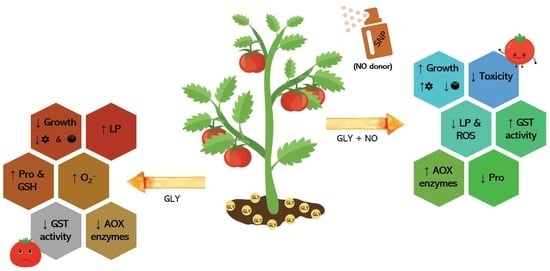
Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways
Abstract
:1. Introduction
2. Results
2.1. Biometric Analysis—Fresh Biomass and Root Length
2.2. Soluble Protein and Nitrate Reductase (NR) Activity
2.3. Biomarkers of Oxidative Stress
2.3.1. Superoxide Anion (O2•−) and Hydrogen Peroxide (H2O2)
2.3.2. Malondialdehyde (MDA) Content
2.4. Evaluation of the Non-Enzymatic Antioxidant Response
2.4.1. Ascorbate (AsA), Glutathione (GSH) and Proline
2.4.2. Total Phenolic Content (TPC), Flavonoids and Total Antioxidant Capacity (TAC)
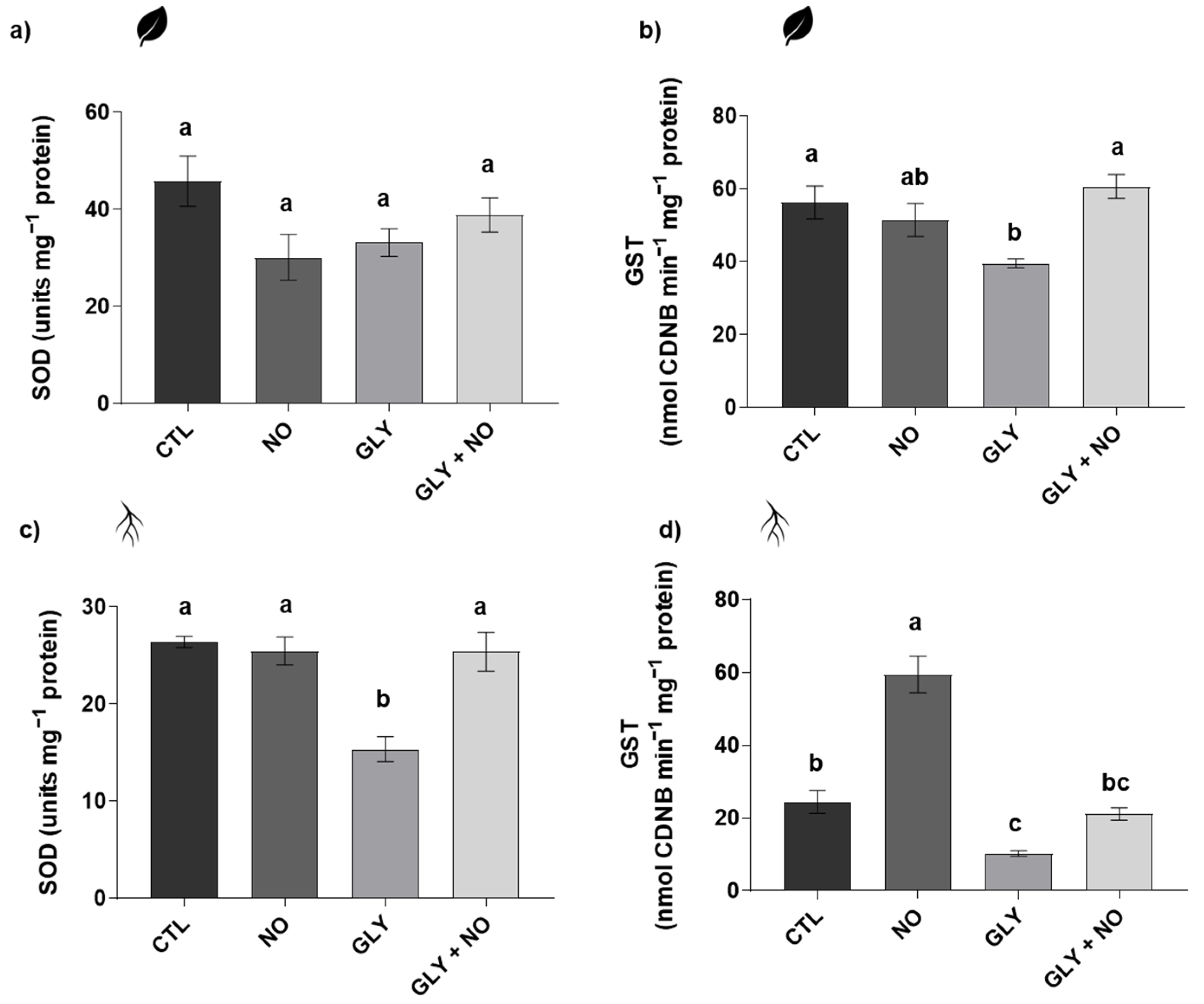
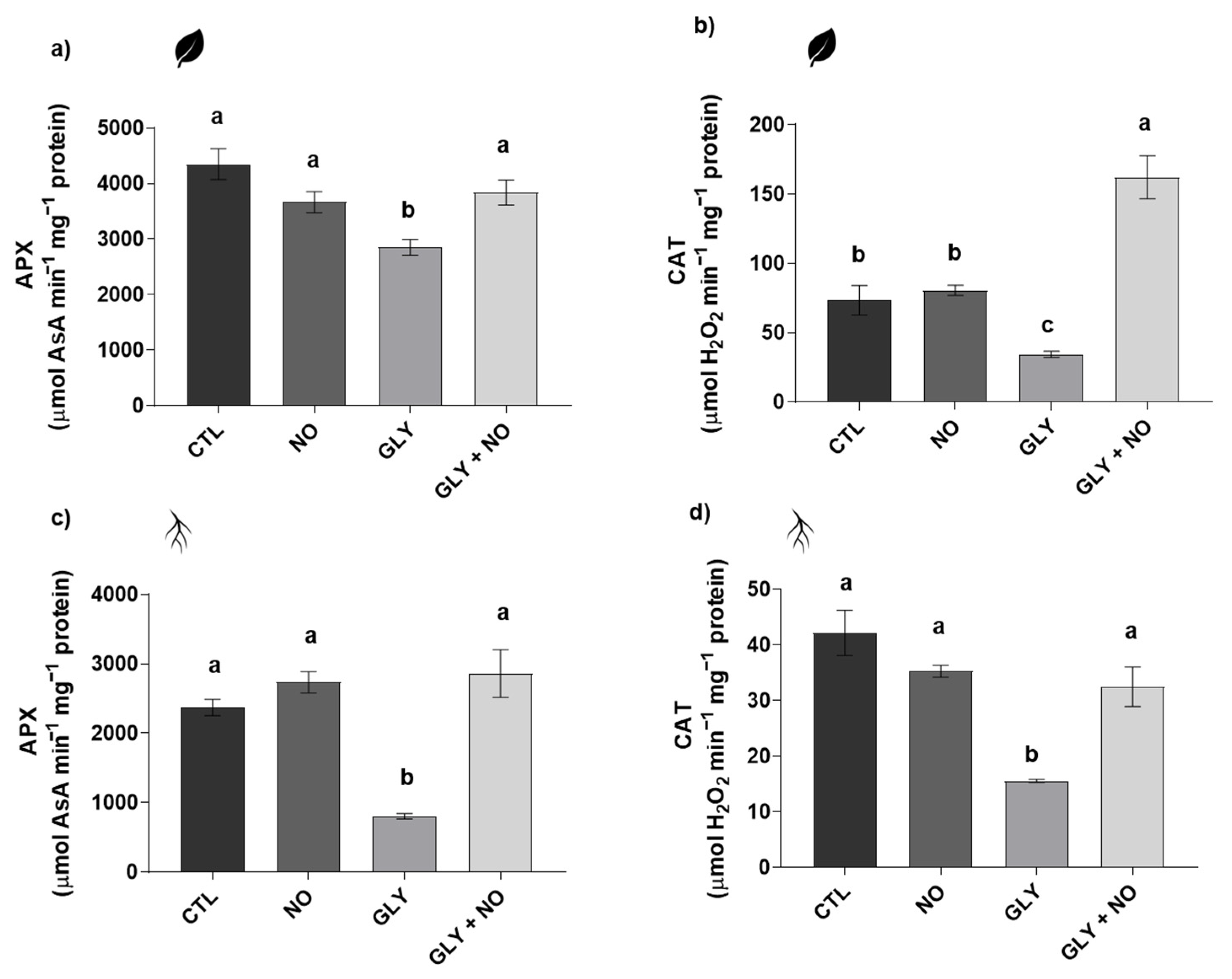
2.5. Antioxidant Enzymes Activity (Superoxide Dismutase (SOD, EC 1.15.11); Glutathione S-Transferase (GST, EC 2.5.1.18); Catalase (CAT, EC 2.5.1.18); Ascorbate Peroxidase (APX, EC 1.11.1.11))
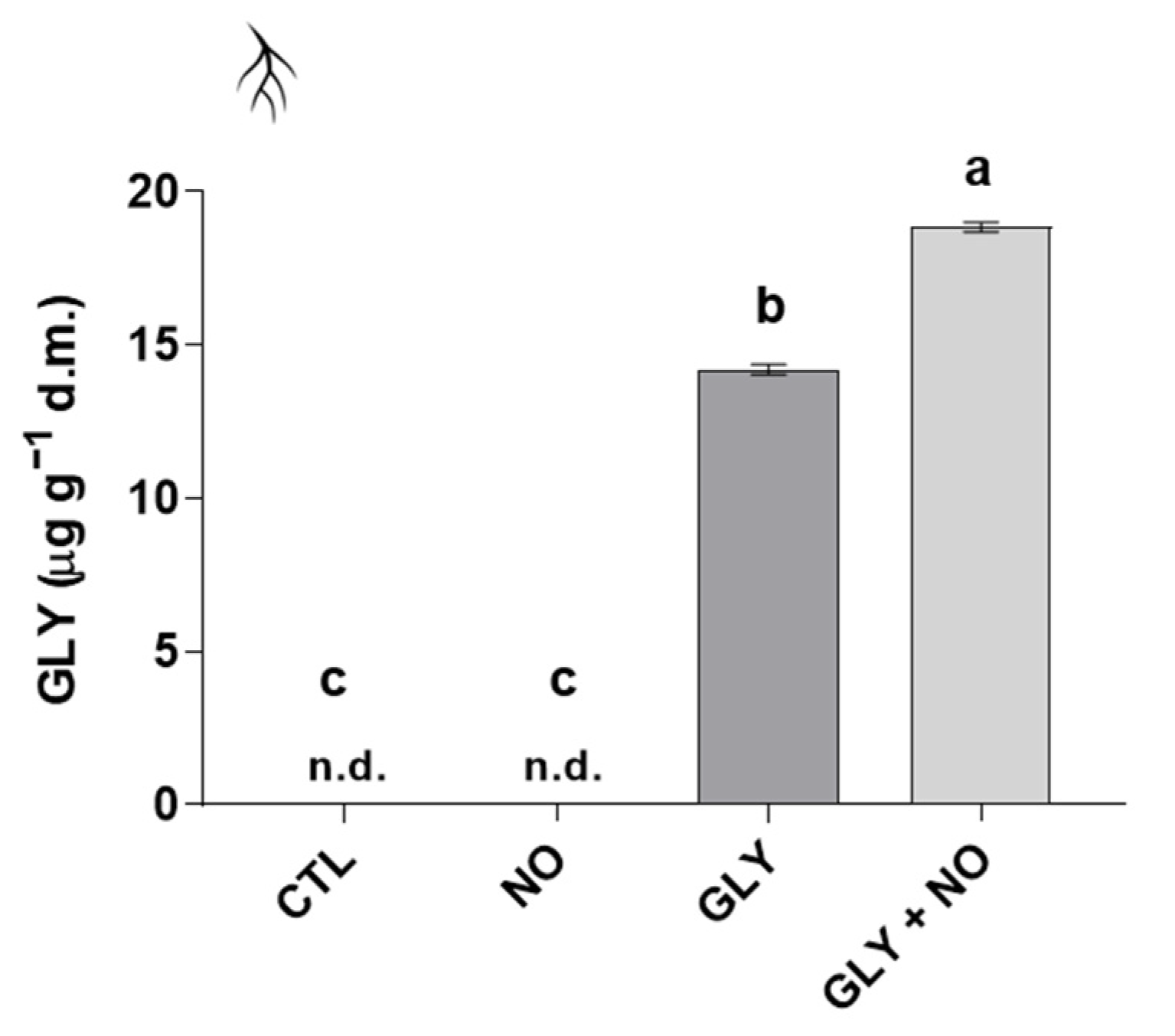
2.6. Bioaccumulation of GLY
2.7. Productivity-Related Traits
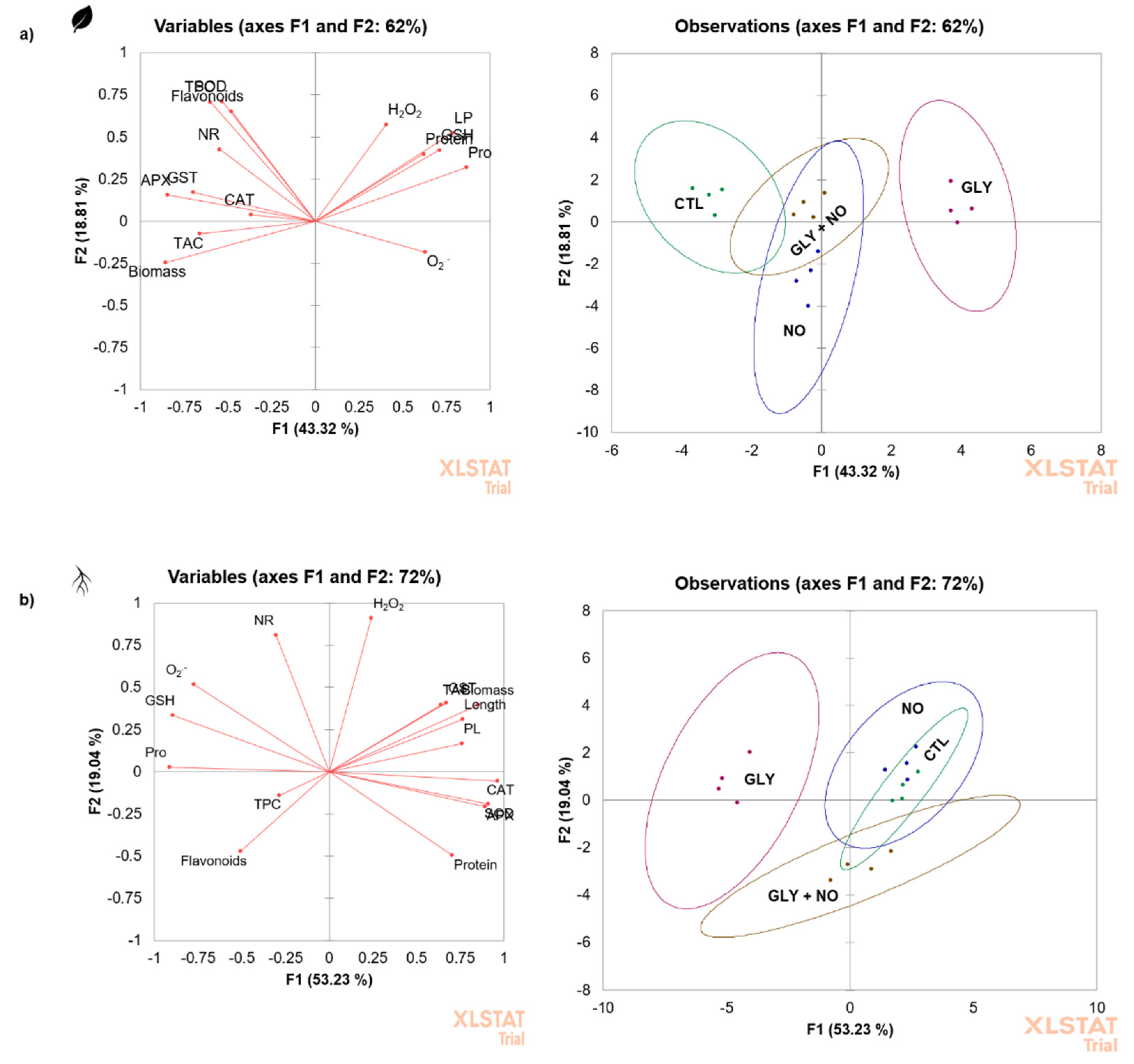
2.8. Principal Component Analysis (PCA)
3. Discussion
3.1. GLY Disrupted Tomato Plants’ Growth, but NO Partially Reduced Its Macroscopic Phytotoxicity
3.2. GLY Disrupted the Cellular Redox State, but NO Managed to Keep ROS under Control
3.3. Antioxidant Metabolites Are Not Directly Related to NO-Mediated Restoration of the Redox Balance Disrupted by GLY
3.4. NO-Mediated Alleviation of GLY Phytotoxicity Involves the Upregulation of the Main Antioxidant Enzymes
3.5. Detoxification Pathways Impaired by GLY Are Stimulated by the Exogenous Application of NO
3.6. GLY-Mediated Effects on Crop Productivity Are Partially Prevented by the Co-Application of NO
4. Materials and Methods
4.1. Chemicals and Test Substrate
4.2. Plant Material, Plant Growth Conditions and Experimental Design
4.3. Biometric and Productivity-Related Analysis
4.4. Total Protein Content and NR (EC 1.7.1.1) Activity
4.5. Biomarkers of Oxidative Stress
4.5.1. O2•− and H2O2
4.5.2. LP
4.6. Evaluation of Antioxidant Metabolites
4.6.1. Quantification of AsA, GSH and Proline
4.6.2. Determination of TPC, Total Flavonoids and TAC
4.7. Extraction of Antioxidant Enzymes
4.8. Spectrophotometric Activity Quantification of SOD (EC 1.15.1.1), CAT (EC.1.11.1.6), APX (EC.1.11.1.11) and GST (EC2.5.1.18)
4.9. Analytical Quantification of GLY and AMPA
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antier, C.; Kudsk, P.; Reboud, X.; Ulber, L.; Baret, P.V.; Messéan, A. Glyphosate Use in the European Agricultural Sector and a Framework for Its Further Monitoring. Sustainability 2020, 12, 5682. [Google Scholar] [CrossRef]
- Ellis, J.M.; Griffin, J.L. Soybean (Glycine max) and Cotton (Gossypium hirsutum) Response to Simulated Drift of Glyphosate and Glufosinate. Weed Technol. 2002, 16, 580–586. [Google Scholar] [CrossRef]
- Neumann, G.; Kohls, S.; Richter, E.; Stock, K.; Souza, O.; Yamada, T.; Römheld, V. Relevance of glyphosate transfer to non-target plants via the rhizosphere. J. Plant Dis. Prot. 2006, 20, 963. [Google Scholar]
- Alves, P.A.d.T.; Ricord, A.; Tornisielo, V.L. Translocação de 14C-Glifosato entre Brachiaria brizantha e mudas de café (Coffea arabica) e citros (Citrus limonia Osbeck). In Proceedings of the Simpósio Internacional de Iniciação Científica da Universidade de São Paulo-SIICUSP, São Paulo, Brazil, 22–26 March 2008. [Google Scholar]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants?—Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Soares, C.; Fidalgo, F. Salicylic acid alleviates glyphosate-induced oxidative stress in Hordeum vulgare L. J. Environ. Manag. 2019, 241, 226–234. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide in plants: The history is just beginning. Plant. Cell Environ. 2001, 24, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric Oxide: A Multitasked Signaling Gas in Plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Krasylenko, Y.; Yemets, A.; Blume, Y. Cell Mechanisms of Nitric Oxide Signaling in Plants Under Abiotic Stress Conditions. In Mechanism of Plant Hormone Signaling under Stress; Pandey, G.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 371–398. ISBN 9781118888926. [Google Scholar]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2019, 168, 318–344. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef]
- Keisham, M.; Jain, P.; Singh, N.; von Toerne, C.; Bhatla, S.C.; Lindermayr, C. Deciphering the nitric oxide, cyanide and iron-mediated actions of sodium nitroprusside in cotyledons of salt stressed sunflower seedlings. Nitric Oxide 2019, 88, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Planchet, E.; Kaiser, W.M. Nitric oxide production in plants: Facts and fictions. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Fancy, N.N.; Bahlmann, A.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant. Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Mohsin, S.M.; Al Mahmud, J.; Parvin, K.; Fujita, M. Exogenous Nitric Oxide- and Hydrogen Sulfide-induced Abiotic Stress Tolerance in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, A., Tripathi, D.K., Eds.; Wiley Online Books: Hoboken, NJ, USA, 2020; pp. 174–213. ISBN 9781119552154. [Google Scholar]
- Arora, D.; Jain, P.; Singh, N.; Kaur, H.; Bhatla, S.C. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic. Res. 2016, 50, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: A synergistic signaling approach. J. Stress Physiol. Biochem. 2011, 7, 34–74. [Google Scholar]
- Singh, H.; Singh, N.B.; Singh, A.; Hussain, I.; Yadav, V. Physiological and biochemical roles of nitric oxide against toxicity produced by glyphosate herbicide in Pisum sativum. Russ. J. Plant Physiol. 2017, 64, 518–524. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Martins, M.; Tamagnini, P.; Serôdio, J.; Moutinho-Pereira, J.; Cunha, A.; Fidalgo, F. Glyphosate-dependent effects on photosynthesis of Solanum lycopersicum L.—An ecophysiological, ultrastructural and molecular approach. J. Hazard. Mater. 2020, 398, 122871. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A Model Fruit-Bearing Crop. CSH Protoc. 2008, 2008, pdb.emo105. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Manac’h, S.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Singh, H.; Singh, N.B.; Singh, A.; Hussain, I. Exogenous Application of Salicylic Acid to Alleviate Glyphosate Stress in Solanum lycopersicum. Int. J. Veg. Sci. 2017, 23, 552–566. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Maccario, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic. Biochem. Physiol. 2016, 130, 65–70. [Google Scholar] [CrossRef]
- Bellaloui, N.; Reddy, K.N.; Zablotowicz, R.M.; Mengistu, A. Simulated Glyphosate Drift Influences Nitrate Assimilation and Nitrogen Fixation in Non-glyphosate-Resistant Soybean. J. Agric. Food Chem. 2006, 54, 3357–3364. [Google Scholar] [CrossRef]
- Reddy, K.N.; Bellaloui, N.; Zablotowicz, R.M. Glyphosate Effect on Shikimate, Nitrate Reductase Activity, Yield, and Seed Composition in Corn. J. Agric. Food Chem. 2010, 58, 3646–3650. [Google Scholar] [CrossRef]
- Vieira, L.A.J.; Alves, R.D.F.B.; Menezes-Silva, P.E.; Mendonça, M.A.C.; Silva, M.L.F.; Silva, M.C.A.P.; Sousa, L.F.; Loram-Lourenço, L.; Alves da Silva, A.; Costa, A.C.; et al. Water contamination with atrazine: Is nitric oxide able to improve Pistia stratiotes phytoremediation capacity? Environ. Pollut. 2021, 272, 115971. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.C.; Cataneo, A.C.; Remaeh, L.M.R.; Corniani, N.; Fumis, T.d.F.; de Souza, Y.A.; Scavroni, J.; Soares, B.J.A. Nitric oxide reduces oxidative stress generated by lactofen in soybean plants. Pestic. Biochem. Physiol. 2010, 97, 47–54. [Google Scholar] [CrossRef]
- Hoagland, R.E. Interaction of indoleacetic acid and glyphosate on phenolic metabolism in soybeans. Pestic. Biochem. Physiol. 1990, 36, 68–75. [Google Scholar] [CrossRef]
- Ulanov, A.; Lygin, A.; Duncan, D.; Widholm, J.; Lozovaya, V. Metabolic effects of glyphosate change the capacity of maize culture to regenerate plants. J. Plant Physiol. 2009, 166, 978–987. [Google Scholar] [CrossRef]
- Fernandes, B.; Soares, C.; Braga, C.; Rebotim, A.; Ferreira, R.; Ferreira, J.; Fidalgo, F.; Pereira, R.; Cachada, A. Ecotoxicological Assessment of a Glyphosate-Based Herbicide in Cover Plants: Medicago sativa L. as a Model Species. Appl. Sci. 2020, 10, 5098. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Sun, H.; Cai, Y.; Mao, W.; Zhang, G.; Vincze, E.; Wu, F. Genotype-Dependent Effect of Exogenous Nitric Oxide on Cd-induced Changes in Antioxidative Metabolism, Ultrastructure, and Photosynthetic Performance in Barley Seedlings (Hordeum vulgare). J. Plant Growth Regul. 2010, 29, 394–408. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhuyan, M.H.M.B.; Oku, H.; Fujita, M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2018, 126, 173–186. [Google Scholar] [CrossRef]
- Qian, H.; Chen, W.; Li, J.; Wang, J.; Zhou, Z.; Liu, W.; Fu, Z. The effect of exogenous nitric oxide on alleviating herbicide damage in Chlorella vulgaris. Aquat. Toxicol. 2009, 92, 250–257. [Google Scholar] [CrossRef]
- Laspina, N.V.; Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005, 169, 323–330. [Google Scholar] [CrossRef]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Hu, K.D.; Hu, L.Y.; Li, Y.H.; Zhang, F.Q.; Zhang, H. Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul. 2007, 53, 173–183. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Peres, L.E.P.; Azevedo, R.A. The Isolation of Antioxidant Enzymes from Mature Tomato (cv. Micro-Tom) Plants. HortSci. Horts 2008, 43, 1608–1610. [Google Scholar] [CrossRef] [Green Version]
- Sandermann, H. Plant metabolism of xenobiotics. Trends Biochem. Sci. 1992, 17, 82–84. [Google Scholar] [CrossRef]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Miteva, L.P.E.; Ivanov, S.V.; Alexieva, V.S. Alterations in glutathione pool and some related enzymes in leaves and roots of pea plants treated with the herbicide glyphosate. Russ. J. Plant Physiol. 2010, 57, 131–136. [Google Scholar] [CrossRef]
- Jain, M.; Bhalla-Sarin, N. Glyphosate-Induced Increase in Glutathione S-Transferase Activity and Glutathione Content in Groundnut (Arachis hypogaea L.). Pestic. Biochem. Physiol. 2001, 69, 143–152. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of ketoprofen toxicity in two freshwater species: Effects on biochemical, physiological and population endpoints. Environ. Pollut. 2020, 265, 114993. [Google Scholar] [CrossRef]
- Letelier, M.E.; Molina-Berríos, A.; Cortés-Troncoso, J.; Jara-Sandoval, J.A.; Müller, A.; Aracena-Parks, P. Comparative Effects of Superoxide Anion and Hydrogen Peroxide on Microsomal and Cytosolic Glutathione S-Transferase Activities of Rat Liver. Biol. Trace Elem. Res. 2010, 134, 203–211. [Google Scholar] [CrossRef]
- Strandberg, B.; Sørensen, P.B.; Bruus, M.; Bossi, R.; Dupont, Y.L.; Link, M.; Damgaard, C.F. Effects of glyphosate spray-drift on plant flowering. Environ. Pollut. 2021, 280, 116953. [Google Scholar] [CrossRef]
- Londo, J.P.; McKinney, J.; Schwartz, M.; Bollman, M.; Sagers, C.; Watrud, L. Sub-lethal glyphosate exposure alters flowering phenology and causes transient male-sterility in Brassica spp. BMC Plant Biol. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pline-Srnic, W. Technical performance of some commercial glyphosate-resistant crops. Pest Manag. Sci. 2005, 61, 225–234. [Google Scholar] [CrossRef]
- Donnini, S.; Tessarin, P.; Ribera-Fonseca, A.; Di Foggia, M.; Parpinello, G.P.; Rombolà, A.D. Glyphosate impacts on polyphenolic composition in grapevine (Vitis vinifera L.) berries and wine. Food Chem. 2016, 213, 26–30. [Google Scholar] [CrossRef]
- Lieten, P. Effect of Metosulam and Glyphosate on Flower Abnormalities of Strawberry. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium; 2006; pp. 401–404. [Google Scholar]
- Owagboriaye, F.; Dedeke, G.; Bamidele, J.; Bankole, A.; Aladesida, A.; Feyisola, R.; Adeleke, M.; Adekunle, O. Wormcasts produced by three earthworm species (Alma millsoni, Eudrilus eugeniae and Libyodrilus violaceus) exposed to a glyphosate-based herbicide reduce growth, fruit yield and quality of tomato (Lycopersicon esculentum). Chemosphere 2020, 250, 126270. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Liu, L.; Liu, X.; Li, B.; Jin, C.; Lin, X. Molecular functions of nitric oxide and its potential applications in horticultural crops. Hortic. Res. 2021, 8, 71. [Google Scholar] [CrossRef]
- OECD. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD: Paris, France, 2006. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- ISO. For ISO Guideline 11269–2: Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015; ISBN 1605353531. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Brendle-Behnisch, E. Rapid Modulation of Spinach Leaf Nitrate Reductase Activity by Photosynthesis 1: I. Modulation in Vivo by CO2 Availability. Plant Physiol. 1991, 96, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO Nanoparticles on Brassica nigra Seedlings and Stem Explants: Growth Dynamics and Antioxidative Response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of Antioxidants to Paraquat in Pea Leaves (Relationships to Resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, J.; de Sousa, A.; Azenha, M.; Moreira, J.T.; Fidalgo, F.; Fernando Silva, A.; Faria, J.L.; Silva, A.M.T. Solanum nigrum L. weed plants as a remediation tool for metalaxyl-polluted effluents and soils. Chemosphere 2011, 85, 744–750. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Soares, A.G.; Ferreira, I.M.P.L.V.O. Quantitative analysis of glyphosate, glufosinate and AMPA in irrigation water by in situ derivatization–dispersive liquid–liquid microextraction combined with UPLC-MS/MS. Anal. Methods 2018, 10, 554–561. [Google Scholar] [CrossRef]


| Parameter | CTL | NO | GLY | GLY + NO |
|---|---|---|---|---|
| Total protein (mg g−1 f.m.) | 3.03 ± 0.20 b | 3.47 ± 0.19 ab | 3.85 ± 0.14 a | 3.68 ± 0.21 ab |
| NR (mmol NADH min−1 mg−1 protein) | 41.67 ± 3.18 a | 26.67 ± 1.76 b | 28.33 ± 3.18 b | 25 ± 2.88 b |
| Proline (μg g−1 f.m.) | 110 ± 8 b | 88 ± 11 b | 587 ± 87 a | 163 ± 26 b |
| Total ascorbate (µmol g−1 f.m.) | 1.52 ± 0.09 bc | 1.29 ± 0.18 c | 2.05 ± 0.18 ab | 2.27 ± 0.21 a |
| AsA/DHA | 2.94 ± 0.66 a | 0.37 ± 0.10 b | 1.47 ± 0.22 a | 2.120 ± 0.40 a |
| GSH (nmol g−1 f.m.) | 288 ± 21 b | 310 ± 20 b | 454 ± 9 a | 426 ± 20 a |
| TAC (μg AsA equivalents g−1 f.m.) | 1067 ± 141 a | 928 ± 133 a | 717 ± 84 a | 895 ± 112 a |
| Total phenols (μg gallic acid equivalents g−1 f.m.) | 960 ± 27 a | 381 ± 10 c | 542 ± 28 bc | 652 ± 54 b |
| Flavonoids (μg quercetin equivalents g−1 f.m.) | 424 ± 43 a | 217 ± 14 b | 298 ± 8 b | 326 ± 16 ab |
| Parameter | CTL | NO | GLY | GLY + NO |
|---|---|---|---|---|
| Total protein (mg g−1 f.m.) | 5.09 ± 0.55 a | 3.66 ± 0.21 b | 2.53 ± 0.10 c | 4.91 ± 0.14 a |
| NR (mmol NADH min−1 mg−1 protein) | 110 ± 12 a | 122 ± 11 a | 132 ± 15 a | 83 ± 10 a |
| Proline (μg g−1 f.m.) | 46.44 ± 1.98 b | 46.38 ± 3.73 b | 95.74 ± 10.68 a | 58.17 ± 7.52 b |
| Total ascorbate (µmol g−1 f.m.) | 0.40 ± 0.05 b | 0.64 ± 0.05 a | 0.41 ± 0.04 b | 0.38 ± 0.03 b |
| AsA/DHA | 0.77 ± 0.05 bc | 0.68 ± 0.12 c | 1.02 ± 0.07 ab | 1.11 ± 0.07 a |
| GSH (nmol g−1 f.m.) | 68.96 ± 1.31 b | 65.69 ± 4.67 b | 139.1 ± 9.53 a | 58.49 ± 2.88 b |
| TAC (μg AsA equivalents g−1 f.m.) | 422 ± 35 a | 362 ± 14 a | 309 ± 21 b | 315 ± 20 b |
| Total phenols (μg gallic acid equivalents g−1 f.m.) | 243 ± 9 a | 273 ± 32 a | 280 ± 38 a | 273 ± 17 a |
| Flavonoids (μg quercetin equivalents g−1 f.m.) | 19.2 ± 0.9 a | 21.0 ± 0.2 a | 24.1 ± 2.8 a | 24.3 ± 4.4 a |
| Parameter | CTL | NO | GLY | GLY + NO |
|---|---|---|---|---|
| Number of flowers per plant | 13.3 ± 2.3 a | 10 ± 3.4 ab | 6.5 ± 1.0 b | 10.0 ± 2.3 ab |
| Number of fruits per plant | 8.0 ± 0.9 a | 3.7 ± 1.2 b | 3.6 ± 0.4 b | 4.3 ± 0.8 b |
| Fruit fresh mass (g) | 3.7 ± 1.1 a | 3.0 ± 0.2 a | 2.3 ± 0.2 a | 3.2 ± 0.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.; Rodrigues, F.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways. Plants 2021, 10, 1862. https://doi.org/10.3390/plants10091862
Soares C, Rodrigues F, Sousa B, Pinto E, Ferreira IMPLVO, Pereira R, Fidalgo F. Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways. Plants. 2021; 10(9):1862. https://doi.org/10.3390/plants10091862
Chicago/Turabian StyleSoares, Cristiano, Francisca Rodrigues, Bruno Sousa, Edgar Pinto, Isabel M. P. L. V. O. Ferreira, Ruth Pereira, and Fernanda Fidalgo. 2021. "Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways" Plants 10, no. 9: 1862. https://doi.org/10.3390/plants10091862
APA StyleSoares, C., Rodrigues, F., Sousa, B., Pinto, E., Ferreira, I. M. P. L. V. O., Pereira, R., & Fidalgo, F. (2021). Foliar Application of Sodium Nitroprusside Boosts Solanum lycopersicum L. Tolerance to Glyphosate by Preventing Redox Disorders and Stimulating Herbicide Detoxification Pathways. Plants, 10(9), 1862. https://doi.org/10.3390/plants10091862