The Combination of Increased Temperatures and High Irradiation Causes Changes in Photosynthetic Efficiency
Abstract
:1. Introduction
2. Results
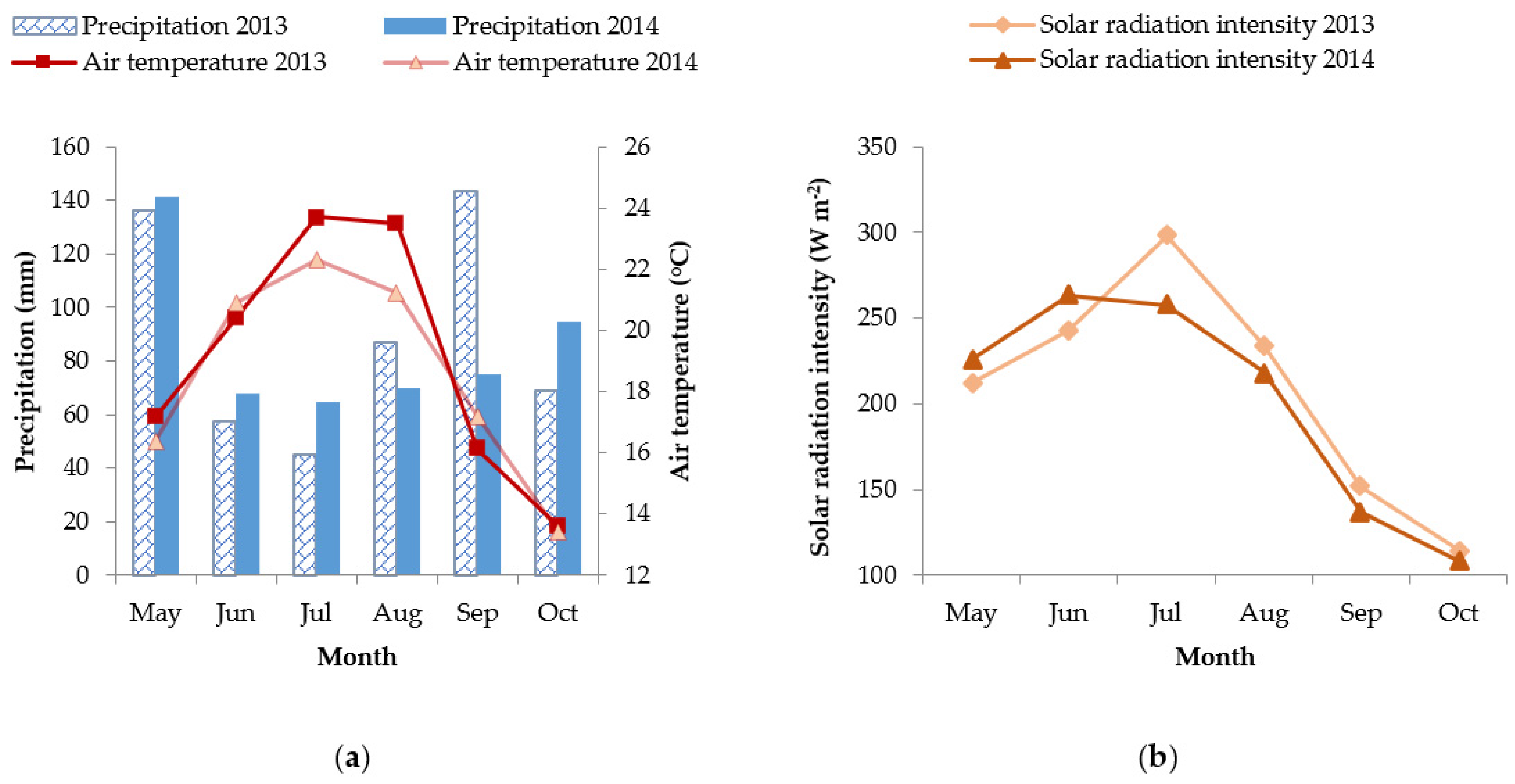
2.1. Climate in the Experimental Site
2.2. Chlorophyll a Fluorescence
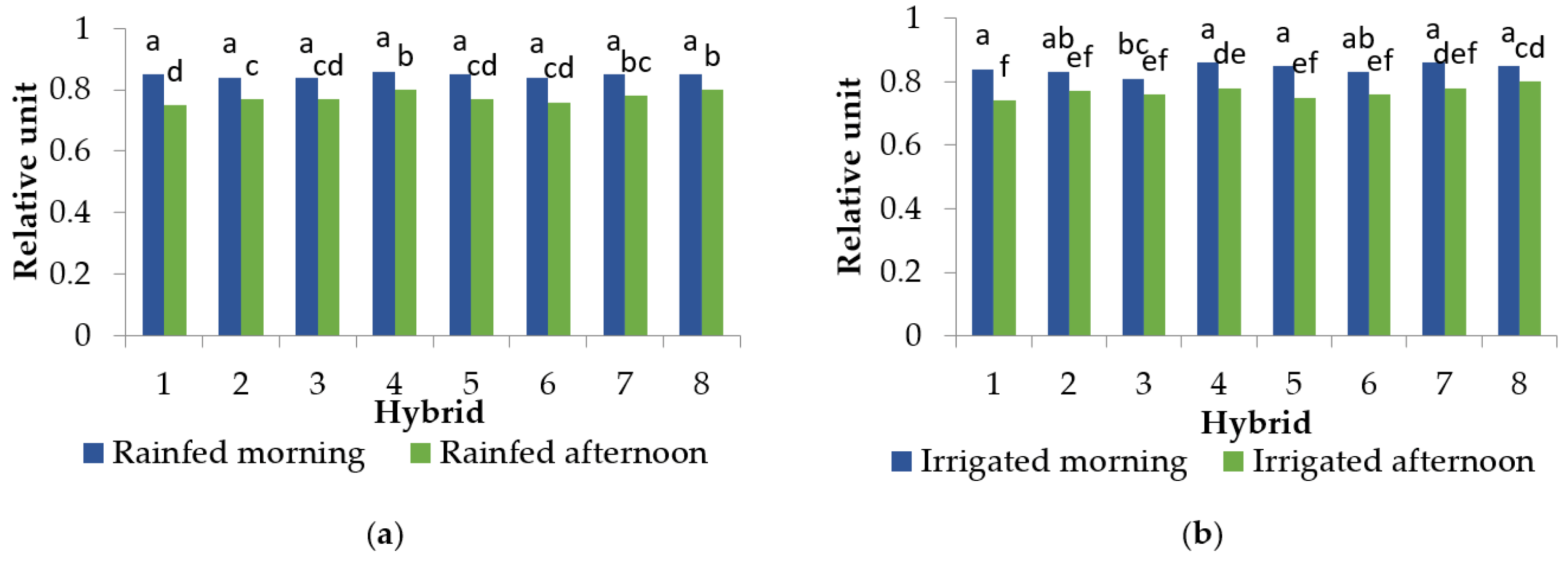
2.3. Relative Water Content
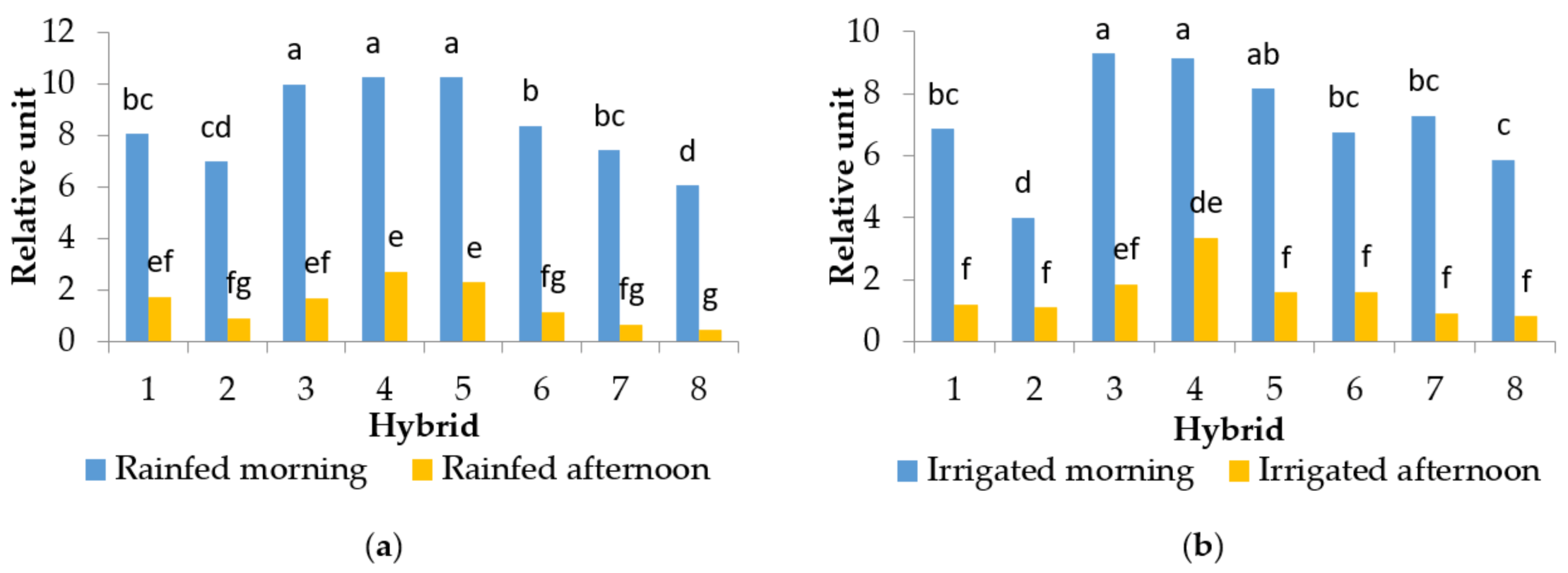
2.4. Enzime Guaiacol Peroxidase
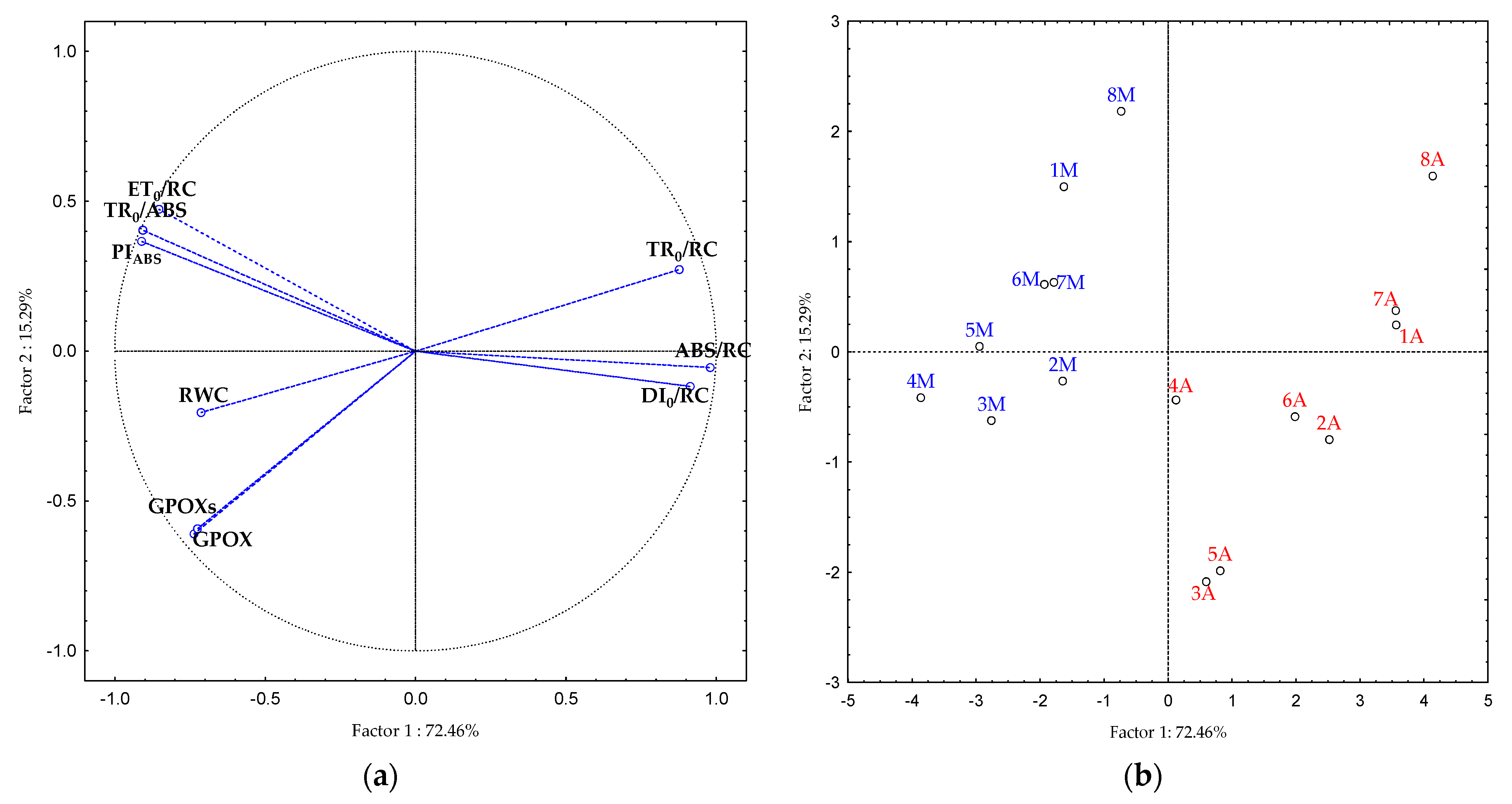
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Plant Growing Conditions
4.2. Irrigation of the Experimental Field
4.3. Field Measurements and Tissue Sampling
4.4. Chlorophyll a Fluorescence (ChlF)
4.5. Relative Water Content (RWC)
4.6. Enzime Guaiacol Peroxidase
4.7. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espino, J.; Sharma, I.; Chatrath, R.; Singh, G.P.; Sohu, V.S.; Mavi, G.S.; Sukaru, V.S.P.; et al. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in south Asia. Field Crops Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Panero, J.L.; Funk, V.A. Toward a phylogenetic subfamilial classification for the Compositae (Asteraceae). P. Biol. Soc. Wash. 2002, 115, 909–922. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donatelli, M.; Srivastava, A.K.; Duveiller, G.; Niemeyer, S.; Fumagalli, D. Climate change impact and potential adaptation strategies under alternate realisations of climate scenarios for three major crops in Europe. Environ. Res. Lett. 2015, 10, 075005. [Google Scholar] [CrossRef] [Green Version]
- Jug, D.; Jug, I.; Brozović, B.; Vukadinović, V.; Stipešević, B.; Đurđević, B. The role of conservation agriculture in mitigation and adaptation to climate change. Poljoprivreda 2018, 24, 35–44. [Google Scholar] [CrossRef]
- Hoang Dinh, T.; Takaragawa, H.; Watanabe, K.; Naka baru, M.; Kawamitsu, Y. Leaf Photosynthesis Response to Change of Soil Moisture Content in Sugarcane. Sugar Tech. 2019, 21, 949–958. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; George, C., Papageorgiou, C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Šimić, D.; Lepeduš, H.; Jurković, V.; Antunović, J.; Cesar, V. Quantitative genetic analysis of chlorophyll a fluorescence parameters in maise in the field environments. J. Integr. Plant. Biol. 2014, 56, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, T. Plant response to environmental conditions: Assessing potential production, water demand, and negative effects of water deficit. Front. Physiol. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Mlinarić, S.; Antunović Dunić, J.; Štolfa, I.; Cesar, V.; Lepeduš, H. High irradiation and increased temperature induce different strategies for competent photosynthesis in young and mature fig leaves. S. Afr. J. Bot. 2016, 103, 25–31. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Strnad, M.; Salopek-Sondi, B. Early Brassica Crops Responses to Salinity Stress: A Comparative Analysis Between Chinese Cabbage, White Cabbage, and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Mihaljević, I.; Lepeduš, H.; Šimić, D.; Viljevac Vuletić, M.; Tomaš, V.; Vuković, D.; Dugalić, K.; Teklić, T.; Skendrović Babojelić, M.; Zdunić, Z. Photochemical efficiency of photosystem II in two apple cultivars affected by elevated temperature and excess light in vivo. S. Afr. J. Bot. 2020, 130, 316–326. [Google Scholar] [CrossRef]
- Pfündel, E. Estimating the contribution of photosystem I to total leaf chlorophyll fluorescence. Photosynth. Res. 1998, 56, 185–195. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.P.; Baker, N.R.; Öquist, G.; Schreiber, U.; Lechner, E.G. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: A review of current Instrumentation. Funct. Ecol. 1989, 3, 497–514. [Google Scholar] [CrossRef]
- Mlinarić, S.; Antunović Dunić, J.; Skendrović Babojelić, M.; Cesar, V.; Lepeduš, H. Differential accumulation of photosynthetic proteins regulates diurnal photochemical adjustments of PSII in common fig (Figus carica L.) leaves. J. Plant Physiol. 2017, 209, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.U.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Franić, M.; Galić, V.; Mazur, M.; Šimić, D. Effects of excess cadmium in soil on JIP-test parameters, hydrogen peroxide content and antioxidant activity in two maise inbred and their hybrid. Photosynthetica 2018, 56, 660–669. [Google Scholar] [CrossRef]
- Kovačević, J.; Mazur, M.; Drezner, G.; Lalić, A.; Sudarić, A.; Dvojsković, K.; Viljevac Vuletić, M.; Josipović, M.; Josipović, A.; Markulj Kulundžić, A.; et al. Photosynthetic efficiency parameters as indicators of agronomic traits of winter wheat cultivars in different soil water conditions. Genetika 2017, 49, 891–910. [Google Scholar] [CrossRef] [Green Version]
- Viljevac, M.; Dugalić, K.; Mihaljević, I.; Šimić, D.; Sudar, R.; Jurković, Z.; Lepeduš, H. Chlorophyll content, photosynthetic efficiency and genetic markers in two sour cherry (Prunus cerasus L.) genotypes under drought stress. Acta Bot. Croat. 2013, 72, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.; Popescu, G.C. Diurnal changes in leaf photosynthesis and relative water content of grapevine. Curr. Trends Nat. Sci. 2014, 3, 74–81. [Google Scholar]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Jifon, J.; Da Silva, J.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant. Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Singh, D.V.; Srivastova, G.C. Changes in activities of antioxidant enzymes in sunflower leaves of different ages. Biol. Plant. 2003, 47, 61–66. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.; Ali, S.; Akram, N.A.; Rizwan, M.; Abbas, F.; Bukhari, S.A.H.; Saeed, R. Phyto-management of Cr-contaminated soils by sunflower hybrids: Physiological and biochemical response and metal extractability under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 16845–16859. [Google Scholar] [CrossRef]
- Jovičić, D.; Štajner, D.; Popović, B.; Marjanović-Jeromela, A.; Nikolić, Z.; Petrović, G.; Ždero-Pavlović, R. Salt-induced changes in the antioxidant system and viability of oilseed rape. Zemdirbyste 2017, 104, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic Acid Improves the Antioxidant Ability against Arsenic-Induced Oxidative Stress in Sunflower (Helianthus Annuus) Seedling. J. Plant Nutr 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Kasraoui, M.F.; Duquesnoy, I.; Winterton, P.; Lamaze, T. Soluble and cell wall bound peroxidase activities are markers of flower bud development stages in lemon (Citrus limon L.). J. Appl. Bot. Food Qual. 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Lu, P.; Sang, W.-G.; Ma, K.-P. Activity of stress-related antioxidative enzymes in the invasive plant crofton weed (Eupatorium adenophorum). J. Integr. Plant Biol. 2007, 49, 1555–1564. [Google Scholar] [CrossRef]
- Yu, C.; Huang, S.J.; Hu, X.M.; Deng, W.; Xiong, C.; Ye, C.H.; Li, Y.; Peng, B. Changes in photosynthesis, chlorophyll fluorescence, and antioxidant enzymes of mulberry (Morus ssp.) in response to salinity and high-temperature stress. Biologia 2013, 68, 404–413. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Lepeduš, H.; Šimić, D.; Lalić, A.; Mlinarić, S.; Kovačević, J.; Cesar, V. Physiological response to different irradiation regimes during barley seedlings growth followed by drought stress under non-photoinhibitory light. JAS 2015, 7, 69–83. [Google Scholar] [CrossRef]
- Viljevac Vuletić, M.; Španić, V. Characterization of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterisation. Photosynthetica 2019, 57, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Schneiter, A.A.; Miller, J.F. Description of sunflower growth stages. Crop. Sci. 1981, 21, 901–903. [Google Scholar] [CrossRef]
- Lisogorov, S.D. Irrigated Agriculture, Textbook, 5th ed.; State Publishing House, Agricultural Literature: Moscow, Russia, 1959. [Google Scholar]
- Poormohammad Kiani, S.; Talia, P.; Maury, P.; Grieu, P.; Heinz, R.; Perrault, A.; Nishinakamasu, V.; Hopp, E.; Gentzbittel, L.; Paniego, N.; et al. Genetic analysis of plant water status and osmotic adjustment in recombinant inbred lines of sunflower under two water treatments. Plant Sci. 2007, 172, 773–787. [Google Scholar] [CrossRef]
- Siegel, B.Z.; Galston, W. The peroxidase of Pisum sativum. Plant Physiol. 1967, 42, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilising principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Hybrid | Parameter/ Year/ Condition | TR0/ABS | ABS/RC | TR0/RC | ET0/RC | DI0/RC | PIABS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | ||
| 1 | M | 0.84 a | 1.83 ghi | 1.81 fg | 1.54 efgh | 1.54 bcd | 1.11 b | 1.11 ab | 0.29 ef | 0.28 fgh | 8.03 d |
| A | 0.75 cd | 2.16 cde | 2.24 a | 1.61 cde | 1.66 a | 0.79 e | 0.73 g | 0.56 cd | 0.57 a | 1.12 g | |
| 2 | M | 0.82 a | 1.94 efghi | 1.85 fg | 1.59 defg | 1.54 bcd | 1.07 bc | 1.12 ab | 0.35 ef | 0.31 fg | 7.55 d |
| A | 0.71 ef | 2.42 bc | 1.98 cde | 1.70 cd | 1.52 cd | 0.80 e | 0.77 fg | 0.72 ab | 0.46 cd | 1.85 fg | |
| 3 | M | 0.85 a | 1.69 i | 1.82 fg | 1.43 h | 1.51 cd | 1.06 bc | 1.11 ab | 0.26 f | 0.31 f | 7.69 d |
| A | 0.75 c | 2.07 defg | 2.06 bc | 1.56 defgh | 1.57 abc | 0.83 de | 0.83 fg | 0.51 cd | 0.49 bc | 1.92 fg | |
| 4 | M | 0.85 a | 1.73 hi | 1.59 hi | 1.46 fgh | 1.36 e | 1.10 b | 1.03 bc | 0.27 f | 0.23 h | 11.55 a |
| A | 0.79 b | 1.99 defgh | 1.92 def | 1.56 defgh | 1.51 cd | 0.96 cd | 0.85 ef | 0.43 de | 0.41 de | 2.68 ef | |
| 5 | M | 0.84 a | 1.73 hi | 1.75 g | 1.45 gh | 1.48 cd | 1.09 bc | 1.11 fgh | 0.28 f | 0.27 fgh | 9.46 b |
| A | 0.76 bc | 2.11 def | 2.14 ab | 1.59 def | 1.63 ab | 0.88 de | 0.77 fg | 0.51 cd | 0.52 ab | 1.39 g | |
| 6 | M | 0.83 a | 1.83 ghi | 1.83 fg | 1.52 efhg | 1.53 bcd | 1.10 b | 1.13 a | 0.31 ef | 0.30 fg | 8.14 cd |
| A | 0.74 cde | 2.20 cde | 2.04 bcd | 1.61 cde | 1.56 bcd | 0.82 e | 0.80 fg | 0.59 bc | 0.49 bc | 1.72 fg | |
| 7 | M | 0.84 a | 1.87 fghi | 1.72 gh | 1.57 defg | 1.47 cd | 1.13 b | 1.07 abc | 0.30 ef | 0.25 gh | 9.12 bc |
| A | 0.70 f | 2.49 b | 2.02 bc | 1.74 c | 1.57 abc | 0.77 e | 0.79 fg | 0.75 a | 0.45 cd | 1.90 fg | |
| 8 | M | 0.85 a | 2.25 bcd | 1.54 i | 1.91 b | 1.31 e | 1.35 a | 0.98 cd | 0.34 ef | 0.23 h | 11.33 a |
| A | 0.72 def | 2.92 a | 1.90 ef | 2.09 a | 1.53 bcd | 0.84 de | 0.93 de | 0.84 a | 0.38 e | 3.39 e | |
| Hybrid | Parameter/ Year/ Condition | RWC | GPOX | GPOXs | |||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | ||
| 1 | M | 65.86 bcd | 73.73 a | 57.51 ef | 5.45 i | 1.49 bcd | 0.25 g |
| A | 66.25 bcd | 63.81 c | 36.82 hi | 15.03 fg | 0.86 ghi | 0.43 efg | |
| 2 | M | 73.18 a | 69.10 abc | 63.21 cde | 19.68 cde | 1.03 fghi | 1.59 a |
| A | 68.50 abc | 68.51 abc | 43.81 gh | 22.23 bcde | 0.74 hi | 0.95 bcd | |
| 3 | M | 66.79 bcd | 67.68 abc | 87.38 a | 20.25 bcde | 1.76 abc | 0.91 bcde |
| A | 66.83 bcd | 68.85 abc | 73.84 bc | 24.22 ab | 1.69 abc | 0.86 bcdef | |
| 4 | M | 66.83 bcd | 72.56 ab | 71.33 cd | 22.59 abcd | 1.92 a | 0.94 bcd |
| A | 64.45 cde | 71.46 ab | 60.84 def | 23.26 abc | 1.23 def | 0.50 defg | |
| 5 | M | 68.28 abc | 71.22 ab | 84.12 ab | 10.78 gh | 1.78 ab | 0.52 cdefg |
| A | 70.95 ab | 71.22 ab | 61.86 de | 26.78 a | 1.43 cde | 1.05 b | |
| 6 | M | 72.93 a | 70.49 ab | 65.59 cde | 17.76 ef | 1.12 efg | 0.64 bcdefg |
| A | 65.10 cde | 70.06 abc | 41.49 ghi | 21.31 bcde | 0.71 i | 1.12 ab | |
| 7 | M | 64.27 cde | 72.86 ab | 64.43 cde | 18.48 def | 1.07 fgh | 1.02 bc |
| A | 59.75 e | 68.67 abc | 39.38 ghi | 19.11 cdef | 0.72 i | 0.42 efg | |
| 8 | M | 66.86 bcd | 70.41 ab | 50.26 fg | 8.43 hi | 1.17 defg | 0.40 fg |
| A | 61.54 de | 66.98 bc | 32.72 i | 9.67 hi | 0.74 hi | 0.47 defg | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Mijić, A.; Varga, I.; Sudarić, A.; Cesar, V.; Lepeduš, H. The Combination of Increased Temperatures and High Irradiation Causes Changes in Photosynthetic Efficiency. Plants 2021, 10, 2076. https://doi.org/10.3390/plants10102076
Markulj Kulundžić A, Viljevac Vuletić M, Matoša Kočar M, Mijić A, Varga I, Sudarić A, Cesar V, Lepeduš H. The Combination of Increased Temperatures and High Irradiation Causes Changes in Photosynthetic Efficiency. Plants. 2021; 10(10):2076. https://doi.org/10.3390/plants10102076
Chicago/Turabian StyleMarkulj Kulundžić, Antonela, Marija Viljevac Vuletić, Maja Matoša Kočar, Anto Mijić, Ivana Varga, Aleksandra Sudarić, Vera Cesar, and Hrvoje Lepeduš. 2021. "The Combination of Increased Temperatures and High Irradiation Causes Changes in Photosynthetic Efficiency" Plants 10, no. 10: 2076. https://doi.org/10.3390/plants10102076






