A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of Grape Pomace Extracts (RP, WP) and Canes Extracts (C) from Vitis Vinifera, with High Polyphenolic Content/DoE for Extraction Efficiency Enhancement
2.3.1. Experimental Design Development
2.3.2. Extract Preparation
2.3.3. Determination of Total Phenolic Contents (Total Polyphenols, Tannins, Flavonoids, Caffeic Acid Derivatives)
2.3.4. Experimental Design Analysis and Extraction Efficiency Enhancement
2.4. LC/MS Analysis of Polyphenols
2.5. Determination of Antioxidant Properties
2.5.1. DPPH Assay
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.3. Nitrite-Induced Autoxidation of Hemoglobinin
2.5.4. Free radical Generation Experiment
2.6. Statistical Analysis
3. Results and Discussion
3.1. DoE for Extraction Efficiency Enhancement
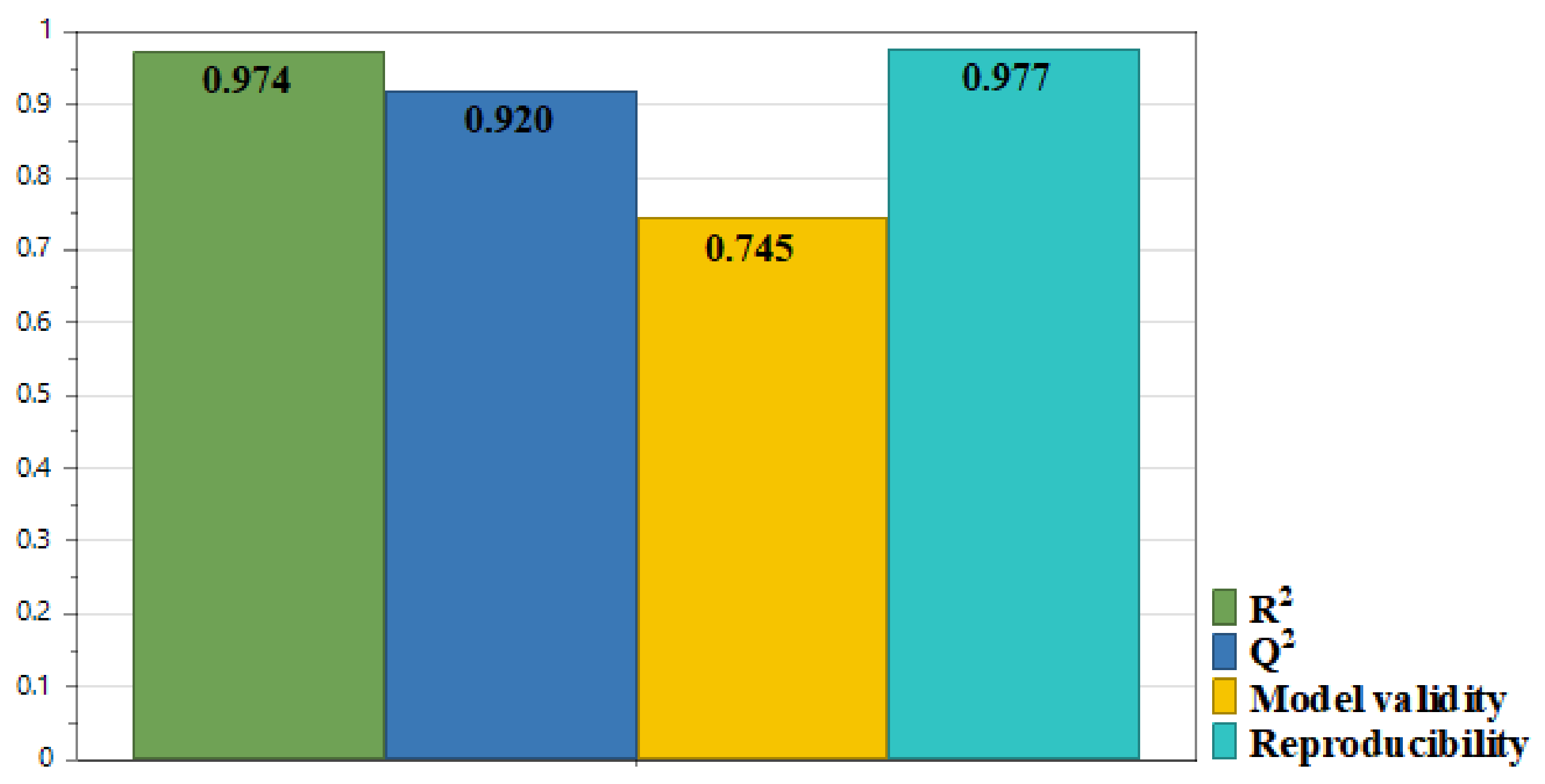
3.1.1. Fitting the Experimental Results with the Model
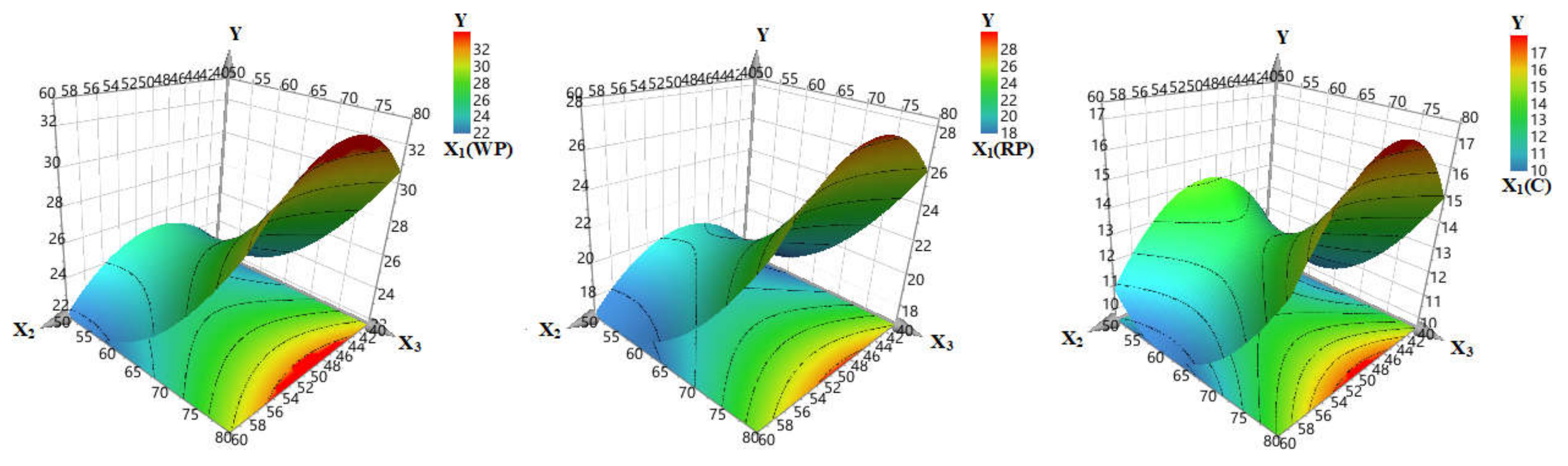
3.1.2. The Effect of Input Variables (X1–3) on TPC (Y)
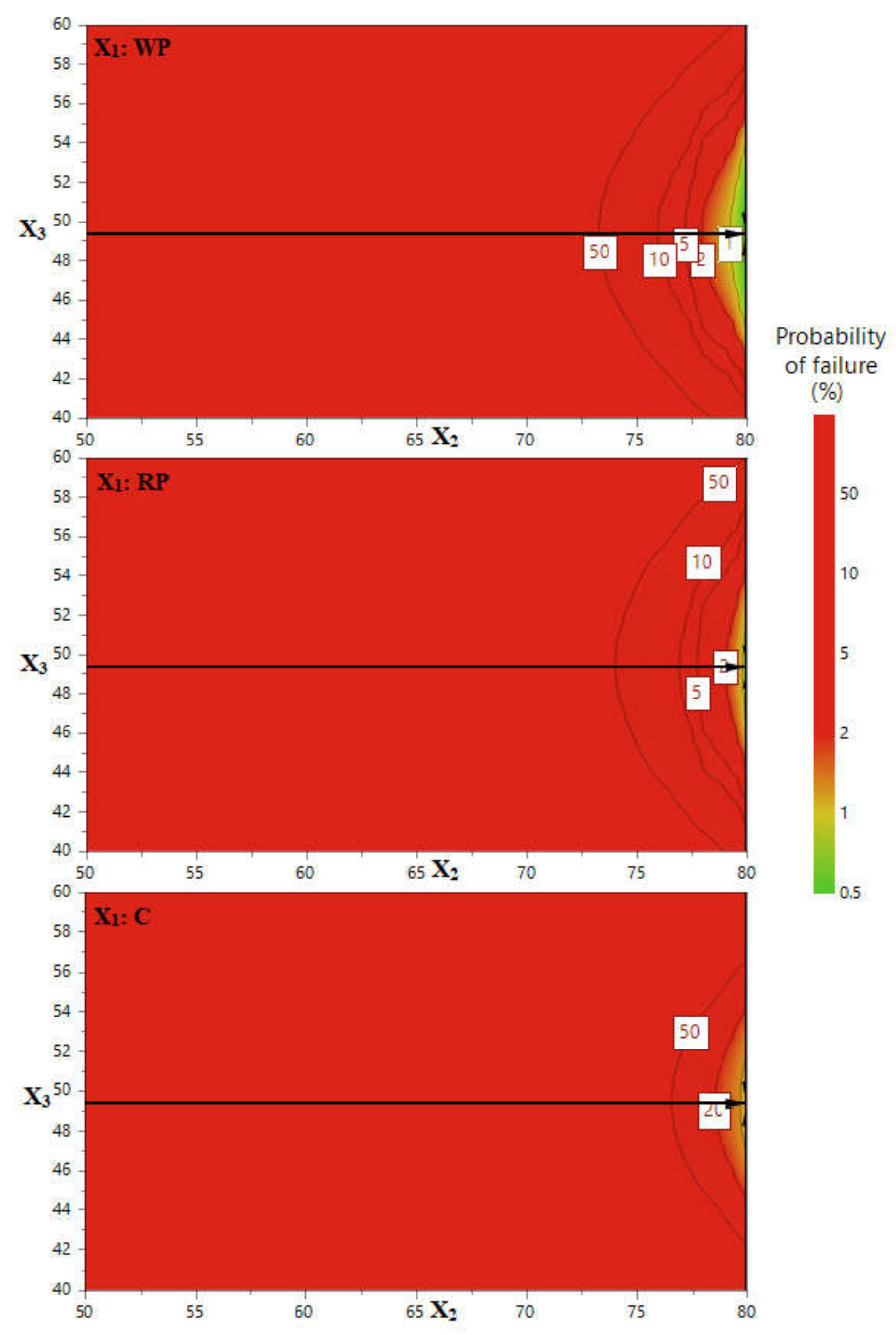
3.1.3. Extraction Method Efficiency Enhancement
3.2. Extract Characterization
3.2.1. Chromatographic Analysis of Phenolic Compounds
3.2.2. Antioxidant Activity
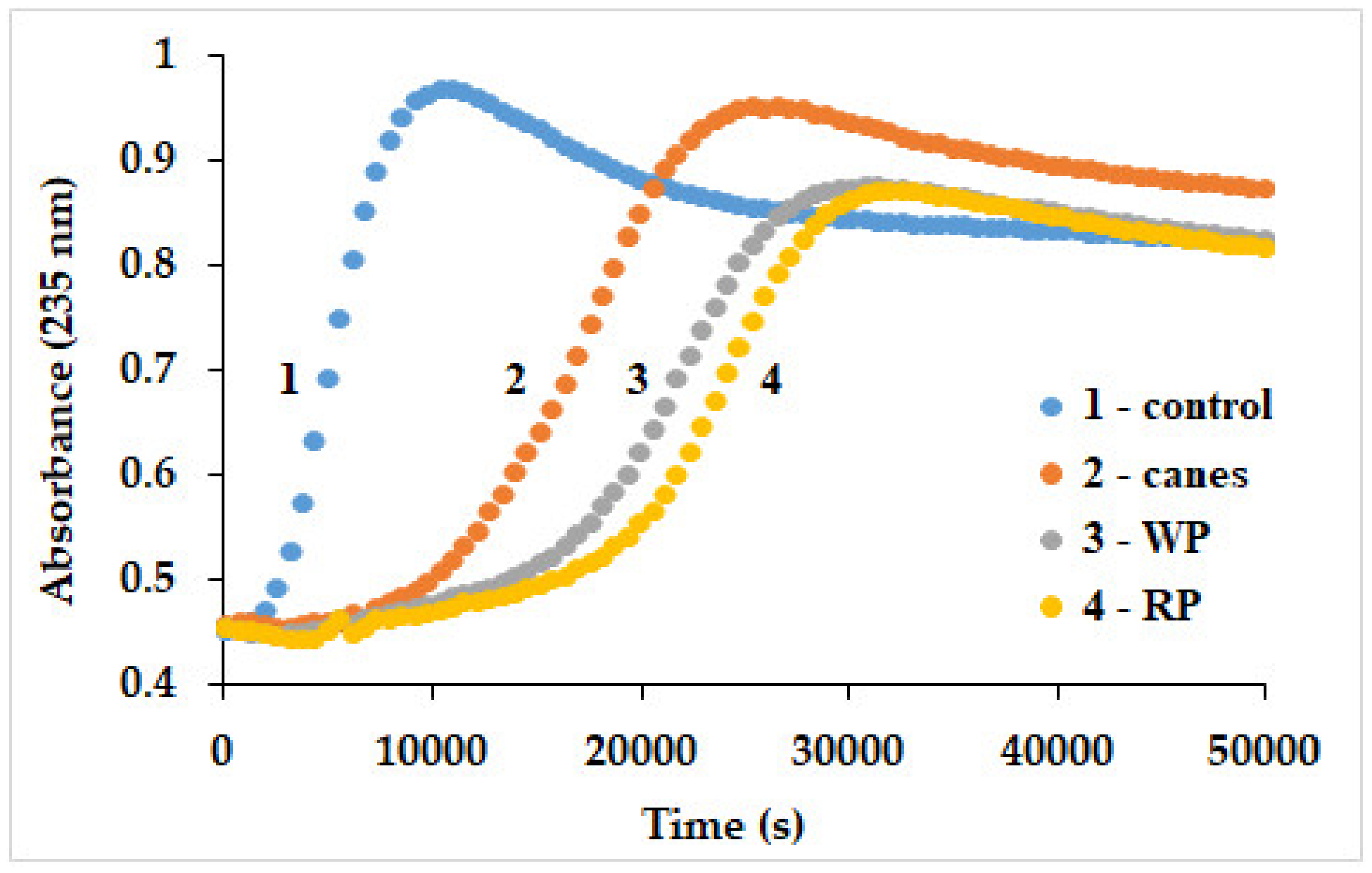
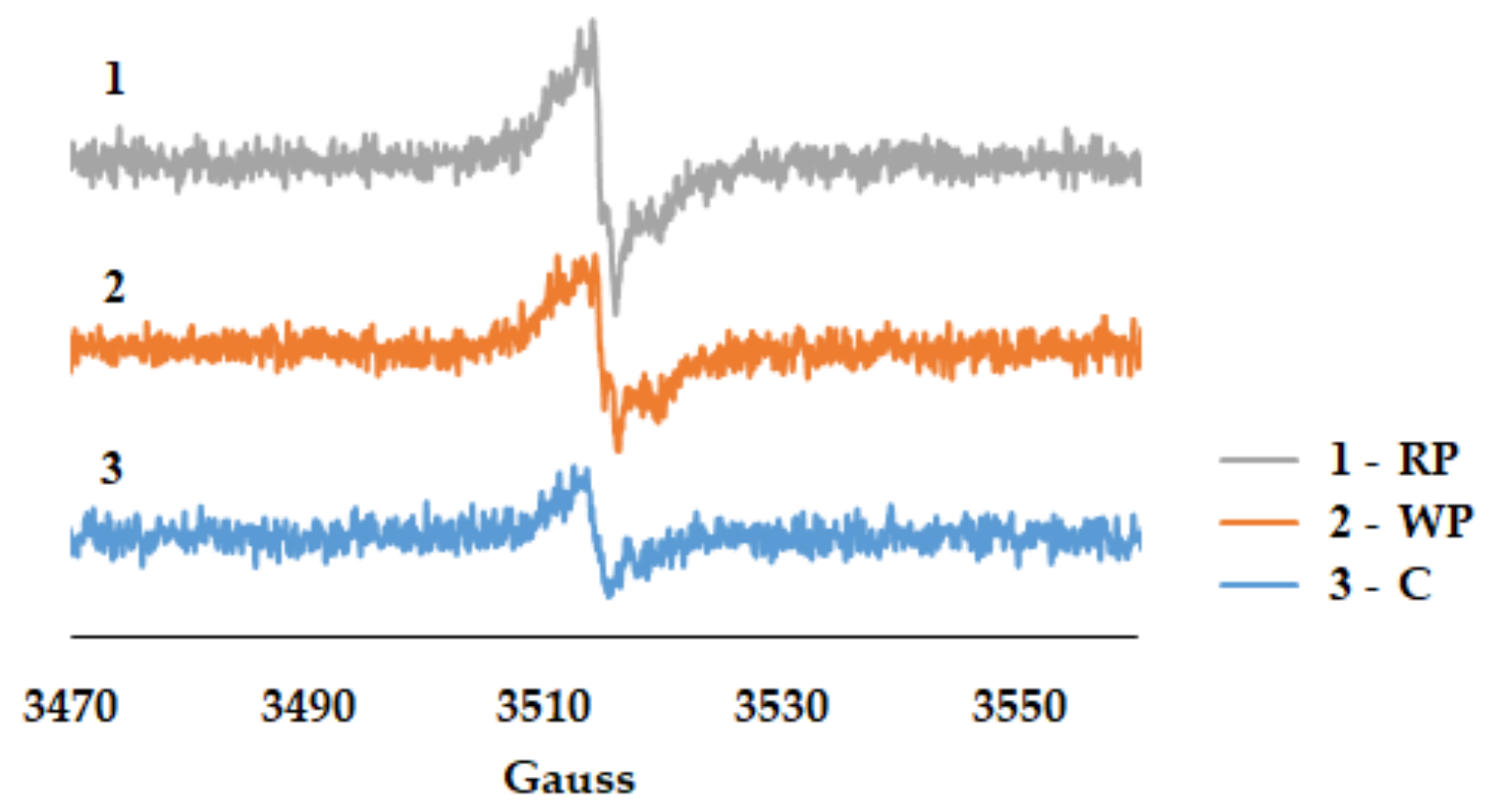
3.2.3. Free Radicals Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2014, 22, 506–514. [Google Scholar] [CrossRef]
- Zilich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food. Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, 12626. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, K.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerge, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 1, 622–646. [Google Scholar] [CrossRef]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Kostić, M.; Kitić, D.; Petrović, M.B.; Jevtović-Stoimenov, T.; Jović, M.; Petrović, A.; Živanović, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59. [Google Scholar] [CrossRef]
- Özden, F.O.; Sakallioğlu, E.E.; Sakallioğlu, U.; Ayas, B.; Erişgin, Z. Effects of grape seed extract on periodontal disease: An experimental study in rats. J. Appl. Oral. Sci. 2017, 25, 121–129. [Google Scholar] [CrossRef]
- Rassameemasmaung, S.; Sirikulsathean, A.; Amornchat, C.; Maungmingsook, P.; Rojanapanthu, P.; Gritsanaphan, W. Topical application of Garcinia mangostana L pericarp gel as an adjunct to periodontal treatment. Complement. Ther. Med. 2008, 16, 262–267. [Google Scholar] [CrossRef]
- Tomaz, I.; Huzanic, N.; Preiner, D.; Stupic, D.; Andabaka, Z.; Maletic, E.; Karoglan Kontic, J.; Asperger, D. Extraction methods of polyphenol from grapes: Extractions of grape polyphenols. In Polyphenols in Plants: Isolation, Purification and Extract Preparation, 2nd ed.; Ross Watson, R., Ed.; Elsevier: City, Switzerland, 2019; pp. 151–167. [Google Scholar]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of Polyphenols and Antioxidant Potential of White Grape Pomace Byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef]
- Gaedcke, F.; Steinhoff, B.; Blasius, H. Definitions. In Herbal Medicinal Products: Scientific and Regulatory Basis for Development, Quality Assurance and Marketing Authorisation; Medpharm GmbH Scientific Publishers: Stuttgart, Germany, 2003; pp. 5–13. [Google Scholar]
- Tournour, H.H.; Segundo, M.A.; Magalhaes, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crop. Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Yang, J.; Ou, X.; Zhang, X.; Zhou, Z.; Ma, L. Effect of Different Solvents on the Measurement of Phenolics and the Antioxidant Activity of Mulberry (Morus atropurpurea Roxb.) with Accelerated Solvent Extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Iurian, S.; Bogdan, C.; Tomuta, I.; Szabo-Revesz, P.; Chvatal, A.; Leucuta, S.; Moldovan, M.; Ambrus, R. Development of oral lyophilisates containing meloxicam nanocrystals using a QbD approach. Eur. J. Pharm. Sci. 2017, 104, 356–365. [Google Scholar] [CrossRef]
- Balea, S.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Andreicuț, A.; Pârvu, M. Phytochemical profiling, antioxidant and cardioprotective properties of Pinot Noir cultivar pomace extracts. Farmacia 2018, 66, 433–441. [Google Scholar] [CrossRef]
- Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from Walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reaction with Ligands; Elsevier: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Hathazi, D.; Scurtu, F.; Bischin, C.; Mot, A.; Attia, A.A.A.; Kongsted, J.; Silaghi-Dumitrescu, R. The reaction of oxy hemoglobin with nitrite: Mechanism, antioxidant-modulated effect, and implications for blood substitute evaluation. Molecules 2018, 23, 350. [Google Scholar] [CrossRef]
- Mot, A.C.; Bischin, C.; Muresan, B.; Parvu, M.; Damian, G.; Vlase, L.; Silaghi Dumitrescu, R. Antioxidant activity evaluation by physiologically relevant assays based on hemoglobin peroxidase activity and cytochrome c-induced oxidation of liposomes. Nat. Prod. Res. 2015, 30, 1–5. [Google Scholar]
- Benedec, D.; Popica, I.-L.; Oniga, I.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R.; Bischin, C.; Vlase, L. Comparative HPLC-MS analysis of phenolics from Achillea distans and Achillea millefolium and their Bioactivity. Stud. Chem. 2015, 4, 257–266. [Google Scholar]
- Mot, A.C.; Bischin, C.; Damian, G.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation involving hemoglobin-related free radical reactivity. Methods Mol. Biol. 2015, 1208, 247–255. [Google Scholar]
- Benedec, D.; Oniga, I.; Muresan, B.; Mot, A.C.; Damian, G.; Nistor, A.; Silaghi-Dumitrescu, R.; Hanganu, D.; Duma, M.; Vlase, L. Contrast between water- and ethanol-based antioxidant assays: Aspen (Populus tremula) and black poplar (Populus nigra) extracts as a case study. J. Food Qual. 2014, 37, 259–267. [Google Scholar] [CrossRef]
- El Hassani, S.E.A.; Driouich, A.; Mellouk, H.; Bejjany, B.; Dani, A.; Digua, K. Extraction of phenolic from Moroccan grape pomace: Optimization of decoction extraction of phenolic compounds using response surface methodology. Mediterr. J. Chem. 2019, 7, 423–432. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005; p. 221. [Google Scholar]
- Benedec, D.; Hanganu, D.; Oniga, I.; Filip, L.; Bischin, C.; Silaghi-Dumitrescu, R.; Tiperciuc, B.; Vlase, L. Achillea schurii flowers: Chemical, antioxidant, and antimicrobial investigations. Molecules 2016, 2, 1050. [Google Scholar] [CrossRef]
- Hanganu, D.; Benedec, D.; Vlase, L.; Popica, I.; Bele, C.; Raita, O.; Gheldiu, A.M.; Mihali, C.V.; Țărmure, V. Polyphenolic content and antioxidant activity of Chrysanthemum parthenium extract. Farmacia 2016, 64, 498–501. [Google Scholar]
- Hanganu, D.; Olah, N.K.; Mocan, A.; Vlase, L.; Benedec, D.; Raita, O.; Toma, C. Comparative polyphenolic content and antioxidant activities of two Romanian Lysimachia species. Rev. Chim. 2016, 67, 227–231. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Olah, N.K.; Osser, G.; Câmpean, R.F.; Furtuna, F.R.; Benedec, D.; Filip, L.; Raita, O.; Hanganu, D. The study of polyphenolic compounds profile of some (Rosmarinus officinalis L.) extracts. Pak. J. Pharm. Sci. 2016, 29, 2355–2361. [Google Scholar]
- Council of Europe. Determination of Tannins in Herbal Drugs. In European Pharmacopoeia, 6th ed.; European Directorate for the Quality of Medicines: Strasbourg, France, 2007; p. A286. [Google Scholar]
- Romanian Pharmacopoeia Commission National Medicines Agency. Romanian Pharmacopoeia; Medical Publishing House: Bucharest, Romania, 1993; p. 335. [Google Scholar]
- Galvão, M.A.M.; Arruda, A.O.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Evaluation of the Folin-Ciocalteu method and quantification of total tannins in stem barks and pods from Libidibia ferrea (Mart. ex Tul) L. P. Queiroz. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Amorim, E.; Nascimento, J.; Monteiro, J.; Peixoto Sobrinho, T.; Araujo, T.; Albuquerque, U. A simple and accurate procedure for the determination of tannin and flavonoid levels and some applications in Ethnobotany and Ethnopharmacology. Funct. Ecosyst. Commun. 2008, 2, 88–94. [Google Scholar]
- Pulipati, S.; Babu, S.; Naveena, U.; Rafeeka Parveen, S.K.; Sumaya Nausheen, S.K.; Tanmai Naga Sai, M. Determination of total phenolic, tannin, flavonoid contents and evaluation of antioxidant property of Amaranthus tricolor (L). Int. J. Pharm. Pharm. Res. 2017, 9, 814–819. [Google Scholar] [CrossRef]
- Debebe, A.; Chandravanshi, B.S.; Abshiro, M.R. Total contents of phenolics, flavonoids, tannins and antioxidant capacity of selected traditional Ethiopian alcoholic beverages. Bull. Chem. Soc. Ethiop. 2016, 30, 27–37. [Google Scholar] [CrossRef]
- Andriamadio, J.H.; Rasoanaivo, L.H.; Benedec, D.; Vlase, L.; Gheldiu, A.M.; Duma, M.; Toiu, A.; Raharisololalao, A.; Oniga, I. HPLC/MS analysis of polyphenols, antioxidant and antimicrobial activities of Artabotrys hildebrandtii O. Hffm. extracts. Nat. Prod. Res. 2015, 29, 2188–2196. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Tiperciuc, B.; Crişan, G. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note, I. Farmacia 2013, 61, 261–267. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Rockenbac, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; de Souza Schmidt Gonçalves, M.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Suciu, S.; Iurian, S.; Bogdan, C.; Iovanov, R.; Rus, L.; Moldovan, M.; Tomuta, I. QbD approach in the development of oral lyophilisates with ibuprofen for paediatric use. Farmacia 2018, 66, 514–523. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Li, Y.P.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodriguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Tech. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Ronghua, L.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2016, 38, 602–611. [Google Scholar] [CrossRef]
- Iurian, S.; Tomuta, I.; Bogdan, C.; Rus, L.; Tokes, T.; Barbu-Tudoran, L.; Achim, M.; Moldovan, M.; Leucuta, S. Defining the design space for freeze-dried orodispersible tablets with meloxicam. Drug. Dev. Ind. Pharm. 2016, 42, 1977–1989. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brigida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crop. Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Gonzalez-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simai, S.; Rossello, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- ICH Q8 (R2) Pharmaceutical Development. 2009. Available online: www.ich.org%2Ffileadmin%2FPublic%0A_Web_Site%2FICH_Products%2FGuidelines%2FQuality%2FQ8_R1%2FStep4%2FQ8_R2_%0AGuideline.pdf (accessed on 12 June 2019).
- Gharwalová, L.; Hutár, D.; Masák, J.; Kolouchová, I. Antioxidant Activity and phenolic content of organic and conventional vine cane extracts. Czech J. Food Sci. 2018, 36, 289–295. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Baydar, N.G. Chemical composition of grape canes. Ind. Crop. Prod. 2011, 134, 994–998. [Google Scholar] [CrossRef]
- Pop, I.M.; Pascariu, S.M.; Simeanu, D.; Radu-Rusu, C.; Albu, A. Determination of the chemical composition of the grape pomace of different varieties of grapes. Lucrări Științifice-Universitatea de Științe Agricole și Medicină Veterinară, Seria Zootehnie 2015, 63, 76–80. Available online: www.uaiasi.ro/zootehnie/Pdf/Pdf_Vol_63/I_Pop.pdf (accessed on 9 May 2019).
- Tassoni, A.; Michelini, E.; Calabretta, M.; Roda, A.; Ferri, M. Recovery of polyphenols having beneficial biological activities from red and white grape pomace. ISANH 2016, 3. [Google Scholar] [CrossRef]
- Frum, A.; Georgescu, C.; Gligor, F.; Lengyel, E.; Stegarus, D.; Dobrea, C.; Tita, O. Identification and quantification of phenolic compounds from red grape pomace. St. Cerc. St. CICBIA 2018, 19, 45–52. [Google Scholar]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Gaita, C.; Alexa, E.; Popescu, I.; Popescu, S.; Negrea, M.; Poiana, M.A. Grape pomace: A potential sustainable resource for natural bioactive compounds recovery. J. Agroaliment. Process. Technol. 2017, 23, 141–147. [Google Scholar]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oprean, R.; Crișan, G. Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers. Molecules 2015, 20, 15060–15071. [Google Scholar] [CrossRef]
- Sťavíková, L.; Polovka, M.; Hohnová, B.; Karásek, P.; Roth, M. Antioxidant activity of grape skin aqueous extracts from pressurized hot water extraction combined with electron paramagnetic resonance spectroscopy. Talanta 2011, 85, 2233–2240. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Gheldiu, A.M.; Puscas, C.; Silaghi-Dumitrescu, R.; Duma, M.; Tiperciuc, B.; Varban, R.; Vlase, L. Sources for developing new medicinal products: Biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement. Altern. Med. 2018, 18, 226. [Google Scholar] [CrossRef]
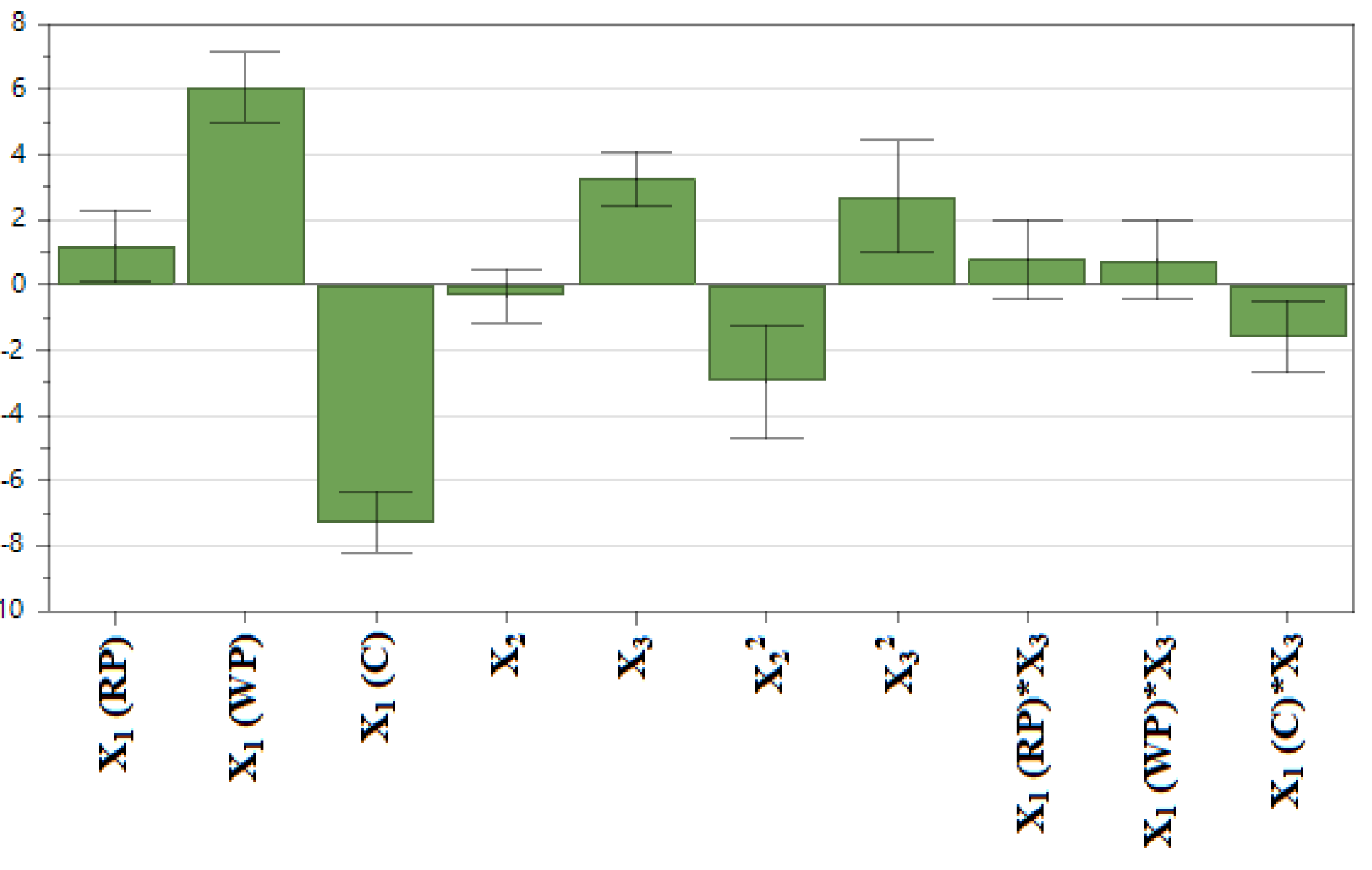
| Independent Variables | Variation Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: Plant material | Red pomace (RP) | White pomace (WP) | Canes (C) |
| X2: Ethanol concentration | 40 | 50 | 60 |
| X3: Temperature | 50 | 65 | 80 |
| Dependent variable | |||
| Y1: Total polyphenolic content (TPC) | |||
| Experiment Name | Plant Material (X1) | Ethanol Ratio (%) (X2) | Temperature (°C) (X3) | TPC (Y) |
|---|---|---|---|---|
| N1 | RP | 40 | 50 | 17.12 ± 0.34 |
| N2 | RP | 60 | 50 | 16.05 ± 0.32 |
| N3 | RP | 40 | 80 | 25.17 ± 0.49 |
| N4 | RP | 60 | 80 | 24.25 ± 0.46 |
| N5 | RP | 50 | 65 | 24.27 ± 0.47 |
| N6 | WP | 40 | 50 | 23.55 ± 0.46 |
| N7 | WP | 60 | 50 | 22.15 ± 0.43 |
| N8 | WP | 40 | 80 | 30.50 ± 0.59 |
| N9 | WP | 60 | 80 | 31.35 ± 0.61 |
| N10 | WP | 50 | 65 | 23.72 ± 0.41 |
| N11 | C | 40 | 50 | 11.82 ± 0.24 |
| N12 | C | 60 | 50 | 10.07 ± 0.21 |
| N13 | C | 40 | 80 | 14.75 ± 0.29 |
| N14 | C | 60 | 80 | 14.45 ± 0.29 |
| N15 | C | 40 | 65 | 10.30 ± 0.21 |
| N16 | C | 60 | 65 | 10.22 ± 0.21 |
| N17 | C | 50 | 50 | 14.55 ± 0.29 |
| N18 | C | 50 | 80 | 17.37 ± 0.34 |
| N19 | C | 50 | 65 | 12.60 ± 0.25 |
| N20 | C | 50 | 65 | 13.05 ± 0.26 |
| N21 | C | 50 | 65 | 13.32 ± 0.27 |
| Samples | TPC (mg GAE/g) | TFC (mg RE/g) | Caffeic acid Derivatives (mg CAE/g) | Tannins (mg GAE/g) |
|---|---|---|---|---|
| RP | 32.00 ± 0.76 | 0.54 ± 0.07 | 9.11 ± 1.38 | 18.26 ± 1.73 |
| WP | 37.80 ± 0.19 | 1.89 ± 0.10 | 17.64 ± 1.35 | 12.56 ± 1.43 |
| C | 18.45 ± 0.48 | 0.33 ± 0.05 | 1.86 ± 0.08 | 13.14 ± 0.35 |
| Polyphenolic Compounds | m/z Value | tR ± SD (min) | WP (µg/g) | RP (µg/g) | C (µg/g) |
|---|---|---|---|---|---|
| Gallic acid | 169 | 1.50 ± 0.01 | 128.19 ± 0.93 | 146.87 ± 1.13 | 39.57 ± 0.30 |
| Protocatechuic acid | 153 | 2.80 ± 0.01 | 57.17 ± 0.24 | 34.88 ± 0.12 | 11.69 ± 0.28 |
| Caftaric acid | 311 | 3.54 ± 0.05 | <0.02 | <0.02 | <0.02 |
| Gentisic acid | 179 | 3.52 ± 0.04 | <0.02 | <0.02 | - |
| Catechin | 289 | 6.00 ± 0.03 | 539.14 ± 1.86 | 561.93 ± 4.07 | 413.40 ± 3.65 |
| Vanillic acid | 167 | 6.70 ± 0.01 | - | 45.00 ± 0.88 | - |
| Syringic acid | 197 | 8.40 ± 0.01 | 4.38 ± 0.12 | 72.22 ± 0.62 | 1.69 ± 0.01 |
| Epicatechin | 289 | 9.00 ± 0.01 | 513.52 ± 2.48 | 425.78 ± 4.22 | 278.90 ± 2.33 |
| p-Coumaric acid | 163 | 9.48 ± 0.08 | - | <0.02 | - |
| Hyperoside | 463 | 18.60 ± 0.12 | <0.02 | - | <0.02 |
| Isoquercitrin | 463 | 19.60 ± 0.10 | 29.70 ± 0.30 | 6.59 ± 0.09 | 3.50 ± 0.05 |
| Rutin | 609 | 20.20 ± 0.15 | 2.63 ± 0.07 | - | 2.63 ± 0.04 |
| Quercitrin | 447 | 23.64 ± 0.13 | 26.08 ± 0.37 | - | - |
| Quercetin | 301 | 26.80 ± 0.15 | 6.69 ± 0.02 | 9.99 ± 0.01 | - |
| Luteolin | 285 | 29.10 ± 0.19 | 3.50 ± 0.04 | - | - |
| Samples | DPPH | FRAP (µmolTE/g) | NHA mg CATE/g | |
|---|---|---|---|---|
| I% (131.25 μg/mL) | IC50 μg/mL (37.5–262.50 μg/mL) | |||
| RP | 57.96 | 112.02 ± 3.98 | 2652.50 ± 6.49 | 16.87 ± 2.71 |
| WP | 80.93 | 76.33 ± 2.67 | 3908.42 ± 12.85 | 13.06 ± 4.40 |
| C | 30.86 | 198.27 ± 4.31 | 997.81 ± 2.18 | 8.27 ± 0.80 |
| Trolox | - | 11.27 ± 0.04 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. https://doi.org/10.3390/biom9100529
Moldovan ML, Iurian S, Puscas C, Silaghi-Dumitrescu R, Hanganu D, Bogdan C, Vlase L, Oniga I, Benedec D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules. 2019; 9(10):529. https://doi.org/10.3390/biom9100529
Chicago/Turabian StyleMoldovan, Mirela L., Sonia Iurian, Cristina Puscas, Radu Silaghi-Dumitrescu, Daniela Hanganu, Catalina Bogdan, Laurian Vlase, Ilioara Oniga, and Daniela Benedec. 2019. "A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction" Biomolecules 9, no. 10: 529. https://doi.org/10.3390/biom9100529
APA StyleMoldovan, M. L., Iurian, S., Puscas, C., Silaghi-Dumitrescu, R., Hanganu, D., Bogdan, C., Vlase, L., Oniga, I., & Benedec, D. (2019). A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules, 9(10), 529. https://doi.org/10.3390/biom9100529










