; Wang, Z.F. Correlations of shearing force and feed nutritional characteristics of crop straws. Sci. Agric. Sin. 2012, 45, 3137–3146. [Google Scholar]
- Feng, G.; Huang, C.L.; Xing, J.F. The research progress in lodging resistance of maize. Crops 2008, 4, 12–14. [Google Scholar]
- Zhao, X.; Zhou, S.L. Research progress on traits and assessment methods of stalk lodging resistance in maize. Acta Agron. Sin. 2022, 48, 15–16. [Google Scholar]
- Li, S.Y.; Ma, W.; Peng, J.Y.; Chen, Z.M. Study on yield loss of summer maize due to lodging at the big flare stage and grain filling stage. Sci. Agric. Sin. 2015, 48, 3952–3964. [Google Scholar]
- Cao, Q.J.; Cao, T.H.; Yang, F.T.; Diallo, L.; Li, G.; Wang, L.C. Effect of wind damage on grain-filling characteristics, grain quality and yield of spring maize (Zea mays L. ) Chin. J. Eco-Agric. 2013, 21, 1107–1113. [Google Scholar]
- Hou, L.Y.; Wang, K.R.; Wang, Y.Z.; Li, L.L.; Ming, B.; Xie, R.Z.; Li, S.K. In-field harvest loss of mechanically-harvested maize grain and affecting factors in china. Int. J. Agric. Biol. Eng. 2021, 14, 29–37. [Google Scholar]
- Wang, X.Q.; Shi, Z.; Zhang, R.Y.; Sun, X.; Wang, J.D.; Wang, S.; Zhang, Y.; Zhao, Y.X.; Su, A.G.; Li, C.H.; et al. Stalk architecture, cell wall composition, and QTL underlying high stalk flexibility for improved lodging resistance in maize. BMC Plant Biol. 2020, 20, 515. [Google Scholar]
- Wang, T.J.; Zhang, L.; Han, Q.; Zheng, F.X.; Wang, T.Q.; Feng, N.N.; Wang, T.X. Effects of stalk cell wall and tissue on the compressive strength of maize. Plant Sci. J. 2015, 33, 109–115. [Google Scholar]
- Ren, B.Z.; Zhang, J.W.; Li, X.; Fan, X.; Dong, S.T.; Liu, P.; Zhao, B. Effects of waterlogging on stem lodging resistance of summer maize under field conditions. Sci. Agric. Sin. 2013, 46, 2440–2448. [Google Scholar]
- Jiang, A.N.; Yan, J.Q.; Lu, H.B.; Zhao, H.C.; Huang, Z.H. Response of stem microstructure of different spring maize varieties to bending strength. J. Maize Sci. 2020, 28, 53–59. [Google Scholar]
- Li, B.; Gao, F.; Ren, B.Z.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density. J. Integr. Agric. 2021, 20, 2077–2089. [Google Scholar]
- Manga-Robles, A.; Santiago, R.; Malvar, R.A.; Moreno-González, V.; Fornalé, S.; López, I.; Centeno, M.L.; Acebes, J.L.; Álvarez, J.M.; Caparros-Ruiz, D.; et al. Elucidating compositional factors of maize cell walls contributing to stalk strength and lodging resistance. Plant Sci. 2021, 307, 110882. [Google Scholar] [PubMed]
- Sekhon, R.S.; Joyner, C.N.; Ackerman, A.J.; McMahan, C.S.; Cook, D.D.; Robertson, D.J. Stalk bending strength is strongly associated with maize stalk lodging incidence across multiple environments. Field Crops Res. 2020, 249, 107737. [Google Scholar]
- Matteo, J.A.D.; Ferreyra, J.M.; Cerrudo, A.A.; Echarte, L.; Andrade, F.H. Yield potential and yield stability of argentine maize hybrids over 45 years of breeding. Field Crops Res. 2016, 197, 107–116. [Google Scholar]
- Wang, K.; Wang, K.R.; Wang, Y.H.; Zhao, J.; Zhao, R.L.; Wang, X.M.; Li, J.; Liang, J.; Li, S.K. Effects of density maize yield and yield components. Sci. Agric. Sin. 2012, 45, 3437–3445. [Google Scholar]
- Li, S.K.; Wang, K.R.; Xie, R.Z.; Hou, P.; Ming, B.; Yang, X.X.; Han, D.S.; Wang, Y.H. Implementing higher population and full mechanization technologies to achieve high yield and high efficiency in maize production. Crops 2016, 4, 1–6. [Google Scholar]
- Koffi, D.; Samuel, A.; Dorlote, S.J.; Komlan, K.; Suat, I.; Naveen, P.; Murali, K.D.; Sangamesh, V.A. Planting date and plant density effects on maize growth, yield and water use efficiency. Environ. Chall. 2022, 6, 100417. [Google Scholar]
- Cazetta, J.O.; Revoredo, M.D. Non-structural carbohydrate metabolism, growth, and productivity of maize by increasing plant density. Agronomy 2018, 8, 243. [Google Scholar]
- Ren, B.Z.; Liu, W.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B. Effects of plant density on the photosynthetic and chloroplast characteristics of maize under high-yielding conditions. Sci. Nat.-Heidelb. 2017, 104, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lv, P.; Su, K.; Yang, J.S.; Zhang, J.W.; Dong, S.T.; Liu, P.; Sun, Q.Q. Effects of planting density on the grain yield and source-sink characteristics of summer maize. Chin. J. Appl. Ecol. 2010, 21, 1737–1743. [Google Scholar]
- Borrás, L.; Maddonni, G.A.; Otegui, M.E. Leaf senescence in maize hybrids: Plant population, row spacing and kernel set effects. Field Crops Res. 2003, 82, 13–26. [Google Scholar]
- Tollenaar, M.; Deen, W.; Echarte, L.; Liu, W.D. Effect of crowding stress on dry matter accumulation and harvest index in maize. Agron. J. 2006, 98, 930–937. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Juntawong, N.; Aekatasanawan, C.; Kermanee, P.; Roytrakul, S.; Xie, J.L.; Yue, H.W.; Jia, Y.S.; Ma, C.H. Effect of planting densities on grain filling and kernel dehydration of maize (Zea mays). Indian J. Agric. Sci. 2020, 90, 133–138. [Google Scholar]
- Hou, Y.; Wang, P.W. Effects of planting density on yield and panicle traits of summer corn. J. Tianjin Agric. Sci. 2014, 20, 94–96. [Google Scholar]
- Shao, H.; Xia, T.T.; Wu, D.L.; Chen, F.J.; Mi, G.H. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar]
- Ren, B.Z.; Li, L.L.; Dong, S.T.; Liu, P.; Zhao, B.; Yang, J.S.; Wang, D.B.; Zhang, J.W. Effects of plant density on stem traits and lodging resistance of summer maize hybrids with different plant heights. Acta Agron. Sin. 2016, 42, 1864–1872. [Google Scholar] [CrossRef]
- Li, S.T.; Bian, D.H.; He, L.; Wang, D.M.; Zheng, X.M.; Cui, Y.H. Lodging characteristics of summer maize and chemical control research progresses preventing lodging in the North China Plain. J. Maize Sci. 2018, 26, 95–101. [Google Scholar]
- Zhang, Y.S.; Wang, Y.B.; Ye, D.L.; Wang, W.; Qiu, X.M.; Duan, L.S.; Li, Z.H.; Zhang, M.C. Ethephon improved stalk strength of maize (Zea Mays L.) mainly through altering internode morphological traits to modulate mechanical properties under field conditions. Agronomy 2019, 9, 186. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Wang, Y.B.; Ye, D.L.; Xing, J.P.; Duan, L.S.; Li, Z.H.; Zhang, M.C. Ethephon-regulated maize internode elongation associated with modulating auxin and gibberellin signal to alter cell wall biosynthesis and modification. Plant Sci. 2020, 290, 110196. [Google Scholar] [PubMed]
- Chai, M.Z.; Li, Z.; Qin, D.L.; Liu, Y.C.; Xv, M.L.; Dong, L.M.; Zhang, Q.; Yang, D.G.; Zhang, P. Effect of ethephon on lodging resistance of maize stem. J. Maize Sci. 2017, 25, 63–72. [Google Scholar]
- Zhao, Y.T.; Lv, Y.J.; Zhang, S.; Ning, F.F.; Cao, Y.B.; Liao, S.H.; Wang, P.; Huang, S.B. Shortening internodes near ear: An alternative to raise maize yield. J. Plant Growth Regul. 2022, 41, 628–638. [Google Scholar]
- Zhang, Z.X.; Zhu, S.Y.; Li, W.Y.; Liu, Z. Effect of chemical control agent-ethephon on main characters and yield of maize. Chin. Agric. Sci. Bull. 2014, 30, 209–213. [Google Scholar]
- Sattin, M.; Zuin, M.C.; Sartorato, I. Light quality beneath field-grown maize, soybean and wheat canopies: Red: Far red variations. Physiol. Plant 1994, 91, 322–328. [Google Scholar] [CrossRef]
- Geng, W.J.; Li, B.; Ren, B.Z.; Zhao, B.; Liu, P.; Zhang, J.W. Regulation mechanism of planting density and spraying ethephon on lignin metabolism and lodging resistance of summar maize. Sci. Agric. Sin. 2022, 55, 307–319. [Google Scholar]
- Rajcan, I.; Swanton, C.T. Understanding maize-weed competition: Resource competition, light quality and the whole plant. Field Crops Res. 2001, 71, 139–150. [Google Scholar]
- Feng, H.J.; Zhang, S.P.; Ma, C.J.; Liu, P.; Dong, S.T.; Zhao, B.; Zhang, J.W.; Yang, J.S. Effect of plant density on microstructure of stalk vascular bundle of summer maize (Zea Mays L.) and its characteristics of sap flow. Acta Agron. Sin. 2014, 40, 1435–1442. [Google Scholar] [CrossRef]
- Dudley, J.W. Selection for rind puncture resistance in two maize populations. Crop Sci. 1994, 34, 1458–1460. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, S.Y.; Julias, M.; Cook, D.D. Maize stalk lodging: Flexural stiffness predicts strength. Crop Sci. 2016, 56, 1711–1718. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.X.; Hou, P.; Zhu, L.Y.; Song, W.B.; Liu, H.; Huang, Y.Q.; Wang, H.; Guo, J.J. Genome-Wide association study of vascular bundle-related traits in maize stalk. Front. Physiol. 2021, 12, 699486. [Google Scholar]
- Tian, H.; Yuan, Z.H.; Chen, Y.; Wu, Y.D.; Du, W.; Zhang, Z.J.; Shi, Y.L. Effect of stalk vascular bundle distribution on wheat lodging. J. Jiangsu Agric. Sci. 2021, 49, 181–186. [Google Scholar]
- Wang, H.Y.; Chen, X.W.; Niu, X.X.; Su, H.; Shen, T.T.; Dong, X.H. Influence of ethephon on maize cluster growth and development and the physiological mechanism. J. Maize Sci. 2014, 22, 64–70. [Google Scholar]
- Zhang, Q.; Zhang, L.Z.; Evers, J.; Werf, W.V.D.; Zhang, W.Q.; Duan, L.S. Maize yield and quality in response to plant density and application of a novel plant growth regulator. Field Crops Res. 2014, 164, 82–89. [Google Scholar]
- Li, C.Q.; Zheng, H.M.; Li, Y.; Li, C.H. Effect of planting density on the yield and development of maize ear. Sci. Agric. Sin. 2010, 43, 2435–2442. [Google Scholar]
- Li, C.Q.; Cao, R.Y.; Zheng, H.M.; Zhang, S.L.; Li, Y.; Li, C.H. Effects of planting density on the development of maize tassel. J. Henan Agric. Univ. 2010, 44, 630–634. [Google Scholar]
- Stubbs, C.J.; Seegmiller, K.; McMahan, C.; Sekhon, R.S.; Robertson, D.J. Diverse maize hybrids are structurally inefficient at resisting wind induced bending forces that cause stalk lodging. Plant Methods 2020, 16, 67. [Google Scholar]
- Cheng, F.L.; Du, X.; Liu, M.X.; Ji, X.L.; Cui, Y.H. Lodging of summer maize and the effects on grain yield. J. Maize Sci. 2011, 19, 105–108. [Google Scholar]
- Li, S.Y.; Wang, Y.X.; Hu, C.D.; Yang, Y. Effects of strong wind lodging at pre- and post- tasseling stages on growth and yield of summer maize. Chin. J. Appl. Ecol. 2015, 26, 2405–2413. [Google Scholar]
- Xue, J.; Li, L.L.; Xie, R.Z.; Wang, K.R.; Hou, P.; Ming, B.; Zhang, W.X.; Zhang, G.Q.; Gao, S.; Bai, S.J.; et al. Effect of lodging on maize grain losing and harvest efficiency in mechanical grain harvest. Acta Agron. Sin. 2018, 44, 1774–1781. [Google Scholar]
- Simmons, S.R.; Jones, R.J. Contributions of pre-silking assimilate to grain yield in maize. Crop Sci. 1985, 25, 1004–1006. [Google Scholar]
- Yang, Y.S.; Guo, X.X.; Liu, G.Z.; Liu, W.M.; Xue, J.; Ming, B.; Xie, R.Z.; Wang, K.R.; Hou, P.; Li, S.K. Solar radiation effects on dry matter accumulations and transfer in maize. Front. Plant Sci. 2021, 12, 727134. [Google Scholar] [PubMed]
- Xu, C.L.; Gao, Y.B.; Tian, B.J.; Ren, J.H.; Meng, Q.F.; Wang, P. Effects of edah, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar]
- Shao, H.; Shi, D.F.; Shi, W.J.; Ban, X.B.; Chen, Y.C.; Ren, W.; Chen, F.J.; Mi, G.H. The impact of high plant density on dry matter remobilization and stalk lodging in maize genotypes with a different stay-green degree. Arch. Agron. Soil Sci. 2021, 67, 504–518. [Google Scholar]
- Cui, H.Y.; Ji, L.B.; Li, B.; Zhang, J.W.; Zhao, B.; Dong, S.T.; Liu, P. Effects of shading on stalks morphology, structure and lodging of summer maize in field. Sci. Agric. Sin. 2012, 45, 3497–3505. [Google Scholar]
- Huang, G.M.; Liu, Y.R.; Guo, Y.L.; Peng, C.X.; Tan, W.M.; Zhang, M.C.; Li, Z.H.; Zhou, Y.Y.; Duan, L.S. A novel plant growth regulator improves the grain yield of high-density maize crops by reducing stalk lodging and promoting a compact plant type. Field Crops Res. 2021, 260, 107982. [Google Scholar]
- Yang, J.S.; Geng, W.J.; Zhang, J.W.; Ren, B.Z.; Wang, L.C. Responses of the lodging resistance of summer maize with different gene types to plant density. Agronomy 2022, 12, 10. [Google Scholar]
- Hong, D.F.; Wei, X.Z.; Ma, J.F.; Ma, Y.; Wei, F.; Wang, J.M.; Zhang, T.Q.; Cheng, D.X.; Tang, Z.H.; Guo, Q.G.; et al. Effects of high density on agronomic traits, yields comments and tolerance of different high density maize genotypes. Chin. Agric. Sci. Bull. 2020, 36, 13–21. [Google Scholar]
- Wei, X.Z.; Zhang, M.C.; Li, Z.H.; Duan, L.S. Differences in responding sensitivity to ethephon among different maize genotypes. Acta Agron. Sin. 2011, 37, 1819–1827. [Google Scholar]
- Zhang, S.L.; Chang, J.Z.; Cheng, X.J.; Qin, G.W.; Lu, H.W.; Zhu, Z.K. Characteristics of new maize variety Xundan No.20 and its high yield cultivation technology. Seed 2007, 11, 98–99. [Google Scholar]
- Zhang, N.X. Characteristics and high-yield cultivation points of maize Xundan20. Seed Sci. Technol. 2011, 29, 44–45. [Google Scholar]


| Treatment | LAI | SPAD | ||
|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |
| LCK | 4.2 d | 4.4 d | 60.4 a | 58.6 a |
| MCK | 5.3 c | 5.7 b | 58.9 b | 56.0 b |
| ME | 5.3 c | 5.0 cd | 59.1 b | 58.4 a |
| HCK | 6.4 a | 6.4 a | 56.5 c | 54.6 c |
| HE | 5.7 b | 5.3 bc | 58.6 b | 56.5 b |
| ANOVA | ||||
| Year (Y) | ns | ** | ||
| Density (D) | ** | ** | ||
| Ethephon (E) | ** | ** | ||
| Y × D | ns | ns | ||
| Y × E | * | * | ||
| D × E | * | ns | ||
| Y × D × E | ns | ** | ||
| Year | Treatment | 2nd | 3rd | 4th | 5th | 6th |
|---|---|---|---|---|---|---|
| 2020 | LCK | 7.5 bc | 10.3 c | 13.4 c | 15.5 d | 17.4 cd |
| MCK | 8.2 b | 11.6 b | 14.2 b | 16.7 b | 18.4 b | |
| ME | 6.9 c | 10.0 c | 12.9 c | 15.5 d | 16.7 d | |
| HCK | 9.4 a | 12.8 a | 15.4 a | 18.0 a | 19.5 a | |
| HE | 7.8 b | 10.4 c | 13.0 c | 16.0 c | 17.7 bc | |
| 2021 | LCK | 5.9 b | 9.2 c | 12.0 c | 14.0 c | 15.4 cd |
| MCK | 6.3 b | 10.4 b | 13.5 b | 15.3 b | 16.5 b | |
| ME | 5.8 b | 8.9 c | 11.9 c | 13.8 c | 14.9 d | |
| HCK | 7.4 a | 11.5 a | 14.5 a | 16.1 a | 17.9 a | |
| HE | 6.2 b | 9.0 c | 12.0 c | 13.9 c | 15.7 b | |
| ANOVA | ||||||
| Year (Y) | ** | ** | ** | ** | ** | |
| Density (D) | ** | ** | ** | ** | ** | |
| Ethephon (E) | ** | ** | ** | ** | ** | |
| Y × D | ns | ns | ns | ns | ns | |
| Y × E | ns | ns | ns | ns | ns | |
| D × E | ns | ** | ** | ** | ns | |
| Y × D × E | ns | ns | ns | ns | ns | |
| Year | Treatment | 2nd | 3rd | 4th | 5th | 6th |
|---|---|---|---|---|---|---|
| 2020 | LCK | 24.8 a | 23.3 a | 22.1 a | 20.9 a | 20.2 a |
| MCK | 23.1 c | 22.0 b | 20.9 b | 20.3 a | 19.3 b | |
| ME | 24.8 a | 23.4 a | 22.1 a | 20.8 a | 20.3 a | |
| HCK | 22.1 d | 21.0 c | 19.9 c | 19.3 b | 18.5 c | |
| HE | 24.0 b | 23.1 a | 22.1 a | 20.7 a | 19.7 ab | |
| 2021 | LCK | 25.0 a | 23.0 ab | 22.1 a | 21.4 a | 20.4 a |
| MCK | 23.7 bc | 22.3 b | 21.1 b | 20.4 c | 19.6 b | |
| ME | 24.8 ab | 23.2 a | 22.2 a | 21.2 a | 20.7 a | |
| HCK | 22.8 c | 20.4 c | 19.8 c | 19.6 d | 18.5 c | |
| HE | 24.3 ab | 22.8 ab | 21.9 ab | 20.9 b | 20.1 ab | |
| ANOVA | ||||||
| Year (Y) | ns | ns | ns | ns | ns | |
| Density (D) | ** | ** | ** | ** | ** | |
| Ethephon (E) | ** | ** | ** | ** | ** | |
| Y × D | ns | ns | ns | ns | ns | |
| Y × E | ns | ns | ns | ns | ns | |
| D × E | ns | ** | * | * | ns | |
| Y × D × E | ns | ns | ns | ns | ns | |
| Year | Treatment | Tissue Thickness | The Area of Vascular Bundles (mm2) | The Number of Vascular Bundles (No.) | |||
|---|---|---|---|---|---|---|---|
| Sclerenchyma (μm) | Lignified Parenchyma (mm) | Small Vascular Bundles | Big Vascular Bundles | Small Vascular Bundles | Big Vascular Bundles | ||
| 2020 | LCK | 52.7 a | 1.67 a | 0.17 a | 0.20 a | 426 a | 199 a |
| MCK | 45.3 b | 1.56 a | 0.16 b | 0.19 b | 365 c | 156 c | |
| ME | 52.6 a | 1.63 a | 0.17 a | 0.20 a | 404 b | 177 b | |
| HCK | 34.2 c | 1.36 b | 0.13 c | 0.16 c | 318 d | 123 d | |
| HE | 52.4 a | 1.57 a | 0.16 b | 0.20 ab | 401 b | 185 ab | |
| 2021 | LCK | 62.0 a | 1.98 a | 0.18 a | 0.21 a | 374 a | 175 a |
| MCK | 53.7 b | 1.77 b | 0.16 b | 0.20 a | 323 c | 143 b | |
| ME | 62.8 a | 1.88 a | 0.18 a | 0.21 a | 329 c | 137 b | |
| HCK | 45.9 c | 1.55 c | 0.15 c | 0.17 b | 283 d | 111 c | |
| HE | 61.3 a | 1.90 a | 0.17 a | 0.21 a | 342 b | 106 c | |
| ANOVA | |||||||
| Year (Y) | ** | ** | ** | ** | ** | ** | |
| Density (D) | ** | ** | ** | ** | ** | ** | |
| Ethephon (E) | ** | ** | ** | ** | ** | ** | |
| Y × D | ns | ns | * | ns | ns | ** | |
| Y × E | ns | ns | ns | ns | ** | ** | |
| D × E | ** | ** | ** | ** | ** | ns | |
| Y × D × E | ns | ns | ns | ns | ns | ** | |
| Year | Treatment | Ears (No. ha−1) | Kernels per Ear | 1000-Grain Weight (g) | Grain Yield (kg ha−1) |
|---|---|---|---|---|---|
| 2020 | LCK | 50,556 d | 598 a | 315.4 a | 9535.3 d |
| MCK | 60,556 c | 545 c | 306.1 b | 10,102.2 cd | |
| ME | 66,667 b | 554 b | 291.1 c | 10,751.3 b | |
| HCK | 69,444 b | 526 e | 291.8 c | 10,658.7 bc | |
| HE | 75,556 a | 538 d | 289.1 c | 11,751.7 a | |
| 2021 | LCK | 56,667 e | 601 a | 253.5 a | 8633.4 c |
| MCK | 70,741 d | 572 b | 245.9 b | 9950.1 b | |
| ME | 73,704 c | 573 b | 237.2 c | 10,017.5 ab | |
| HCK | 77,593 b | 546 c | 234.8 d | 9947.5 b | |
| HE | 88,148 a | 528 c | 226.1 e | 10,523.2 a | |
| ANOVA | |||||
| Year (Y) | ** | ** | ** | ** | |
| Density (D) | ** | ** | ** | ** | |
| Ethephon (E) | ** | ns | ** | ** | |
| Y × D | ns | ** | * | ns | |
| Y × E | ns | * | ns | * | |
| D × E | * | ns | ** | ns | |
| Y × D × E | * | ns | ** | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, W.; Sun, Z.; Ren, B.; Ren, H.; Zhao, B.; Liu, P.; Zhang, J. Spraying Ethephon Effectively Increased Canopy Light Transmittance of Densely Planted Summer Maize, Thus Achieving Synergistic Improvement in Stalk Lodging Resistance and Grain Yield. Plants 2022, 11, 2219. https://doi.org/10.3390/plants11172219
Geng W, Sun Z, Ren B, Ren H, Zhao B, Liu P, Zhang J. Spraying Ethephon Effectively Increased Canopy Light Transmittance of Densely Planted Summer Maize, Thus Achieving Synergistic Improvement in Stalk Lodging Resistance and Grain Yield. Plants. 2022; 11(17):2219. https://doi.org/10.3390/plants11172219
Chicago/Turabian StyleGeng, Wenjie, Zhichao Sun, Baizhao Ren, Hao Ren, Bin Zhao, Peng Liu, and Jiwang Zhang. 2022. "Spraying Ethephon Effectively Increased Canopy Light Transmittance of Densely Planted Summer Maize, Thus Achieving Synergistic Improvement in Stalk Lodging Resistance and Grain Yield" Plants 11, no. 17: 2219. https://doi.org/10.3390/plants11172219
APA StyleGeng, W., Sun, Z., Ren, B., Ren, H., Zhao, B., Liu, P., & Zhang, J. (2022). Spraying Ethephon Effectively Increased Canopy Light Transmittance of Densely Planted Summer Maize, Thus Achieving Synergistic Improvement in Stalk Lodging Resistance and Grain Yield. Plants, 11(17), 2219. https://doi.org/10.3390/plants11172219








