Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe—Part I
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield
2.2. Seed Size and Weight
| Year | Variety | Length (mm) 1 | Width (mm) 1 | TSW (g) 1,2 | Test Weight (kg/hL) 1 | Saponins (mg/g) 1,2 |
|---|---|---|---|---|---|---|
| 2017 | Atlas | 1.97 ± 0.00 d,B | 1.80 ± 0.00 f,B | 2.85 ± 0.01 e,B | 75.9 ± 0.1 e,C | <LoQ |
| Bastille | - | - | - | - | - | |
| Dutchess | 1.96 ± 0.00 d,B | 1.80 ± 0.01 f,B | 2.94 ± 0.01 f,g,C | 71.9 ± 0.2 a,B | 0.1 ± 0.0 a,A | |
| Faro | 1.86 ± 0.01 c,B | 1.69 ± 0.00 c,B | 2.56 ± 0.01 c,B | 74.9 ± 0.2 c,d,A | 1.7 ± 0.6 c,d,A | |
| Jessie | 1.95 ± 0.01 d,B | 1.79 ± 0.01 e,f,C | 2.76 ± 0.02 d,C | 75.3 ± 0.2 d,B | <LoQ | |
| Oro de Valle | 1.97 ± 0.01 d,C | 1.76 ± 0.01 e,C | 2.98 ± 0.01 g,B | 74.6 ± 0.1 b,c,B | 1.1 ± 0.2 b,c,A | |
| Pasto | 1.77 ± 0.00 b,A | 1.61 ± 0.01 b,A | 2.37 ± 0.01 b,B | 74.7 ± 0.2 b,c,A | < LoQ | |
| Puno | 1.72 ± 0.01 a,C | 1.56 ± 0.01 a,B | 2.27 ± 0.01 a,C | 81.7 ± 0.2 g,B | 2.2 ± 0.2 d,A | |
| Rouge Marie | - | - | - | - | - | |
| Summer Red | 2.24 ± 0.01 e,B | 1.99 ± 0.00 g,B | 3.47 ± 0.02 h,C | 74.5 ± 0.1 b,B | <LoQ | |
| Titicaca | 1.88 ± 0.01 c,A | 1.69 ± 0.01 c,A | 2.87 ± 0.03 e,f,B | 82.5 ± 0.1 h,C | 0.4 ± 0.1 a,b,A | |
| Vikinga | 1.89 ± 0.00 c,C | 1.72 ± 0.00 d,C | 2.70 ± 0.01 d,C | 77.2 ± 0.1 f,B | < LoQ | |
| Zwarte | 2.25 ± 0.02 e,B | 1.98 ± 0.02 g,A | 3.68 ± 0.02 I,B | 77.6 ± 0.2 f,B | 0.8 ± 0.2 a,b,c,A | |
| 2018 | Atlas | 1.89 ± 0.01 d,A | 1.74 ± 0.01 d,A | 2.32 ± 0.02 c,d,A | 75.6 ± 0.1 a,B | <LoQ |
| Bastille | 1.87 ± 0.00 c,d,A | 1.68 ± 0.01 c,A | 2.49 ± 0.02 f,A | 78.1 ± 0.3 d,B | <LoQ | |
| Dutchess | 1.86 ± 0.01 c,A | 1.69 ± 0.01 c,A | 2.38 ± 0.04 d,e,A | 75.3 ± 0.2 a,C | <LoQ | |
| Faro | 1.80 ± 0.01 b,A | 1.64 ± 0.00 b,A | 2.28 ± 0.03 c,A | 79.6 ± 0.1 e,C | 5.2 ± 0.6 c,B | |
| Jessie | 1.90 ± 0.02 d,A | 1.75 ± 0.02 d,B | 2.24 ± 0.02 c,A | 76.8 ± 0.1 b,C | <LoQ | |
| Oro de Valle | 1.85 ± 0.00 c,A | 1.67 ± 0.01 c,A | 2.46 ± 0.01 f,A | 77.1 ± 0.1 b,C | 2.8 ± 0.9 b,B | |
| Pasto | 1.78 ± 0.01 b,A | 1.62 ± 0.01 b,A | 2.00 ± 0.03 b,A | 76.7 ± 0.1 b,B | <LoQ | |
| Puno | 1.68 ± 0.01 a,B | 1.54 ± 0.01 a,B | 1.90 ± 0.03 a,A | 81.6 ± 0.1 f,B | 5.1 ± 1.2 c,B | |
| Rouge Marie | - | - | - | - | - | |
| Summer Red | 2.05 ± 0.01 e,A | 1.88 ± 0.01 e,A | 2.95 ± 0.04 g,A | 78.3 ± 0.2 d,C | <LoQ | |
| Titicaca | 2.03 ± 0.02 e,B | 1.85 ± 0.02 e,C | 2.93 ± 0.04 g,C | 79.9 ± 0.1 e,B | 1.3 ± 0.4 a,B | |
| Vikinga | 1.85 ± 0.02 c,B | 1.68 ± 0.02 c,B | 2.43 ± 0.01 e,f,A | 77.6 ± 0.2 c,C | <LoQ | |
| Zwarte | - | - | - | - | - | |
| 2019 | Atlas | 1.89 ± 0.01 c,A | 1.73 ± 0.00 c,d,A | 2.33 ± 0.02 b,A | 73.6 ± 0.2 c,d,A | <LoQ |
| Bastille | 1.97 ± 0.01 d,B | 1.76 ± 0.01 d,B | 2.90 ± 0.06 e,B | 71.9 ± 0.2 b,A | <LoQ | |
| Dutchess | 2.00 ± 0.01 d,C | 1.83 ± 0.01 e,C | 2.77 ± 0.04 d,B | 68.9 ± 0.1 a,A | <LoQ | |
| Faro | 1.79 ± 0.01 b,A | 1.64 ± 0.01 b,A | 2.75 ± 0.02 d,C | 75.5 ± 0.3 g,B | 7.9 ± 0.1 e,C | |
| Jessie | 1.89 ± 0.01 c,A | 1.72 ± 0.00 c,A | 2.48 ± 0.02 c,B | 73.9 ± 0.2 d,e,A | < LoQ | |
| Oro de Valle | 1.89 ± 0.01 c,B | 1.73 ± 0.00 c,B | 3.04 ± 0.02 f,C | 73.4 ± 0.2 c,A | 5.4 ± 0.4 c,d,C | |
| Pasto | - | - | - | - | - | |
| Puno | 1.65 ± 0.01 a,A | 1.50 ± 0.01 a,A | 2.15 ± 0.03 a,B | 74.6 ± 0.3 f,A | 6.3 ± 0.5 d,C | |
| Rouge Marie | 1.98 ± 0.01 d,A | 1.83 ± 0.01 e,A | 3.27 ± 0.05 g,h,A | 73.5 ± 0.3 c,d,A | <LoQ | |
| Summer Red | 2.05 ± 0.02 e,A | 1.88 ± 0.01 f,A | 3.23 ± 0.02 g,B | 73.5 ± 0.2 c,d,A | <LoQ | |
| Titicaca | 1.88 ± 0.01 c,A | 1.71 ± 0.01 c,B | 2.79 ± 0.01 d,A | 74.5 ± 0.2 f,A | 3.2 ± 0.4 b,C | |
| Vikinga | 1.78 ± 0.01 b,C | 1.61 ± 0.01 b,A | 2.55 ± 0.03 c,B | 74.2 ± 0.2 e,f,A | 0.9 ± 0.4 a,A | |
| Zwarte | 2.19 ± 0.01 f,A | 1.99 ± 0.01 g,A | 3.35 ± 0.05 h,A | 75.9 ± 0.1 g,A | 4.8 ± 0.4 c,B |
2.3. Seed Color
2.4. Saponins
2.5. Chemical Composition
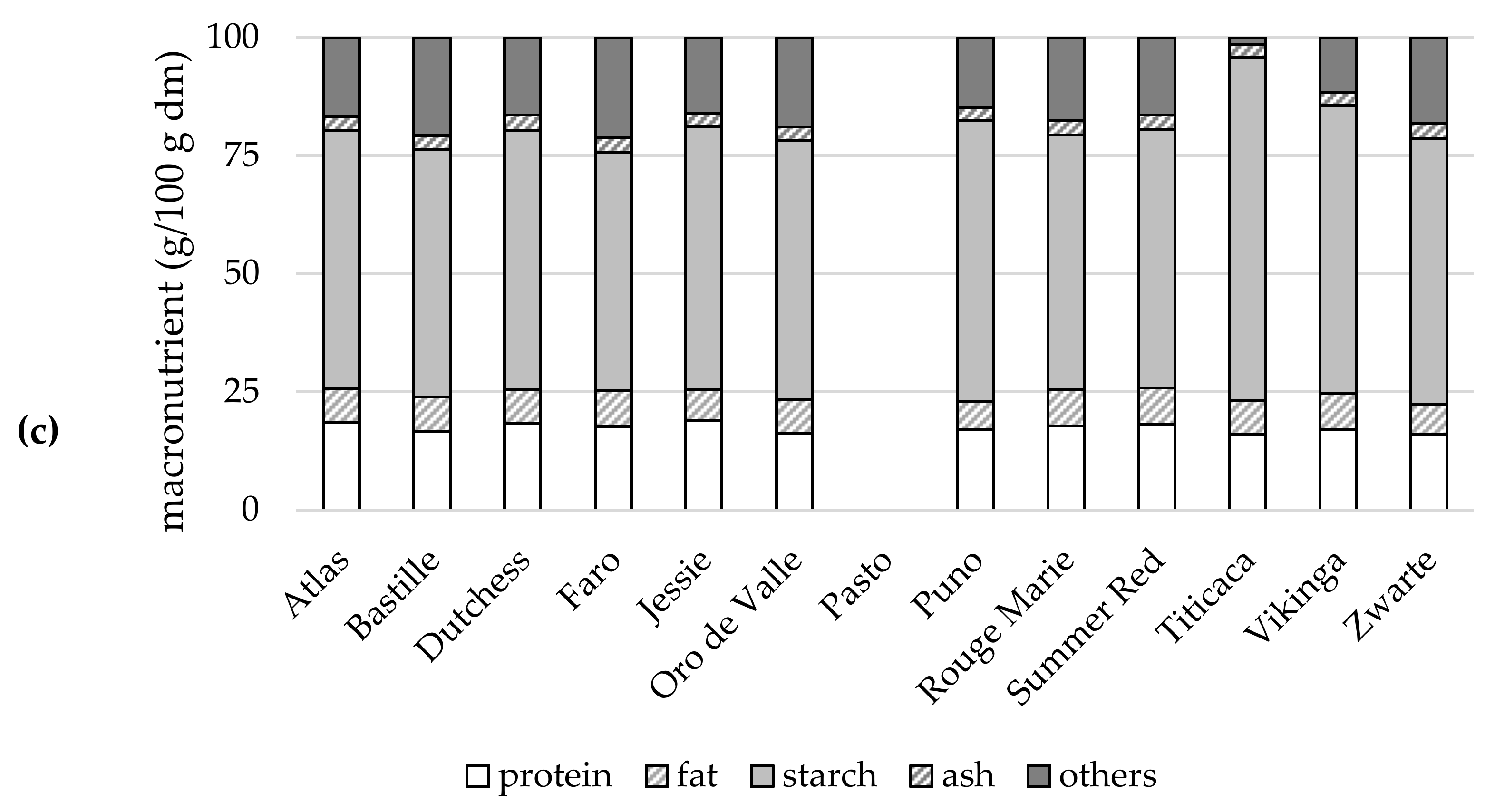
2.5.1. Macronutrients
2.5.2. Protein and Amino Acids
2.5.3. Fat, Fatty Acids, and Triacylglycerols
2.5.4. Starch
2.5.5. Minerals
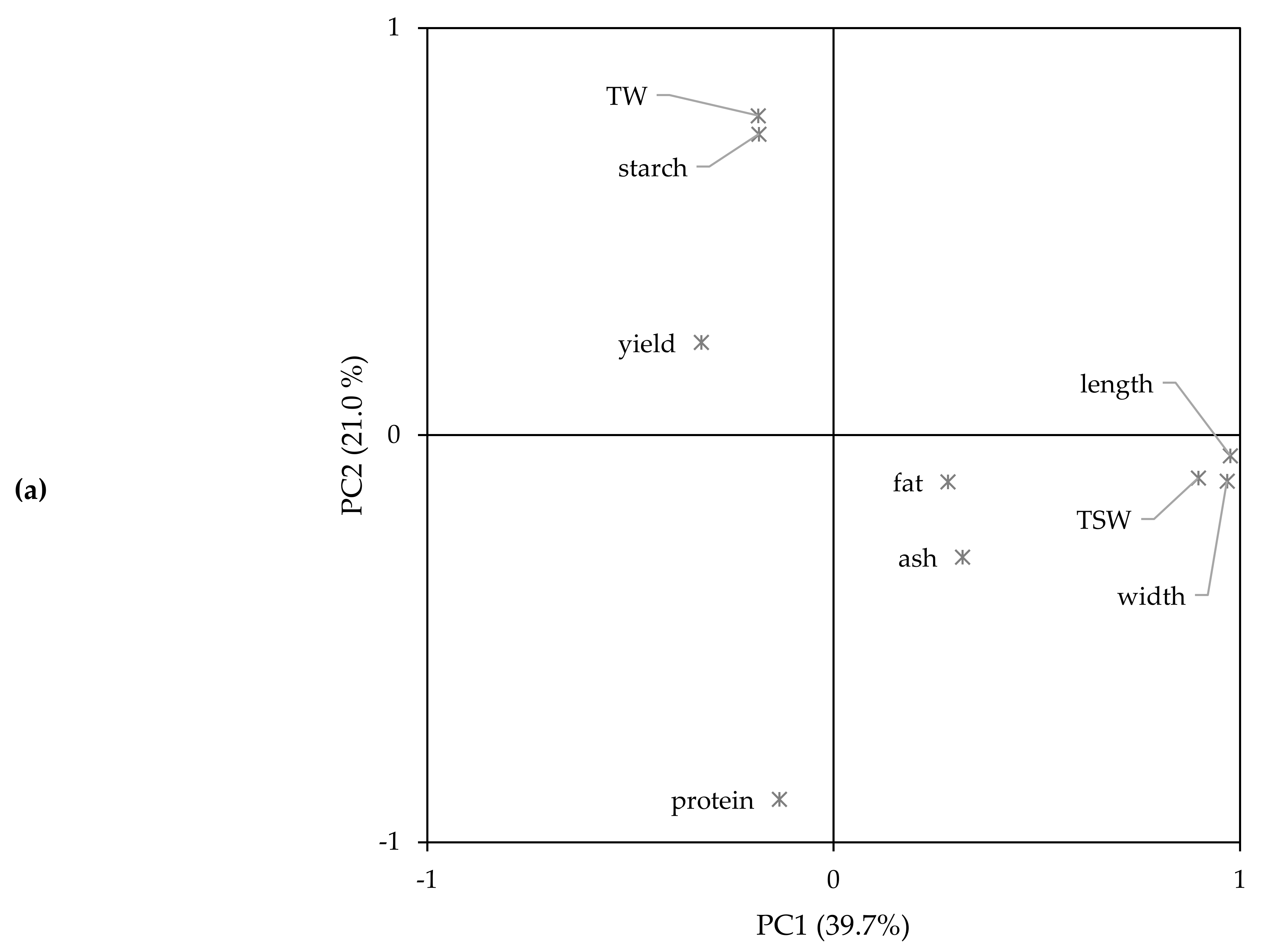
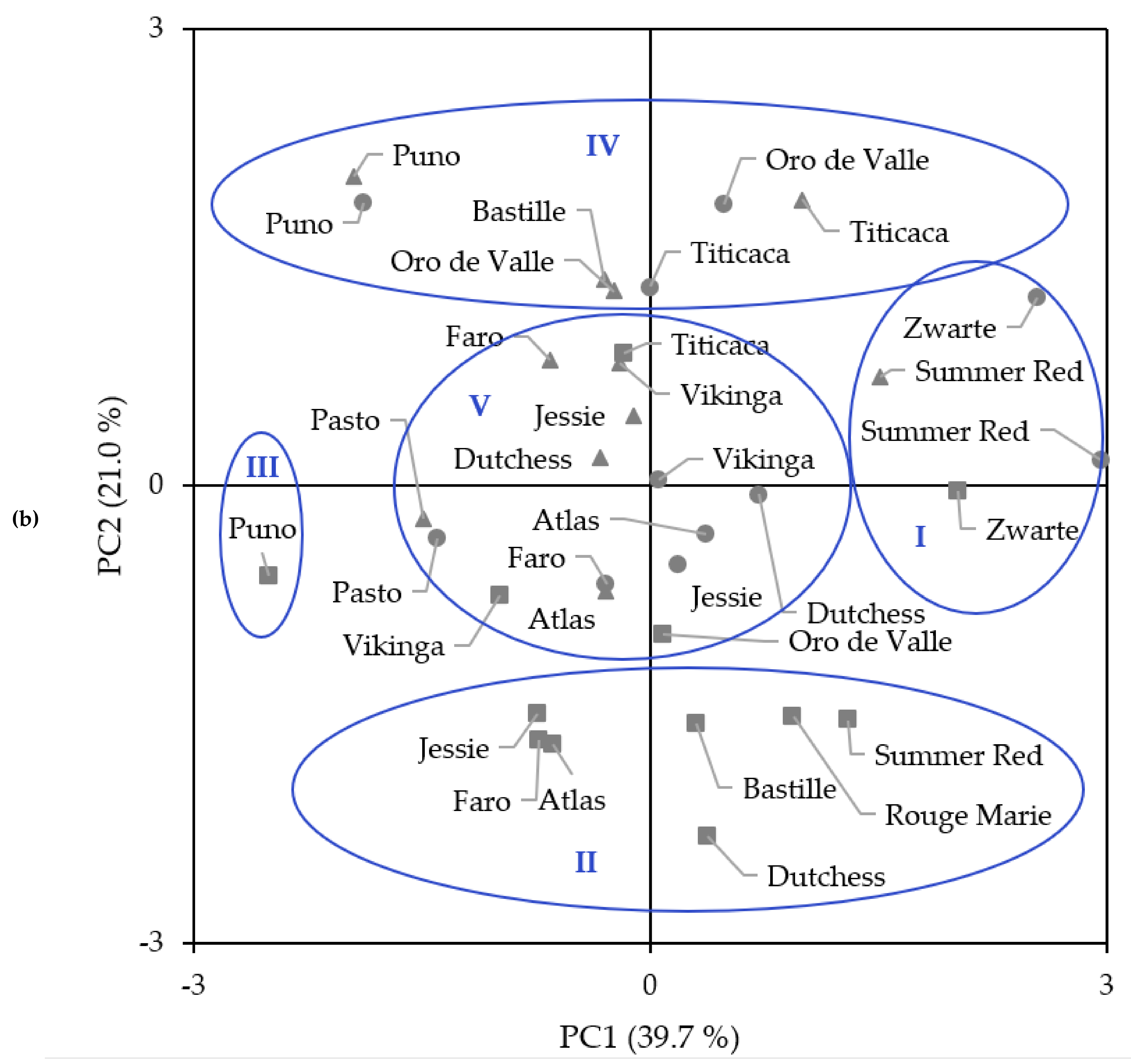
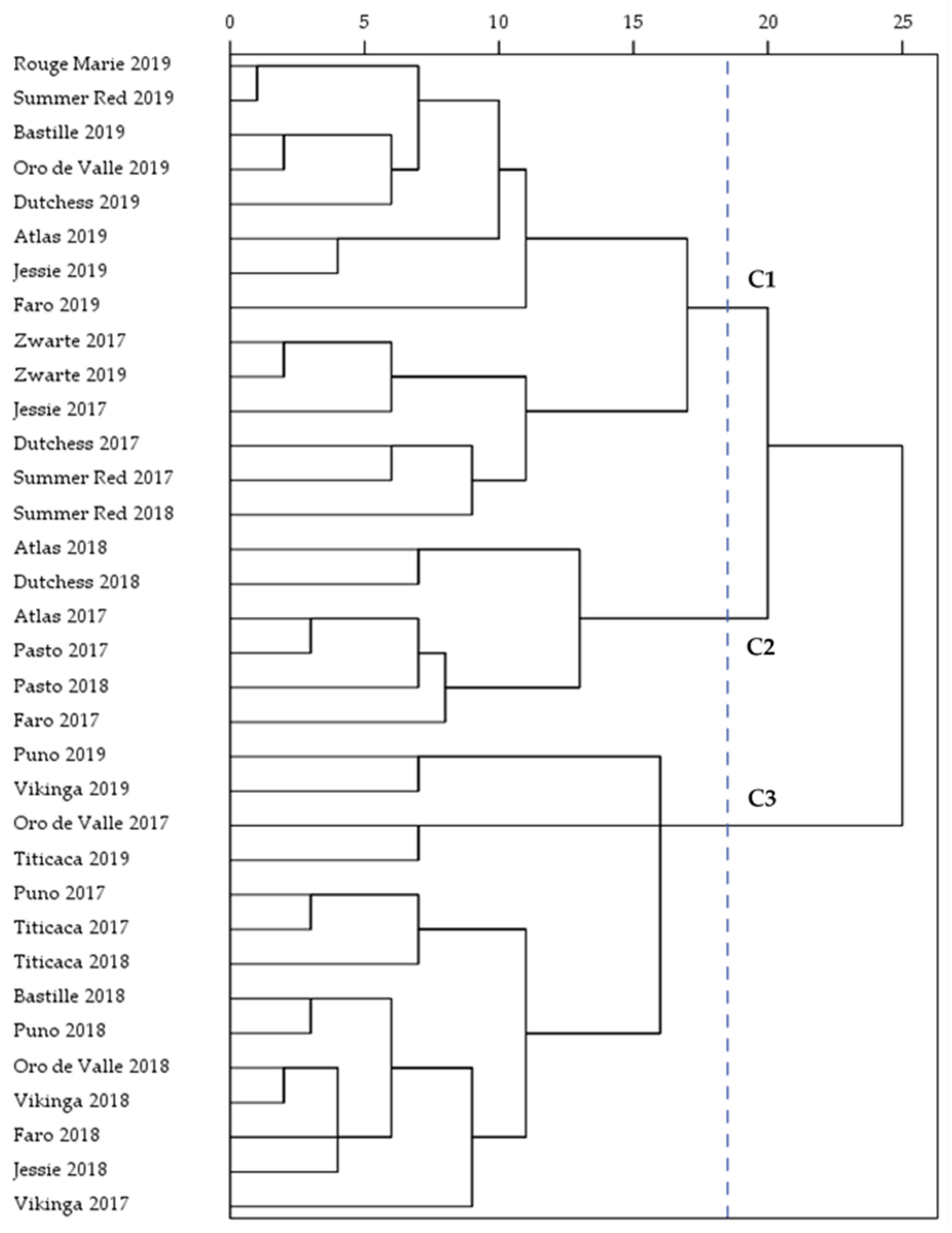
2.6. Principal Components Analysis and Hierarchical Cluster Analysis
3. Materials and Methods
3.1. Plant Varieties
3.2. Field Trials
3.3. Seed Characteristics
3.4. Chemical Composition
3.4.1. Macronutrients
3.4.2. Amino Acids
3.4.3. Fatty Acids
3.4.4. Triacylglycerols
3.4.5. Minerals
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galwey, N.W. The potential of quinoa as a multi-purpose crop for agricultural diversification: A review. Ind. Crops Prod. 1992, 1, 101–106. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa willd.) cultivars grown under field conditions in southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Pineli, L.L.O.; Botelho, R.B.A.; Zandonadi, R.P.; Solorzano, J.L.; de Oliveira, G.T.; Reis, C.E.G.; Teixeira, D.d.S. Low glycemic index and increased protein content in a novel quinoa milk. LWT-Food Sci. Technol. 2015, 63, 1261–1267. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crops Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- CBI The European Market Potential for Quinoa. Available online: https://www.cbi.eu/market-information/grains-pulses-oilseeds/quinoa-grains/market-potential (accessed on 31 August 2021).
- Craine, E.B.; Murphy, K.M. Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Mastebroek, H.D.; Van Loo, E.N.; Dolstra, O. Combining ability for seed yield traits of Chenopodium quinoa breeding lines. Euphytica 2002, 125, 427–432. [Google Scholar] [CrossRef]
- Patiranage, D.S.R.; Asare, E.; Maldonado-Taipe, N.; Rey, E.; Emrani, N.; Tester, M.; Jung, C. Haplotype variations of major flowering time genes in quinoa unveil their role in the adaptation to different environmental conditions. Plant. Cell Environ. 2021, 44, 2565–2579. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, A.; Srivastava, S. Quinoa: Botany, Production and Uses; CABI: Wallingford, UK, 2013; ISBN 1780642261. [Google Scholar]
- Zevallos, V.F.; Ellis, H.J.; Šuligoj, T.; Herencia, L.I.; Ciclitira, P.J. Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease. Am. J. Clin. Nutr. 2012, 96, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; FAO: Rome, Italy; CIRAD: Rome, Italy, 2015. [Google Scholar]
- Reguera, M.; Conesa, C.M.; Gil-Gómez, A.; Haros, C.M.; Pérez-Casas, M.Á.; Briones-Labarca, V.; Bolaños, L.; Bonilla, I.; Álvarez, R.; Pinto, K.; et al. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef]
- Aluwi, N.A.; Murphy, K.M.; Ganjyal, G.M. Physicochemical characterization of different varieties of quinoa. Cereal Chem. 2017, 94, 847–856. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and interrelationship among various morphological and quality traits in quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2007, 101, 104–116. [Google Scholar] [CrossRef]
- Murphy, K.M.; Bazile, D.; Kellogg, J.; Rahmanian, M. Development of a Worldwide Consortium on Evolutionary Participatory Breeding in Quinoa. Front. Plant Sci. 2016, 7, 608–614. [Google Scholar] [CrossRef]
- Granado-Rodríguez, S.; Aparicio, N.; Matías, J.; Pérez-Romero, L.F.; Maestro, I.; Gracés, I.; Pedroche, J.J.; Haros, C.M.; Fernandez-Garcia, N.; Navarro del Hierro, J.; et al. Studying the Impact of Different Field Environmental Conditions on Seed Quality of Quinoa: The Case of Three Different Years Changing Seed Nutritional Traits in Southern Europe. Front. Plant Sci. 2021, 12, 649132. [Google Scholar] [CrossRef] [PubMed]
- Crop Production—Protein2Food. Available online: https://www.protein2food.eu/research-activities/crop-production/ (accessed on 29 September 2021).
- Bertero, H.D.; De La Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crops Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; D’Andria, R.; Iafelice, G.; Marconi, E. Field Trial Evaluation of Two Chenopodium quinoa Genotypes Grown Under Rain-Fed Conditions in a Typical Mediterranean Environment in South Italy. J. Agron. Crop Sci. 2010, 196, 407–411. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martinez, E.; López, J.; Rodríguez, M.J.; Henríquez, K.; Fuentes, F. Genetic diversity and comparison of physicochemical and nutritional characteristics of six quinoa (Chenopodium quinoa willd.) genotypes cultivated in Chile. Food Sci. Technol. 2012, 32, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Pando, L. Quinoa breeding. Quinoa Improv. Sustain. Prod. 2015, 42, 87–108. [Google Scholar]
- Wu, G.; Morris, C.F.; Murphy, K.M. Evaluation of texture differences among varieties of cooked Quinoa. J. Food Sci. 2014, 79, S2337–S2345. [Google Scholar] [CrossRef]
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Wieme, R.A.; Carpenter-Boggs, L.A.; Crowder, D.W.; Murphy, K.M.; Reganold, J.P. Agronomic and economic performance of organic forage, quinoa, and grain crop rotations in the Palouse region of the Pacific Northwest, USA. Agric. Syst. 2020, 177, 102709. [Google Scholar] [CrossRef]
- Peterson, A.; Jacobsen, S.E.; Bonifacio, A.; Murphy, K. A crossing method for Quinoa. Sustainability 2015, 7, 3230–3243. [Google Scholar] [CrossRef] [Green Version]
- Sobota, A.; Świeca, M.; Gęsiński, K.; Wirkijowska, A.; Bochnak, J. Yellow-coated quinoa (Chenopodium quinoa Willd)—Physicochemical, nutritional, and antioxidant properties. J. Sci. Food Agric. 2020, 100, 2035–2042. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Medina-Meza, I.G.; Aluwi, N.A.; Saunders, S.R.; Ganjyal, G.M. GC–MS Profiling of Triterpenoid Saponins from 28 Quinoa Varieties (Chenopodium quinoa Willd.) Grown in Washington State. J. Agric. Food Chem. 2016, 64, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Gómez, M.J.; Matías Prieto, J.; Cruz Sobrado, V.; Calvo Magro, P. Nutritional characterization of six quinoa (Chenopodium quinoa Willd) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 103876. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Konishi, Y.; Bruno, M.; Valoy, M.; Prado, F.E. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J. Sci. Food Agric. 2012, 92, 1222–1229. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; FAO: Rome, Italy, 2013; Volume 92, ISBN 978-92-5-107417-6. [Google Scholar]
- Vidueiros, S.M.; Curti, R.N.; Dyner, L.M.; Binaghi, M.J.; Peterson, G.; Bertero, H.D.; Pallaro, A.N. Diversity and interrelationships in nutritional traits in cultivated quinoa (Chenopodium quinoa Willd.) from Northwest Argentina. J. Cereal Sci. 2015, 62, 87–93. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martínez, E.A.; López, J.; Marín, R.; Aranda, M.; Fuentes, F. Influence of contrasting environments on seed composition of two quinoa genotypes: Nutritional and functional properties. Chil. J. Agric. Res. 2013, 73, 108–116. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Fanali, C.; Beccaria, M.; Salivo, S.; Tranchida, P.; Tripodo, G.; Farnetti, S.; Dugo, L.; Dugo, P.; Mondello, L. Non-polar lipids characterization of Quinoa (Chenopodium quinoa) seed by comprehensive two-dimensional gas chromatography with flame ionization/mass spectrometry detection and non-aqueous reversed-phase liquid chromatography with atmospheric pressure chemical ionization mass spectrometry detection. J. Sep. Sci. 2015, 38, 3151–3160. [Google Scholar] [CrossRef] [PubMed]
- Jahaniaval, F.; Kakuda, Y.; Marcone, M.F. Fatty acid and triacylglycerol compositions of seed oils of five Amaranthus accessions and their comparison to other oils. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 847–852. [Google Scholar] [CrossRef]
- Dziki, D.; Laskowski, J. Influence of kernel size on grinding process of wheat at respective grinding stages. Pol. J. Food Nutr. Sci. 2004, 13, 29–33. [Google Scholar]
- De Bock, P.; Van Bockstaele, F.; Raes, K.; Vermeir, P.; Van der Meeren, P.; Eeckhout, M. Impact of tempering process on yield and composition of quinoa flour. LWT-Food Sci. Technol. 2021, 140, 110808. [Google Scholar] [CrossRef]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [CrossRef]
- FAO; WHO. Energy and Protein Requirements; FAO: Rome, Italy; WHO: Geneva, Switzerland, 1973. [Google Scholar]
- Foubert, I.; Dewettinck, K.; Huyghebaert, A. Modelling Isothermal Cocoa Butter Crystallization: Influence of Temperature and Chemical Composition. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2003. [Google Scholar]
- Rombaut, R.; De Clercq, N.; Foubert, I.; Dewettinck, K. Triacylglycerol analysis of fats and oils by evaporative light scattering detection. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 19–25. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]



| Variety 1/Year 2 | n | Amino Acid (mg/g Protein) 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Leu | Met | Ile | Val | Thr | His | Lys | Trp | ||
| Atlas | 3 | 45 ± 3 a | 76 ± 9 a | 20 ± 3 a | 45 ± 8 a | 58 ± 15 a | 36 ± 3 a | 30 ± 3 a | 54 ± 7 a | 17 ± 2 a |
| Bastille | 2 | 42 ± 1 | 71 ± 12 | 22 ± 2 | 42 ± 5 | 53 ± 9 | 36 ± 1 | 25 ± 7 | 51 ± 2 | 19 ± 5 |
| Dutchess | 3 | 42 ± 4 a | 74 ± 6 a | 20 ± 3 a | 42 ± 5 a | 56 ± 13 a | 38 ± 6 a | 30 ± 3 a | 52 ± 4 a | 17 ± 2 a |
| Faro | 3 | 42 ± 6 a | 75 ± 4 a | 23 ± 3 a | 44 ± 2 a | 60 ± 7 a | 39 ± 4 a | 29 ± 5 a | 55 ± 4 a | 17 ± 2 a |
| Jessie | 3 | 43 ± 6 a | 76 ± 5 a | 24 ± 2 a | 44 ± 2 a | 61 ± 7 a | 41 ± 3 a | 30 ± 6 a | 56 ± 1 a | 17 ± 2 a |
| Oro de Valle | 3 | 43 ± 6 a | 74 ± 5 a | 24 ± 2 a | 43 ± 3 a | 60 ± 6 a | 41 ± 3 a | 30 ± 6 a | 57 ± 2 a | 15 ± 4 a |
| Pasto 4 | 2 | 42 ± 4 | 70 ± 12 | 24 ± 1 | 41 ± 3 | 58 ± 17 | 43 ± 11 | 29 ± 10 | 59 ± 7 | 16 |
| Puno | 3 | 44 ± 6 a | 74 ± 5 a | 26 ± 5 a | 42 ± 4 a | 59 ± 7 a | 41 ± 3 a | 30 ± 6 a | 58 ± 3 a | 15 ± 4 a |
| Rouge Marie | 1 | 41 | 82 | 21 | 48 | 64 | 36 | 30 | 46 | 16 |
| Summer Red | 3 | 44 ± 6 a | 74 ± 5 a | 25 ± 6 a | 42 ± 3 a | 59 ± 7 a | 40 ± 4 a | 30 ± 6 a | 57 ± 4 a | 15 ± 4 a |
| Titicaca | 3 | 42 ± 8 a | 71 ± 7 a | 24 ± 7 a | 40 ± 5 a | 56 ± 6 a | 38 ± 2 a | 29 ± 5 a | 54 ± 5 a | 15 ± 3 a |
| Vikinga | 3 | 42 ± 7 a | 73 ± 7 a | 24 ± 6 a | 42 ± 6 a | 59 ± 4 a | 40 ± 4 a | 28 ± 5 a | 56 ± 7 a | 16 ± 4 a |
| Zwarte | 2 | 36 ± 1 | 73 ± 1 | 23 ± 1 | 39 ± 0 | 64 ± 2 | 44 ± 1 | 32 ± 2 | 57 ± 3 | 15 ± 2 |
| 2017 | 9 | 38 ± 3 A | 74 ± 6 A,B | 23 ± 1 A | 41 ± 5 A | 63 ± 5 B | 43 ± 3 B | 34 ± 2 C | 57 ± 4 A,B | 15 ± 2 A |
| 2018 | 9 | 48 ± 2 C | 69 ± 5 A | 28 ± 5 B | 41 ± 3 A | 51 ± 6 A | 38 ± 3 A | 23 ± 2 A | 58 ± 4 B | 21 ± 2 B |
| 2019 | 9 | 43 ± 3 B | 81 ± 7 B | 22 ± 3 A | 47 ± 3 B | 65 ± 4 B | 36 ± 2 A | 32 ± 2 B | 53 ± 3 A | 15 ± 1 A |
| Variety 1/Year 2 | n | Fatty Acid (% FAME) 3,4 | ||||
|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1c | C18:2 n-6 | C18:3 n-3 | ||
| Atlas | 3 | 9.3 ± 0.5 a,b | 0.7 ± 0.1 e | 20.9 ± 0.3 c | 55.3 ± 0.4 a,b | 5.4 ± 0.3 a,b |
| Bastille | 2 | 9.4 ± 0.1 | 0.6 ± 0.0 | 19.9 ± 0.6 | 54.6 ± 0.2 | 7.9 ± 0.3 |
| Dutchess | 3 | 9.4 ± 0.6 a,b | 0.6 ± 0.1 d,e | 20.6 ± 0.7 c | 54.6 ± 1.0 a | 5.6 ± 0.4 a,b |
| Faro | 3 | 8.9 ± 0.1 a | 0.6 ± 0.1 c,d,e | 19.9 ± 0.8 b,c | 57.4 ± 0.6 c | 6.1 ± 0.7 b,c |
| Jessie | 3 | 9.1 ± 0.1 a,b | 0.5 ± 0.0 a,b | 18.7 ± 1.3 b | 57.3 ± 0.5 c | 6.3 ± 0.7 b,c |
| Oro de Valle | 3 | 9.1 ± 0.3 a,b | 0.5 ± 0.0 a,b,c | 20.6 ± 0.3 c | 56.3 ± 0.9 b,c | 6.7 ± 0.2 c,d |
| Pasto | 2 | 9.6 ± 0.2 | 0.8 ± 0.2 | 19.4 ± 0.2 | 54.8 ± 0.7 | 6.8 ± 0.3 |
| Puno | 3 | 9.2 ± 0.3 a,b | 0.5 ± 0.0 a | 16.8 ± 1.2 a | 59.0 ± 0.6 d | 7.2 ± 0.4 d |
| Rouge Marie | 1 | 10.0 | 0.6 | 19.7 | 56.3 | 4.8 |
| Summer Red | 3 | 9.5 ± 0.7 a,b | 0.6 ± 0.1 a,b,c,d,e | 20.2 ± 1.1 b,c | 55.8 ± 0.7 a,b | 4.8 ± 0.2 a |
| Titicaca | 3 | 10.3 ± 1.4 b | 0.6 ± 0.1 b,c,d,e | 19.7 ± 0.8 b,c | 56.7 ± 0.9 b,c | 5.5 ± 0.6 a,b |
| Vikinga | 3 | 10.4 ± 1.2 b | 0.6 ± 0.1 a,b,c,d | 21.2 ± 1.0 c | 54.7 ± 0.9 a | 5.8 ± 0.7 b,c |
| Zwarte | 2 | 9.5 ± 0.2 | 0.7 ± 0.1 | 18.9 ± 0.5 | 56.6 ± 0.6 | 7.1 ± 0.0 |
| 2017 | 9 | 9.2 ± 0.4 A | 0.6 ± 0.1 B | 19.6 ± 1.3 A | 55.9 ± 1.6 A | 6.0 ± 0.7 A,B |
| 2018 | 9 | 9.8 ± 1.2 A | 0.6 ± 0.1 A,B | 20.8 ± 1.2 B | 56.4 ± 1.5 A | 5.5 ± 0.8 A |
| 2019 | 9 | 9.5 ± 0.4 A | 0.5 ± 0.1 A | 19.2 ± 1.6 A | 56.8 ± 1.4 A | 6.3 ± 0.8 B |
| Variety 1/Year 2 | n | Triacylglycerol (Area %) 3,4 | |||||
|---|---|---|---|---|---|---|---|
| LLLn | LLL | LLO | LLP | OOL | OLP | ||
| Atlas | 3 | 6.08 ± 0.66 a,b,c | 20.29 ± 3.46 a | 24.00 ± 3.05 a | 11.30 ± 1.04 a | 8.71 ± 0.71 b | 7.74 ± 1.55 a |
| Bastille | 2 | 7.49 ± 0.54 | 17.96 ± 1.55 | 22.04 ± 1.46 | 11.98 ± 0.26 | 8.13 ± 0.33 | 8.34 ± 0.60 |
| Dutchess | 3 | 5.67 ± 0.88 a,b | 19.70 ± 3.76 a | 25.01 ± 5.65 a | 11.53 ± 1.61 a | 8.73 ± 0.60 b | 8.16 ± 1.89 a |
| Faro | 3 | 7.11 ± 1.05 b,c,d | 20.04 ± 2.21 a | 22.23 ± 1.40 a | 11.57 ± 0.47 a | 8.50 ± 0.59 b | 7.78 ± 0.43 a |
| Jessie | 3 | 7.24 ± 1.08 c,d | 20.23 ± 1.62 a | 21.88 ± 0.59 a | 12.41 ± 0.20 a | 7.68 ± 1.10 a,b | 8.15 ± 0.81 a |
| Oro de Valle | 3 | 8.26 ± 1.06 d,e | 22.05 ± 1.38 a | 22.41 ± 3.12 a | 12.02 ± 1.55 a | 7.51 ± 1.50 a,b | 7.06 ± 0.57 a |
| Pasto | 2 | 7.47 ± 0.60 | 19.79 ± 3.28 | 23.29 ± 3.15 | 11.47 ± 1.39 | 7.19 ± 0.52 | 7.20 ± 1.51 |
| Puno | 3 | 8.92 ± 0.67 e | 23.28 ± 0.94 a | 21.19 ± 1.33 a | 13.18 ± 0.61 a | 6.12 ± 0.67 a | 6.93 ± 0.53 a |
| Rouge Marie | 1 | 5.20 | 18.77 | 22.37 | 13.29 | 8.61 | 9.35 |
| Summer Red | 3 | 5.30 ± 0.26 a | 20.98 ± 4.08 a | 24.83 ± 3.31 a | 12.36 ± 1.15 a | 8.23 ± 1.16 b | 8.27 ± 1.94 a |
| Titicaca | 3 | 6.39 ± 0.58 a,b,c | 20.62 ± 0.92 a | 22.76 ± 0.62 a | 13.16 ± 0.29 a | 8.38 ± 0.45 b | 8.59 ± 0.47 a |
| Vikinga | 3 | 6.26 ± 0.90 a,b,c | 19.04 ± 1.47 a | 23.33 ± 1.68 a | 12.08 ± 1.08 a | 8.81 ± 1.26 b | 8.41 ± 1.48 a |
| Zwarte | 2 | 8.29 ± 0.28 | 20.36 ± 1.05 | 21.93 ± 1.67 | 12.02 ± 1.13 | 7.39 ± 0.07 | 7.63 ± 0.63 |
| 2017 | 9 | 6.98 ± 1.21 A,B | 22.36 ± 2.92 B | 25.38 ± 3.76 B | 11.24 ± 1.28 A | 7.65 ± 0.72 A | 6.85 ± 1.04 A |
| 2018 | 9 | 6.01 ± 1.08 A | 19.40 ± 1.73 A | 22.52 ± 0.52 A | 12.42 ± 0.44 B | 8.92 ± 0.91 B | 8.87 ± 0.82 C |
| 2019 | 9 | 7.41 ± 1.47 B | 20.32 ± 2.17 A | 21.32 ± 1.28 A | 12.87 ± 0.84 B | 7.65 ± 1.43 A | 7.97 ± 1.01 B |
| Variety 1/Year 2 | n | Mineral (mg/kg dm) 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Fe | K | Mg | Na | P | Zn | ||
| Atlas | 3 | 613 ± 54 a,b | 77 ± 3 a | 11055 ± 1232 a,b | 2299 ± 186 a | 97 ± 28 a | 5716 ± 370 a | 60 ± 4 b |
| Bastille | 2 | 636 ± 56 | 392 ± 468 | 11397 ± 460 | 2166 ± 138 | 74 ± 30 | 4492 ± 410 | 76 ± 47 |
| Dutchess | 3 | 686 ± 84 b | 110 ± 67 a | 12055 ± 1233 a,b | 2308 ± 123 a | 160 ± 129 a | 5344 ± 584 a | 45 ± 3 a |
| Faro | 3 | 686 ± 50 b | 203 ± 236 a | 10634 ± 846 a,b | 2486 ± 191 a | 73 ± 26 a | 5324 ± 560 a | 48 ± 5 a |
| Jessie | 3 | 507 ± 25 a,b | 73 ± 8 a | 10222 ± 415 a,b | 2314 ± 149 a | 73 ± 21 a | 5533 ± 653 a | 47 ± 3 a |
| Oro de Valle | 3 | 633 ± 59 a,b | 85 ± 36 a | 11029 ± 537 a,b | 2090 ± 241 a | 74 ± 18 a | 4597 ± 705 a | 48 ± 4 a |
| Pasto | 2 | 777 ± 41 | 161 ± 99 | 12539 ± 396 | 2706 ± 50 | 122 ± 61 | 5281 ± 130 | 67 ± 0 |
| Puno | 3 | 576 ± 70 a,b | 66 ± 5 a | 9844 ± 1303 a | 2164 ± 177 a | 124 ± 90 a | 4322 ± 633 a | 44 ± 1 a |
| Rouge Marie | 1 | 515 | 64 | 12025 | 2000 | 57 | 4766 | 44 |
| Summer Red | 3 | 474 ± 66 a | 58 ± 4 a | 12550 ± 409 b | 2157 ± 100 a | 88 ± 39 a | 5103 ± 378 a | 44 ± 3 a |
| Titicaca | 3 | 450 ± 45 a | 176 ± 142 a | 9869 ± 954 a | 2152 ± 152 a | 80 ± 24 a | 4671 ± 542 a | 45 ± 6 a |
| Vikinga | 3 | 604 ± 97 a,b | 73 ± 12 a | 10168 ± 399 a,b | 2203 ± 106 a | 71 ± 17 a | 4630 ± 370 a | 47 ± 4 a |
| Zwarte | 2 | 658 ± 107 | 55 ± 7 | 13717 ± 476 | 2049 ± 35 | 143 ± 138 | 4515 ± 1 | 41 ± 2 |
| 2017 | 9 | 564 ± 81 A | 101 ± 90 A | 11076 ± 1596 A | 2197 ± 187 A | 137 ± 75 B | 4959 ± 608 A,B | 48 ± 5 A |
| 2018 | 9 | 545 ± 98 A | 138 ± 133 A | 10259 ± 922 A | 2396 ± 139 B | 91 ± 22 A,B | 5556 ± 444 B | 46 ± 5 A |
| 2019 | 9 | 634 ± 105 A | 69 ± 7 A | 11140 ± 764 A | 2132 ± 92 A | 53 ± 7 A | 4564 ± 503 A | 49 ± 7 A |
| Variety | Seed Source 1 | Origin | Year Data |
|---|---|---|---|
| Atlas | Gilbel | Wageningen University & Research, Wageningen, NL | 3 |
| Dutchess | Gilbel | Wageningen University & Research, Wageningen, NL | 3 |
| Pasto | Gilbel | Wageningen University & Research, Wageningen, NL | 2 |
| Bastille | Gilbel | AbbottAgra, Longué-Jumelles, FR | 2 |
| Jessie | Gilbel | AbbottAgra, Longué-Jumelles, FR | 3 |
| Rouge Marie | Gilbel | AbbottAgra, Longué-Jumelles, FR | 1 |
| Summer Red | Gilbel | AbbottAgra, Longué-Jumelles, FR | 3 |
| Puno | Quinoa Quality | University of Copenhagen, Copenhagen, DK [16] | 3 |
| Titicaca | Quinoa Quality | University of Copenhagen, Copenhagen, DK [16] | 3 |
| Vikinga | Quinoa Quality | University of Copenhagen, Copenhagen, DK [45] | 3 |
| Faro | De Nieuwe Tuin | Redwood Seeds, Manton, CA, USA | 3 |
| Oro de Valle | De Nieuwe Tuin | Wild Garden Seeds, Philomath, OR, USA [16] | 3 |
| Zwarte 2 | De Nieuwe Tuin | White Mountain Farm, Mosca, CO, USA [16] | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bock, P.; Van Bockstaele, F.; Muylle, H.; Quataert, P.; Vermeir, P.; Eeckhout, M.; Cnops, G. Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe—Part I. Plants 2021, 10, 2689. https://doi.org/10.3390/plants10122689
De Bock P, Van Bockstaele F, Muylle H, Quataert P, Vermeir P, Eeckhout M, Cnops G. Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe—Part I. Plants. 2021; 10(12):2689. https://doi.org/10.3390/plants10122689
Chicago/Turabian StyleDe Bock, Phara, Filip Van Bockstaele, Hilde Muylle, Paul Quataert, Pieter Vermeir, Mia Eeckhout, and Gerda Cnops. 2021. "Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe—Part I" Plants 10, no. 12: 2689. https://doi.org/10.3390/plants10122689
APA StyleDe Bock, P., Van Bockstaele, F., Muylle, H., Quataert, P., Vermeir, P., Eeckhout, M., & Cnops, G. (2021). Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe—Part I. Plants, 10(12), 2689. https://doi.org/10.3390/plants10122689







