Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit
Abstract
1. Introduction
2. Results
2.1. Effect of Methods and Durations of Extraction on TFCs and TPCs of A. compactum Extracts
2.2. Analysis of Antioxidant Activity of A. compactum Extracts
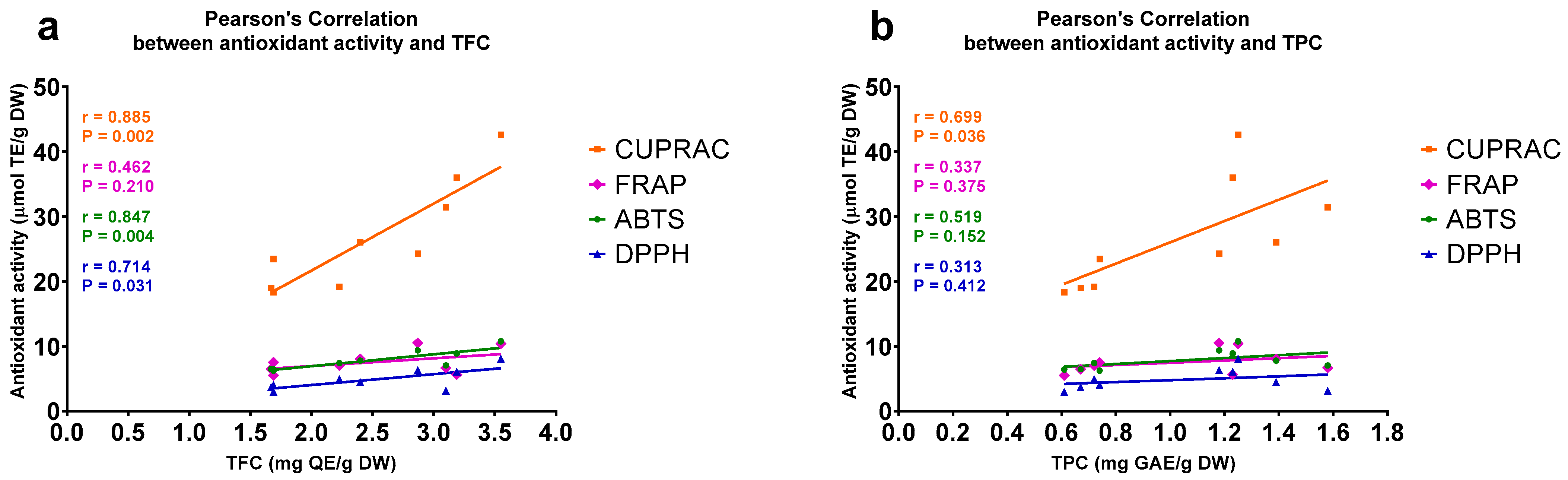
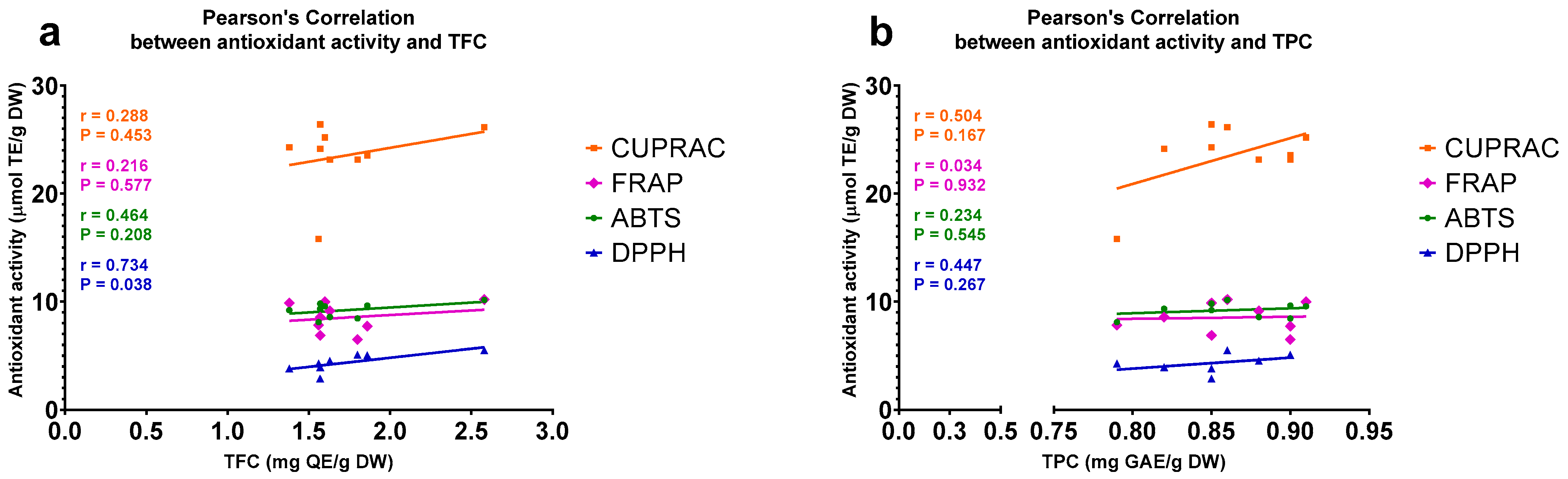
2.3. Antioxidant Activities of A. compactum Extracts Correlate with Their TFCs and TPCs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. CSE
4.4. MAE
4.5. UAE
4.6. Quantification of TFC
4.7. Quantification of TPC
4.8. Quantification of Antioxidant Activities
4.8.1. ABTS Assay
4.8.2. CUPRAC Assay
4.8.3. DPPH Assay
4.8.4. FRAP Assay
4.9. Statistics and Figure Preparation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Chaudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef]
- Arshiya, S. The Antioxidant Effect of Certain Fruits: A Review. J. Pharm. Sci. Res. 2013, 5, 265–268. [Google Scholar]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.R.; Moyo, M.; Van Staden, J. Natural Antioxidants: Fascinating or Mythical Biomolecules? Molecules 2010, 15, 6905–6930. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of Polyphenols Extraction from Dried Chokeberry Using Maceration as Traditional Technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Droop, J.; Kaewsri, W.; Lamxay, V.; Poulsen, A.D.; Newman, M. Identity and Lectotypification of Amomum compactum and Amomum kepulaga (Zingiberaceae). Taxon 2013, 62, 1287–1294. [Google Scholar] [CrossRef]
- Setyawan, A.D.; Wiryanto, W.; Suranto, S.; Bermawie, N.; Sudarmono, S. Short Communication: Comparisons of Isozyme Diversity in Local Java Cardamom (Amomum compactum) and True Cardamom (Elettaria cardamomum). Nusant. Biosci. 2014, 6, 94–101. [Google Scholar] [CrossRef]
- Pujiarti, R.; Kusumadewi, A. Chemical Compounds, Physicochemical Properties, and Antioxidant Activity of A. cardamomum Leaves and Rhizomes Oils on Different Distillation Time. Wood Res. J. 2020, 11, 35–40. [Google Scholar] [CrossRef]
- Yu, J.G.; Feng, H.J.; Li, J.T. Essential oil of fruits and leaves of A. kravanh and A. compactum. Chin. Trad. Herb. Drugs 1982, 13, 4–7. [Google Scholar]
- Feng, X.; Jiang, Z.T.; Wang, Y.; Li, R. Composition comparison of Essential Oils Extracted by Hydrodistillation and Microwave-Assisted Hydrodistillation from Amomum kravanh and Amomum compactum. J. Essent. Oil Bear. Plants 2013, 14, 354–359. [Google Scholar] [CrossRef]
- Alkandahri, M.Y.; Shafirany, M.Z.; Rusdin, A.; Agustina, L.S.; Pangaribuan, F.; Fitrianti, F.; Farhamzah, K.A.; Sugiharta, S.; Arfania, M.; Mardiana, L.A. Amomum compactum: A Review of Pharmacological Studies. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 61–69. [Google Scholar]
- Hartady, T.; Balia, R.L.; Syamsunarno, M.R.A.A.; Jasni, S.; Priosoeryanto, B.P. Bioactivity of Amomum Compactum Soland Ex Maton (Java Cardamom) as a Natural Antibacterial. Sys. Rev. Pharm. 2020, 11, 384–387. [Google Scholar]
- Chismirina, S.; Aulia, C.P. Konsentrasi Hambat dan Bunuh Minimum Ekstrak Buah Kapulaga (Amomum compactum) terhadap Aggregatibacter actinomycetemcomitans. J. Syiah Kuala Dent. Soc. 2016, 1, 192–200. [Google Scholar]
- Widowati, W.; Ratnawati, H.; Husin, W.; Maesaroh, M. Antioxidant Properties of Spice Extracts. Biol. Eng. 2015, 1, 24–29. [Google Scholar]
- Amma, K.P.A.P.; Rani, M.P.; Sasidharan, I.; Nisha, V.N.P. Chemical Composition, Flavonoid—Phenolic Contents and Radical Scavenging Activity of Four Major Varieties of Cardamom. Int. J. Biol. Med. Res. 2010, 1, 20–24. [Google Scholar]
- Nurcholis, W.; Sya’bani Putri, D.N.; Husnawati, H.; Aisyah, S.I.; Priosoeryanto, B.P. Total Flavonoid Content and Antioxidant Activity of Ethanol and Ethyl Acetate Extracts from Accessions of Amomum Compactum Fruits. Ann. Agric. Sci. 2021, 66, 58–62. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1, 1000106. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Coelho, J.; Veiga, J.; Karmali, A.; Nicolai, M.; Reis, C.P.; Nobre, B.; Palavra, A. Supercritical CO2 Extracts and Volatile Oil of Basil (Ocimum Basilicum L.) Comparison with Conventional Methods. Separations 2018, 5, 21. [Google Scholar] [CrossRef]
- Khatri, P.; Rana, J.S.; Jamdagni, P.; Sindhu, A. Phytochemical Screening, Gc-Ms and Ft-Ir Analysis of Methanolic Extract Leaves of Elettaria Cardamomum. Int. J. Res.-GRANTHAALAYAH 2017, 5, 213–224. [Google Scholar] [CrossRef]
- Gosu, V.; Sasidharan, S.; Saudagar, P.; Lee, H.K.; Shin, D. Computational Insights into the Structural Dynamics of Mda5 Variants Associated with Aicardi–Goutières Syndrome and Singleton–Merten Syndrome. Biomolecules 2021, 11, 1251. [Google Scholar] [CrossRef]
- Upadhya, V.; Pai, S.R.; Hegde, H.V. Effect of Method and Time of Extraction on Total Phenolic Content in Comparison with Antioxidant Activities in Different Parts of Achyranthes Aspera. J. King Saud Univ. Sci. 2015, 27, 204–208. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus Persica L.) Fruit; Elsevier, B.V.: Amsterdam, The Netherlands, 2016; Volume 162, ISBN 2135415065. [Google Scholar]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-Assisted Extraction of Phenolics from Pomegranate Peels: Optimization, Kinetics, and Comparison with Ultrasounds Extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS Scavenging and Antioxidant Signalling by Redox Metallic and Fullerene Nanomaterials: Potential Implications in ROS Associated Degenerative Disorders. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food. Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, L.G.; Kria, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of Sonication on the Phenolic Content and Antioxidant Activity of Terminalia Catappa L. Leaves. Pharmacogn. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A Systematic Survey of Antioxidant Activity of 30 Chinese Medicinal Plants Using the Ferric Reducing Antioxidant Power Assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Hassanbaglou, B.; Hamid, A.A.; Roheeyati, A.M.; Saleh, N.M.; Abdulamir, A.; Khatib, A.; Sabu, M.C. Antioxidant Activity of Different Extracts from Leaves of Pereskia Bleo (Cactaceae). J. Med. Plants Res. 2012, 6, 2932–2937. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In Vitro Antioxidant Capacity and Free Radical Scavenging Evaluation of Standardized Extract of Costus Afer Leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of Antioxidant and Antimicrobial Activities of Tilia (Tilia Argentea Desf Ex DC), Sage (Salvia Triloba L.), and Black Tea (Camellia Sinensis) Extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Calvindi, J.; Syukur, M.; Nurcholis, W. Investigation of Biochemical Characters and Antioxidant Properties of Different Winged Bean (Psophocarpus Tetragonolobus) Genotypes Grown in Indonesia. Biodiversitas 2020, 21, 2420–2424. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, L. Effects of Extraction Solvent on Wheat Bran Antioxidant Activity Estimation. LWT-Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Irshad, M.; Zafaryab, M.; Singh, M.; Rizvi, M.M. Comparative Analysis of the Antioxidant Activity of Cassia fistula Extracts. Int. J. Med. Chem. 2012, 2012, 157125. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Nurcholis, W.; Ma’rifah, K.; Artika, I.M.; Aisyah, S.I.; Priosoeryanto, B.P. Optimization of Total Flavonoid Content from Cardamom Fruits Using a Simplex-Centroid Design, Along with the Evaluation of the Antioxidant Properties. Trop. J. Nat. Prod. Res. 2021, 5, 1382–1388. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.F.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant Capacity of Phytochemicals and Their Potential Effects on Oxidative Status in Animals—A Review. Asian-Aust. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Arpitha, S.; Srinivasan, K.; Sowbhagya, H.B. Anti-inflammatory Effect of Resin Fraction of Cardamom (Elettaria Cardamomum) in Carrageenan-Induced Rat Paw Edema. PharmaNutrition 2019, 10, 100165. [Google Scholar] [CrossRef]
- Winarsi, H.; Yuniaty, A.; Nuraeni, I. Improvement of Antioxidant and Immune Status of Atherosclerotic Rats Adrenaline And Egg-Yolks -Induced Using Cardamom-Rhizome-Ethanolic-Extract: An Initial Study of Functional Food. Agric. Agric. Sci. Procedia 2016, 9, 264–270. [Google Scholar] [CrossRef][Green Version]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition Mechanism of Cardamom Essential Oil on Methicillin-Resistant Staphylococcus Aureus Biofilm. LWT Food Sci. Technol. 2020, 122, 109057. [Google Scholar] [CrossRef]
- Ashokumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, Traditional Uses, Phytochemistry and Biological Activities of Cardamom [Elettaria Cardamomum (L.) Maton]—A Critical Review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Lee, J.A.; Lee, M.Y.; Seo, C.S.; Jung, D.Y.; Lee, N.H.; Kim, J.H.; Ha, H.; Shin, H.K. Anti-Asthmatic Effects of an Amomum compactum Extract on an Ovalbumin (OVA)-Induced Murine Asthma Model. Biosci. Biotechnol. Biochem. 2010, 74, 1814–1818. [Google Scholar] [CrossRef]
- Lee, J.A.; Lee, M.Y.; Shin, I.S.; Seo, C.S.; Ha, H.; Shin, H.K. Anti-Inflammatory Effects of Amomum compactum on RAW 264.7 Cells via Induction of Heme Oxygenase-1. Arch. Pharmacal. Res. 2010, 35, 739–746. [Google Scholar] [CrossRef]
- Qonita, A.; Riptanti, E.W.; Uchyani, R. Sustainability of Cardamom Comparative Advantage Incentral Java Province, Indonesia. Eco. Env. Cons. 2019, 25, 145–151. [Google Scholar]
- Bobo-garcía, G.; Davidov-pardo, G.; Arroqui, C.; Marín-arroyo, M.R. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2014, 95, 204–209. [Google Scholar] [CrossRef]

| Extraction Method | Duration (min) | TFC (mg QE g−1 DW) | TPC (mg GAE g−1 DW) |
|---|---|---|---|
| CSE | 30 | 1.684 ± 0.010 c | 0.672 ± 0.063 c |
| 180 | 2.575 ± 0.459 b | 1.233 ± 0.451 a | |
| 360 | 3.202 ± 0.337 a | 1.221 ± 0.034 a | |
| MAE | 1 | 1.729 ± 0.333 c | 0.895 ± 0.054 b,c |
| 2 | 1.809 ± 0.228 c | 1.000 ± 0.008 a,b | |
| 3 | 3.187 ± 0.232 a | 1.263 ± 0.029 a | |
| UAE | 20 | 1.904 ± 0.582 c | 0.841 ± 0.021 b,c |
| 40 | 1.611 ± 0.240 c | 0.889 ± 0.031 b,c | |
| 60 | 1.661 ± 0.123 c | 0.858 ± 0.057 b,c |
| Extraction Method | Duration (min) | ABTS (µmol TE g−1 DW) | DPPH (µmol TE g−1 DW) | CUPRAC (µmol TE g−1 DW) | FRAP (µmol TE g−1 DW) |
|---|---|---|---|---|---|
| CSE | 30 | 6.380 ± 0.114 e | 3.537 ± 0.502 e | 20.280 ± 2.791 c | 6.497 ± 1.000 b |
| 180 | 7.409 ± 0.384 d | 4.158 ± 0.936 e | 25.547 ± 6.113 b,c | 7.251 ± 0.707 b | |
| 360 | 9.683 ± 0.974 b | 6.187 ± 1.081 a,b | 34.302 ± 9.286 a,b | 8.848 ± 2.796 b | |
| MAE | 1 | 9.263 ± 0.703 b | 6.177 ± 0.794 b,c | 25.658 ± 2.329 b,c | 9.041 ± 2.052 b |
| 2 | 9.441 ± 0.515 b | 5.982 ± 0.669 b,c,d | 34.791 ± 8.859 a,b | 9.462 ± 5.652 b | |
| 3 | 12.933 ± 0.209 a | 8.078 ± 0.960 a | 38.147 ± 3.710 a | 18.637± 4.631 a | |
| UAE | 20 | 9.772 ± 0.414 b | 4.100 ± 1.318 e | 25.569 ± 1.239 b,c | 8.549 ± 1.658 b |
| 40 | 9.478 ± 0.230 b | 4.420 ± 0.864 d,e | 24.347 ± 0.835 c | 9.198 ± 1.277 b | |
| 60 | 8.379 ± 0.241 c | 4.624 ± 0.419 c,d,e | 20.702 ± 4.234 c | 7.830 ± 1.315 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurcholis, W.; Alfadzrin, R.; Izzati, N.; Arianti, R.; Vinnai, B.Á.; Sabri, F.; Kristóf, E.; Artika, I.M. Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit. Plants 2022, 11, 2221. https://doi.org/10.3390/plants11172221
Nurcholis W, Alfadzrin R, Izzati N, Arianti R, Vinnai BÁ, Sabri F, Kristóf E, Artika IM. Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit. Plants. 2022; 11(17):2221. https://doi.org/10.3390/plants11172221
Chicago/Turabian StyleNurcholis, Waras, Rahma Alfadzrin, Nurul Izzati, Rini Arianti, Boglárka Ágnes Vinnai, Fadillah Sabri, Endre Kristóf, and I Made Artika. 2022. "Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit" Plants 11, no. 17: 2221. https://doi.org/10.3390/plants11172221
APA StyleNurcholis, W., Alfadzrin, R., Izzati, N., Arianti, R., Vinnai, B. Á., Sabri, F., Kristóf, E., & Artika, I. M. (2022). Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit. Plants, 11(17), 2221. https://doi.org/10.3390/plants11172221








