Col11a1a Expression Is Required for Zebrafish Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance, Care, and Staging
2.2. PCR
2.3. Cloning and Riboprobe Synthesis
2.4. In Situ Hybridization
2.5. Antisense Morpholino Oligonucleotide Injection
2.6. CRISPR/Cas9 Gene Editing
2.7. Statistical Analysis
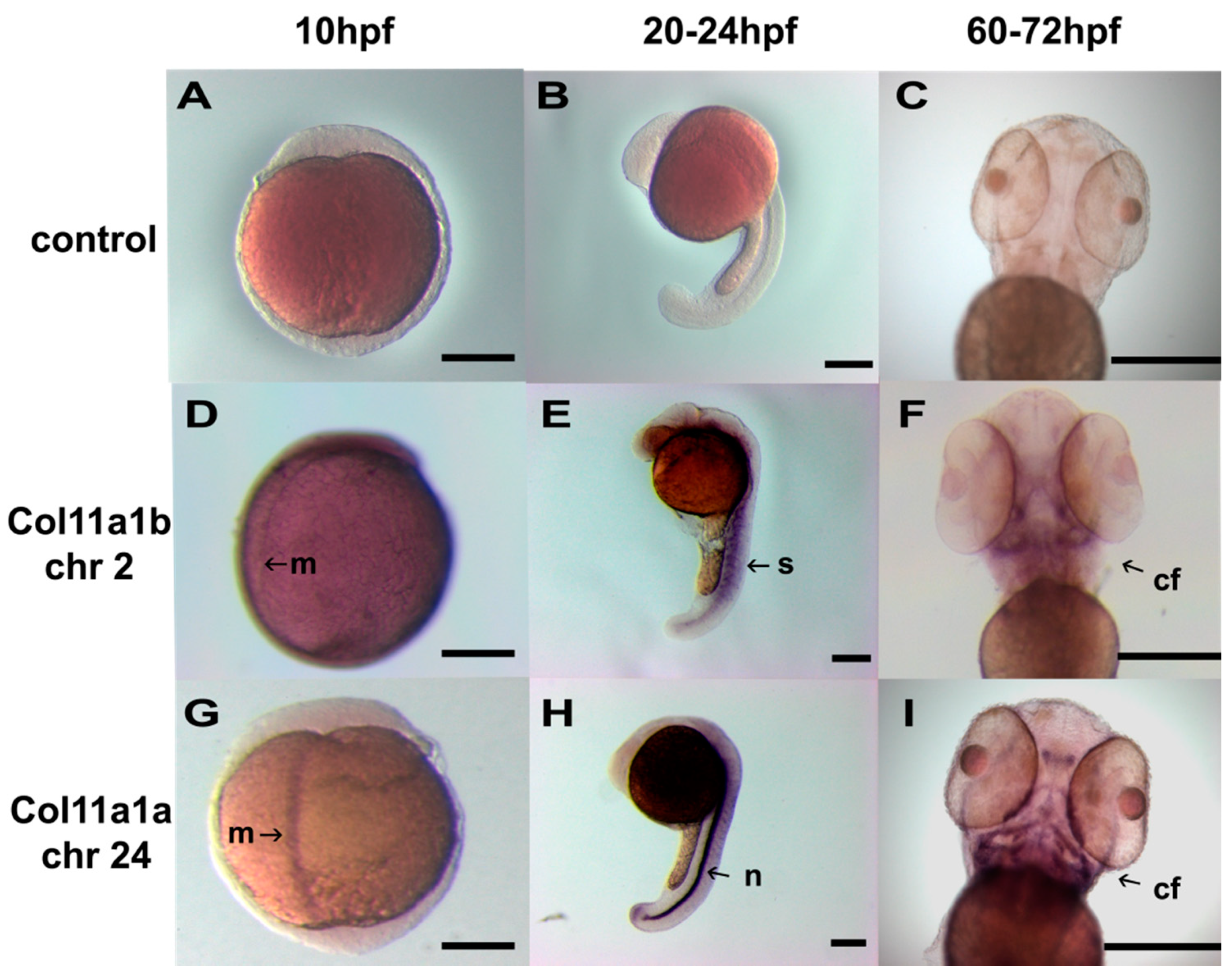
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar] [PubMed]
- Glenister, T.W. An embryological view of cartilage. J. Anat. 1976, 122, 323–330. [Google Scholar]
- Zylińska, B.; Silmanowicz, P.; Sobczyńska-Rak, A.; Jarosz, Ł.; Szponder, T. Treatment of articular cartilage defects: Focus on tissue engineering. In Vivo 2018, 32, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.B.; Phillips, B.T. Ringing in the new ear: Resolution of cell interactions in otic development. Dev. Biol. 2003, 261, 289–312. [Google Scholar] [CrossRef]
- Whitfield, T.T.; Riley, B.B.; Chiang, M.Y.; Phillips, B. Development of the zebrafish inner ear. Dev. Dyn. 2002, 223, 427–458. [Google Scholar] [CrossRef]
- Whitfield, T.T.; Granato, M.; Van Eeden, F.J.M.; Schach, U.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.P.; Jiang, Y.J.; et al. Mutations affecting development of the zebrafish inner ear and lateral line. Development 1996, 123, 241–254. [Google Scholar]
- Nicolson, T. The Genetics of Hearing and Balance in Zebrafish. Annu. Rev. Genet. 2005, 39, 9–22. [Google Scholar] [CrossRef]
- Stickler, G.B.; Belau, P.G.; Farrell, F.J.; Jones, J.D.; Pugh, D.G.; Steinberg, A.G.; Ward, L.E. Hereditary progressive artho-opthalmopathy. Mayo Clin. Proc. 1965, 40, 433–455. [Google Scholar]
- Yelick, P.C.; Schilling, T.F. Molecular dissection of craniofacial development using zebrafish. Crit. Rev. Oral Biol. Med. 2002, 13, 308–322. [Google Scholar] [CrossRef]
- Mundlos, S.; Olsen, B.R. Heritable diseases of the skeleton. Part II: Molecular insights into skeletal development-matrix components and their homeostasis. FASEB J. 1997, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Todhunter, R.J.; Garrison, S.J.; Jordan, J.; Hunter, L.; Castelhano, M.G.; Ash, K.; Meyers-Wallen, V.; Krotscheck, U.; Hayward, J.J.; Grenier, J. Gene expression in hip soft tissues in incipient canine hip dysplasia and osteoarthritis. J. Orthop. Res. 2019, 37, 313–324. [Google Scholar] [CrossRef]
- Marshall, D. Ectodermal dysplasia. Report of kindred with ocular abnormalities and hearing defect. Am. J. Ophthalmol. 1958, 45, 143–156. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lufkin, T. The Sound of Silence: Mouse Models for Hearing Loss. Genet. Res. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Seegmiller, R.; Fraser, F.C.; Sheldon, H. A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis. J. Cell Biol. 1971, 48, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.W.R.J.; Cornelius, W.R.J.; Smith, R. Genetic Hearing Impairment: Its Clinical Presentations; Karger: Basel, Switzerland, 2002; ISBN 9783805574495. [Google Scholar]
- Griffith, A.J.; Sprunger, L.K.; Sirko-Osadsa, D.A.; Tiller, G.E.; Meisler, M.H.; Warman, M.L. Marshall syndrome associated with a splicing defect at the COL11A1 locus. Am. J. Hum. Genet. 1998, 62, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Szymko-Bennett, Y.M.; Kurima, K.; Olsen, B.; Seegmiller, R.; Griffith, A.J. Auditory function associated with Col11a1 haploinsufficiency in chondrodysplasia (cho) mice. Hear. Res. 2003, 175, 178–182. [Google Scholar] [CrossRef]
- Hufnagel, S.B.; Weaver, K.N.; Hufnagel, R.B.; Bader, P.I.; Schorry, E.K.; Hopkin, R.J. A novel dominant COL11A1 mutation resulting in a severe skeletal dysplasia. Am. J. Med. Genet. Part A 2014, 164, 2607–2612. [Google Scholar] [CrossRef]
- Acke, F.R.E.; Dhooge, I.J.M.; Malfait, F.; De Leenheer, E.M.R. Hearing impairment in Stickler syndrome: A systematic review. Orphanet J. Rare Dis. 2012, 7, 84. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar] [CrossRef]
- Ahmed, S.; Nowlan, N.C. Initiation and emerging complexity of the collagen network during prenatal skeletal development. Eur. Cells Mater. 2020, 39, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Jacenko, O.; Olsen, B.; LuValle, P. Organization and regulation of collagen genes. In Critical Reviews in Eukaryotic Gene Expression; Stein, G.S., Stein, J., Lians, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 1991; Volume 1, pp. 327–353. [Google Scholar]
- Eyre, D.R.; Wu, J.J.; Fernandes, R.J.; Pietka, T.A.; Weis, M.A. Recent developments in cartilage research: Matrix biology of the collagen II/IX/XI heterofibril network. Biochem. Soc. Trans. 2002, 30, 893–899. [Google Scholar] [CrossRef]
- Mendler, M.; Eich-Bender, S.G.; Vaughan, L.; Winterhalter, K.H.; Bruckner, P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J. Cell Biol. 1989, 108, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.D.; Barembaum, M.; Upholt, W.B. The chicken α1(XI) collagen gene is widely expressed in embryonic tissues. J. Biol. Chem. 1992, 267, 22581–22586. [Google Scholar]
- Mayne, R.; Brewton, R.G.; Mayne, P.M.; Baker, J.R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J. Biol. Chem. 1993, 268, 9381–9386. [Google Scholar] [PubMed]
- Fang, M.; Adams, J.S.; McMahan, B.L.L.; Brown, R.J.R.J.; Oxford, J.T. The expression patterns of minor fibrillar collagens during development in zebrafish. Gene Expr. Patterns 2010, 10, 315–322. [Google Scholar] [CrossRef]
- Gagnon, J.A.; Valen, E.; Thyme, S.B.; Huang, P.; Ahkmetova, L.; Pauli, A.; Montague, T.G.; Zimmerman, S.; Richter, C.; Schier, A.F. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 2014, 9, e98186. [Google Scholar] [CrossRef]
- Moradi-Améli, M.; Deléage, G.; Geourgjon, C.; van der Rest, M. Common topology within a non-collagenous domain of several different collagen types. Matrix Biol. 1994, 14, 233–239. [Google Scholar] [CrossRef]
- Tisi, D.; Talts, J.; Timpl, R.; Hohenester, E. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 2009, 19, 1432–1440. [Google Scholar] [CrossRef]
- Timpl, R.; Tisi, D.; Talts, J.F.; Andac, Z.; Sasaki, T.; Hohenester, E. Structure and function of laminin LG modules. Matrix Biol. 2000, 19, 309–317. [Google Scholar] [CrossRef]
- Hohenester, E.; Tisi, D.; Talts, J.F.; Timpl, R. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell 1999, 4, 783–792. [Google Scholar] [CrossRef]
- Fallahi, A.; Kroll, B.; Warner, L.R.; Oxford, R.J.; Irwin, K.M.; Mercer, L.M.; Shadle, S.E.; Oxford, J.T. Structural model of the amino propeptide of collagen XI alpha1 chain with similarity to the LNS domains. Protein Sci. 2005, 14, 1526–1537. [Google Scholar] [CrossRef]
- Wälchi, C.; Trueb, J.; Kessler, B.; Winterhalter, K.H.; Trueb, B. Complete primary structure of chicken collagen XIV. Eur. J. Biochem. 1993, 212, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bork, P. The modular architecture of vertebrate collagens. FEBS Lett. 1992, 307, 49–54. [Google Scholar] [CrossRef]
- Tillet, E.; Mann, K.; Nischt, R.; Pan, T.-C.; Chu, M.-L.; Timpl, R. Recombinant Analysis of Human α1(XVI) Collagen: Evidence for Processing of the N-Terminal Globular Domain. Eur. J. Biochem. 1995, 228, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, K.; Yoshioka, H.; Khaleduzzaman, M.; Ninomiya, Y. The mrna for α1(XIX) collagen chain, a new member of FACITs, contains a long unusual 3′ untranslated region and displays many unique splicing variants. J. Biochem. 1995, 117, 137–146. [Google Scholar] [CrossRef]
- Van Der Rest, M.; Garrone, R. Collagen family of proteins. FASEB J. 1991, 5, 2814–2823. [Google Scholar] [CrossRef]
- Fang, M.; Jacob, R.; McDougal, O.; Oxford, J.T. Minor fibrillar collagens, variable regions alternative splicing, intrinsic disorder, and tyrosine sulfation. Protein Cell 2012, 3, 419–433. [Google Scholar] [CrossRef]
- Tsumaki, N.; Kimura, T. Differential expression of an acidic domain in the amino-terminal propeptide of mouse pro-α2(XI) collagen by complex alternative splicing. J. Biol. Chem. 1995, 270, 2372–2378. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ramirez, F. Pro-alpha 1(XI) collagen. Structure of the amino-terminal propeptide and expression of the gene in tumor cell lines. J. Biol. Chem. 1990, 265, 6423–6426. [Google Scholar] [PubMed]
- Gregory, K.E.; Oxford, J.T.; Chen, Y.; Gambee, J.E.; Gygi, S.P.; Aebersold, R.; Neame, P.J.; Mechling, D.E.; Bächinger, H.P.; Morris, N.P. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J. Biol. Chem. 2000, 275, 11498–11506. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, N.I.; Justice, S.K.; Mayne, R. Alternative mRNA processing occurs in the variable region of the pro- α1(XI) and pro-α2(XI) collagen chains. J. Biol. Chem. 1995, 270, 9486–9493. [Google Scholar] [CrossRef]
- Oxford, J.T.; Doege, K.J.; Morris, N.P. Alternative exon splicing within the amino-terminal nontriple-helical domain of the rat pro-α1(XI) collagen chain generates multiple forms of the mRNA transcript which exhibit tissue-dependent variation. J. Biol. Chem. 1995, 270, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.G.; Branam, A.M.; Huang, G.; Pelegri, F.; Cole, W.G.; Wenstrup, R.M.; Greenspan, D.S. Characterization of the six zebrafish clade B fibrillar procollagen genes, with evidence for evolutionarily conserved alternative splicing within the pro-α1(V) C-propeptide. Matrix Biol. 2010, 29, 261–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davies, G.B.; Oxford, J.T.; Hausafus, L.C.; Smoody, B.F.; Morris, N.P. Temporal and spatial expression of alternative splice-forms of the alpha1(XI) collagen gene in fetal rat cartilage. Dev. Dyn. 1998, 213, 12–26. [Google Scholar] [CrossRef]
- Richards, A.J.; Martin, S.; Nicholls, A.C.; Harrison, J.B.; Pope, F.M.; Burrows, N.P. A single base mutation in COL5A2 causes Ehlers-Danlos syndrome type II. J. Med. Genet. 1998, 35, 846–848. [Google Scholar] [CrossRef]
- Annunen, S.; Körkkö, J.; Czarny, M.; Warman, M.L.; Brunner, H.G.; Kääriäinen, H.; Mulliken, J.B.; Tranebjærg, L.; Brooks, D.G.; Cox, G.F.; et al. Splicing Mutations of 54-bp Exons in the COL11A1 Gene Cause Marshall Syndrome, but Other Mutations Cause Overlapping Marshall/Stickler Phenotypes. Am. J. Hum. Genet. 1999, 65, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Robin, N.H.; Moran, R.T.; Ala-Kokko, L. Gene Reviews: Stickler Syndrome; University of Washington: Seattle, WA, USA, 2017. [Google Scholar]
- Seegmiller, R.E.; Foster, C.; Burnham, J.L. Understanding chondrodysplasia (cho): A comprehensive review of cho as an animal model of birth defects, disorders, and molecular mechanisms. Birth Defects Res. 2019, 111, 237–247. [Google Scholar] [CrossRef]
- Schilling, T.F.; Walker, C.; Kimmel, C.B. The chinless mutation and neural crest cell interactions in zebrafish jaw development. Development 1996, 122, 1417–1426. [Google Scholar]
- Newgreen, D.F.; Erickson, C.A. The migration of neural crest cells. Int. Rev. Cytol. 1986, 103, 89–145. [Google Scholar] [PubMed]
- Perris, R.; Krotoski, D.; Bronner-Fraser, M. Collagens in avian neural crest development: Distribution in vivo and migration-promoting ability in vitro. Development 1991, 113, 969–984. [Google Scholar] [PubMed]
- Lallier, T.; Leblanc, G.; Artinger, K.B.; Bronner-Fraser, M. Cranial and trunk neural crest cells use different mechanisms for attachment to extracellular matrices. Development 1992, 116, 531–541. [Google Scholar] [PubMed]
- Maxwell, G.D. Substrate dependence of cell migration from explanted neural tubes in vitro. Cell Tissue Res. 1976, 172, 325–330. [Google Scholar] [CrossRef]
- Seufert, D.W.; Hanken, J.; Klymkowsky, M.W. Type II collagen distribution during cranial development in Xenopus laevis. Anat. Embryol. 1994, 189, 81–89. [Google Scholar] [CrossRef]







| Name | Target | Guide Sequence 1 | Forward Primer | Reverse Primer |
|---|---|---|---|---|
| E101 | Exon 1 | ATTTAGGTGACACTATAGGCCAAGGTGGTCCCCAATGGTTTTAGAGCTAGAAATAGCAAG | GGCACTTTTGGGATTGTAGAAG | CATCTCCTCTTAGAAAGCCCCT |
| E201 | Exon 2 | ATTTAGGTGACACTATAAAGAGCATCACAGCCAGACGGTTTTAGAGCTAGAAATAGCAAG | CTGCTGACATTTTGCATGTCTT | CATTTAAACGCAGCTGAACGTA |
| E301 | Exon 3 | ATTTAGGTGACACTATAAGGCGTCCAGCAGCTGGGCGGTTTTAGAGCTAGAAATAGCAAG | GTAAGAAGAAGCTGACCAAGCC | CCCGTTTATTTCTACCTCATGC |
| E401 | Exon 4 | ATTTAGGTGACACTATATGGCACCAGGATCCTGGATGGTTTTAGAGCTAGAAATAGCAAG | GTAAGAAGAAGCTGACCAAGCC | CCCGTTTATTTCTACCTCATGC |
| E501 | Exon 5 | ATTTAGGTGACACTATAGCCTGCAGTGTGTCCTTGTGGTTTTAGAGCTAGAAATAGCAAG | GCTCTGTTTTTGGTCTCCTCAG | AGACGTCCAGAAGCGTTTAGTC |
| E2701 | Exon 27 | ATTTAGGTGACACTATAGGTGTCCGTGGTCTAAAGGGGTTTTAGAGCTAGAAATAGCAAG | TTCACTGTTGTCATTTTCAGGG | ACGTGTGACGATTTCTCCATTA |
| AMO | N | Lethality |
|---|---|---|
| Col11a1b-MOe1 | 54 | 14 (26%) |
| Col11a1a-MOe1 | 87 | 50 (57%) |
| Col11a1a-MOe6a | 80 | 24 (30%) |
| Col11a1a-MOe8 | 56 | 19 (34%) |
| Std. AMO control | 32 | 11 (34%) |
| AMO | Length (mm) | % Decrease |
|---|---|---|
| Col11a1b-MOe1 | 2.67 ± 0.16 | −13.1% |
| Col11a1a-MOe1 | 2.81 ± 0.12 | −7.5% |
| Col11a1a-MOe6a | 2.85 ± 0.12 | −6.0% |
| Col11a1a-MOe8 | 2.85 ± 0.18 | −6.0% |
| Std. AMO control | 3.02 ± 0.17 | 0 |
| AMO | n | Missing or Extra Otoliths | Pericardial Edema | Curved Notochord | Smaller Meckel’s Cartilage |
|---|---|---|---|---|---|
| Col11a1b-MOe1 | 40 | 40 (100%) | 39 (98%) | 30 (75%) | 40 (100%) |
| Col11a1a-MOe1 | 34 | 18 (53%) | 28 (82%) | 25 (74%) | 33 (97%) |
| Col11a1a-MOe6a | 44 | 30 (68%) | 42 (95%) | 41 (93%) | 44 (100%) |
| Col11a1a-MOe8 | 18 | 0 | 0 | 1 | 18 (100%) |
| Std. AMO control | 32 | 0 | 0 | 0 | 0 |
| CRISPR/Cas9 | N | Lethality |
|---|---|---|
| Col11a1a −/− | 303 | 299 (98%) |
| Col11a1a +/− | 299 | 152 (50%) |
| Wild-type control | 311 | 25 (8%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardy, M.J.; Reeck, J.C.; Fang, M.; Adams, J.S.; Oxford, J.T. Col11a1a Expression Is Required for Zebrafish Development. J. Dev. Biol. 2020, 8, 16. https://doi.org/10.3390/jdb8030016
Hardy MJ, Reeck JC, Fang M, Adams JS, Oxford JT. Col11a1a Expression Is Required for Zebrafish Development. Journal of Developmental Biology. 2020; 8(3):16. https://doi.org/10.3390/jdb8030016
Chicago/Turabian StyleHardy, Makenna J., Jonathon C. Reeck, Ming Fang, Jason S. Adams, and Julia Thom Oxford. 2020. "Col11a1a Expression Is Required for Zebrafish Development" Journal of Developmental Biology 8, no. 3: 16. https://doi.org/10.3390/jdb8030016
APA StyleHardy, M. J., Reeck, J. C., Fang, M., Adams, J. S., & Oxford, J. T. (2020). Col11a1a Expression Is Required for Zebrafish Development. Journal of Developmental Biology, 8(3), 16. https://doi.org/10.3390/jdb8030016







