Anti-Inflammatory Effect of Liverwort (Marchantia polymorpha L.) and Racomitrium Moss (Racomitrium canescens (Hedw.) Brid.) Growing in Korea
Abstract
1. Introduction
2. Results and Discussion
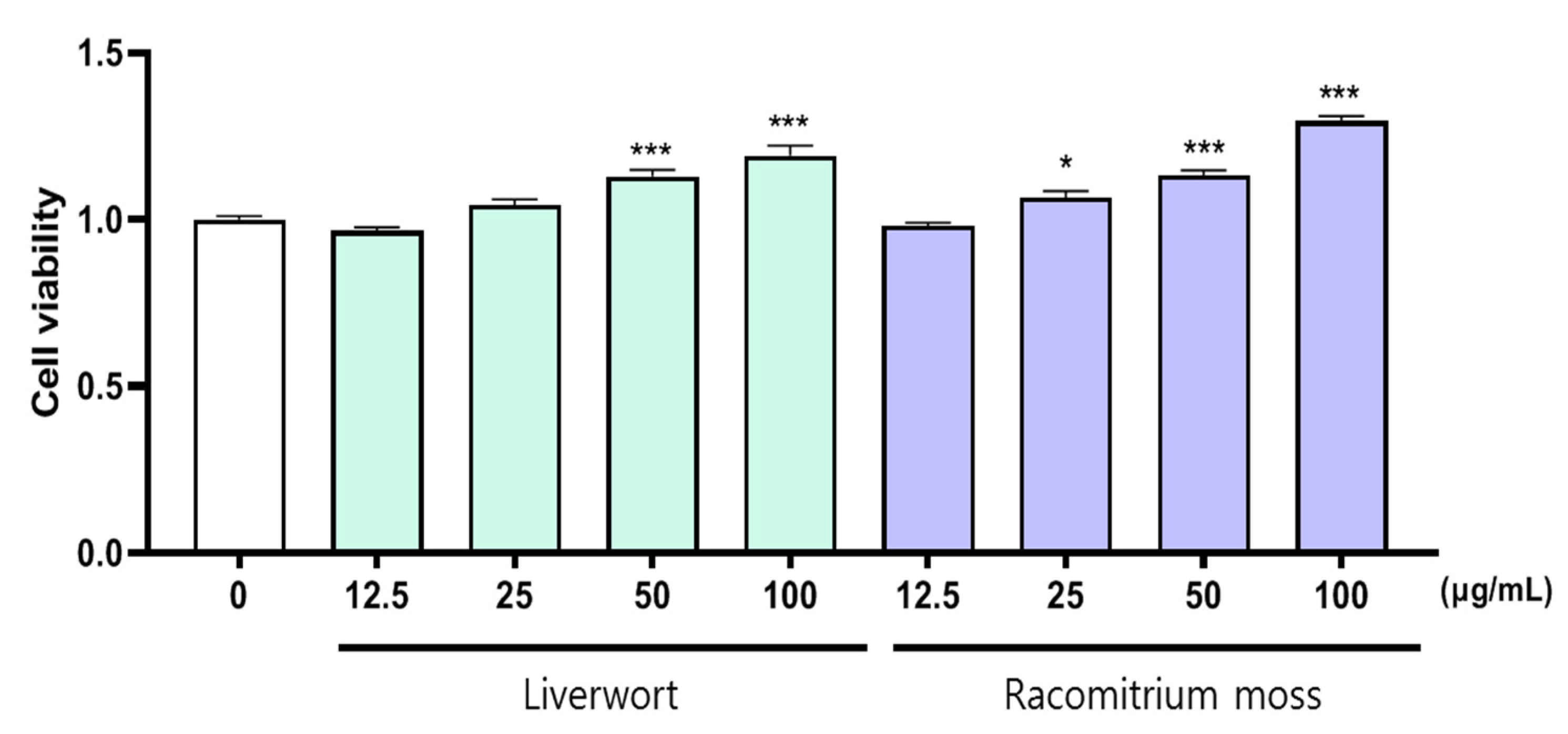
2.1. The Effect of Extract on the Viability of HaCaT Cells
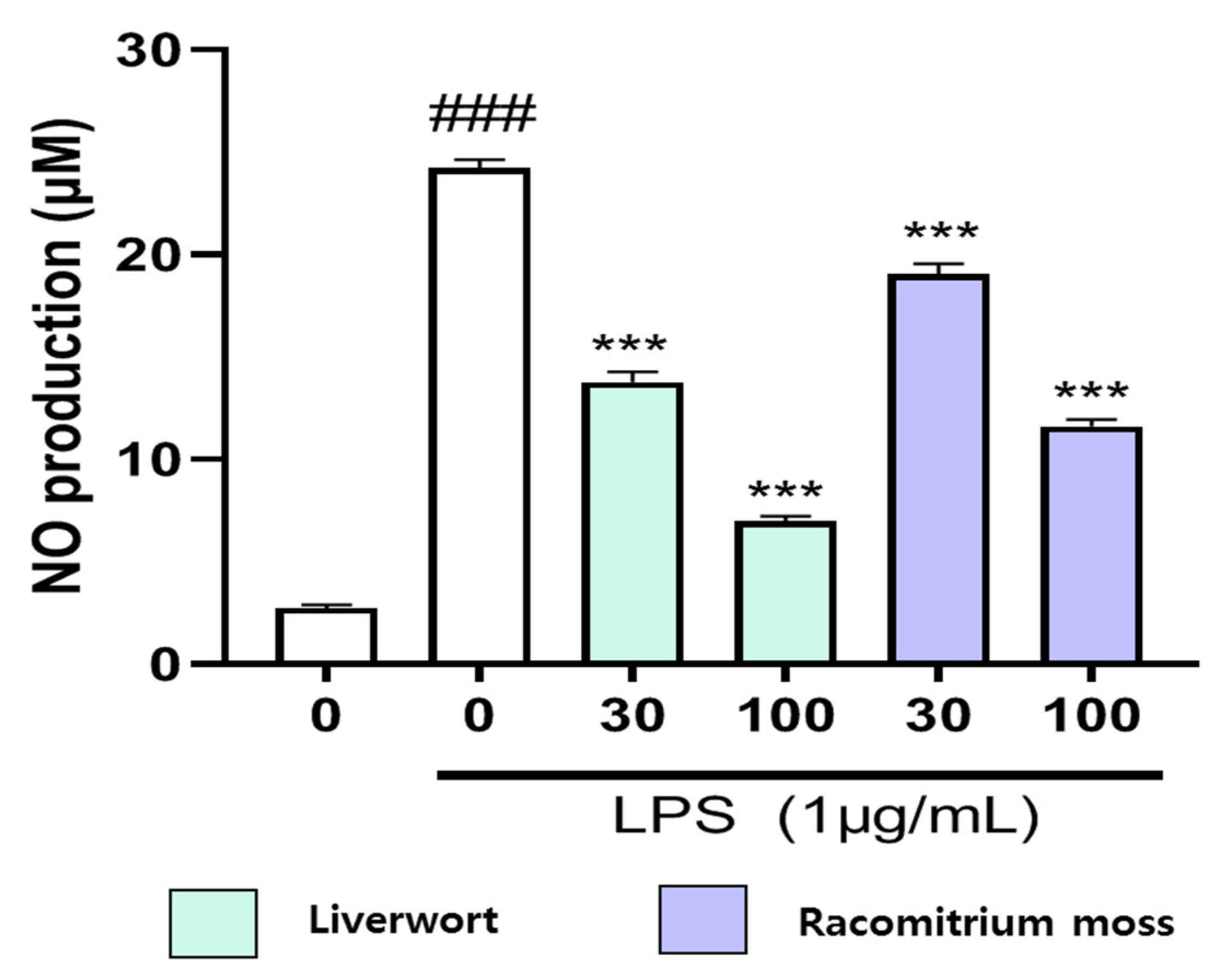
2.2. Inhibition of Nitric Oxide Production in LPS-Stimulated HaCaT Cells
2.3. The Effect of Extracts on mRNA Expression of iNOS, COX-2, TNF-α, IL-6, and IL-1β
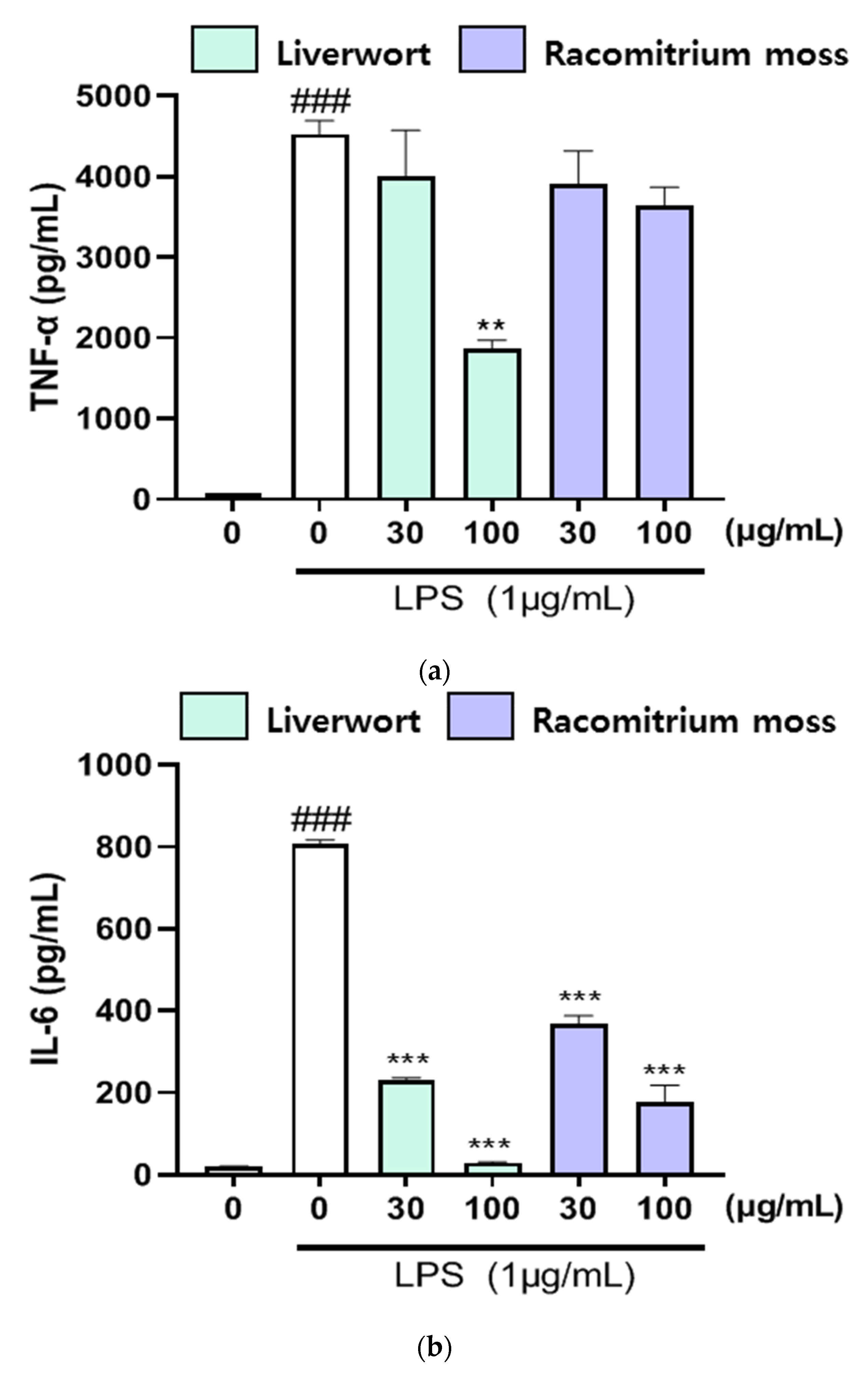
2.4. The Effect of Extracts on the Production of TNF-α, IL-16, and IL-1β
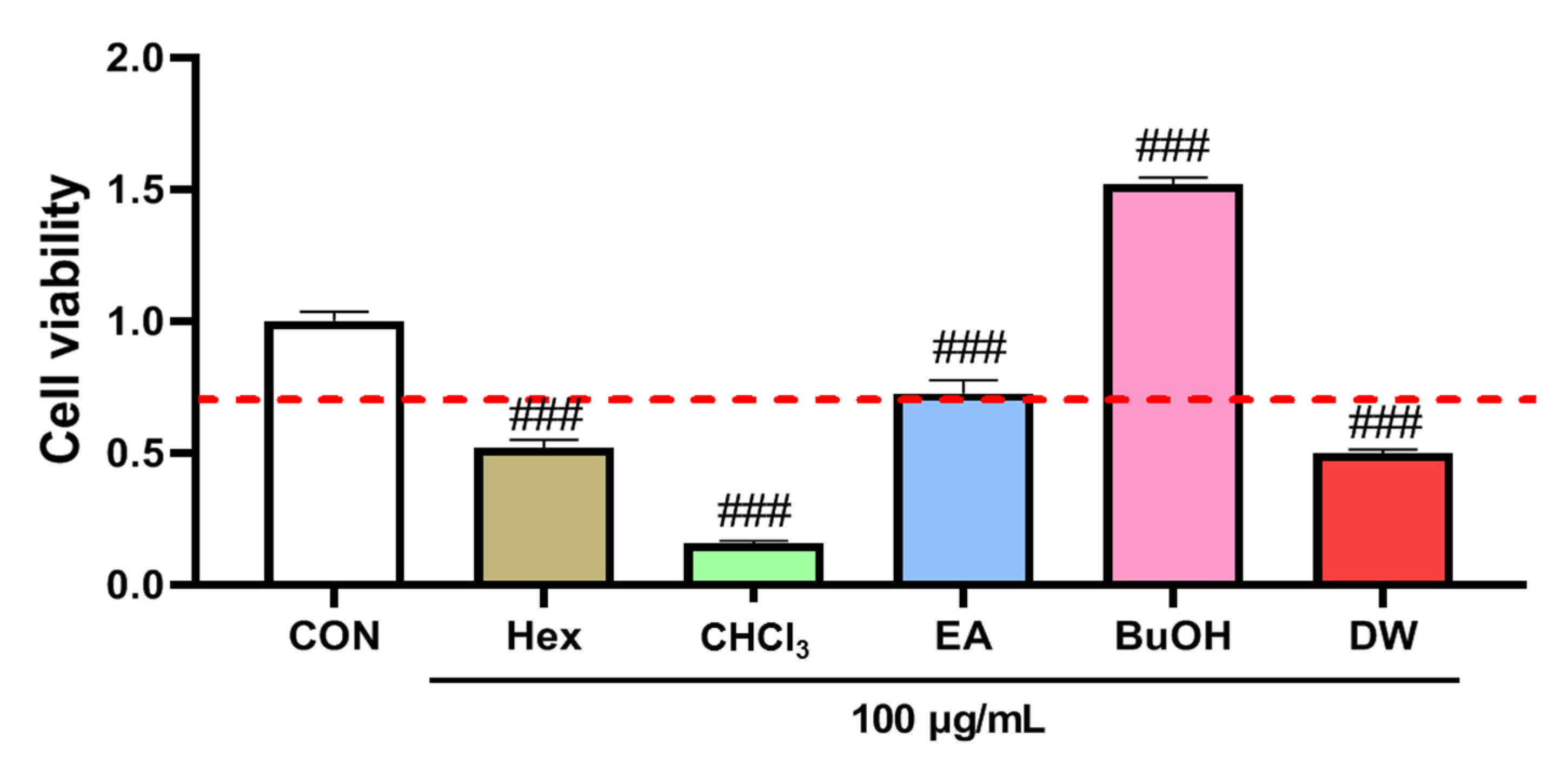
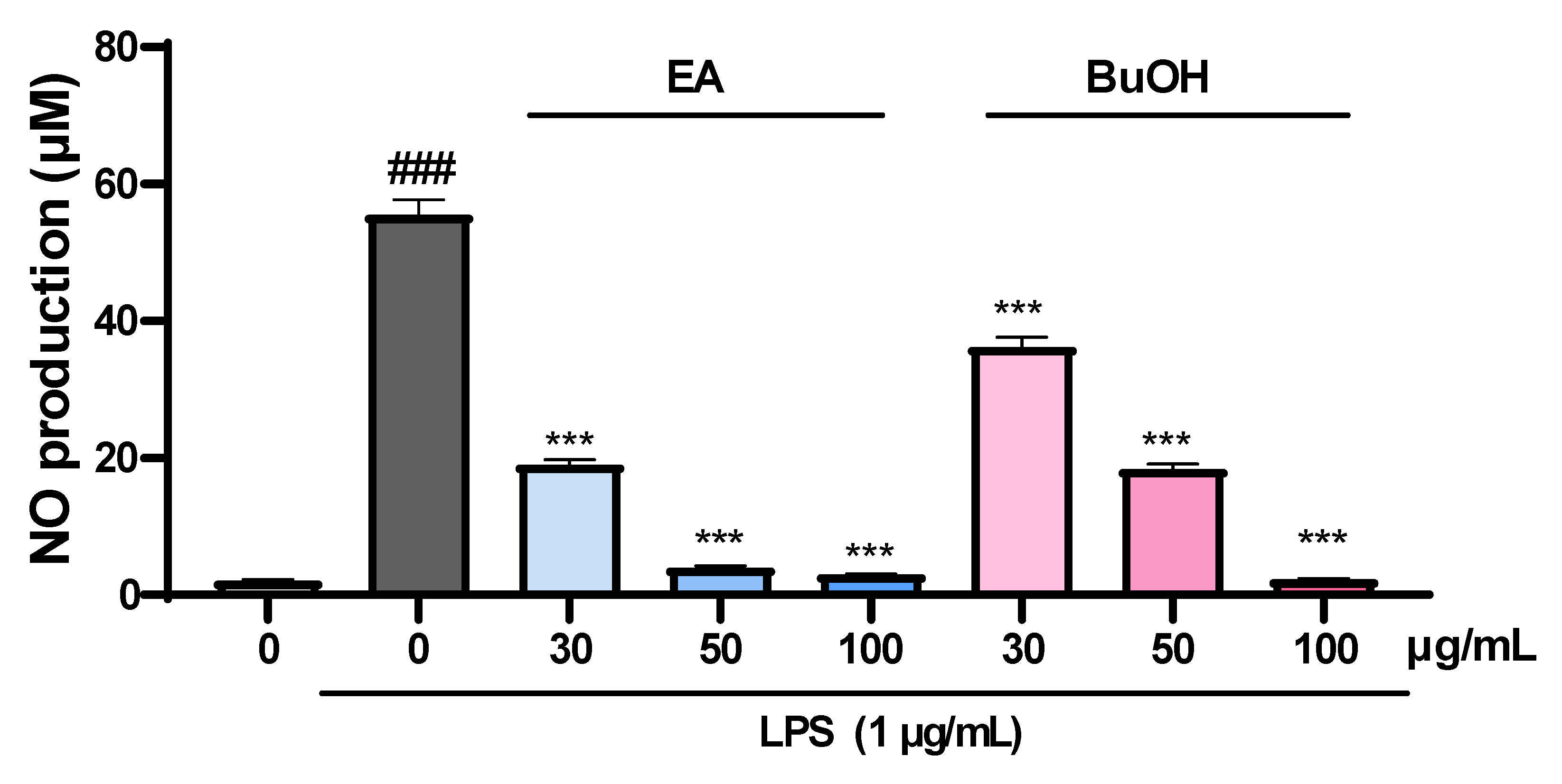
2.5. The Effect of Fractions of Liverwort on Nitric Oxide Production
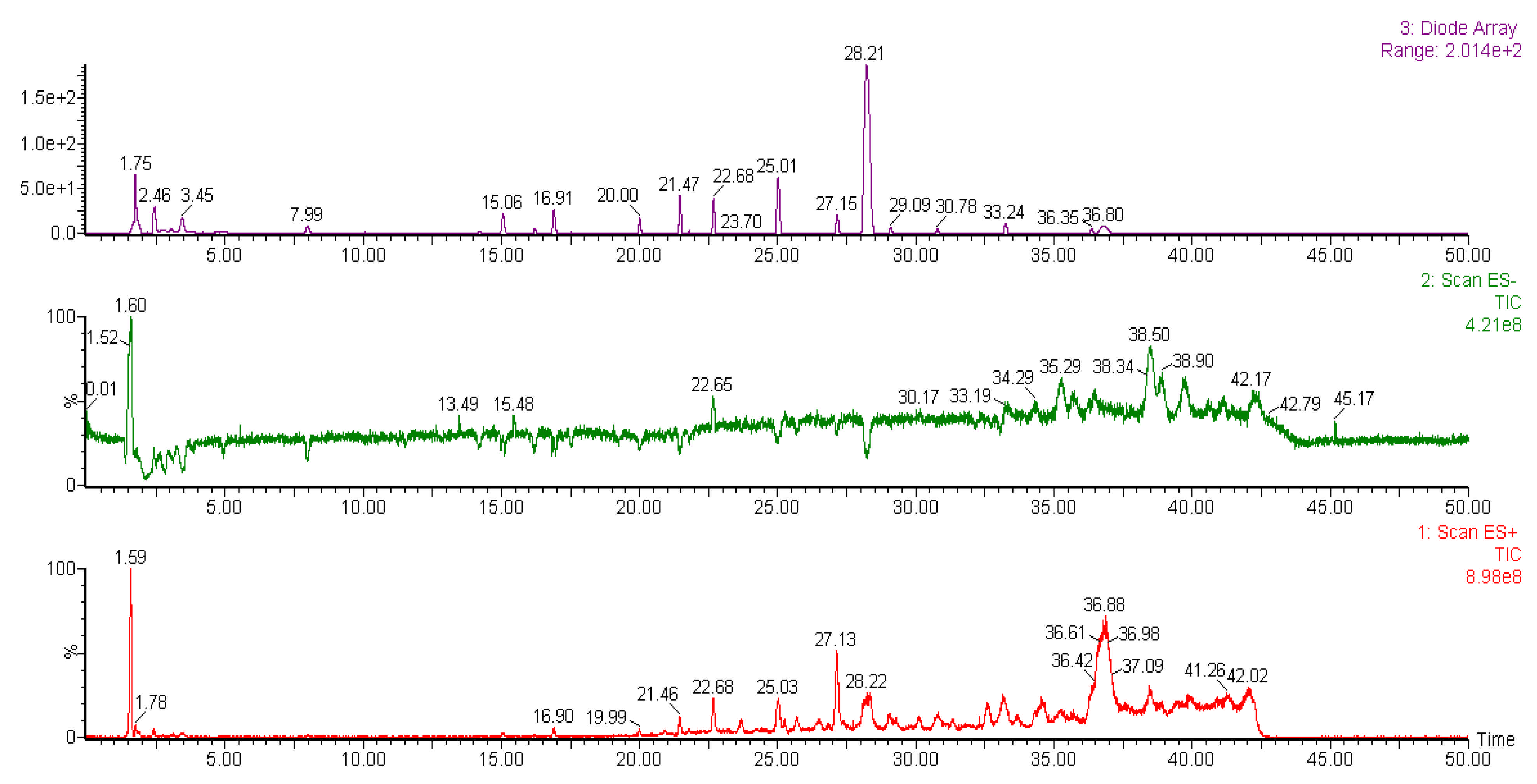
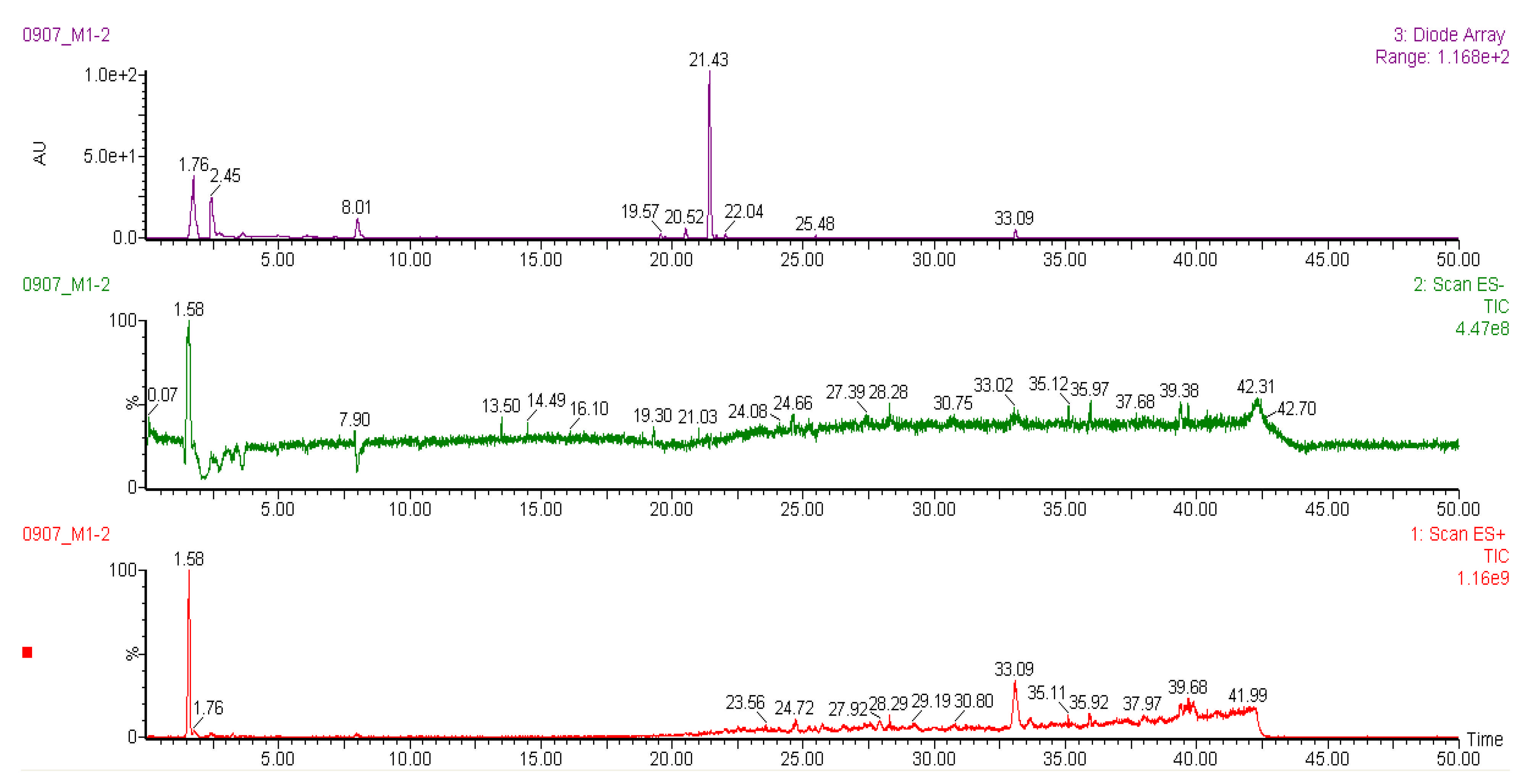
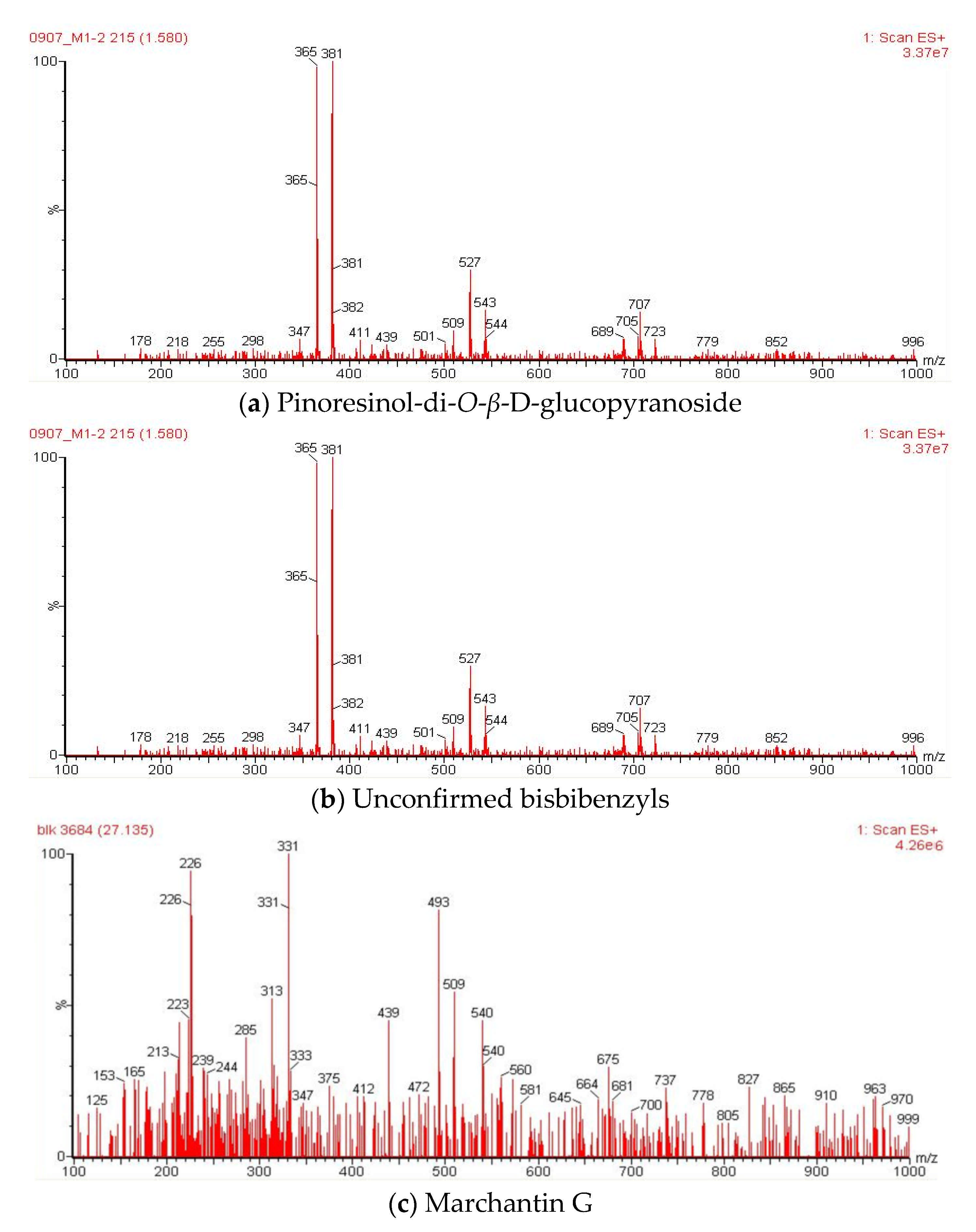
2.6. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Methanol Extracts
3. Materials and Methods
3.1. Sample Collection
3.2. Preparation of Methanol Extract
3.3. Cell Culture
3.4. Cell Viability Analysis
3.5. Measurement of Nitric Oxide
3.6. RNA Isolation and Real Time-Polymerase Chain Reaction (RT-PCR)
3.7. Enzyme-Linked Immunosorbent Assay (ELISA)
3.8. Fractionation of Methanol Extract of Liver Wort and Nitric Oxide Measurement
3.9. Liquid Chromatography-Mass Spectrometry (LCMS) Analysis of Methanol Extracts
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Han, T.H.; Lee, S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocytes HaCaT cells. J. Ethnopharmacol. 2009, 122, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, H.S.; Lee, W.; Han, E.J.; Kim, S.Y.; Fernando, I.P.S.; Ahn, G.; Kim, K.N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Wnorowska, U.; Cieśluk, M.; Deptula, P.; Pogoda, K.; Misztalewska-Turkowicz, I.; Paprocka, P.; Niemirowicz-Laskowska, K.; Wilczewska, A.Z.; Janmey, P.A. Inhibition of inflammatory response in human keratinocytes by magnetic nanoparticles functionalized with PBP10 peptide derived from the PIP2-binding site of human plasma gelsolin. J. Nanobiotechnol. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef]
- Liang, Q.; Chalamaiah, M.; Liao, W.; Ren, X.; Ma, H.; Wu, J. Zein hydrolysate and its peptides exert anti-inflammatory activity on endothelial cells by preventing TNF-alpha-induced NF-kappa B activation. J. Funct. Foods 2020, 64, 103598. [Google Scholar] [CrossRef]
- Zhao, B.; Li, R.; Yang, F.; Yu, F.; Xu, N.; Zhang, F.; Ge, X.; Du, J. LPS-induced vitamin D receptor decrease in oral Keratinocytes is associated with oral lichen planus. Sci. Rep. 2018, 8, 763. [Google Scholar] [CrossRef]
- Lu, Y.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Simonsen, H.T. Valuable fatty acids in bryophytes—Production, biosynthesis, analysis and applications. Plants 2019, 8, 524. [Google Scholar] [CrossRef]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberg, I. Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef]
- Tosun, A.; Nagashima, F.; Asakawa, Y. Terpenoid and steroid components of selected liverworts. Chem. Nat. Compd. 2015, 51, 387–391. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.K. Bryophytes: Hoard of remedies, an ethno-medicinal review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Veldman, S.; Larraın, J.; Munoz, J.; Quandt, D.; Hassel, K.; Kruijer, H. Molecular species delimitation in the Racomitrium canescens Complex (Grimmiaceae) and implications for DNA barcoding of species complexes in mosses. PLoS ONE 2013, 8, e53134. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef]
- Tosun, A.; Küpeli Akkol, E.; Süntar, I.; Özenoğlu Kiremit, H.; Asakawa, Y. Phytochemical investigations and bioactivity evaluation of liverworts as a function of anti-inflammatory and antinociceptive properties in animal models. Pharm. Biol. 2013, 51, 1008–1013. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Asakawa, Y. Chemotaxonomic value of essential oil components in liverwort species. A review. Flavour Fragr. J. 2015, 30, 189–196. [Google Scholar] [CrossRef]
- Romani, F.; Banić, E.; Florent, S.N.; Kanazawa, T.; Goodger, J.Q.D.; Mentink, R.A.; Dierschke, T.; Zachgo, S.; Ueda, T.; Bowman, J.L. Oil body formation in Marchantia polymorpha is controlled by MpC1HDZ and serves as a defense against arthropod herbivores. Curr. Biol. 2020, 30, 2815–2828. [Google Scholar] [CrossRef]
- Asakawa, Y. Biologically active compounds from bryophytes. Pure Appl. Chem. 2007, 79, 557–580. [Google Scholar] [CrossRef]
- Mewari, N.; Kumar, P. Antimicrobial activity of extracts of Marchantia polymorpha. Pharm. Biol. 2008, 46, 819–822. [Google Scholar] [CrossRef]
- Guo, X.L.; Leng, P.; Yang, Y.; Yu, L.G.; Lou, H.X. Plagiochin E, a botanic-derived phenolic compound, reverses fungal resistance to fluconazole relating to the efflux pump. J. Appl. Microbiol. 2008, 104, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gökbulut, A.; Satilmi, B.; Batçioğlu, K.; Çetin, B.; Şarer, E. Antioxidant activity and luteolin content of Marchantia polymorpha L. Turk. J. Biol. 2012, 36, 381–385. [Google Scholar]
- Rana, M.; Pant, J.; Jantwal, A.; Rana, A.J.; Upadhyay, J.; Bisht, S.S. In vitro anti-inflammatory and antioxidant activity of ethanolic extract of Marchantia polymorpha in Kumaun region. World J. Pharm. Res. 2018, 7, 864–875. [Google Scholar]
- Jensen, J.S.R.E.; Omarsdottir, S.; Thorsteinsdottir, J.B.; Ogmundsdottir, H.M.; Olafsdottir, E.S. Synergistic Cytotoxic Effect of the Micro tubule Inhibitor Marchantin A from Marchantia polymorpha and the Aurora Kinase Inhibitor MLN 8237 on Breast Cancer Cells In Vitro. Planta Med. 2012, 78, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.R.; Lee, T.H.; Bang, M.H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-β-d-glucopyanosylspinasterol via blocking NF-κB and STAT1 signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, M.S.; Jeong, G.S.; Yoon, J. Xanthii fructus extract inhibits TNF-α/ IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M. Protective effect of strawberry extract against inflammatory stress induced in human dermal fibroblasts. Molecules 2017, 22, 164. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Zhao, B.T.; Lee, J.H.; Kim, Y.H.; Min, B.S.; Woo, M.H. Isolation of benzoic and cinnamic acid derivatives from the grains of Sorghum bicolor and their inhibition of lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells. Food Chem. 2015, 168, 512–519. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Quang, D.N.; Nishizawa, T.; Hashimoto, T.; Kohichi, C.; Soma, G.I.; Asakawa, Y. Bioactive compounds from liverworts: Inhibition of lipopolysaccharide-induced inducible NOS mRNA in RAW 264.7 cells by herbertenoids and cuparenoids. Phytomedicine 2007, 14, 486–491. [Google Scholar] [CrossRef]
- Quang, D.N.; Asakawa, Y. Chemical constituents of the Vietnamese liverwort Porella densifolia. Fitoterapia 2010, 81, 659–661. [Google Scholar] [CrossRef]
- Li, S.; Niu, H.; Qiao, Y.; Zhu, R.; Sun, Y.; Ren, Z.; Yuan, H.; Gao, Y.; Li, Y.; Chen, W.; et al. Terpenoids isolated form Chinese liverworts Lepidozia reptans and their anti-inflammatory activity. Bioorg. Med. Chem. 2018, 26, 2392–2400. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, R.; Zhang, J.; Wu, X.; Shen, T.; Zhou, J.; Qiao, Y.; Gao, Y.; Lou, H. Clerodane diterpenoids from the Chinese liverwort Jamesoniella autumnalis and their anti-inflammatory activity. Phytochemistry 2018, 154, 85–93. [Google Scholar] [CrossRef]
- Choi, W.S.; Jeong, J.W.; Kim, S.O.; Kim, G.Y.; Kim, B.W.; Kim, C.M.; Seo, Y.B.; Kim, W.Y.; Lee, S.Y.; Jo, K.H. Anti-inflammatory potential of peat moss extracts in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2014, 34, 1101–1109. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Kim, D.H.; An, B.J.; Kim, S.G.; Park, T.S.; Park, G.H.; Son, J.H. Anti-inflammatory effect of Ligularia fischeri, Solidago virga-aurea and Aruncus dioicus complex extracts in Raw 264.7 cells. J. Life Sci. 2011, 21, 678–683. [Google Scholar] [CrossRef]
- Liu, Y.W.; Mei, H.C.; Su, Y.W.; Fan, H.T.; Chen, C.C.; Tsai, Y.C. Inhibitory effects of Pleurotus tuber-regium mycelia and bioactive constituents on LPS-treated RAW 264.7 cells. J. Funct. Foods 2014, 7, 662–670. [Google Scholar] [CrossRef]
- He, Y.; Kim, B.; Kim, H.E.; Sun, Q.; Shi, S.; Ma, G.; Kim, Y.; Kim, O.; Kim, O. The protective role of feruloylserotonin in LPS-induced HaCaT Cells. Molecules 2019, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Kang, N.J.; Han, S.C.; Koo, D.H.; Kang, H.K.; Yoo, B.S.; Yoo, E.S. The chloroform fraction of Carpinus tschonoskii leaves inhibits the production of inflammatory mediators in HaCaT keratinocytes and RAW264.7 macrophages. Toxicol. Res. 2012, 28, 255–262. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Xie, R.; Jiang, C.; Liu, M. Schizandrin A alleviates LPS-Induced injury in human keratinocyte cell HaCaT through a microRNA-127-dependent regulation. Cell Physiol. Biochem. 2018, 49, 2229–2239. [Google Scholar] [CrossRef]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Asakawa, Y. Bryophytes as a source of bioactive volatile terpenoids—A review. Food Chem. Toxicol. 2019, 132, 110649. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.-S.; Xu, L.-L.; Zhang, J.-Y.; Wang, Z.-J.; Jiang, Y.-Y.; Liu, B. Rapid Characterization and identification of non-diterpenoid constituents in Tinospora sinensis by HPLC-LTQ-Orbitrap MSn. Molecules 2018, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Choi, S.I.; Son, S.R.; Han, H.S.; Ahn, H.S.; Shin, Y.K.; Lee, S.H.; Lee, K.T.; Kwon, H.C.; Jang, D.S. Chemical constituents of the leaves of Campanula takesimana (Korean Bellflower) and their inhibitory effects on LPS-induced PGE2 production. Plants 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Sabovljević, M.S.; Vujičić, M.; Wang, X.; Garraffo, H.M.; Bewley, C.A.; Sabovljević, A. Production of the macrocyclic bis-bibenzyls in axenically farmed and wild liverwort Marchantia polymorpha L. subsp. ruderalis Bischl. et Boisselier. Plant Biosyst. 2017, 151, 414–418. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Lv, B.B.; Sun, B.; Zhu, C.J.; Lou, H.X. LC-DAD/MS/MS detection of macrocyclic bisbibenzyls from the liverwort Reboulia hemisphaerica and the cell-based screening of their microtubule inhibitory effects. Chin. J. Nat. Med. 2009, 7, 123–128. [Google Scholar] [CrossRef]
- Xing, J.; Xie, C.; Qu, J.; Guo, H.; Lv, B.; Lou, H. Rapid screening for bisbibenzyls in bryophyte crude extracts using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2467–2476. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Wu, Z.; Ding, L. ESI-QqTOF-MS/MS and APCI-IT-MS/MS analysis of steroid saponins from the rhizomes of Dioscorea panthaica. J. Mass Spectrom. 2006, 41, 1–22. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Quang, D.N.; Takeshi, N.; Hashimoto, T.; Kohchi, C.; Soma, G.; Asakawa, Y. Bis(bibenzyls) from liverworts inhibit lipopolysaccharide-induced inducible NOS in RAW 264.7 cells: A study of structure-activity relationships and molecular mechanism. J. Nat. Prod. 2005, 68, 1779–1781. [Google Scholar] [CrossRef]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective effect of brown alga Phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NF-κB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Gair, B.P.; Kang, M.G.; Choi, J.W. S1P2 contributes to microglial activation and M1 polarization following cerebral ischemia through ERK1/2 and JNK. Sci. Rep. 2019, 9, 12106. [Google Scholar] [CrossRef] [PubMed]




| Peak | Tentative Identification | Retention Time (min) | Molecular Mass (Da) | Fragments (m/z) | References |
|---|---|---|---|---|---|
| Liverwort | |||||
| 1 | Pinoresinol-di-O-β-D-glucopyranoside | 1.59 | 705 | 707, 543, 527, 381, 365 | [40] |
| 2 | Unknown | 1.78 | 517 | 826, 625, 517, 381, 204 | - |
| 3 | Unconfirmed bisbibenzyl | 15.05 | 421 | 463, 423, 411, 309, 213 | [43] |
| 4 | Unknown | 16.90 | 447 | 621, 447, 271, 145 | - |
| 5 | Unknown | 19.90 | 674 | 970, 806, 772, 674, 597 | - |
| 6 | Unknown | 21.46 | 565 | 903, 858, 565, 431, 322, | - |
| 7 | Unknown | 22.68 | 658 | 917, 833, 658, 483, 158 | - |
| 8 | Unconfirmed bisbibenzyl | 25.03 | 424 | 479, 452, 417, 239, 142 | [44] |
| 9 | Marchantin G | 27.13 | 454 | 493, 439, 331, 313, 226 | [42] |
| 10 | Unconfirmed bisbibenzyl | 28.22 | 454 | 463, 458, 333, 224, 197 | [44] |
| 11 | Unknown | 36.88 | 599 | 975, 907, 599, 256, 157 | - |
| Racomitrium moss | |||||
| 1 | Pinoresinol-di-O-β-D-glucopyranoside | 1.58 | 705 | 707, 543, 527, 381, 365 | [40] |
| 2 | Dioscoreside A | 7.94 | 784 | 931, 793, 767, 753, 268 | [45] |
| 3 | Unknown | 25.75 | 748 | 748, 657, 603, 505, 411 | - |
| 4 | Unknown | 33.09 | 518 | 518, 497, 263, 215, 205 | - |
| Target Gene | Primer Sequence | |
|---|---|---|
| iNOS | Forward | 5′-CATTGATCTCCGTGACAGCC-3′ |
| Reverse | 5′-CATGCTACTGGAGGTGGGTG-3′ | |
| COX-2 | Forward | 5′-GCAGCCATTTCCTTCTCTCC-3′ |
| Reverse | 5′-TGCTGTACAAGCAGTGGCAA-3′ | |
| TNF-α | Forward | 5′-CTG ATG AGA GGG AGG CCA TT-3′ |
| Reverse | 5′- AGC ACA GAA AGC ATG ATC CG-3′ | |
| IL-6 | Forward | 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| Reverse | 5′-GAGGATACCACTCCCAACAGACC-3′ | |
| IL-1β | Forward | 5′-TCGTTGCTTGGTTCTCCTTG-3′ |
| Reverse | 5′-ACCTGCTGGTGTGTGACGTT-3′ | |
| β-actin | Forward | 5′-TCAGCAATGCCTGGGTACAT-3′ |
| Reverse | 5′-ATCACTATTGGCAACGAGCG-3′ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Hong, M.; Kim, T.-H.; Lee, K.Y.; Park, S.J.; Hong, S.H.; Sowndhararajan, K.; Kim, S. Anti-Inflammatory Effect of Liverwort (Marchantia polymorpha L.) and Racomitrium Moss (Racomitrium canescens (Hedw.) Brid.) Growing in Korea. Plants 2021, 10, 2075. https://doi.org/10.3390/plants10102075
Kim S-Y, Hong M, Kim T-H, Lee KY, Park SJ, Hong SH, Sowndhararajan K, Kim S. Anti-Inflammatory Effect of Liverwort (Marchantia polymorpha L.) and Racomitrium Moss (Racomitrium canescens (Hedw.) Brid.) Growing in Korea. Plants. 2021; 10(10):2075. https://doi.org/10.3390/plants10102075
Chicago/Turabian StyleKim, So-Yeon, Minji Hong, Tae-Hee Kim, Ki Yeon Lee, Se Jin Park, Sun Hee Hong, Kandhasamy Sowndhararajan, and Songmun Kim. 2021. "Anti-Inflammatory Effect of Liverwort (Marchantia polymorpha L.) and Racomitrium Moss (Racomitrium canescens (Hedw.) Brid.) Growing in Korea" Plants 10, no. 10: 2075. https://doi.org/10.3390/plants10102075
APA StyleKim, S.-Y., Hong, M., Kim, T.-H., Lee, K. Y., Park, S. J., Hong, S. H., Sowndhararajan, K., & Kim, S. (2021). Anti-Inflammatory Effect of Liverwort (Marchantia polymorpha L.) and Racomitrium Moss (Racomitrium canescens (Hedw.) Brid.) Growing in Korea. Plants, 10(10), 2075. https://doi.org/10.3390/plants10102075







