Cell Wall Calcium and Hemicellulose Have a Role in the Fruit Firmness during Storage of Blueberry (Vaccinium spp.)
Abstract
1. Introduction
2. Results
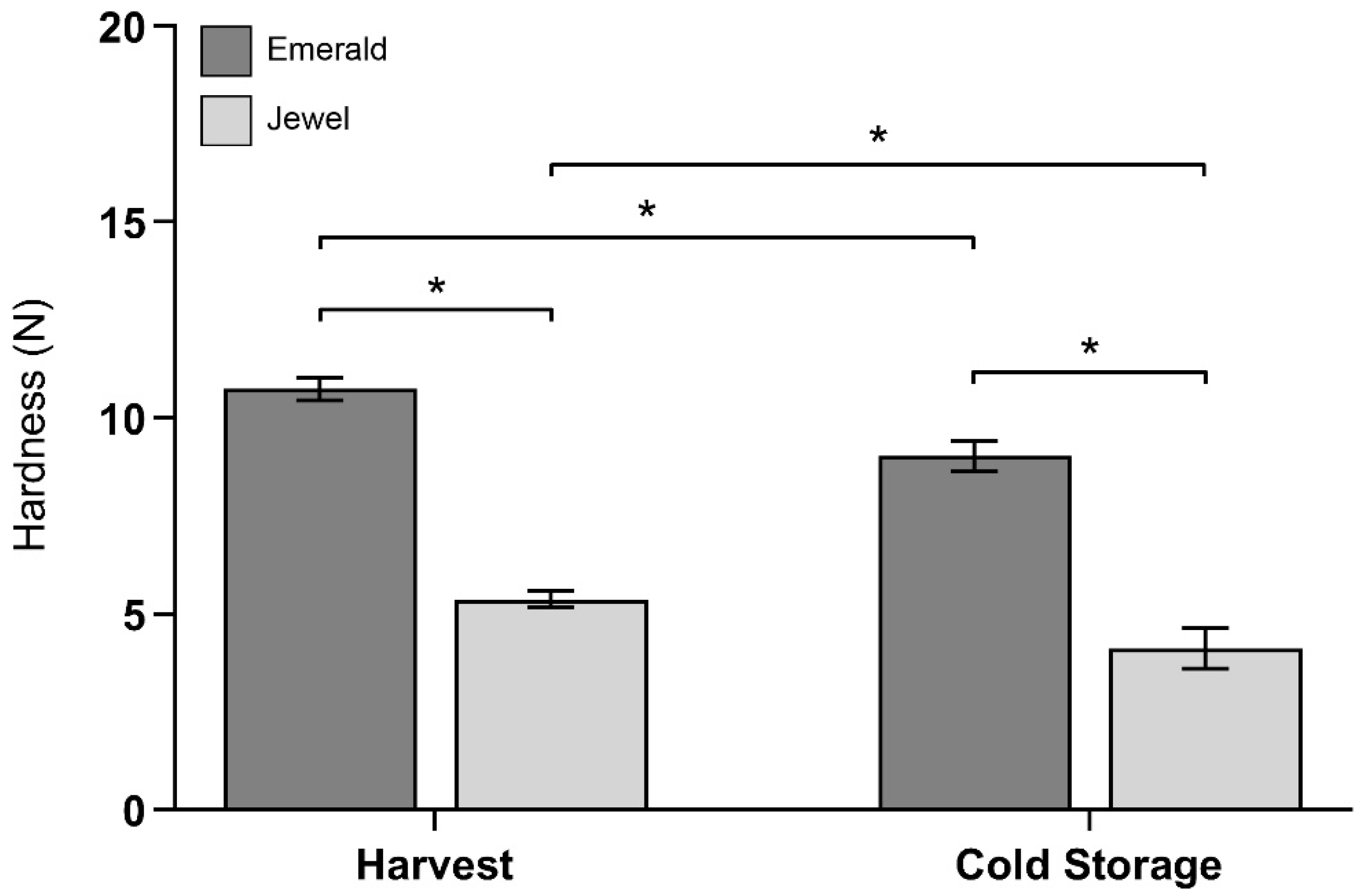
2.1. Phenotype and Firmness Properties of Blueberry Cultivars at Harvest and after Cold Storage
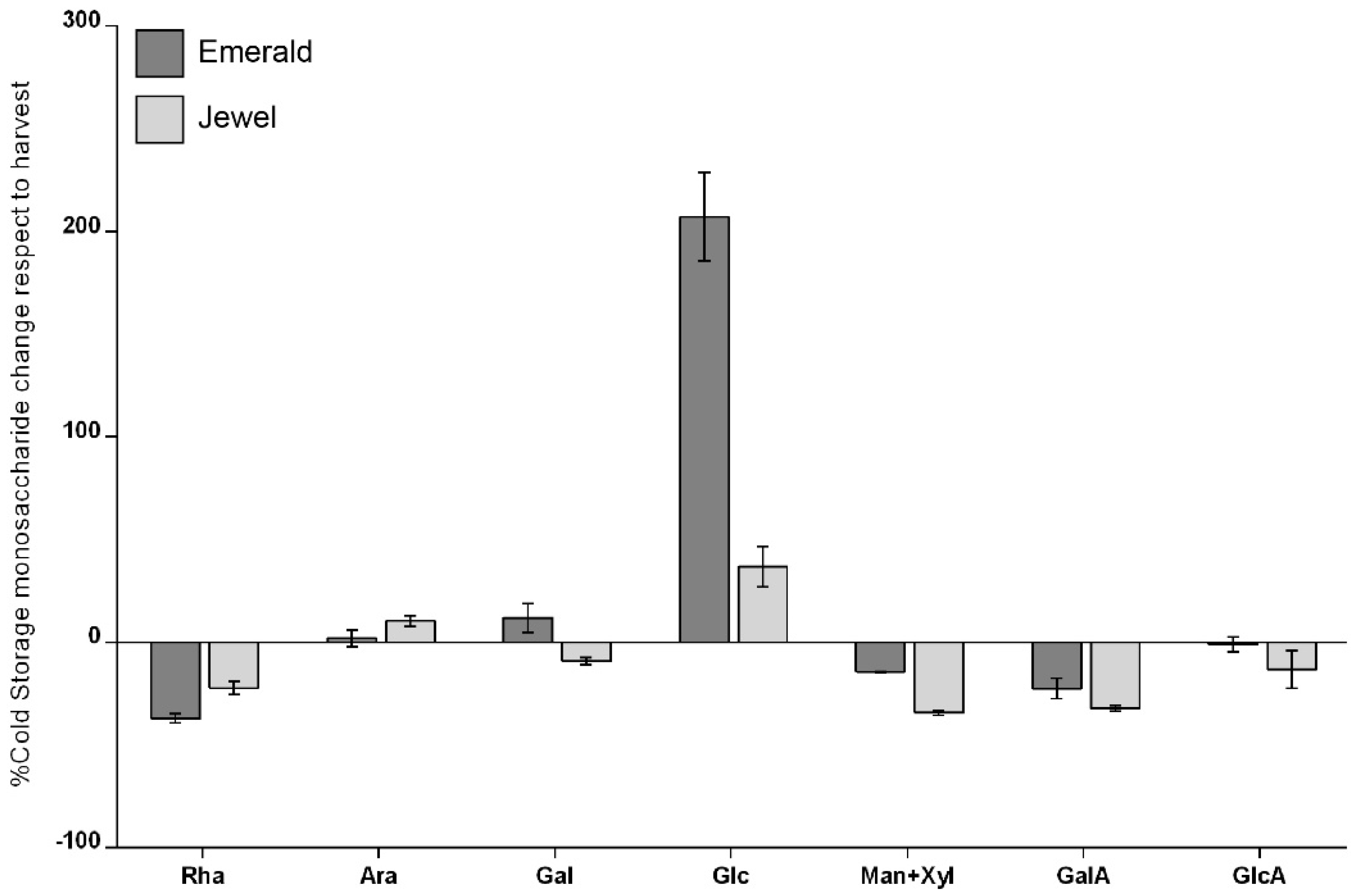
2.2. Global Cell Wall Noncellulosic Monosaccharide Composition Analysis of Emerald and Jewel Blueberry Cultivars by HPAEC-PAD
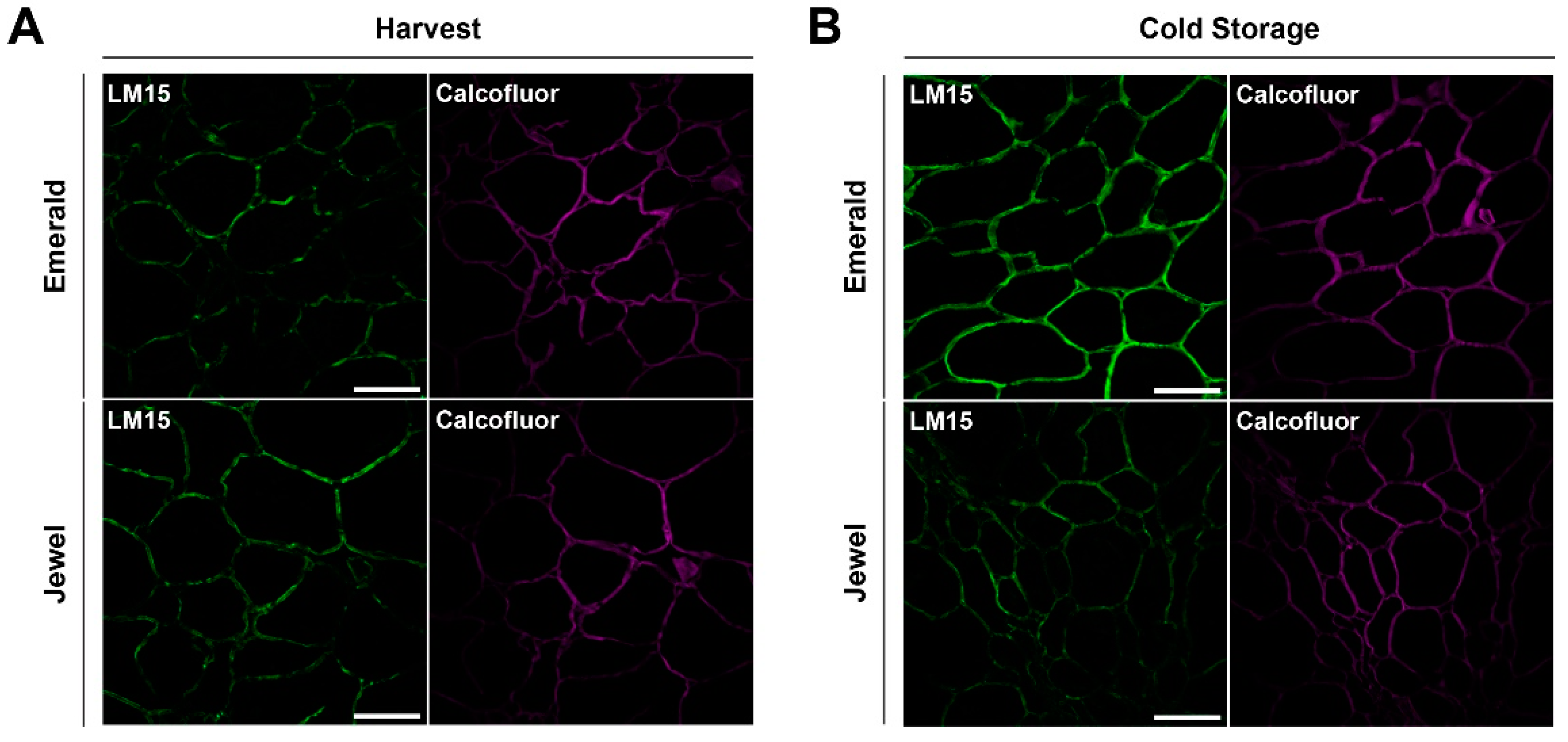
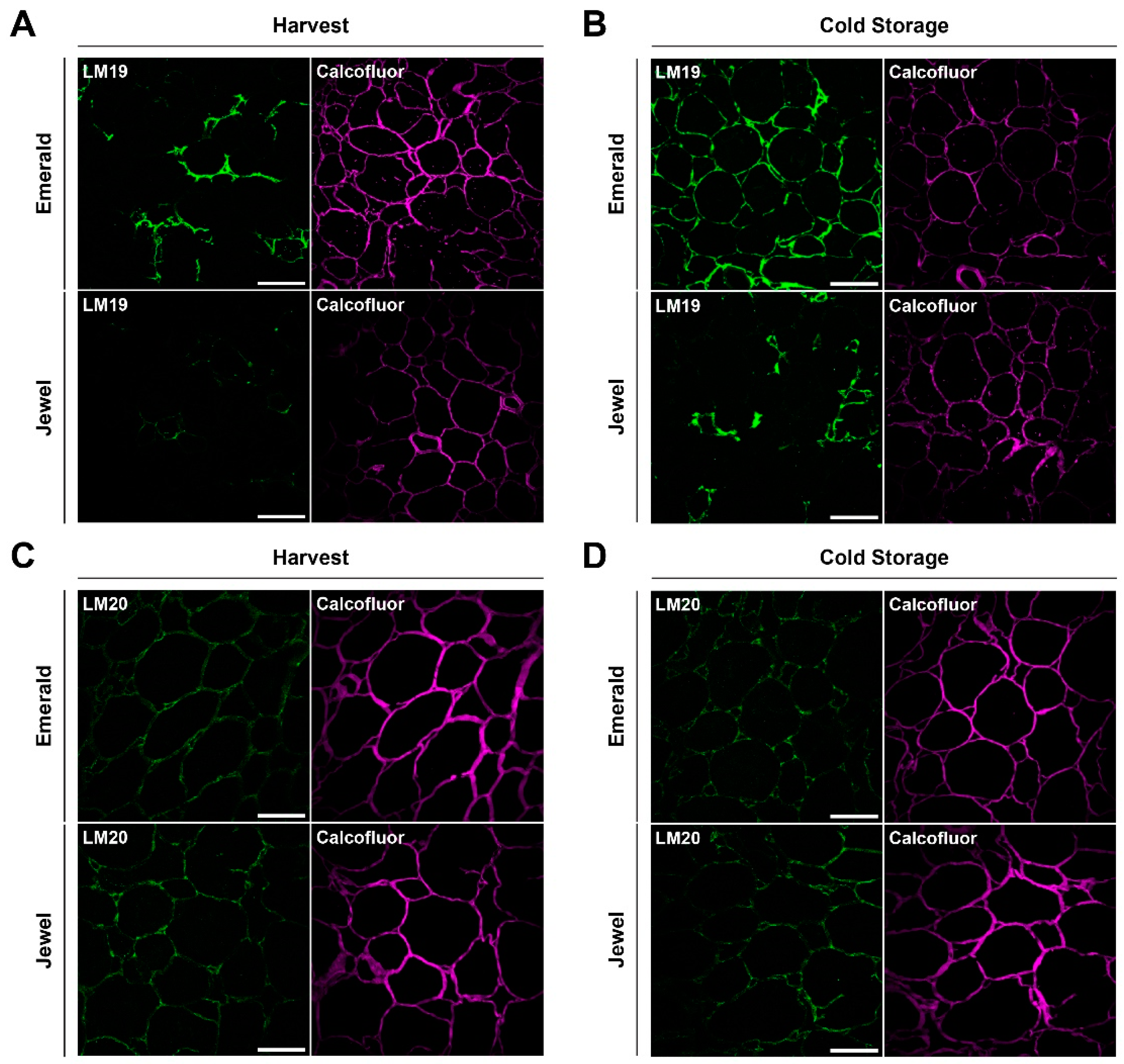
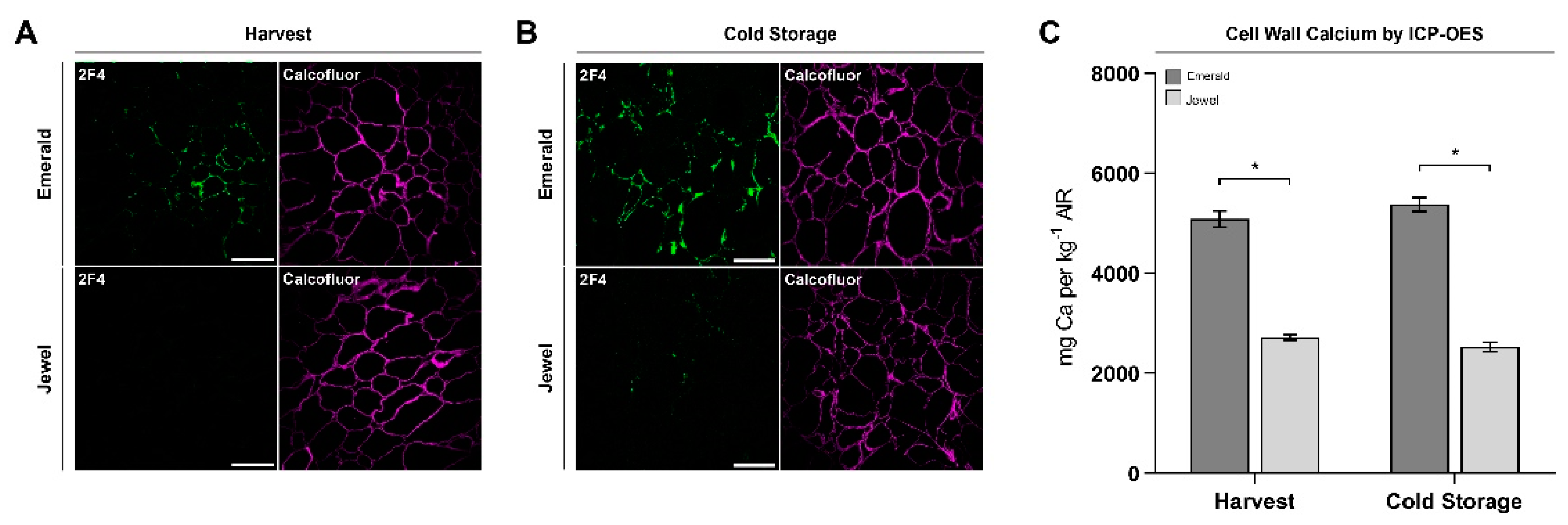
2.3. Immunohistochemical and Analytical Assays of Emerald and Jewel Blueberries
2.4. Firmer Blueberries Are Associated with HG Methylesterification Status and a Higher Cell Wall Calcium Content
3. Discussion
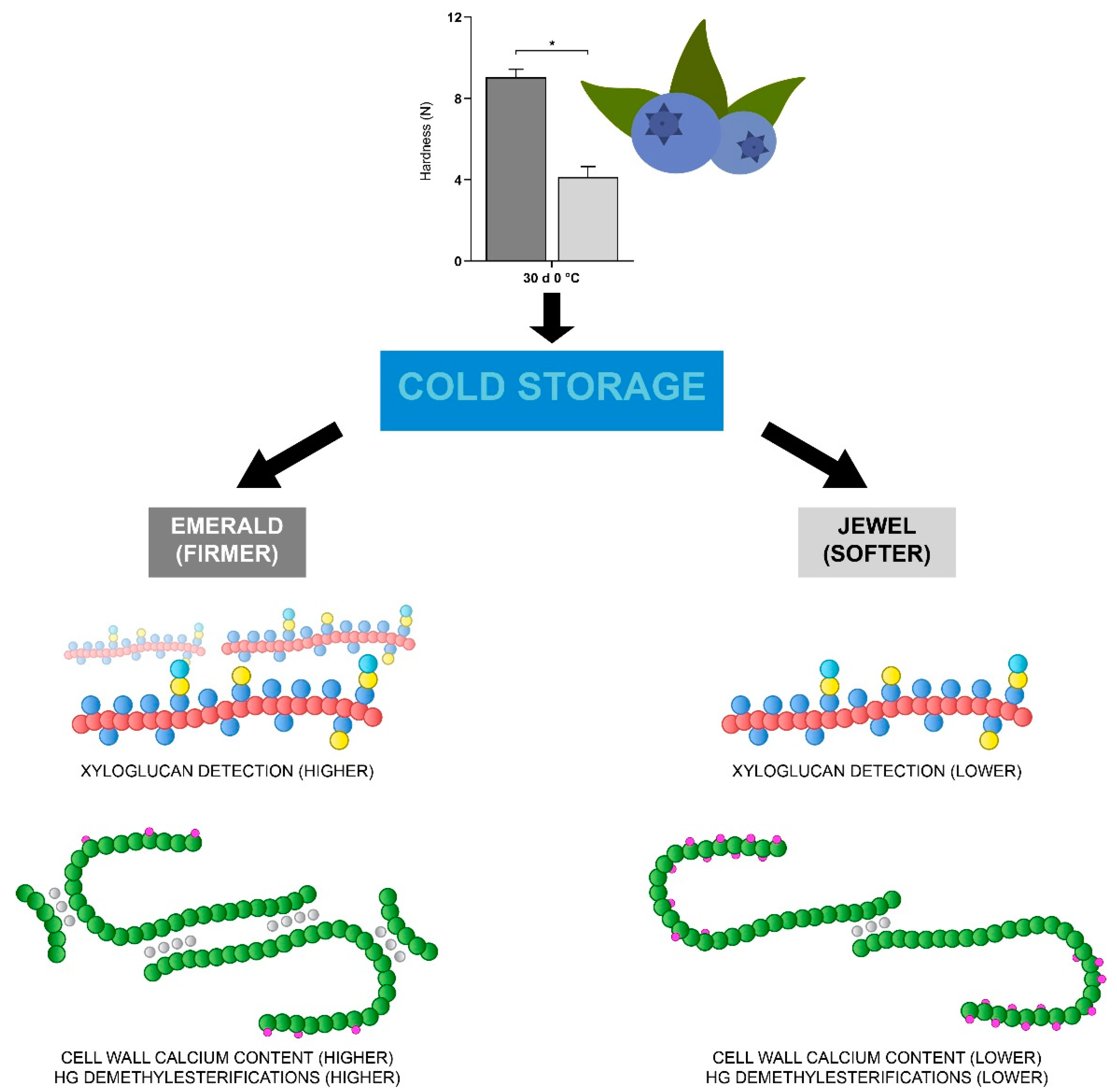
3.1. Increased Xyloglucan Amount Is Associated with Blueberry Firmness during Cold Storage
3.2. Homogalacturonan Methylesterification Status and Calcium Dynamics Are Correlated to Blueberry Firmness
4. Materials and Methods
4.1. Plant Material and Phenotypic Analysis
4.2. Texture Analysis
4.3. Determining Cell Wall Monosaccharide Composition
4.4. Immunohistochemistry Assay
4.5. Quantification of Cell Wall Calcium
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, Phenolics, and Antioxidant Capacity in Diverse Small Fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Davidson, K.T.; Zhu, Z.; Balabanov, D.; Zhao, L.; Wakefield, M.R.; Bai, Q.; Fang, Y. Beyond Conventional Medicine—A Look at Blueberry, a Cancer-Fighting Superfruit. Pathol. Oncol. Res. 2017, 24, 733–738. [Google Scholar] [CrossRef]
- Seeram, N.P.; Burton-Freeman, B. The Seventh Biennial Berry Health Benefits Symposium. Food Funct. 2018, 9, 20–21. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Martin, R.B., Jr. A Survey of Fruit Firmness in Highbush Blueberry and Species-Introgressed Blueberry Cultivars. HortScience 2002, 37, 386–389. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G.; Rolle, L. Mechanical behaviour and quality traits of highbush blueberry during postharvest storage. J. Sci. Food Agric. 2009, 89, 989–992. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Olmstead, J.W.; Colquhoun, T.A.; Levin, L.A.; Clark, D.G.; Moskowitz, H.R. Consumer-assisted Selection of Blueberry Fruit Quality Traits. HortScience 2014, 49, 864–873. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Cameron, A.C.; Shirazi, A.; Dostal-Lange, D.L. Modified-atmosphere Packaging of Blueberry Fruit: Effect of Temperature on Package O2 and CO2. J. Am. Soc. Hortic. Sci. 1992, 117, 436–441. [Google Scholar] [CrossRef]
- Lobos, G.A.; Callow, P.; Hancock, J.F. The effect of delaying harvest date on fruit quality and storage of late highbush blueberry cultivars (Vaccinium corymbosum L.). Postharvest Biol. Technol. 2014, 87, 133–139. [Google Scholar] [CrossRef]
- Montecchiarini, M.; Bello, F.; Rivadeneira, M.; Vazquez, D.; Podesta, F.; Tripodi, K. Metabolic and physiologic profile during the fruit ripening of three blueberries highbush (Vaccinium corymbosum) cultivars. J. Berry Res. 2018, 8, 177–192. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef]
- Paniagua, A.; East, A.; Hindmarsh, J.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Ayala, I.L.; Beaudry, R.M.; Moggia, C. Within-plant variability in blueberry (Vaccinium corymbosum L.): Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. In Plant Cell Walls; Popper, Z., Ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2001; pp. 311–340. [Google Scholar]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.-P.; Schols, H.A.; Oomen, R.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.; Visser, R.G. If Homogalacturonan Were a Side Chain of Rhamnogalacturonan I. Implications for Cell Wall Architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Bangerth, F. Calcium-Related Physiological Disorders of Plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- Brady, C.J. Fruit Ripening. Annu. Rev. Plant Physiol. 1987, 38, 155–178. [Google Scholar] [CrossRef]
- Hepler, P.K.; Wayne, R.O. Calcium and Plant Development. Annu. Rev. Plant Physiol. 1985, 36, 397–439. [Google Scholar] [CrossRef]
- Ehocking, B.; Tyerman, S.D.; Burton, R.A.; Egilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Reid, M.S.; Prasad, M. Calcium analysis and the prediction of bitter pit in apple fruit. N. Z. J. Agric. Res. 1979, 22, 485–490. [Google Scholar] [CrossRef]
- Wójcik, P. Quality and ‘Conference’ Pear Storability as Influenced by Preharvest Sprays of Calcium Chloride. J. Plant Nutr. 2012, 35, 1970–1983. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Ejsmentewicz, T.; Balic, I.; Sanhueza, D.; Barria, R.; Meneses, C.; Orellana, A.; Prieto, H.; Defilippi, B.G.; Campos-Vargas, R. Comparative Study of Two Table Grape Varieties with Contrasting Texture during Cold Storage. Molecules 2015, 20, 3667–3680. [Google Scholar] [CrossRef] [PubMed]
- Val, J.; Fernández, V. In-season calcium-spray formulations improve calcium balance and fruit quality traits of peach. J. Plant Nutr. Soil Sci. 2011, 174, 465–472. [Google Scholar] [CrossRef]
- Langer, S.E.; Marina, M.; Burgos, J.L.; Martínez, G.A.; Civello, P.M.; Villarreal, N.M. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria × ananassa, Duch). J. Sci. Food Agric. 2019, 99, 4003–4010. [Google Scholar] [CrossRef]
- Lv, J.; Han, X.; Bai, L.; Xu, D.; Ding, S.; Ge, Y.; Li, C.; Li, J. Effects of calcium chloride treatment on softening in red raspberry fruit during low-temperature storage. J. Food Biochem. 2020, 44, 1–8. [Google Scholar] [CrossRef]
- Hanson, E.J.; Beggs, J.L.; Beaudry, R.M. Applying Calcium Chloride Postharvest to Improve Highbush Blueberry Firmness. HortScience 1993, 28, 1033–1034. [Google Scholar] [CrossRef]
- Hanson, E.J.; Berkheimer, S.F. Effect of Soil Calcium Applications on Blueberry Yield and Quality. Small Fruits Rev. 2004, 3, 133–139. [Google Scholar] [CrossRef]
- Stückrath, R.; Quevedo, R.; de la Fuente, L.; Hernández, A.; Sepúlveda, V. Effect of Calcium Foliar Application on the Characteristics of Blueberry Fruit during Storage. J. Plant Nutr. 2008, 31, 849–866. [Google Scholar] [CrossRef]
- Koron, D.; Sturm, K.; Pavlin, S. Effects of CA Foliar Fertilizers on Fruit Quality of Highbush Blueberry. Acta Hortic. 2009, 810, 705–708. [Google Scholar] [CrossRef]
- Vance, A.J.; Jones, P.; Strik, B.C. Foliar Calcium Applications Do Not Improve Quality or Shelf Life of Strawberry, Raspberry, Blackberry, or Blueberry Fruit. HortScience 2017, 52, 382–387. [Google Scholar] [CrossRef]
- Arrington, M.; Devetter, L.W. Foliar Applications of Calcium and Boron Do Not Increase Fruit Set or Yield in Northern Highbush Blueberry (Vaccinium corymbosum). HortScience 2017, 52, 1259–1264. [Google Scholar] [CrossRef]
- Rivera, S.; Sofkova-Bobcheva, S.; East, A.; Hutchins, D.; Kerckhoffs, H. The effect of foliar calcium application on key (post) harvest quality attributes in Rabbit-eye blueberry (Vaccinium ashei). Acta Hortic. 2019, 1265, 135–144. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J. Foliar applications of calcium do not impact on fruit and leaf nutrient concentration or quality of ‘O’Neal’ blueberry. J. Hortic. Sci. Biotechnol. 2019, 94, 676–684. [Google Scholar] [CrossRef]
- Lobos, T.; Retamales, J.; Hanson, E. Early preharvest calcium sprays improve postharvest fruit quality in ‘Liberty’ highbush blueberries. Sci. Hortic. 2021, 277, 109790. [Google Scholar] [CrossRef]
- Manríquez, T.L.; Quezada, H.P.; Alvarez, W.L. Efecto de aplicaciones de calcio en la calidad de la fruta de arándano alto (Vaccinium corymbosum L.) cv. Elliot. Idesia 2011, 29, 59–64. [Google Scholar] [CrossRef]
- Ochmian, I.D. The Impact of Foliar Application of Calcium Fertilizers on the Quality of Highbush Blueberry Fruits Belonging to the ‘Duke’ Cultivar. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 163–169. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Silva, J.L.; Marroquin, E.; Matta, F.B.; Garner, J.O.; Stojanovic, J. Physicochemical, carbohydrate and sensory characteristics of highbush and rabbiteye blueberry cultivars. J. Sci. Food Agric. 2005, 85, 1815–1821. [Google Scholar] [CrossRef]
- Vicente, A.R.; Ortugno, C.; Rosli, H.; Powell, A.L.T.; Greve, L.C.; Labavitch, J.M. Temporal Sequence of Cell Wall Disassembly Events in Developing Fruits. 2. Analysis of Blueberry (Vaccinium Species). J. Agric. Food Chem. 2007, 55, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Amelung, W.; Cheshire, M.V.; Guggenberger, G. Determination of neutral and acidic sugars in soil by capillary gas-liquid chromatography after trifluoroacetic acid hydrolysis. Soil Biol. Biochem. 1996, 28, 1631–1639. [Google Scholar] [CrossRef]
- Petridis, A.; van der Kaay, J.; Sungurtas, J.; Verrall, S.R.; McCallum, S.; Graham, J.; Hancock, R.D. Photosynthetic plasticity allows blueberry (Vaccinium corymbosum L.) plants to compensate for yield loss under conditions of high sink demand. Environ. Exp. Bot. 2020, 174, 104031. [Google Scholar] [CrossRef]
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 3 February 2021).
- Quesada, M.A.; Blanco-Portales, R.; Posé, S.; García-Gago, J.A.; Jiménez-Bermúdez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Muñoz-Blanco, J. Antisense Down-Regulation of the FaPG1 Gene Reveals an Unexpected Central Role for Polygalacturonase in Strawberry Fruit Softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of Polygalacturonase1 alters firmness, tensile strength and water loss in apple (Malus x domestica) fruit. BMC Plant Biol. 2012, 12, 129. [Google Scholar] [CrossRef]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, G.A.R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the Nanostructure of Pectin, Hemicellulose and Cellulose in the Cell Walls of Pears of Different Texture and Firmness. Food Bioprocess Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Wu, C.; Fan, G.; Li, T.; Dong, C. Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]
- Cappai, F.; Benevenuto, J.; Ferrão, L.F.V.; Munoz, P. Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review. Agronomy 2018, 8, 174. [Google Scholar] [CrossRef]
- Murayama, H.; Katsumata, T.; Endou, H.; Fukushima, T.; Sakurai, N. Effect of storage period on the molecular-mass distribution profile of pectic and hemicellulosic polysaccharides in pears. Postharvest Biol. Technol. 2006, 40, 141–148. [Google Scholar] [CrossRef]
- Fullerton, C.G.; Prakash, R.; Ninan, A.S.; Atkinson, R.G.; Schaffer, R.J.; Hallett, I.C.; Schröder, R. Fruit from Two Kiwifruit Genotypes With Contrasting Softening Rates Show Differences in the Xyloglucan and Pectin Domains of the Cell Wall. Front. Plant Sci. 2020, 11, 964. [Google Scholar] [CrossRef]
- Dheilly, E.; le Gall, S.; Guillou, M.-C.; Renou, J.-P.; Bonnin, E.; Orsel, M.; Lahaye, M. Cell wall dynamics during apple development and storage involves hemicellulose modifications and related expressed genes. BMC Plant Biol. 2016, 16, 201. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Tong, C.B.S. Identification of Candidate Genes Involved in Fruit Ripening and Crispness Retention Through Transcriptome Analyses of a ‘Honeycrisp’ Population. Plants 2020, 9, 1335. [Google Scholar] [CrossRef]
- Miedes, E.; Herbers, K.; Sonnewald, U.; Lorences, E.P. Overexpression of a Cell Wall Enzyme Reduces Xyloglucan Depolymerization and Softening of Transgenic Tomato Fruits. J. Agric. Food Chem. 2010, 58, 5708–5713. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.; Ban, Q.; He, Y.; Jin, M.; Rao, J. Overexpression of persimmon DkXTH1 enhanced tolerance to abiotic stress and delayed fruit softening in transgenic plants. Plant Cell Rep. 2017, 36, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.; Chin, L.-H.; Lazan, H. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci. 2004, 167, 317–327. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Montecchiarini, M.; Margarit, E.; Morales, L.; Rivadeneira, M.; Bello, F.; Gollán, A.; Vázquez, D.; Podestá, F.; Tripodi, K. Proteomic and metabolomic approaches unveil relevant biochemical changes in carbohydrate and cell wall metabolisms of two blueberry (Vaccinium corymbosum) varieties with different quality attributes. Plant Physiol. Biochem. 2019, 136, 230–244. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Orfila, C. Altered Middle Lamella Homogalacturonan and Disrupted Deposition of (1right-arrow5)-alpha-L-Arabinan in the Pericarp of Cnr, a Ripening Mutant of Tomato. Plant Physiol. 2001, 126, 210–221. [Google Scholar] [CrossRef]
- Zepeda, B.; Olmedo, P.; Ejsmentewicz, T.; Sepúlveda, P.; Balic, I.; Balladares, C.; Delgado-Rioseco, J.; Fuentealba, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell wall and metabolite composition of berries of Vitis vinifera (L.) cv. Thompson Seedless with different firmness. Food Chem. 2018, 268, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanzana, C.; Celiz-Balboa, J.; Garzo, E.; Marcus, S.E.; Parra-Rojas, J.P.; Rojas, B.; Olmedo, P.; Rubilar, M.A.; Rios, I.; Chorbadjian, R.A.; et al. Pectin Methylesterases Modulate Plant Homogalacturonan Status in Defenses against the Aphid Myzus persicae. Plant Cell 2019, 31, 1913–1929. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.T.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef]
- Liners, F.; Letesson, J.-J.; Didembourg, C.; van Cutsem, P. Monoclonal Antibodies against Pectin. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef]
- Balic, I.; Ejsmentewicz, T.; Sanhueza, D.; Silva, C.; Peredo, T.; Olmedo, P.; Barros, M.; Verdonk, J.C.; Paredes, R.; Meneses, C.; et al. Biochemical and physiological study of the firmness of table grape berries. Postharvest Biol. Technol. 2014, 93, 15–23. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo, P.; Zepeda, B.; Rojas, B.; Silva-Sanzana, C.; Delgado-Rioseco, J.; Fernández, K.; Balic, I.; Arriagada, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell Wall Calcium and Hemicellulose Have a Role in the Fruit Firmness during Storage of Blueberry (Vaccinium spp.). Plants 2021, 10, 553. https://doi.org/10.3390/plants10030553
Olmedo P, Zepeda B, Rojas B, Silva-Sanzana C, Delgado-Rioseco J, Fernández K, Balic I, Arriagada C, Moreno AA, Defilippi BG, et al. Cell Wall Calcium and Hemicellulose Have a Role in the Fruit Firmness during Storage of Blueberry (Vaccinium spp.). Plants. 2021; 10(3):553. https://doi.org/10.3390/plants10030553
Chicago/Turabian StyleOlmedo, Patricio, Baltasar Zepeda, Bárbara Rojas, Christian Silva-Sanzana, Joaquín Delgado-Rioseco, Kamila Fernández, Iván Balic, César Arriagada, Adrián A. Moreno, Bruno G. Defilippi, and et al. 2021. "Cell Wall Calcium and Hemicellulose Have a Role in the Fruit Firmness during Storage of Blueberry (Vaccinium spp.)" Plants 10, no. 3: 553. https://doi.org/10.3390/plants10030553
APA StyleOlmedo, P., Zepeda, B., Rojas, B., Silva-Sanzana, C., Delgado-Rioseco, J., Fernández, K., Balic, I., Arriagada, C., Moreno, A. A., Defilippi, B. G., & Campos-Vargas, R. (2021). Cell Wall Calcium and Hemicellulose Have a Role in the Fruit Firmness during Storage of Blueberry (Vaccinium spp.). Plants, 10(3), 553. https://doi.org/10.3390/plants10030553








