Transcriptome Analysis Reveals Dynamic Cultivar-Dependent Patterns of Gene Expression in Potato Spindle Tuber Viroid-Infected Pepper
Abstract
:1. Introduction
2. Results
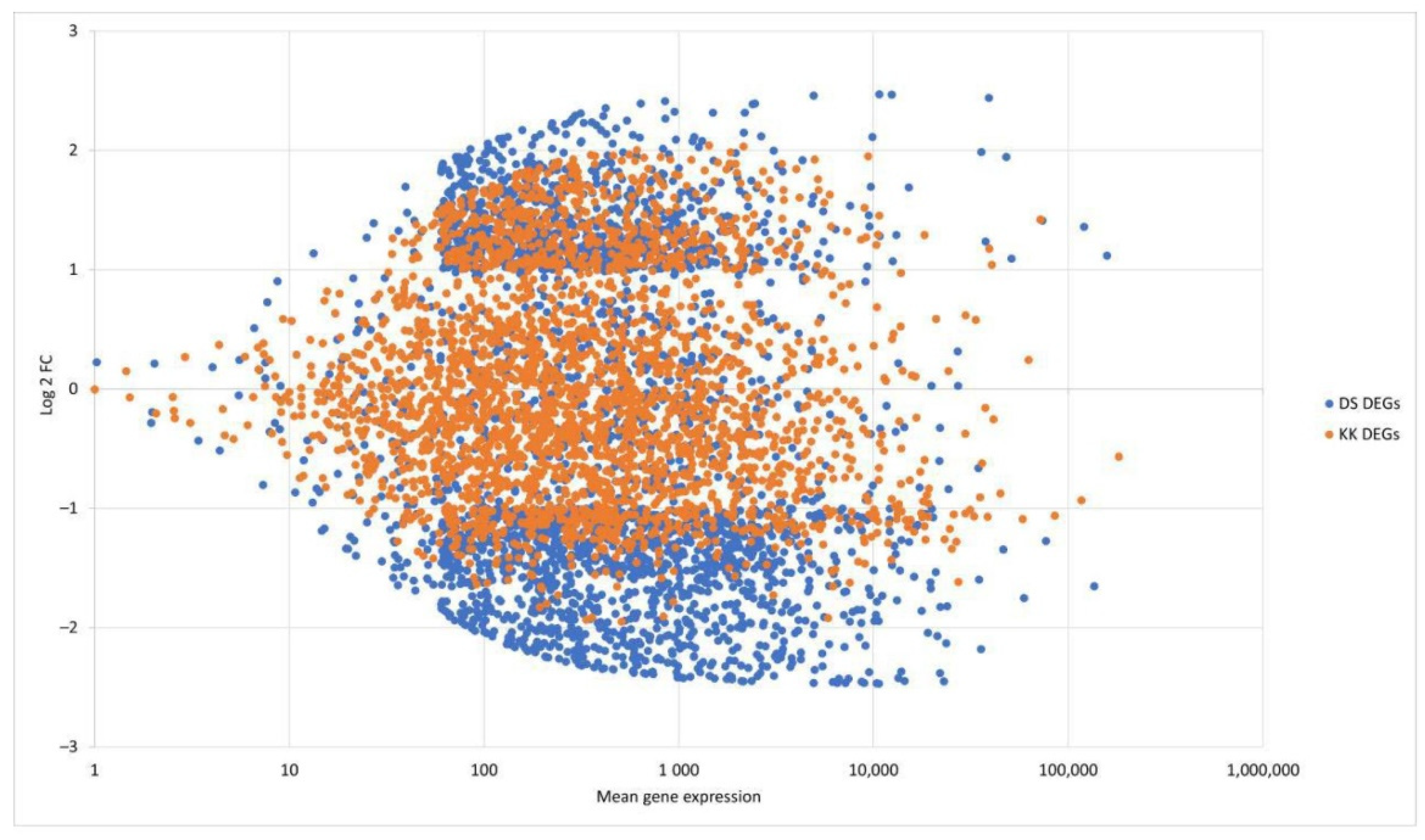
2.1. Analysis of RNA-Seq Data
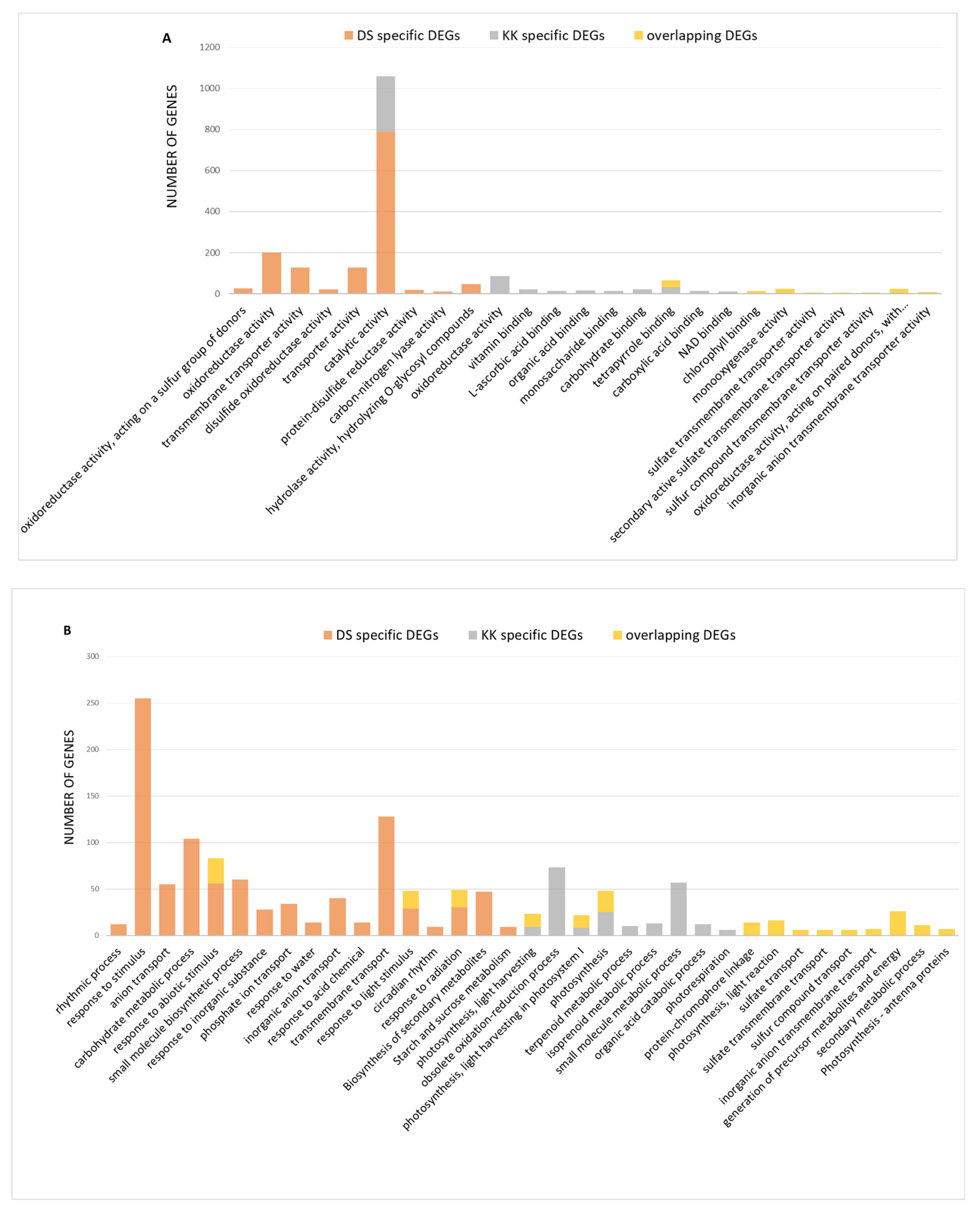
2.2. Functional Annotation of DEGs
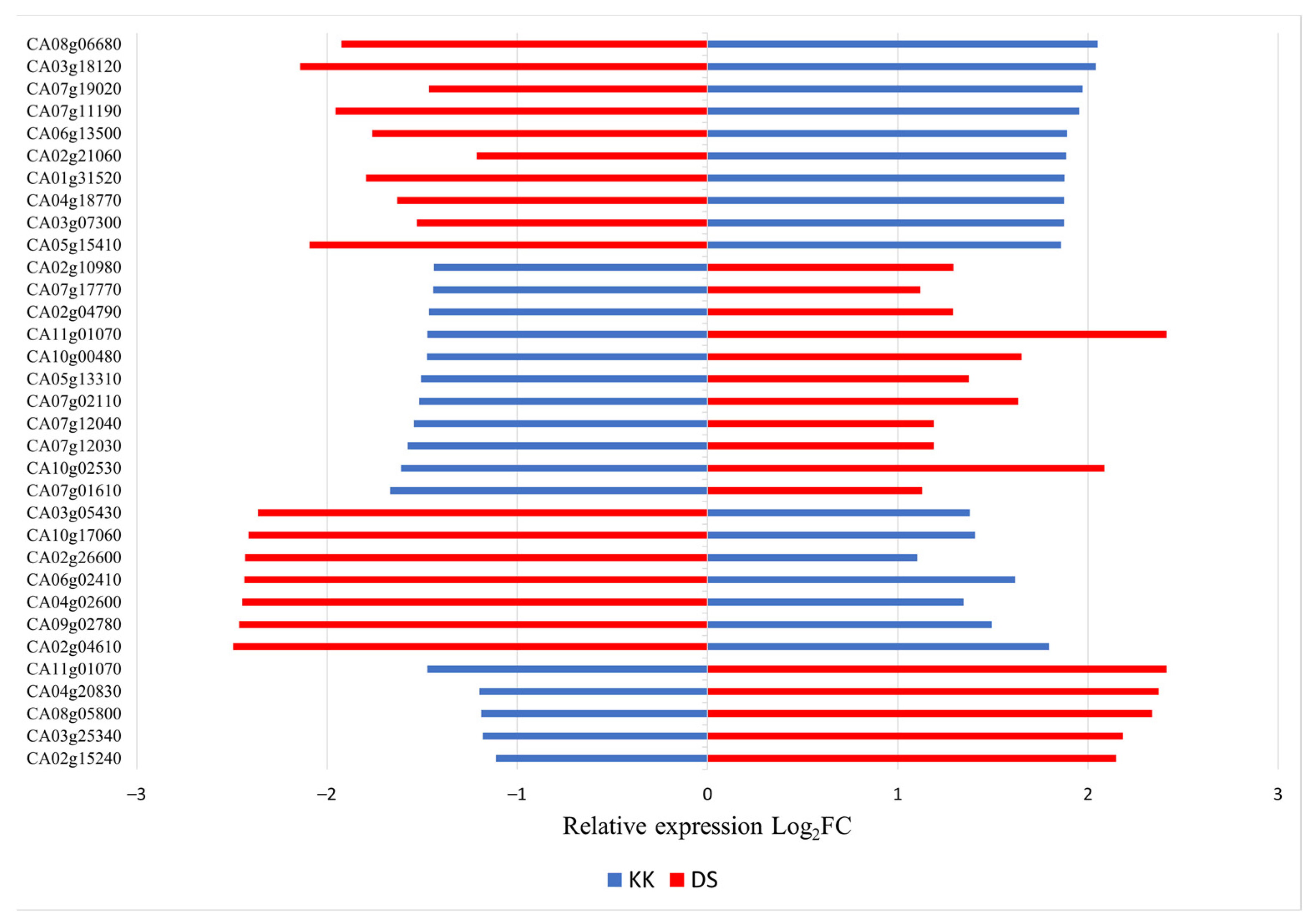
2.3. Comparative Expression Analysis of Overlapping DEGs in Two Pepper Cultivars Infected with PSTVd
3. Discussion
4. Materials and Methods
4.1. Plant Material and PSTVd Inoculation
4.2. RNA Extraction, cDNA Synthesis, and Illumina Sequencing
4.3. Identification and Functional Analysis of DEGs
4.4. Semi-Quantitative RT-PCR and RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diener, T.O. Viroids: The Smallest Known Agents of Infectious Disease. Annu. Rev. Microbiol. 1974, 28, 23–39. [Google Scholar] [CrossRef]
- Flores, R.; Hernández, C.; Martínez De Alba, A.E.; Daròs, J.A.; di Serio, F. Viroids and Viroid-Host Interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef]
- Tsagris, E.M.; de Alba, Á.E.M.; Gozmanova, M.; Kalantidis, K. Viroids. Cell. Microbiol. 2008, 10, 2168–2179. [Google Scholar] [CrossRef]
- Keese, P.; Symons, R.H. Domains in Viroids: Evidence of Intermolecular RNA Rearrangements and Their Contribution to Viroid Evolution. Proc. Natl. Acad. Sci. USA 1985, 82, 4582–4586. [Google Scholar] [CrossRef] [Green Version]
- Steger, G.; Perreault, J.P. Structure and Associated Biological Functions of Viroids. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2016; Volume 94, pp. 141–172. [Google Scholar] [CrossRef]
- Flores, R.; Serra, P.; Minoia, S.; di Serio, F.; Navarro, B. Viroids: From Genotype to Phenotype Just Relying on RNA Sequence and Structural Motifs. Front. Microbiol. 2012, 3, 217. [Google Scholar] [CrossRef] [Green Version]
- di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.F.; Pallás, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current Status of Viroid Taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef]
- Matsushita, Y.; Tsuda, S. Host Ranges of Potato Spindle Tuber Viroid, Tomato Chlorotic Dwarf Viroid, Tomato Apical Stunt Viroid, and Columnea Latent Viroid in Horticultural Plants. Eur. J. Plant Pathol. 2015, 141, 193–197. [Google Scholar] [CrossRef]
- Kovalskaya, N.; Hammond, R.W. Molecular Biology of Viroid-Host Interactions and Disease Control Strategies. Plant Sci. 2014, 228, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Mackie, A.E.; Barbetti, M.J.; Rodoni, B.; McKirdy, S.J.; Jones, R.A.C. Effects of a Potato Spindle Tuber Viroid Tomato Strain on the Symptoms, Biomass, and Yields of Classical Indicator and Currently Grown Potato and Tomato Cultivars. Plant Dis. 2019, 103, 3009–3017. [Google Scholar] [CrossRef]
- Navarro, B.; Gisel, A.; Rodio, M.E.; Delgado, S.; Flores, R.; di Serio, F. Viroids: How to Infect a Host and Cause Disease without Encoding Proteins. Biochimie 2012, 94, 1474–1480. [Google Scholar] [CrossRef]
- di Serio, F.; de Stradis, A.; Delgado, S.; Flores, R.; Navarro, B. Cytopathic Effects Incited by Viroid RNAs and Putative Underlying Mechanisms. Front. Plant Sci. 2013, 3, 288. [Google Scholar] [CrossRef] [Green Version]
- Mudiyanselage, S.D.D.; Qu, J.; Tian, N.; Jiang, J.; Wang, Y. Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation. Viruses 2018, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.; Minoia, S.; Carbonell, A.; Gisel, A.; Delgado, S.; López-Carrasco, A.; Navarro, B.; di Serio, F. Viroids, the Simplest RNA Replicons: How They Manipulate Their Hosts for Being Propagated and How Their Hosts React for Containing the Infection. Virus Res. 2015, 209, 136–145. [Google Scholar] [CrossRef]
- Navarro, B.; Flores, R.; di Serio, F. Advances in Viroid-Host Interactions. Annu. Rev. Virol. 2021, 8, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Alba, A.E.; Sägesser, R.; Tabler, M.; Tsagris, M. A Bromodomain-Containing Protein from Tomato Specifically Binds Potato Spindle Tuber Viroid RNA In Vitro and In Vivo. J. Virol. 2003, 77, 9685–9694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, Y.; Ding, B.; Fei, Z. Comprehensive Transcriptome Analyses Reveal That Potato Spindle Tuber Viroid Triggers Genome-Wide Changes in Alternative Splicing, Inducible Trans-Acting Activity of Phased Secondary Small Interfering RNAs, and Immune Responses. J. Virol. 2017, 91, 247–264. [Google Scholar] [CrossRef] [Green Version]
- Prol, F.V.; Márquez-Molins, J.; Rodrigo, I.; López-Gresa, M.P.; Bellés, J.M.; Gómez, G.; Pallás, V.; Lisón, P. Symptom Severity, Infection Progression and Plant Responses in Solanum Plants Caused by Three Pospiviroids Vary with the Inoculation Procedure. Int. J. Mol. Sci. 2021, 22, 6189. [Google Scholar] [CrossRef]
- Hiddinga, H.J.; Crum, C.J.; Hu, J.; Roth, D.A. Viroid-Induced Phosphorylation of a Host Protein Related to a DsRNA-Dependent Protein Kinase. Science 1988, 241, 451–453. [Google Scholar] [CrossRef]
- Hammond, R.W.; Zhao, Y. Characterization of a Tomato Protein Kinase Gene Induced by Infection by Potato Spindle Tuber Viroid. Mol. Plant-Microbe Interact. 2000, 13, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Diener, T.O.; Hammond, R.W.; Black, T.; Katze, M.G. Mechanism of Viroid Pathogenesis: Differential Activation of the Interferon-Induced, Double-Stranded RNA-Activated, Mr 68 000 Protein Kinase by Viroid Strains of Varying Pathogenicity. Biochimie 1993, 75, 533–538. [Google Scholar] [CrossRef]
- Nath, V.S.; Shrestha, A.; Awasthi, P.; Mishra, A.K.; Kocábek, T.; Matoušek, J.; Sečnik, A.; Jakše, J.; Radišek, S.; Hallan, V. Mapping the Gene Expression Spectrum of Mediator Subunits in Response to Viroid Infection in Plants. Int. J. Mol. Sci. 2020, 21, 2498. [Google Scholar] [CrossRef] [Green Version]
- Adkar-Purushothama, C.R.; Iyer, P.S.; Perreault, J.P. Potato Spindle Tuber Viroid Infection Triggers Degradation of Chloride Channel Protein CLC-b-like and Ribosomal Protein S3a-like MRNAs in Tomato Plants. Sci. Rep. 2017, 7, 8341. [Google Scholar] [CrossRef] [Green Version]
- Adkar-Purushothama, C.R.; Brosseau, C.; Gigu È Re, T.; Sano, T.; Moffett, P.; Perreaulta, J.P. Small RNA Derived from the Virulence Modulating Region of the Potato Spindle Tuber Viroid Silences Callose Synthase Genes of Tomato Plants. Plant Cell 2015, 27, 2178–2194. [Google Scholar] [CrossRef] [Green Version]
- Diermann, N.; Matoušek, J.; Junge, M.; Riesner, D.; Steger, G. Characterization of Plant MiRNAs and Small RNAs Derived from Potato Spindle Tuber Viroid (PSTVd) in Infected Tomato. Biol. Chem. 2010, 391, 1379–1390. [Google Scholar] [CrossRef]
- Avina-Padilla, K.; Martinez de la Vega, O.; Rivera-Bustamante, R.; Martinez-Soriano, J.P.; Owens, R.A.; Hammond, R.W.; Vielle-Calzada, J.P. In Silico Prediction and Validation of Potential Gene Targets for Pospiviroid-Derived Small RNAs during Tomato Infection. Gene 2015, 564, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Katsarou, K.; Wu, Y.; Zhang, R.; Bonar, N.; Morris, J.; Hedley, P.E.; Bryan, G.J.; Kalantidis, K.; Hornyik, C. Insight on Genes Affecting Tuber Development in Potato upon Potato Spindle Tuber Viroid (PSTVd) Infection. PLoS ONE 2016, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Góra-Sochacka, A.; Wiesyk, A.; Fogtmann, A.; Lirski, M.; Zagórski-Ostoja, W. Root Transcriptomic Analysis Reveals Global Changes Induced by Systemic Infection of Solanum Lycopersicum with Mild and Severe Variants of Potato Spindle Tuber Viroid. Viruses 2019, 11, 992. [Google Scholar] [CrossRef] [Green Version]
- Więsyk, A.; Iwanicka-Nowicka, R.; Fogtman, A.; Zagórski-Ostoja, W.; Góra-Sochacka, A. Time-Course Microarray Analysis Reveals Differences between Transcriptional Changes in Tomato Leaves Triggered by Mild and Severe Variants of Potato Spindle Tuber Viroid. Viruses 2018, 10, 257. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, J.T.J.; Koenraadt, H.M.S.; Jodlowska, A.; Hüner, L.; Roenhorst, J.W. Pospiviroid Infections in Capsicum Annuum: Disease Symptoms and Lack of Seed Transmission. Eur. J. Plant Pathol. 2020, 156, 21–29. [Google Scholar] [CrossRef]
- Apostolova, E.; Hadjieva, N.; Ivanova, D.P.; Yahubyan, G.; Baev, V.; Gozmanova, M. MicroRNA Expression Dynamics Reshape the Cultivar-Specific Response of Pepper (Capsicum Annuum L.) to Potato Spindle Tuber Viroid (PSTVd) Infection. Sci. Hortic. 2021, 278, 109845. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanović, J.; Oklestkova, J.; Novák, O.; Mihaljević, S. Effects of Potato Spindle Tuber Viroid Infection on Phytohormone and Antioxidant Responses in Symptomless Solanum Laxum Plants. J. Plant Growth Regul. 2019, 38, 325–332. [Google Scholar] [CrossRef]
- Itaya, A.; Matsuda, Y.; Gonzales, R.A.; Nelson, R.S.; Ding, B. Potato Spindle Tuber Viroid Strains of Different Pathogenicity Induces and Suppresses Expression of Common and Unique Genes in Infected Tomato. Mol. Plant-Microbe Interact. 2002, 15, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global Analysis of Tomato Gene Expression during Potato Spindle Tuber Viroid Infection Reveals a Complex Array of Changes Affecting Hormone Signaling. Mol. Plant-Microbe Interact. 2012, 25, 582–598. [Google Scholar] [CrossRef] [Green Version]
- Bagherian, S.A.A.; Hamzehzarghani, H.; Izadpanah, K.; Djavaheri, M. Effects of Potato Spindle Tuber Viroid Infection on Tomato Metabolic Profile. J. Plant Physiol. 2016, 201, 42–53. [Google Scholar] [CrossRef]
- Milanović, J.; Oklestkova, J.; Majdandžić, A.; Novák, O.; Mihaljević, S. Organ-Specific Differences in Endogenous Phytohormone and Antioxidative Responses in Potato upon PSTVd Infection. J. Plant Physiol. 2019, 232, 107–114. [Google Scholar] [CrossRef]
- Ji, M.; Wang, K.; Wang, L.; Chen, S.; Li, H.; Ma, C.; Wang, Y. Overexpression of a S-Adenosylmethionine Decarboxylase from Sugar Beet M14 Increased Araidopsis Salt Tolerance. Int. J. Mol. Sci. 2019, 20, 1990. [Google Scholar] [CrossRef] [Green Version]
- Mo, H.J.; Sun, Y.X.; Zhu, X.L.; Wang, X.F.; Zhang, Y.; Yang, J.; Yan, G.J.; Ma, Z.Y. Cotton S-Adenosylmethionine Decarboxylase-Mediated Spermine Biosynthesis Is Required for Salicylic Acid- and Leucine-Correlated Signaling in the Defense Response to Verticillium Dahliae. Planta 2016, 243, 1023–1039. [Google Scholar] [CrossRef]
- Kumar, A.; Taylor, M.A.; Mad Arif, S.A.; Davies, H.V. Potato Plants Expressing Antisense and Sense S-Adenosylmethionine Decarboxylase (SAMDC) Transgenes Show Altered Levels of Polyamines and Ethylene: Antisense Plants Display Abnormal Phenotypes. Plant J. 1996, 9, 147–158. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wen, P.F.; Kong, W.F.; Pan, Q.H.; Zhan, J.C.; Li, J.M.; Wan, S.B.; Huang, W.D. Effect of Salicylic Acid on Phenylpropanoids and Phenylalanine Ammonia-Lyase in Harvested Grape Berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z.; et al. Maize Phenylalanine Ammonia-Lyases Contribute to Resistance to Sugarcane Mosaic Virus Infection, Most Likely through Positive Regulation of Salicylic Acid Accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Hwang, B.K. An Important Role of the Pepper Phenylalanine Ammonia-Lyase Gene (PAL1) in Salicylic Acid-Dependent Signalling of the Defence Response to Microbial Pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Kalunke, R.M.; Tundo, S.; Benedetti, M.; Cervone, F.; de Lorenzo, G.; D’Ovidio, R. An Update on Polygalacturonase-Inhibiting Protein (PGIP), Aleucine-Rich Repeat Protein That Protects Crop Plants against Pathogens. Front. Plant Sci. 2015, 6, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhu, X.; Tooley, P.; Zhang, X. Cloning and Functional Analysis of Three Genes Encoding Polygalacturonase-Inhibiting Proteins from Capsicum Annuum and Transgenic CaPGIP1 in Tobacco in Relation to Increased Resistance to Two Fungal Pathogens. Plant Mol. Biol. 2013, 81, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Owens, R.A.; Sun, Q.; Song, H.; Liu, Y.; Eamens, A.L.; Feng, H.; Tian, H.; Wang, M.B.; Zhang, R. Silencing of Transcription Factor Encoding Gene StTCP23 by Small RNAs Derived from the Virulence Modulating Region of Potato Spindle Tuber Viroid Is Associated with Symptom Development in Potato. PLoS Pathog. 2019, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Ech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. Revigo Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, 21800. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting Functional Regulatory Maps in Plants. Nucleic Acids Res. 2020, 48, 1104–1113. [Google Scholar] [CrossRef] [PubMed]



| Sample | Raw Reads | Clean Reads | Mapped Reads |
|---|---|---|---|
| KKH | 40,435,192 | 98.99% | 74.64% |
| KKI | 40,171,400 | 98.22% | 73.8% |
| DSH | 49,817,968 | 98.50% | 76.19% |
| DSI | 42,984,488 | 98.52% | 74.08% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjieva, N.; Apostolova, E.; Baev, V.; Yahubyan, G.; Gozmanova, M. Transcriptome Analysis Reveals Dynamic Cultivar-Dependent Patterns of Gene Expression in Potato Spindle Tuber Viroid-Infected Pepper. Plants 2021, 10, 2687. https://doi.org/10.3390/plants10122687
Hadjieva N, Apostolova E, Baev V, Yahubyan G, Gozmanova M. Transcriptome Analysis Reveals Dynamic Cultivar-Dependent Patterns of Gene Expression in Potato Spindle Tuber Viroid-Infected Pepper. Plants. 2021; 10(12):2687. https://doi.org/10.3390/plants10122687
Chicago/Turabian StyleHadjieva, Nikol, Elena Apostolova, Vesselin Baev, Galina Yahubyan, and Mariyana Gozmanova. 2021. "Transcriptome Analysis Reveals Dynamic Cultivar-Dependent Patterns of Gene Expression in Potato Spindle Tuber Viroid-Infected Pepper" Plants 10, no. 12: 2687. https://doi.org/10.3390/plants10122687
APA StyleHadjieva, N., Apostolova, E., Baev, V., Yahubyan, G., & Gozmanova, M. (2021). Transcriptome Analysis Reveals Dynamic Cultivar-Dependent Patterns of Gene Expression in Potato Spindle Tuber Viroid-Infected Pepper. Plants, 10(12), 2687. https://doi.org/10.3390/plants10122687








