Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack
Abstract
1. Introduction
2. Results
2.1. Yield of Extraction of P. malayana Leaves
2.2. AGI Activity of P. malayana Leaf Extracts
2.3. 2-NBDG Uptake in 3T3-L1 Cell Assay
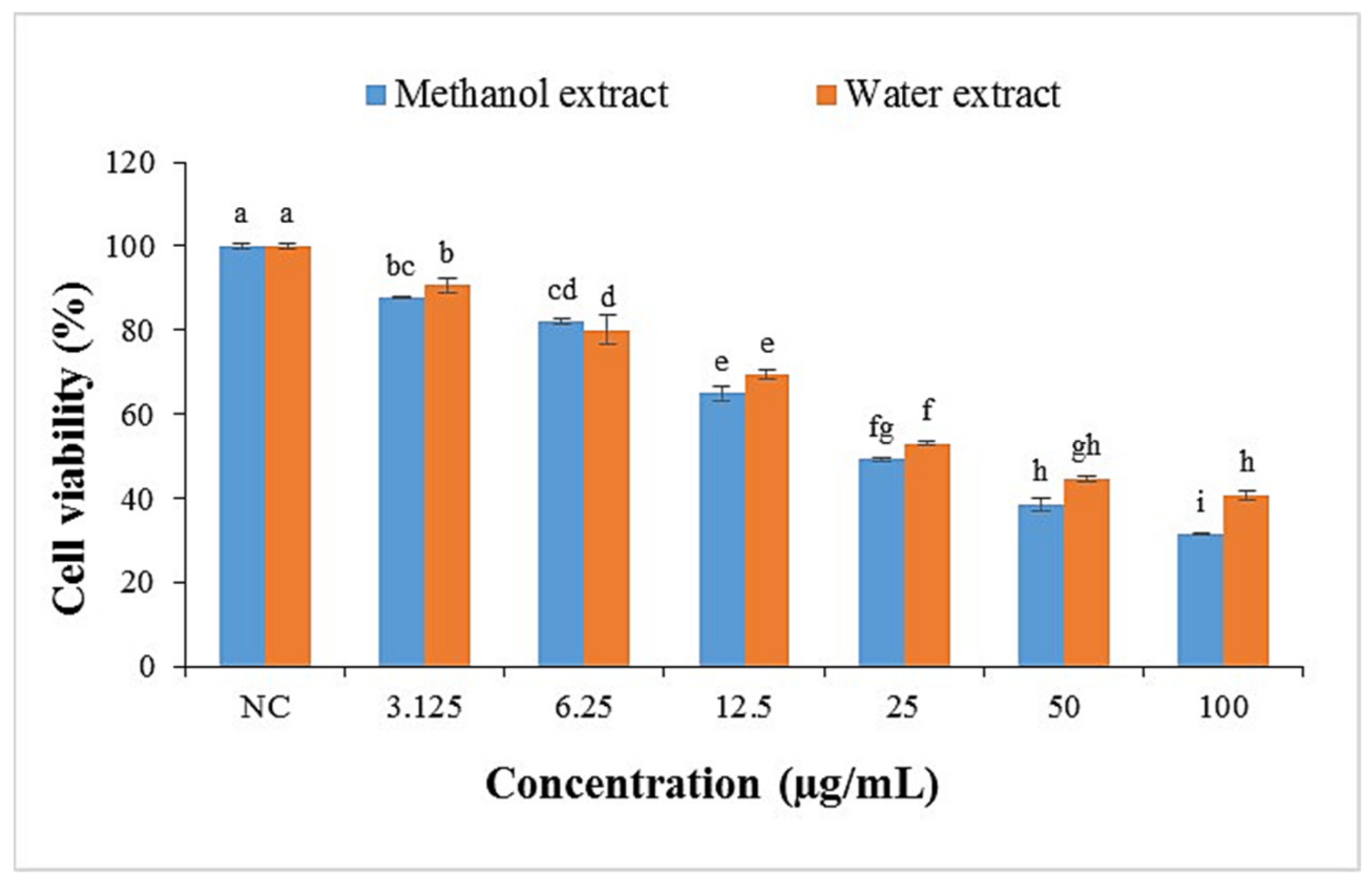
2.3.1. Cell Viability
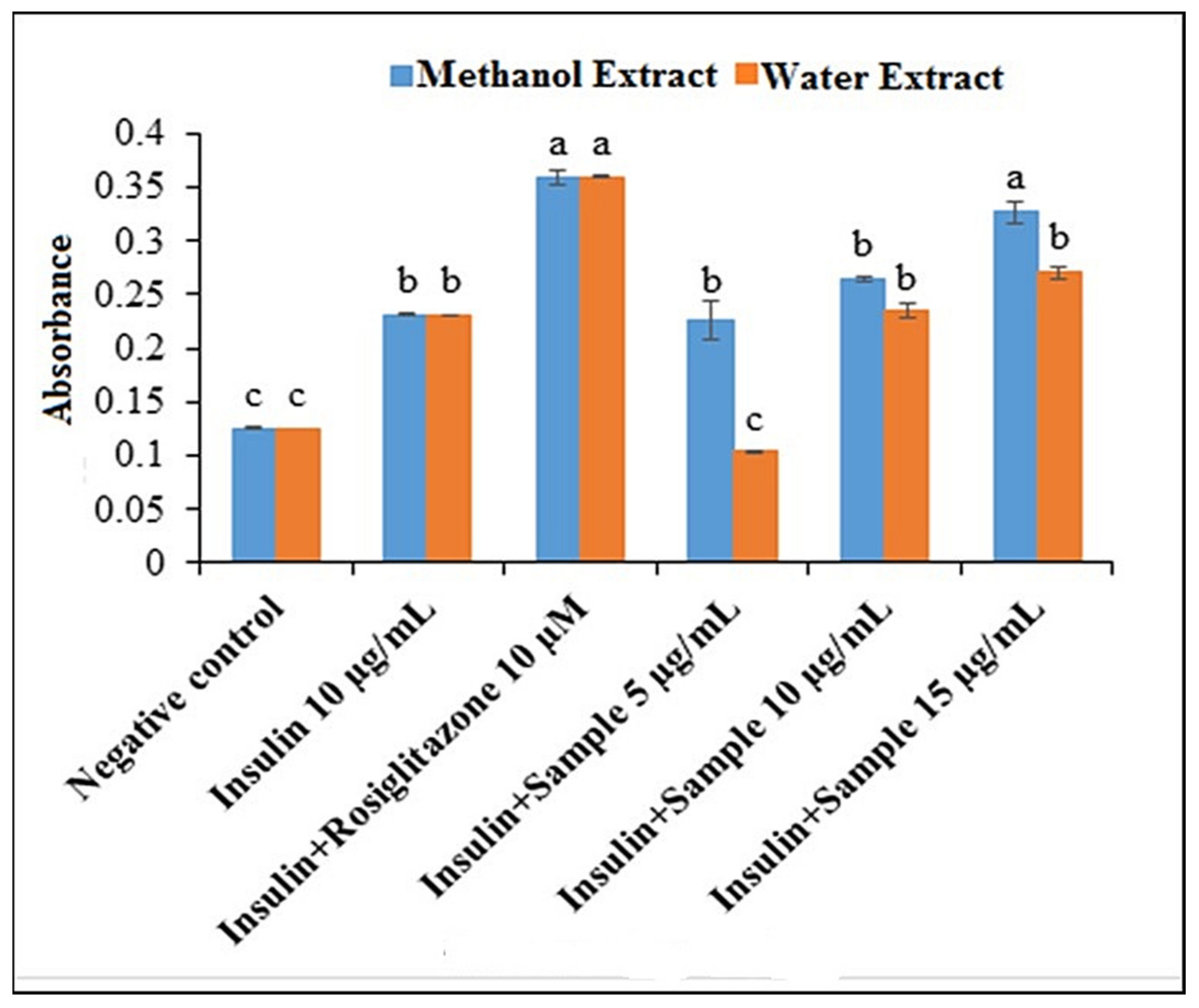
2.3.2. 2-NBDG Uptake in 3T3-L1 Cells
2.4. DPPH and FRAP Assay
2.5. Toxicity Study
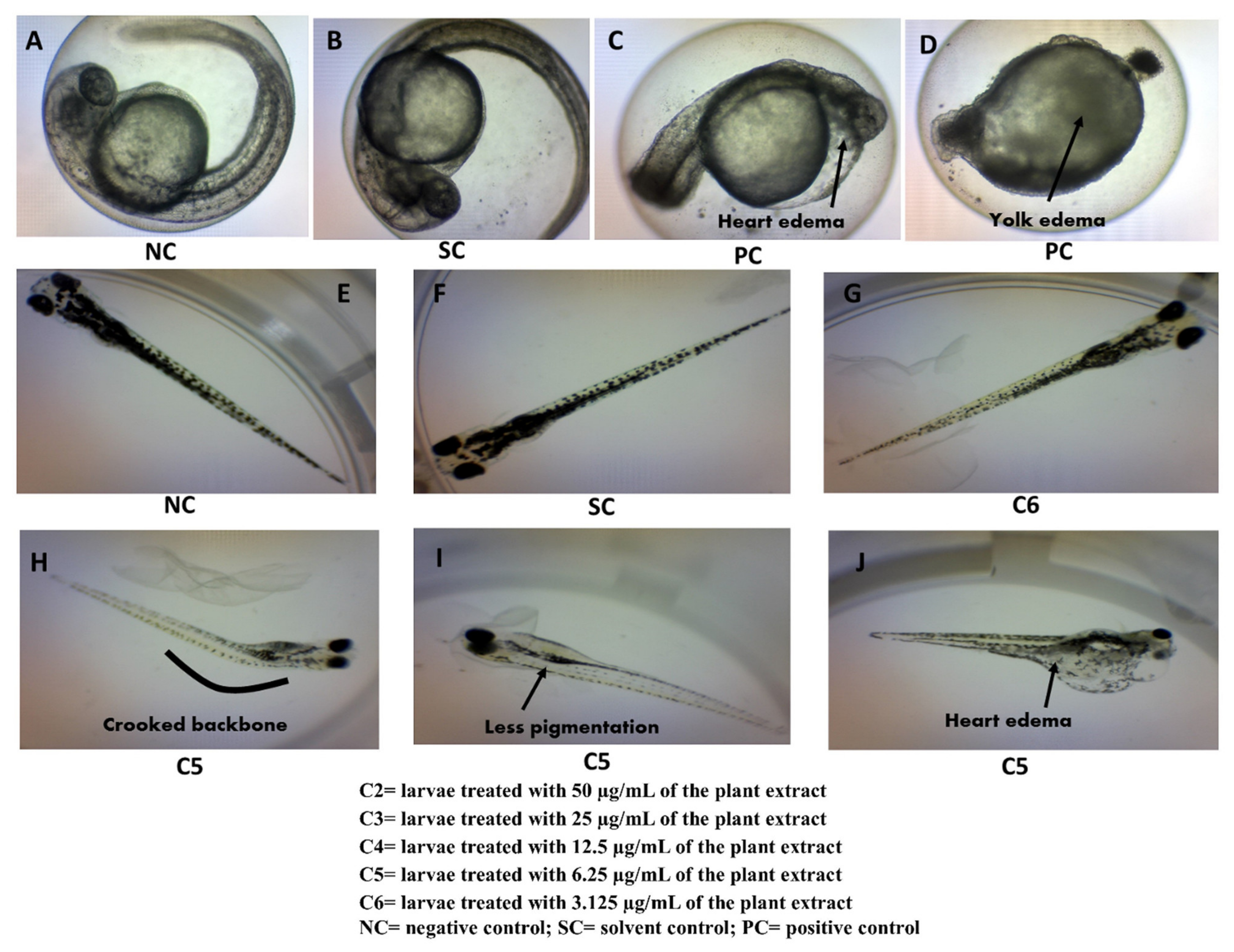
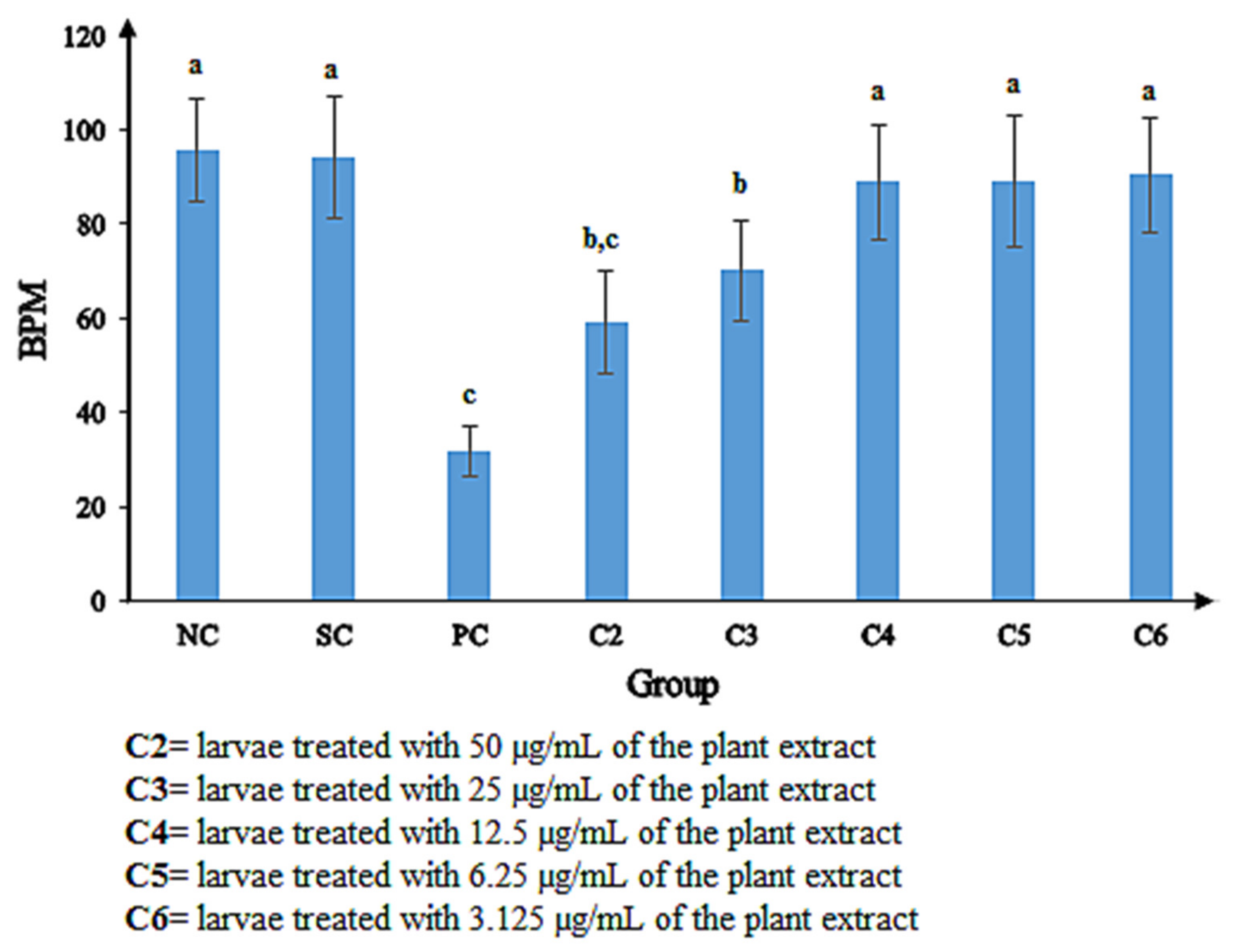
2.5.1. Morphological Defects Related to Mortality of Zebrafish Embryos Exposed to the P. malayana Extracts
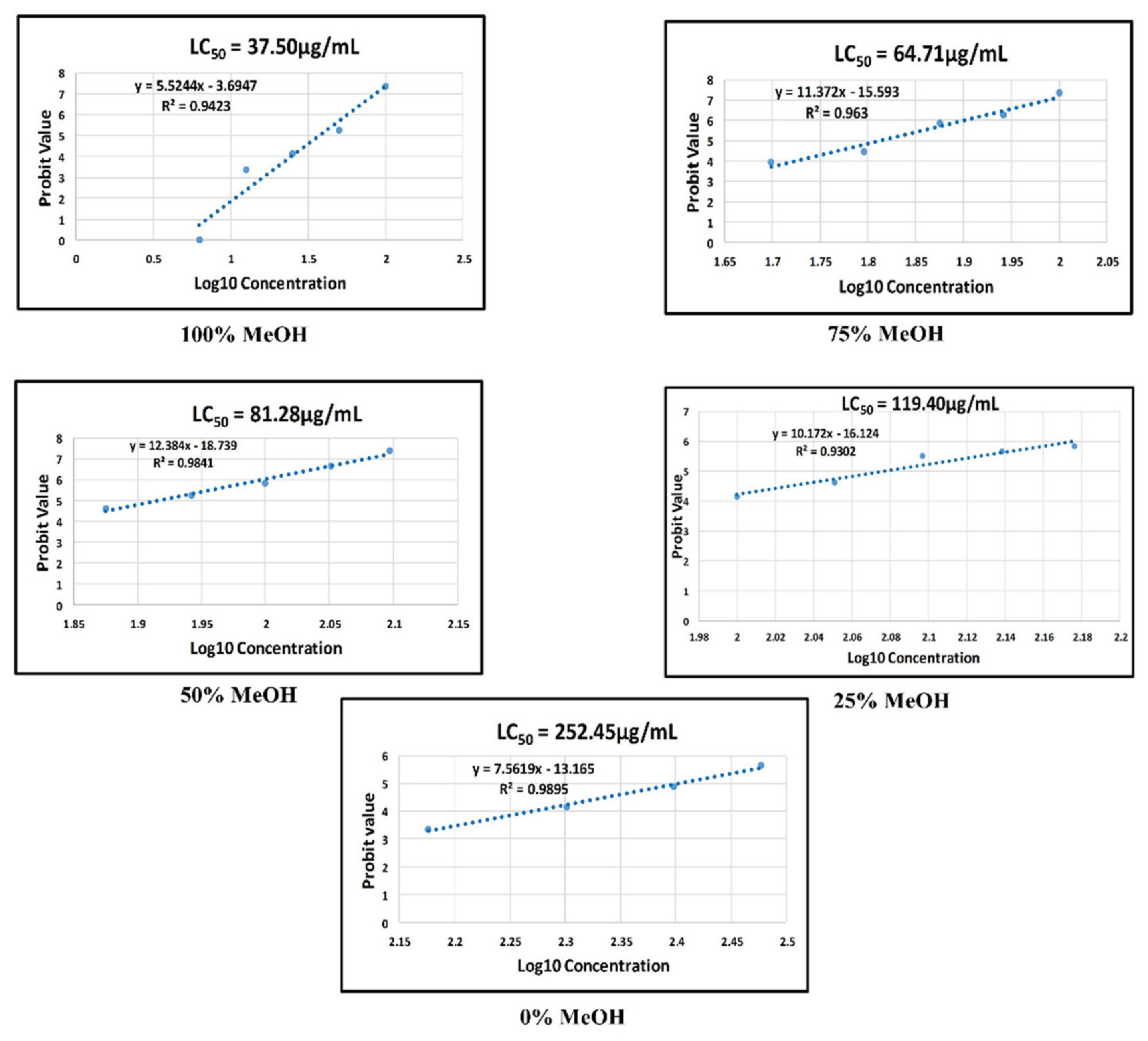
2.5.2. Mortality and Other Morphological Defects of Zebrafish Embryo Exposed to 100% Methanol Extract
Mortality
Hatchability
Yolk and Eye Size
Pigmentation Defect, Crooked Backbone, and Heart Edema
Body Length
2.6. GC-MS Analysis after Derivatization
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagent
4.2. Collection of Sample and Plant Extract Preparation
4.3. AGI Activity
4.4. 2-NBDG Uptake in 3T3-L1 Cells Assay
4.4.1. Cell Culture
4.4.2. Cell Viability Assay
4.4.3. 2-NBDG Uptake in 3T3-L1 Adipocyte Cells
4.5. In Vitro Analysis of Antioxidant Activity
4.5.1. DPPH Assay
4.5.2. FRAP Assay
4.6. Toxicity Test
4.6.1. Maintenance of Zebrafish
4.6.2. The Breeding Procedure of Danio rerio and Maintenance of Embryo
4.6.3. Preparation of the Sample and Experimental Procedure
4.6.4. Microscopic Observations
4.7. Identification of Compounds from Methanol Extracts of P. malayana Leaves by GC-MS Analysis after Derivatization
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, A.C.; D’Alessio, D. Endocrine Pancreas and Pharmacotherapy of Diabetes Mellitus and Hypoglycemia. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017; pp. 863–886. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Asnawati, D.; Ersalena, V.F.; Azwari, A.; Rahmawati, K.P. Characterization of Alkaloids from The Leaves of Psychotria malayana Jack of Lombok Island on The Basis of Gas Chromatography-Mass Spectroscopy. J. Pure Appl. Chem. Res. 2014, 3, 108–113. [Google Scholar] [CrossRef]
- Fairuz, F.; Dewi, H.; Humaryanto, H. Antidiabetic Effect of Psychotria malayana Jack in Induced Type 1 Diabetic Rat. Medisains 2020, 18, 19–23. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ahmed, Q.U.; Redzwan, I.E.; Ibrahim, Z.; Khan, A.Y.F.; Primaharinastiti, R.; Khalifa, S.A.M.; El-Seedi, H.R. Alpha-Glucosidase Inhibitory Effect of Psychotria malayana Jack Leaf: A Rapid Analysis Using Infrared Fingerprinting. Molecules 2020, 25, 4161. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ibrahim, Z.; Ahmed, Q.U.; Redzwan, I.E.; Primaharinastiti, R.; Saiman, M.Z.; Fairuza, R.; Widyaningsih, T.D.; AlAjmi, M.F.; et al. GC-MS- and NMR-Based Metabolomics and Molecular Docking Reveal the Potential Alpha-Glucosidase Inhibitors from Psychotria malayana Jack Leaves. Pharmaceuticals 2021, 14, 978. [Google Scholar] [CrossRef]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Mohamad, C.A.C.; Sulaiman, W.M.A.W.; Wahab, R.A.; Ahmed, Q.U.; Ghaffar, M.A.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Bray, G.A.; Bouchard, C. Handbook of Obesity, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Sadie-Van Gijsen, H. Adipocyte biology: It is time to upgrade to a new model. J. Cell. Physiol. 2019, 234, 2399–2425. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.-L.; Iwata, Y.; Sato, F. Dihydrosanguinarine Enhances Glucose Uptake in Mouse 3T3-L1 Cells. ACS Omega 2017, 2, 6916–6925. [Google Scholar] [CrossRef] [PubMed]
- Riess, M.L.; Elorbany, R.; Weihrauch, D.; Stowe, D.F.; Camara, A.K. PPARγ-Independent Side Effects of Thiazolidinediones on Mitochondrial Redox State in Rat Isolated Hearts. Cells 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Oh, J. Evaluation of Berry Extracts on Intestinal Digestive Enzymes and Sugar Transporters. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 2017. [Google Scholar]
- Abbas, G.; Al-Harrasi, A.; Hussain, H. α-Glucosidase Enzyme Inhibitors from Natural Products. In Discovery and Development of Antidiabetic Agents from Natural Products; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 251–269. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Emad, S., Ed.; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Azat Aziz, M.; Shehab Diab, A.; Abdulrazak Mohammed, A. Antioxidant Categories and Mode of Action. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. ISBN 9781789239195. [Google Scholar] [CrossRef]
- Fatima, N.; Nayeem, N. Toxic Effects as a Result of Herbal Medicine Intake. In Toxicology—New Aspects to This Scientific Conundrum; Larramendy, M.L., Soloneski, S., Eds.; InTech Open: London, UK, 2016; pp. 193–207. [Google Scholar] [CrossRef]
- Murugesu, S.; Khatib, A.; Ahmed, Q.U.; Ibrahim, Z.; Uzir, B.F.; Benchoula, K.; Yusoff, N.I.N.; Perumal, V.; Alajmi, M.F.; Salamah, S.; et al. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicol. Rep. 2019, 6, 1148–1154. [Google Scholar] [CrossRef]
- Arome, D.; Chinedu, E. The Importance of Testing Equipment. J. Pharm. Biosci. 2014, 4, 146–148. [Google Scholar]
- Akhila, J.S.; Shyamjith, D.; Alwar, M.C. Acute Toxicity Studies and Determination of Median Lethal Dose. Curr. Sci. 2007, 93, 917–920. [Google Scholar]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to the aspects of the biology and welfare of animals used for experimental and other scientific purposes. EFSA J. 2005, 3, 292. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Analysis of Lethality and Malformations During Zebrafish. In Teratogenicity Testing-Methods and Protocols; Humana Press: New York, NY, USA, 2018; Volume 1797, pp. 337–363. [Google Scholar] [CrossRef]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar]
- Braunbeck, T.; Boettcher, M.; Hollert, H.; Kosmehl, T.; Lammer, E.; Leist, E.; Rudolf, M.; Seitz, N. Towards an alternative for the acute fish LC50 test in chemical assessment: The fish embryo toxicity test goes multi-species—An update. ALTEX 2005, 22, 87–102. [Google Scholar]
- Busquet, F.; Halder, B.T.; Gourmelon, L.A.; Kleensang, A.; Belanger, S.E.; Carr, G.J.; Walter-rohde, S. OECD Guidelines for the Testing of Chemicals 236—Fish Embryo Acute Toxicity (FET) Test. Organ. Econ. Coop. Dev. 2013, 1–22. [Google Scholar]
- Jemain, M.R.M.; Musa’adah, M.N.; Rohaya, A.; Rashid, L.A.; Hadiani, I.N. In Vitro Antihyperglycaemic Effects of Some Malaysian Plants. J. Trop. For. Sci. 2011, 23, 467–472. [Google Scholar]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Yan, J.; Shen, Y.; Wang, Y.; Luan, X.; Guo, M.; Li, C. The effects of different extraction methods on the physicochemical property and antioxidant activity of Amygdalus pedunculatus seed oil. J. Appl. Bot. Food Qual. 2016, 89, 135–141. [Google Scholar] [CrossRef]
- Sharif, M.F.; Bennett, M.T. The Effect of Different Methods and Solvents on the Extraction of Polyphenols in Ginger (Zingiber officinale. J. Teknol. 2016, 78, 49–54. [Google Scholar] [CrossRef][Green Version]
- Thouri, A.; Chahdoura, H.; El Arem, A.; Hichri, A.O.; Ben Hassin, R.; Achour, L. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Kong, S.; Ding, C.; Huang, L.; Bai, Y.; Xiao, T.; Guo, J.; Su, Z. The effects of COST on the differentiation of 3T3-L1 preadipocytes and the mechanism of action. Saudi J. Biol. Sci. 2017, 24, 251–255. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.G.; Kim, J.; Yeo, S.; Kim, J.A.; Choi, C.W.; Jeong, S.-Y. Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules 2020, 25, 2453. [Google Scholar] [CrossRef] [PubMed]
- Litwack, G. Glycolysis and Gluconeogenesis. In Human Biochemistry; Academic Press: Boston, MA, USA, 2018; pp. 183–198. [Google Scholar] [CrossRef]
- Fukunaga, T.; Zou, W.; Rohatgi, N.; Colca, J.R.; Teitelbaum, S. An insulin-sensitizing thiazolidinedione, which minimally activates PPARγ, does not cause bone loss. J. Bone Miner. Res. 2014, 30, 481–488. [Google Scholar] [CrossRef]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-Based Metabolite Profiling ofCosmos caudatusLeaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
- Nokhala, A.; Ahmed, Q.U.; Saleh, M.S.; Nipun, T.S.; Khan, A.Y.F.; Siddiqui, M.J. Characterization of α-glucosidase inhibitory activity of Tetracera scandens leaves by Fourier transform infrared spectroscopy-based metabolomics. Adv. Tradit. Med. 2020, 20, 169–180. [Google Scholar] [CrossRef]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 273–286. [Google Scholar] [CrossRef]
- Hidajati, N.; Tukiran, T.; Setiabudi, D.A.; Wardana, A.P. Antioxidant Activity of Palmitic Acid and Pinostrobin from Methanol Extract of Syzygium litoralle (Myrtaceae). In Proceedings of the International Conference on Science and Technology (ICST 2018); Atlantis Press: Paris, France, 2018; Volume 1, pp. 183–187. [Google Scholar] [CrossRef]
- Aguirrezábal, L.; Martre, P.; Pereyra-Irujo, G.; Echarte, M.M.; Izquierdo, N. Improving grain quality: Ecophysiological and modeling tools to develop management and breeding strategies. In Crop Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 423–465. [Google Scholar] [CrossRef]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final Amended Safety Assessment of Hydroquinone as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 274S–287S. [Google Scholar] [CrossRef]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, J.K.R.; Lambré, C.; Larsen, J.-C.; et al. Scientific Opinion on the Use of Resorcinol as a Food Additive. EFSA J. 2010, 8, 1441. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant Effects of Phytosterol and Its Components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.J.; Sutherland, W.H.; Walker, R.J.; Williams, S.M.; de Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of High-Dose Vitamin E on Insulin Resistance and Associated Parameters in Overweight Subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Porto, D.D.; Fett-Neto, A.G. Bioactive Alkaloids from South American Psychotria and Related Rubiaceae. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 119–147. [Google Scholar] [CrossRef]
- Hadi, S. Bioactive Alkaloids from Medicinal Plants of Lombok. Ph.D. Thesis, University of Wollongong, Wollongong, NSW, Australia, 2002. [Google Scholar]
- Chebib, M.; Duke, R.K.; Duke, C.C.; Connor, M.; Mewett, K.N.; Johnston, G.A.R. Convulsant Actions of Calycanthine. Toxicol. Appl. Pharmacol. 2003, 190, 58–64. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int. J. Food Sci. Nutr. 2013, 64, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.D.S.; de Lima, T.M.; Curi, R.; Castrucci, A.M.D.L. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. Vitr. 2005, 19, 553–560. [Google Scholar] [CrossRef]
- Kučera, O.; Lotkova, H.; Sobotka, O.; Červinková, Z. The Effect of D-Galactosamine on Lean and Steatotic Rat Hepatocytes in Primary Culture. Physiol. Res. 2015, 64, S637–S646. [Google Scholar] [CrossRef] [PubMed]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Keaney, J.F.; Gaziano, J.M.; Xu, A.; Frei, B.; Curran-Celentano, J.; Shwaery, G.T.; Loscalzo, J.; Vita, J.A. Low-dose alpha-tocopherol improves and high-dose alpha-tocopherol worsens endothelial vasodilator function in cholesterol-fed rabbits. J. Clin. Investig. 1994, 93, 844–851. [Google Scholar] [CrossRef]
- Tsuchikane, E.; Katoh, O.; Sumitsuji, S.; Fukuhara, A.; Funamoto, M.; Otsuji, S.; Tateyama, H.; Awata, N.; Kobayashi, T. Impact of cilostazol on intimal proliferation after directional coronary atherectomy. Am. Heart J. 1998, 135, 495–502. [Google Scholar] [CrossRef]
- Li, Z.; Ptak, D.; Zhang, L.; Walls, E.K.; Zhong, W.; Leung, Y.F. Phenylthiourea Specifically Reduces Zebrafish Eye Size. PLoS ONE 2012, 7, e40132. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2009, 12, 1645–1654. [Google Scholar] [CrossRef]
- Lin, C.; Hui, M.N.; Cheng, S.H. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol. Appl. Pharmacol. 2007, 222, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pamanji, R.; Yashwanth, B.; Bethu, M.; Leelavathi, S.; Ravinder, K.; Rao, J.V. Toxicity effects of profenofos on embryonic and larval development of Zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2015, 39, 887–897. [Google Scholar] [CrossRef]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. Phytochemical Evaluation of Polyherbal Formulation of Clinacanthus nutans and Elephantopus scaber to Identify Flavonoids. Pharmacogn. J. 2016, 8, 534–541. [Google Scholar] [CrossRef]
- McCollum, C.W.; Ducharme, N.; Bondesson, M.; Gustafsson, J.-A. Developmental toxicity screening in zebrafish. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 67–114. [Google Scholar] [CrossRef]
- Zoupa, M.; Machera, K. Zebrafish as an Alternative Vertebrate Model for Investigating Developmental Toxicity—The Triadimefon Example. Int. J. Mol. Sci. 2017, 18, 817. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Ahmed, Q.U.; Uzir, B.F.; Yusoff, N.I.N.; Perumal, V.; Ibrahim, Z.; Abas, F.; Saari, K.; Khatib, A. Rapid investigation of α-glucosidase inhibitory activity of Clinacanthus nutans leaf using infrared fingerprinting. Vib. Spectrosc. 2018, 100, 22–29. [Google Scholar] [CrossRef]
- Dhanawansa, R.; Faridmoayer, A.; van der Merwe, G.; Li, Y.X.; Scaman, C.H. Overexpression, purification, and partial characterization of Saccharomyces cerevisiae processing alpha glucosidase I. Glycobiology 2002, 12, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aykul, S.; Martinez-Hackert, E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal. Biochem. 2016, 508, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Burlingham, B.T.; Widlanski, T.S. An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. J. Chem. Educ. 2003, 80, 214–218. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Smith, A.; La Merrill, M.A. Triphenyl phosphate enhances adipogenic differentiation, glucose uptake and lipolysis via endocrine and noradrenergic mechanisms. Toxicol. Vitr. 2017, 40, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Al-Zikri, P.N.H.; Taher, M.; Susanti, D.; Ichwan, S.J.A. Cytotoxic Activity of Luvunga Scandens against Human Cancer Cell Lines. J. Teknol. 2016, 78, 153–157. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmed, Q.U.; Soad, S.Z.M.; Latip, J.; Taher, M.; Syafiq, T.M.F.; Sarian, M.N.; Alhassan, A.M.; Zakaria, Z.A. Flavonoids from Tetracera indica Merr. induce adipogenesis and exert glucose uptake activities in 3T3-L1 adipocyte cells. BMC Complement. Altern. Med. 2017, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.; Perumal, V.; Ahmed, Q.U.; Uzir, B.F.; Abas, F.; Murugesu, S. Characterization of Antioxidant Activity ofMomordica CharantiaFruit by Infrared-Based Fingerprinting. Anal. Lett. 2017, 50, 1977–1991. [Google Scholar] [CrossRef]
- Paatero, I.; Alve, S.; Gramolelli, S.; Ivaska, J.; Ojala, P. Zebrafish Embryo Xenograft and Metastasis Assay. Bio-Protocol 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]

| Sample | AGI Activity IC50 (μg/mL) | % Yield (w/w) |
|---|---|---|
| Hexane extract | ND | 1.06 ± 0.13 c |
| Ethyl acetate extract | 5.37 ± 0.14 b | 18.87 ± 0.93 b |
| Methanol extract | 2.71 ± 0.11 c | 31.51 ± 1.24 a |
| Water extract | 6.75 ± 0.16 a | 20.40 ± 1.47 b |
| Quercetin | 1.88 ± 0.003 d | ND |
| Sample | DPPH IC50 (μg/mL) | FRAP (mg AAE/g) |
|---|---|---|
| Methanol extract | 10.85 ± 0.34 b | 72.53 ± 1.33 a |
| Water extract | 27.12 ± 0.95 a | 33.71 ± 1.84 b |
| Ascorbic acid | 5.08 ± 0.02 c | ND |
| Methanol-Water Extract | Extracts Concentration (µg/mL) | Coagulation | Non-Detachment of the Tail | Lack of Somite Formation | Lack of Heartbeat |
|---|---|---|---|---|---|
| 100% | 100, 50, 25, 12.5 | + | + | − | + |
| 6.25, 3.125 | − | − | − | − | |
| 75% | 100, 87.5, 75, 62.5, 50, 37.5, 25 | + | − | − | − |
| 12.5 | − | − | − | − | |
| 50% | 125, 112.5, 100, 87.5, 75, 62.5, 50, 37.5 | + | − | − | − |
| 25, 12.5 | − | − | − | − | |
| 25% | 175, 162.5, 150, 137.5, 125, 112.5, 100, 87.5 | + | − | − | − |
| 75, 62.5 | − | − | − | − | |
| 0% | 350, 300, 250, 200, 150 | + | − | − | − |
| 100, 50 | − | − | − | − | |
| Controls | Negative control | − | − | − | − |
| Solvent control | − | − | − | − | |
| Positive control | + | + | − | + |
| Plant Extract Concentration (µg/mL) | Mortality Rate (%) at 24 to 96 hpf | Hatching Rate (%) at 72 hpf |
|---|---|---|
| 100 | 100 | 0 |
| 50 | 60 | 0 |
| 25 | 20 | 0 |
| 12.5 | 5 | 10 |
| 6.25 | 0 | 70 |
| 3.125 | 0 | 100 |
| NC | 0 | 100 |
| SC | 0 | 100 |
| PC | 90 | 5 |
| 100% Methanol Extract (µg/mL) | Eye Size × 104 (µm2) 96 hpf | Yolk Size × 105 (µm2) 24 hpf | Body Length (mm) 96 hpf | Hatching Defect | Less Pigmentation | Yolk Edema | Crooked Backbone | Heart Edema |
|---|---|---|---|---|---|---|---|---|
| 6.25 | 6.1 ± 0.3 a | 2.5 ± 0.2 b | 2.5 ± 0.1 b | + | + | − | + | + |
| 3.125 | 5.6 ± 0.5 b | 2.6 ± 0.1 b | 2.5 ± 0.2 b | − | − | − | − | − |
| NC | 5.7 ± 0.3 b | 2.6 ± 0.2 b | 2.8 ± 0.2 a | − | − | − | − | − |
| SC | 5.7 ± 0.3 a,b | 2.6 ± 0.2 b | 2.8 ± 0.2 a | − | − | − | − | − |
| PC | 2.4 ± 0.7 c | 3.2 ± 0.3 a | NA | + | NA | + | NA | + |
| Extracts | Putative Compounds | Retention Time (min) | % of Area | Similarity Index | Molecular Formula |
|---|---|---|---|---|---|
| Methanol | Phosphonic acid | 16.261 | 0.03 | 93 | H2O3P+ |
| Resorcinol | 19.941 | 0.34 | 95 | C6H6O2 | |
| 1,3,5-benzenetriol | 37.213 | 0.61 | 98 | C6H6O3 | |
| DL-arabinose | 49.569 | 0.81 | 93 | C5H10O5 | |
| D-ribose | 50.112 | 3.86 | 93 | C5H10O5 | |
| D-mannose | 50.815 | 2.87 | 91 | C6H12O6 | |
| D-psicose | 51.393 | 0.12 | 90 | C6H12O6 | |
| L-sorbose | 52.941 | 1.47 | 91 | C6H12O6 | |
| Palmitic acid | 58.142 | 0.04 | 98 | C16H32O2 | |
| Myo-inositol | 60.234 | 0.15 | 94 | C6H12O6 | |
| 1-monopalmitin | 81.935 | 0.17 | 93 | C19H38O4 | |
| Sucrose | 83.878 | 4.21 | 93 | C12H22O11 | |
| Beta-tocopherol | 100.870 | 0.10 | 93 | C28H48O2 | |
| Alpha-tocopherol | 101.104 | 0.24 | 98 | C29H50O2 | |
| 24-epicampesterol | 104.362 | 0.03 | 90 | C28H48O | |
| Stigmast-5-ene | 107.083 | 0.31 | 99 | C29H50 | |
| Water | Resorcinol | 19.941 | 0.21 | 91 | C6H6O2 |
| Hydroquinone | 21.690 | 0.42 | 94 | C6H6O2 | |
| D-ribino-1,4-lactone | 36.384 | 0.11 | 97 | C5H8O5 | |
| 1,3,5-benezenetriol | 37.213 | 0.45 | 98 | C6H6O3 |
| Groups | 2-NBDG (µg/mL) | Insulin (µg/mL) | Rosiglitazone (µM) | Methanol Extract (µg/mL) | Water Extract (µg/mL) |
|---|---|---|---|---|---|
| NC | 100 | - | - | - | - |
| IC | 100 | 10 | - | - | - |
| PC | 100 | 10 | 10 | - | - |
| Insulin-sensitizing activity of methanol extract | 100 | 10 | - | 5, 10, 15 | - |
| Insulin-sensitizing activity of water extract | 100 | 10 | - | - | 5, 10, 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nipun, T.S.; Khatib, A.; Ahmed, Q.U.; Nasir, M.H.M.; Supandi, F.; Taher, M.; Saiman, M.Z. Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack. Plants 2021, 10, 2688. https://doi.org/10.3390/plants10122688
Nipun TS, Khatib A, Ahmed QU, Nasir MHM, Supandi F, Taher M, Saiman MZ. Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack. Plants. 2021; 10(12):2688. https://doi.org/10.3390/plants10122688
Chicago/Turabian StyleNipun, Tanzina Sharmin, Alfi Khatib, Qamar Uddin Ahmed, Mohd Hamzah Mohd Nasir, Farahaniza Supandi, Muhammad Taher, and Mohd Zuwairi Saiman. 2021. "Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack" Plants 10, no. 12: 2688. https://doi.org/10.3390/plants10122688
APA StyleNipun, T. S., Khatib, A., Ahmed, Q. U., Nasir, M. H. M., Supandi, F., Taher, M., & Saiman, M. Z. (2021). Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack. Plants, 10(12), 2688. https://doi.org/10.3390/plants10122688